Abstract
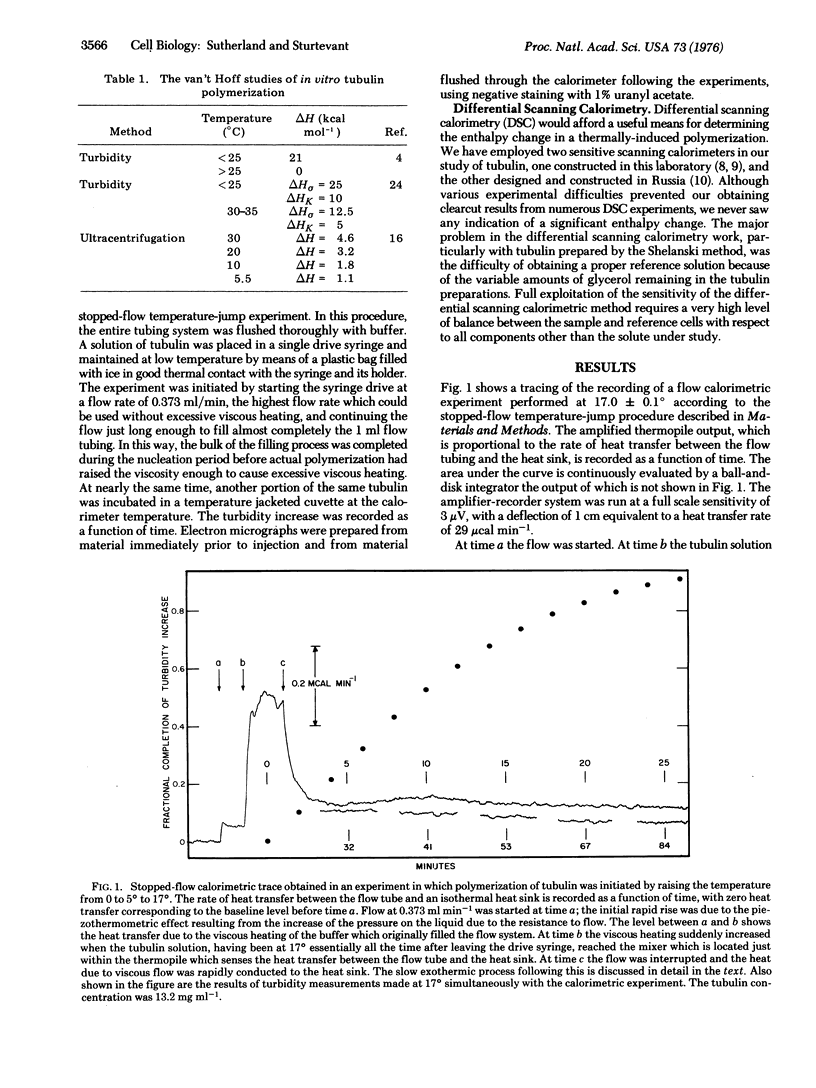
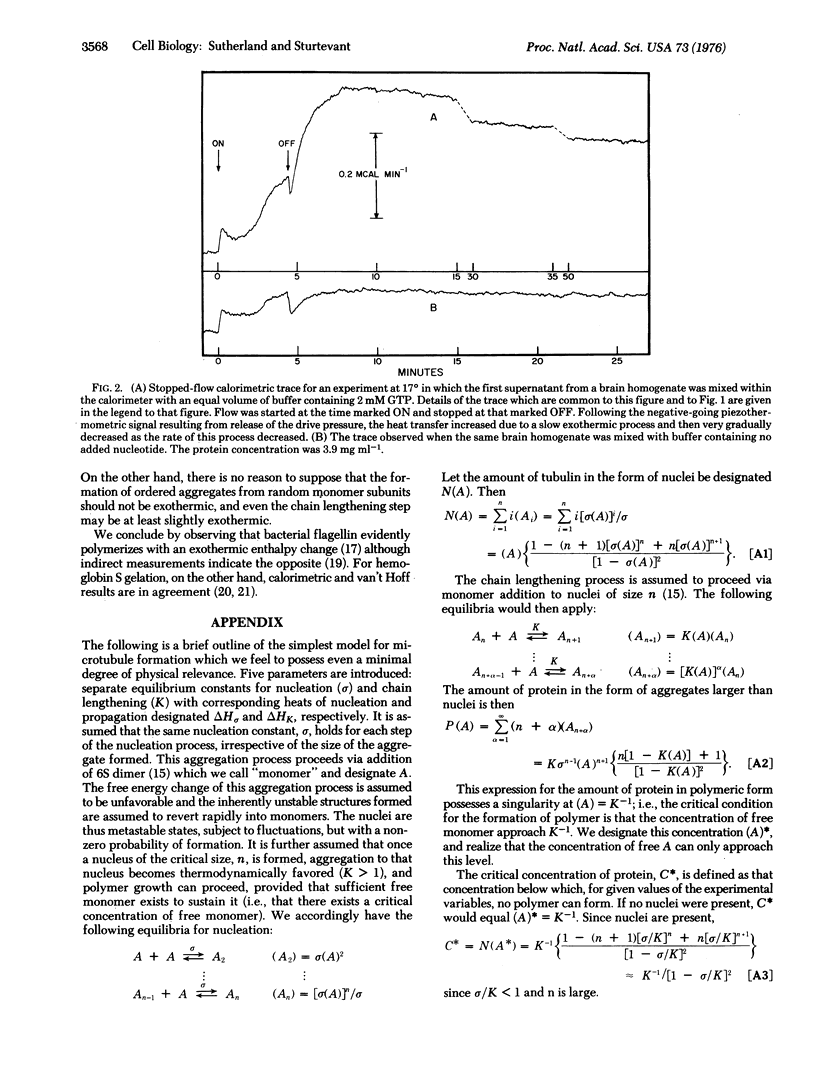
The enthalpy change for chain propagation in the polymerization of bovine tubulin has been studied directly by stopped-flow microcalorimetry at 17 degrees and 25 degrees, and found to be 0 +/- 1 kcal per mol of 6S tubulin dimer at both temperatures. Substantial heat evolution with a half-time of decay of approximately 1 hr was observed w-en tubulin was injected into the calorimeter. This heat was shown to result from contamination of the tublin by small amounts of some material from the crude brain homogenate from which the tubulin was prepared, and to be totally unconnected with microtubule assembly. Model calculations of nucleated polymerization processes reveal that the van't Hoff enthalpy calculated from the temperature dependence of the critical polymerization concentration is a complicated function of the separate enthalpy changes for nucleation and chain propagation. The published values of this quantity for tubulin probably pertain primarily to the nucleation process. It is shown that our observation of a propagation enthalpy change of vanishingly small size is not necessarily inconsistent with the reported van't Hoff enthalpies.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberty R. A. Standard Gibbs free energy, enthalpy, and entropy changes as a function of pH and pMg for several reactions involving adenosine phosphates. J Biol Chem. 1969 Jun 25;244(12):3290–3302. [PubMed] [Google Scholar]
- Dentler W. L., Granett S., Witman G. B., Rosenbaum J. L. Directionality of brain microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1974 May;71(5):1710–1714. doi: 10.1073/pnas.71.5.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelborghs Y., Heremans K. A., De Maeyer L. C., Hoebeke J. Effect of temperature and pressure on polymerisation equilibrium of neuronal microtubules. Nature. 1976 Feb 26;259(5545):686–689. doi: 10.1038/259686a0. [DOI] [PubMed] [Google Scholar]
- Frigon R. P., Timasheff S. N. Magnesium-induced self-association of calf brain tubulin. II. Thermodynamics. Biochemistry. 1975 Oct 21;14(21):4567–4573. doi: 10.1021/bi00692a002. [DOI] [PubMed] [Google Scholar]
- Gaskin F., Cantor C. R., Shelanski M. L. Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol. 1974 Nov 15;89(4):737–755. doi: 10.1016/0022-2836(74)90048-5. [DOI] [PubMed] [Google Scholar]
- Gerber B. R., Asakura S., Oosawa F. Effect of temperature on the in vitro assembly of bacterial flagella. J Mol Biol. 1973 Mar 15;74(4):467–487. doi: 10.1016/0022-2836(73)90040-5. [DOI] [PubMed] [Google Scholar]
- Gerlt J. A., Westheimer F. H., Sturtevant J. M. The enthalpies of hydrolysis of acyclic, monocyclic, and glycoside cyclic phosphate diesters. J Biol Chem. 1975 Jul 10;250(13):5059–5067. [PubMed] [Google Scholar]
- Jacobs M., Smith H., Taylor E. W. Tublin: nucleotide binding and enzymic activity. J Mol Biol. 1974 Nov 5;89(3):455–468. doi: 10.1016/0022-2836(74)90475-6. [DOI] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Magdoff-Fairchild B., Poillon W. N., Li T., Bertles J. F. Thermodynamic studies of polymerization of deoxygenated sickle cell hemoglobin. Proc Natl Acad Sci U S A. 1976 Apr;73(4):990–994. doi: 10.1073/pnas.73.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Borisy G. G. Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2696–2700. doi: 10.1073/pnas.72.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Characterization of microtubule assembly in porcine brain extracts by viscometry. Biochemistry. 1973 Oct 9;12(21):4282–4289. doi: 10.1021/bi00745a037. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Marcum J. M., Johnson K. A., Allen C., Borisy G. G. Microtuble assembly: some possible regulatory mechanisms. J Supramol Struct. 1974;2(2-4):429–450. doi: 10.1002/jss.400020230. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Hofrichter J., Eaton W. A. Calorimetric and optical characterization of sickle cell hemoglobin gelation. J Mol Biol. 1975 Aug 5;96(2):239–253. doi: 10.1016/0022-2836(75)90345-9. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong T. Y., Hearn R. P., Wrathall D. P., Sturtevant J. M. A calorimetric study of thermally induced conformational transitions of ribonuclease A and certain of its derivatives. Biochemistry. 1970 Jun 23;9(13):2666–2677. doi: 10.1021/bi00815a015. [DOI] [PubMed] [Google Scholar]
- Valdes R., Ackers G. K. Calorimetric determination of polymerization enthalpy for bacterial flagellin (Salmonella). Biochem Biophys Res Commun. 1974 Oct 23;60(4):1403–1409. doi: 10.1016/0006-291x(74)90354-4. [DOI] [PubMed] [Google Scholar]


