Abstract
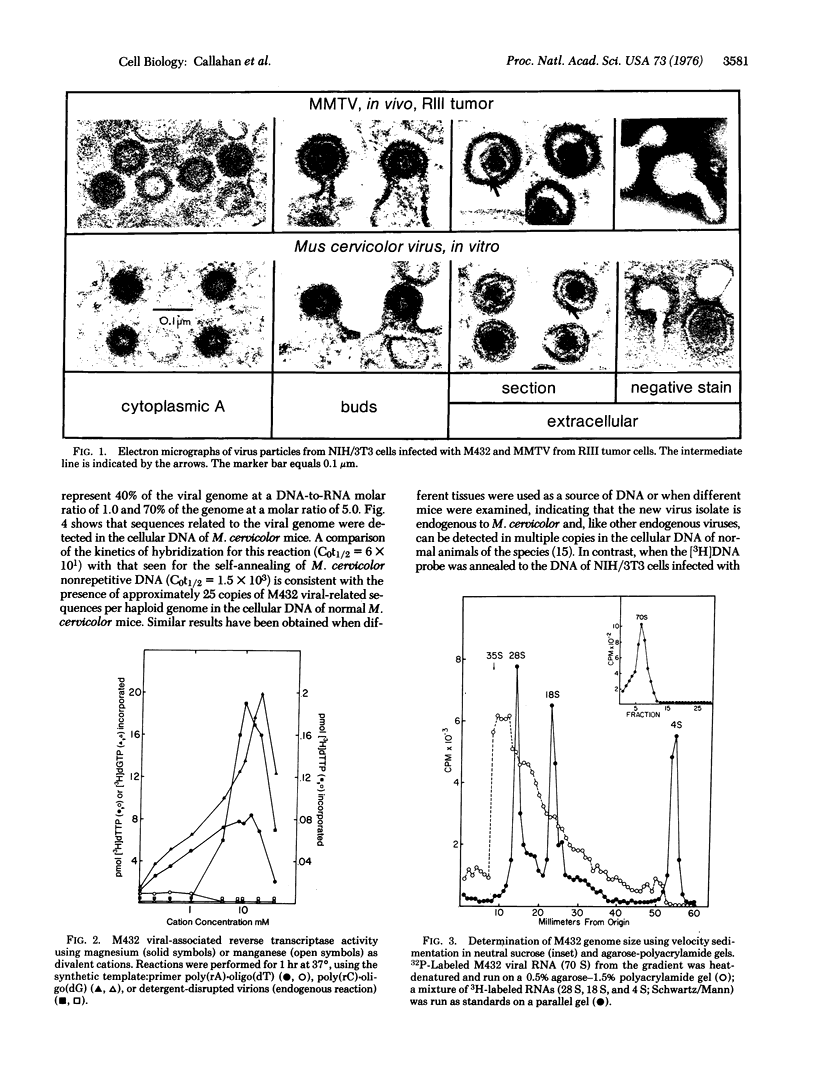
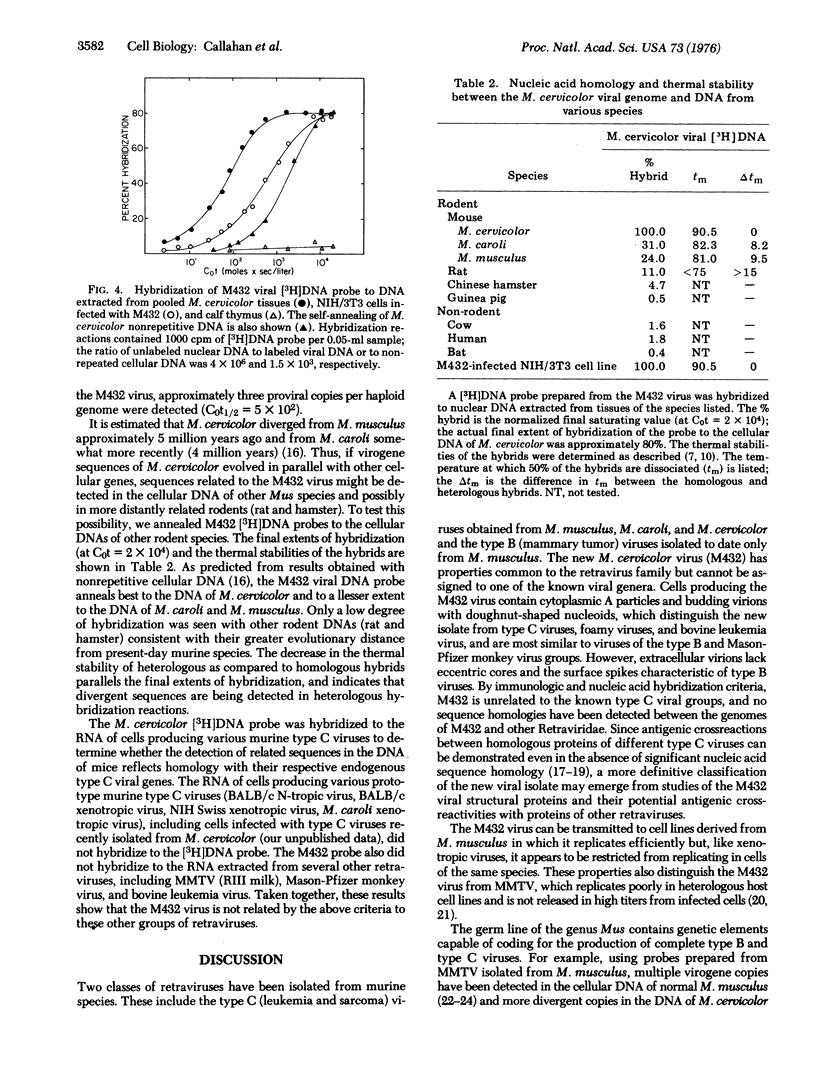
The cocultivation of spleen cells from the Southeast Asian mouse, Mus cervicolor, with heterologous cell lines has permitted the isolation of a new retravirus (designated M432) that can be transmitted to tissue culture cells of the laboratory mouse, M. musculus. Cells infected with M432 contain cytoplasmic type A particles and budding forms with compact,spherical nucleoids; extracellular virions lack surface spikes and have a condensed, central core surrounded by an intermediate line. Like other retraviruses, M432 bands isopycnically in sucrose at 1.16-1.17 g/cm3 and contains a 70S RNA genome composed of 35S subunits and an RNA-dependent DNA polymerase (RNA-dependent DNA nucleotidyltransferase). The viral reverse transcriptase requires magnesium as a cofactor and transcribes the synthetic template:primer poly(rC)-oligo(dG) more efficiently than poly(rA)-oligo(dT). [3H]DNA transcripts of the viral RNA genome detect multiple copies of endogenous virogene sequences in the cellular DNA of normal M. cervicolor, and fewer copies in heterologous cells infected with M432. Partially related nucleic acid sequences are also detected in the DNA of M. caroli and M. musculus as well as in more distantly related species (rat and hamster), reflecting the evolutionary conservation of these gene sequences in rodents. Although the virus from M. cervicolor shares certain morphologic and biochemical properties with murine type B viruses, the new isolate is unrelated by nucleic acid hybridization criteria to the mouse mammary tumor virus, the bovine leukemia virus, the Mason-Pfizer monkey virus, or known murine type C viruses, including endogenous type C viruses isolated from M. cervicolor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basch R. S. An improved method for counting tritium and carbon-14 in acrylamide gels. Anal Biochem. 1968 Oct 10;26(1):184–188. doi: 10.1016/0003-2697(68)90044-4. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Heinemann R., Wilson G. L., Callahan R., Todaro G. J. Detection of baboon type C viral sequences in various primate tissues by molecular hybridization. J Virol. 1974 Jul;14(1):56–67. doi: 10.1128/jvi.14.1.56-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Lieber M. M., Todaro G. J. A distinct class of inducible murine type-C viruses that replicates in the rabbit SIRC cell line. Proc Natl Acad Sci U S A. 1974 Mar;71(3):602–606. doi: 10.1073/pnas.71.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Sherr C. J., Todaro G. J. Evolution of type C viral genes: origin of feline leukemia virus. Science. 1975 Nov 28;190(4217):886–888. doi: 10.1126/science.52892. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: I. Nucleic acid from baboon type C virus as a measure of divergence among primate species. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4513–4518. doi: 10.1073/pnas.71.11.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: evidence for an Asian origin of man. Nature. 1976 May 13;261(5556):101–108. doi: 10.1038/261101a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Multiple divergent copies of endogenous C-type virogenes in mammalian cells. Nature. 1974 Nov 8;252(5479):170–173. doi: 10.1038/252170a0. [DOI] [PubMed] [Google Scholar]
- Bittner J. J. SOME POSSIBLE EFFECTS OF NURSING ON THE MAMMARY GLAND TUMOR INCIDENCE IN MICE. Science. 1936 Aug 14;84(2172):162–162. doi: 10.1126/science.84.2172.162. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Dalton A. J., Melnick J. L., Bauer H., Beaudreau G., Bentvelzen P., Bolognesi D., Gallo R., Graffi A., Haguenau F., Heston W. The case for a family of reverse transcriptase viruses: Retraviridae. Intervirology. 1974;4(4):201–206. doi: 10.1159/000149963. [DOI] [PubMed] [Google Scholar]
- Hall W. T., Schidlovsky G. Typical type-C virus in human leukemia. J Natl Cancer Inst. 1976 Mar;56(3):639–642. doi: 10.1093/jnci/56.3.639. [DOI] [PubMed] [Google Scholar]
- Lasfargues E. Y., Lasfargues J. C., Dion A. S., Greene A. E., Moore D. H. Experimental infection of a cat kidney cell line with the mouse mammary tumor virus. Cancer Res. 1976 Jan;36(1):67–72. [PubMed] [Google Scholar]
- Lieber M. M., Sherr C. J., Todaro G. J., Benveniste R. E., Callahan R., Coon H. G. Isolation from the asian mouse Mus caroli of an endogenous type C virus related to infectious primate type C viruses. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2315–2319. doi: 10.1073/pnas.72.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M. M., Sherr C. J., Todaro G. J. S-tropic murine type-C viruses: frequency of isolation from continuous cell lines, leukemia virus preparations and normal spleens. Int J Cancer. 1974 May 15;13(5):587–598. doi: 10.1002/ijc.2910130503. [DOI] [PubMed] [Google Scholar]
- Michalides R., Schlom J., Dahlberg J., Perk K. Biochemical properties of the bromodeoxyuridine-induced guinea pig virus. J Virol. 1975 Oct;16(4):1039–1050. doi: 10.1128/jvi.16.4.1039-1050.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Schlom J. Relationship in nucleic acid sequences between mouse mammary tumor virus variants. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4635–4639. doi: 10.1073/pnas.72.11.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Scholom J., Pearson J., Perk K., Dahlberg J. Characterization of the oncornavirus particles in the plasma of guinea pigs with L2C leukemia. J Virol. 1976 Jun;18(3):1120–1130. doi: 10.1128/jvi.18.3.1120-1130.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opler S. R. Observations on a new virus associated with guinea pig leukemia: preliminary note. J Natl Cancer Inst. 1967 May;38(5):797–800. [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Nowinski R. C., Reznikoff C. A., Heidelberger C. Endogenous oncornaviruses in chemically induced transformation. I. Transformation independent of virus production. Virology. 1975 Jun;65(2):392–409. doi: 10.1016/0042-6822(75)90045-8. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Straus N. A. Relatedness of mouse satellite deoxyribonucleic acid to deoxyribonucleic acid of various Mus species. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3546–3550. doi: 10.1073/pnas.70.12.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Blair P. B., Bishop J. M., Varmus H. E. Nucleotide sequence homologies among mouse mammary tumor viruses. Virology. 1976 Apr;70(2):550–553. doi: 10.1016/0042-6822(76)90297-x. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Benveniste R. E., Todaro G. J. Interspecies antigenic determinants of the reverse transcriptases and p30 proteins of mammalian type C viruses. J Virol. 1975 Jun;15(6):1440–1448. doi: 10.1128/jvi.15.6.1440-1448.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Lieber M. M., Benveniste R. E., Sherr C. J. Infectious primate type C viruses: Three isolates belonging to a new subgroup from the brains of normal gibbons. Virology. 1975 Oct;67(2):335–343. doi: 10.1016/0042-6822(75)90435-3. [DOI] [PubMed] [Google Scholar]
- Vaidya A. B., Lasfargues E. Y., Heubel G., Lasfargues J. C., Moore D. H. Murine mammary tumor virus: characterization of infection of nonmurine cells. J Virol. 1976 Jun;18(3):911–917. doi: 10.1128/jvi.18.3.911-917.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Bishop J. M., Nowinski R. C., Sarker N. H. Mammary tumour virus specific nucleotide sequences in mouse DNA. Nat New Biol. 1972 Aug 9;238(84):189–191. doi: 10.1038/newbio238189a0. [DOI] [PubMed] [Google Scholar]



