Abstract
Mesorhizobium loti MAFF303099 has a functional type III secretion system (T3SS) that is involved in the determination of nodulation competitiveness on Lotus. The M. loti T3SS cluster contains gene y4yS (mlr8765) that codes for a protein of unknown function (Y4yS). A mutation in the y4yS gene favors the M. loti symbiotic competitive ability on Lotus tenuis cv. Esmeralda and affects negatively the secretion of proteins through T3SS. Here we localize Y4yS in the bacterial membrane using a translational reporter peptide fusion. In silico analysis indicated that this protein presents a tetratricopeptide repeat (TPR) domain, a signal peptide and a canonical lipobox LGCC in the N-terminal sequence. These features that are shared with proteins required for the formation of the secretin complex in type IV secretion systems and in the Tad system, together with its localization, suggest that the y4yS-encoded protein is required for the formation of the M. loti T3SS secretin (RhcC2) complex. Remarkably, analysis of RhcC2 in the wild-type and M. loti y4yS mutant strains indicated that the absence of Y4yS affects negatively the accumulation of normal levels of RhcC2 in the membrane.
Keywords: symbiosis, rhizobium, rhizobia, secretion system, secretin, pilotin
Introduction
Type III secretion systems (T3SSs) are present in several pathogenic bacteria (Viprey et al., 1998; Cornelis, 2002). The T3SS apparatus is a multiprotein complex that delivers effector proteins into the host cell and participates in virulence determination (Galán, 2001; Cornelis, 2002; Alfano and Collmer, 2004). Several of the core protein constituents of the complex are secreted into the bacterial envelope via the universal sec-dependent secretion pathway (Francis, 2010). Type III secretion systems also present T3SS-dependent extracellular appendages that link bacteria to their hosts (Saad et al., 2008). In animal pathogens these appendages are called needle structures. When the needle comes into contact with a host cell, synthesis of a translocation pore composed of different bacterial proteins (termed translocators) occurs in the host plasma membrane (Saad et al., 2008). T3SSs are also present in some rhizobia species (Krause et al., 2002; Marie et al., 2004; Sánchez et al., 2009). Flavonoids and NodD induce the expression of rhizobial T3SS components and effectors since the gene encoding the transcriptional factor TtsI contains a nod box consensus sequence in its promoter region (Krause et al., 2002; Marie et al., 2004). TtsI binds to tts boxes (TB motifs) in the promoter regions of genes encoding T3SS components, inducing their transcription (Wassem et al., 2008). Mesorhizobium loti MAFF303099 has a functional T3SS (Sánchez et al., 2009; Okazaki et al., 2010). The T3SS gene cluster is part of the symbiotic island (Kaneko et al., 2000a,b). Regulation of the M. loti MAFF303099 T3SS is similar to that of other rhizobia; a nod box precedes its ttsI gene homolog (Figure 1) (Sánchez et al., 2009). The cluster of T3SS genes of MAFF303099 also contains conserved TB motifs upstream of the orthologs of nopC (mlr8768), nopX (mll6337), and nopB (mlr8763) (Krause et al., 2002) (Figure 1). Several proteins secreted through the rhizobial T3SS have been shown to affect the nodulation process (Bartsev et al., 2004; Skorpil et al., 2005; Rodrigues et al., 2007; Dai et al., 2008; Kambara et al., 2009; Sánchez et al., 2012). Evidence for T3SS effector translocation to the plant host cell cytoplasm has been observed in the case of several proteins (Schechter et al., 2010; Wenzel et al., 2010; Kimbrel et al., 2013). However, translocation during rhizobial nodulation has been observed only for Sinorhizobium fredii USDA257 NopP and Bradyrhizobium japonicum NopE1/NopE2 (Schechter et al., 2010; Wenzel et al., 2010). Depending on the nodulated legume, a mutation affecting M. loti T3SS functionality can alter its nodulation competitiveness (Sánchez et al., 2012). Genes that code for proteins secreted by this system in M. loti and with functionality in nodulation competitiveness (mlr6316, mlr6331, mlr6361, and mlr6358) were localized in the symbiotic island, outside of the T3SS cluster (Hubber et al., 2004; Sánchez et al., 2009, 2012).
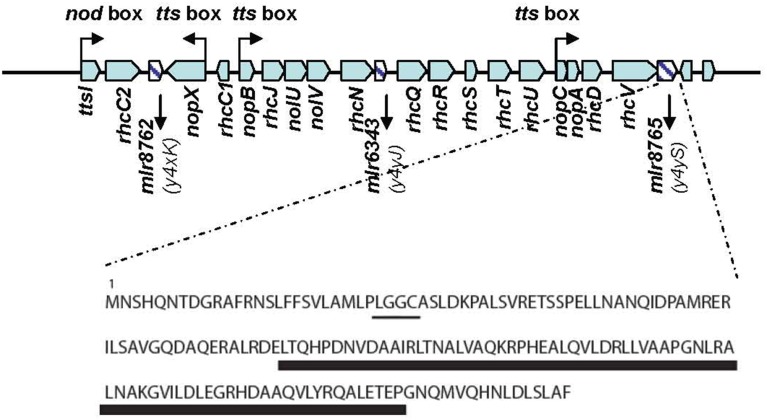
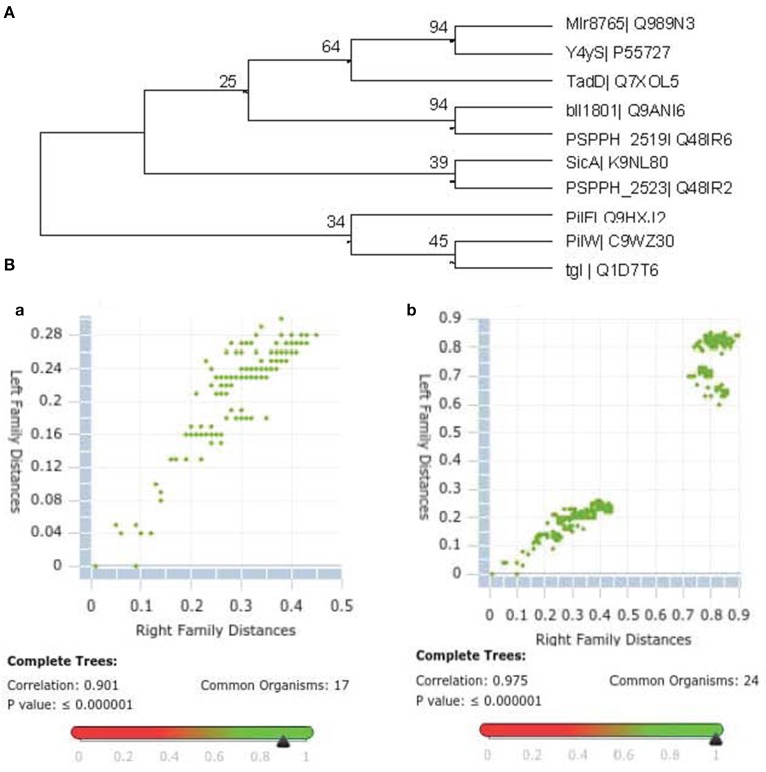
Figure 1.
The T3SS locus of MAFF303099. Predicted cis-elements are shown. Three open reading frames (ORFs) corresponding to genes coding for unknown proteins are shown as hatched bars. Characteristic features of the protein coded by the y4yS gene are shown. The lipobox and the region containing the TPR domain are underlined by a thin and a wide line respectively.
The M. loti MAFF303099 T3SS cluster, which contains all the conserved genes required for the formation of the T3SS apparatus, also harbors an additional three genes, mlr8762, mlr6343, and mlr8765, to which no function has yet been demonstrated (Figure 1). mlr8762 codes for a putative lipoprotein with homology to a protein of Caulobacter crescentus involved in the assembly of the extracellular filament (CpaD) (Skerker and Shapiro, 2000; Tampakaki, 2014; Rhizobase data bank). mlr6343 codes for a protein similar to members of the T3SS SctO protein family with unknown function. mlr8765 is a homolog to the y4yS gene of Rhizobium sp. NGR234, B. japonicum USDA110, and S. fredii (Marie et al., 2001; Gazi et al., 2012). The M. loti y4yS (mlr8765) gene belongs to a cluster of open reading frames (ORFs) that present a tts box upstream the nopC gene (Figure 1). The gene codes for a small unknown protein (165 aa) with a tetratricopeptide repeat (TPR) domain. TPR domains are imperfect 34-amino acid repeats often arranged in tandem arrays (Edqvist et al., 2006) that are involved in protein-protein interactions and the assembly of multiprotein complexes (D'Andrea and Regan, 2003). TPR domains were described in several T3SS proteins such as chaperones, regulators and exceptionally in one T3SS effector. TPR domains are found in class II and class V T3SS chaperones. Class II T3SS chaperones are translocator-chaperones and class V T3SS chaperones are required for T3SS needle formation in pathogens (Sun et al., 2008; Francis, 2010). T3SS of rhizobia have pili instead of a needle (Saad et al., 2008; Abby and Rocha, 2012). NopX, NopA, and NopB have been described as components of rhizobial T3SS pili where NopX has been suggested to be the translocator protein in the system (Marie et al., 2001; Saad et al., 2008). No chaperone for T3SS effectors (named class I chaperones) or for pili components has been described for M. loti T3SS until now. The existence of tetratricopeptide-like repeats has also been reported in transcriptional regulators of T3SS such as HilA from Salmonella enterica and HrpB from Ralstonia solanacearum (Pallen et al., 2003). Also a T3SS effector of Xanthomonas (PthA) was found to have a TPR domain (Murakami et al., 2010). It has also been reported that TPR proteins are involved in the functionality of other secretion systems, including pilotins and some accessory proteins of type IV secretion systems (T4SS) (Korotkov et al., 2011; Koo et al., 2012). Pilotins are small membrane lipoproteins required for the localization and/or stability of the secretin complex formed at the outer membrane (OM) in T2SS, T3SS, and T4SS (Koo et al., 2012). The secretin complex is a homo-multimeric complex that forms a gated channel in the OM, which opens to allow passage of proteins (Koo et al., 2012). Very much as every known OM protein, secretins are synthesized in the cytoplasm as precursors with N-terminal signal sequence, which is essential for translocation across inner membrane by the Sec system (Bos and Tommassen, 2004). Several integral OM proteins are targeted to and insert into this membrane through a cascade of interactions with periplasmic chaperones, with peripheral lipoproteins and with an integral OM lipoprotein complex called the BamA complex (Collin et al., 2011). However, the targeting to the OM of some secretins is independent of the BamA complex and only requires the binding to a specific pilotin (Collin et al., 2011). Pilotins have a type II N-terminal signal sequence followed by a conserved cysteine, which allows the protein to be lipidated and transferred from the inner membrane to the inner leaflet of the OM by the Lol system (Okuda and Tokuda, 2011). Then, some secretins transit to the OM together with pilotin and the corresponding Lol protein (LolA) (Collin et al., 2011). Some secretins are indeed lipoproteins that are directly transported by the Lol system without the requirement of pilotins (Viarre et al., 2009). As was mentioned earlier, pilotins and some accessory proteins were also described as required for secretin monomer and/or secretin complex stability (Koo et al., 2012). Putative TPR domains were also described in an inner membrane accessory protein for type IV pili secretin complex formation FimV (Wehbi et al., 2011). A TPR protein (TadD) appeared to be also required for the formation of the secretin complex in the Tad system of Aggregatibacter actinomycetemcomitans (Clock et al., 2008). T3SSs have a secretin complex at the OM and require pilotins for their formation. However, no T3SS pilotin or accessory protein has been described to have TPR domains (Koo et al., 2012).
The aim of the present work was to determine the function of the protein encoded by the M. loti y4yS gene.
Materials and methods
Bacterial strains and growth media
The bacterial strains and plasmids used in this study are listed in Supplemental Table 1. Escherichia coli strains were grown at 37°C in Luria-Bertani media. MAFF303099 strains were grown at 28°C in AB minimal medium (Douglas et al., 1985) supplemented with sucrose (0.5% wt/vol). When necessary, antibiotics were added to the following final concentrations: gentamicin (Gm) at 30 μg/ml, ampicillin (Amp) at 100 μg/ml, neomycin (Nm) at 100 μg/ml, and tetracycline (Tc) at 10 μg/ml for E. coli or 1 μg/ml for M. loti. For T3SS induction, naringenin was added to cultures at an OD 600 nm of 0.1, to a final concentration of 1 μM.
Generation of M. loti y4yS mutant and complementation
The oligonucleotide primers mlr8765UpF, mlr8765UpR, mlr8765DwF, and mlr8765DwR (Supplemental Table 1) were designed to amplify the flanking regions of mlr8765. The HindIII and BamH1 restriction endonuclease sites for the upstream flanking region and BamH1 and XbaI sites for the downstream flanking region were incorporated into the respective forward and reverse primers. The PCR products were ligated to the pGEMTeasy vector and the appropriate orientation for each was selected, resulting in the generation of pGEMUp8765 and pGEMDw8765. pGEMDw8765 was digested with BamHI plus SpeI and the insert was ligated into pGEMUp8765 digested with the same enzymes. Clones containing pGEMTeasy with the two inserts were selected (pGEMUpDw8765). A Gm cassette devoid of the transcriptional terminator sequence (Ugalde et al., 2000) was introduced using the created BamH1 site into plasmid pGEMUpDw8765, resulting in the generation of pGEMUpDw8765::Gm. Gm cassette orientation was selected in the mlr8765 gene orientation. The gene fragment containing the Gm cassette was cut out of the plasmid with HindIII and XbaI and ligated with pK18mobTc (Sánchez et al., 2009). The resulting plasmid (pK18mobTc-UpDwy4yS::Gm) was used to transform the E. coli S17 λ pir strain and then introduced by biparental conjugation into M. loti MAFF303099. Gm-resistant clones were isolated and double recombination events were selected by testing sensitivity to Nm and Tc. On this basis, the mutant named y4yS strain was selected. By means of PCR, we also confirmed that the mutant generated was the result of a double crossover event.
For mutant complementation, oligonucleotide primers mlr8765UpComp and mlr8765DwComp (Supplemental Table 1) were designed to amplify the entire mlr8765 gene. HindIII and BamH1 restriction endonuclease sites were incorporated into the forward and reverse primers respectively. The amplified fragment was cloned into plasmid pBBR1MCS-4 under the lac promoter activity (constitutive in rhizobium), and then introduced by triparental conjugation into the y4yS strain.
Plasmid pMP2112 was introduced by triparental conjugation into the MAFF303099 and y4yS strains.
Construction of 3xFlag translational fusions
The mlr8765 gene was amplified with oligonucleotide primers mlr8765FlagUp and mlr8765-FlagDw. The BamH1 and NcoI restriction endonuclease sites were incorporated into the forward and reverse primers respectively. pBAD-y4yS-1 was constructed by cloning the amplified gene sequence into vector pBAD 3x FLAG (Supplemental Table 1). The amplified fragment was sequenced to eliminate any possible alteration. The fragment containing the fusion to the 3x FLAG sequence was cut with restriction enzymes BamH1 and HindIII, and then cloned into pBBR1MCS-4 in the orientation of the lac promoter. The resulting plasmid (Supplemental Table 1) was transferred to y4yS pMP2112 by triparental mating.
Oligonucleotide primers mlr8765Up and mlr8765-FlagDw were used to integrate the y4yS-FLAG fusion into the chromosome. The amplified fragment in this case did not contain the N-terminal gene sequence. This eliminates the expression of y4yS that has not been fused to Flag. pBAD-y4yS-2 was constructed by cloning the amplified gene sequence into the vector pBAD 3x FLAG (Supplemental Table 1). The amplified fragment was sequenced to eliminate any possible alteration. The fragment containing the fusion to the 3x FLAG sequence was cut with restriction enzymes BamH1 and HindIII, and then cloned into pK18mobTc (Sánchez et al., 2009). The plasmid was introduced by biparental mating into the MAFF303099 pMP2112 strain. The single homologous recombination event was selected by searching Tc resistant strains and confirmed by PCR using oligonucleotides complementary to the vector sequence.
For chromosomal integration of the mlr6335-FLAG fusion, the C-terminal fragment of the gene was amplified with oligonucleotide primers mlr6335-FlagUp and mlr6335-FlagDw. The same procedure described above for integration of the mlr8765 fusion was carried out. The only difference was that the fragment containing the fusion to the 3xFlag sequence was cloned into pK18mob (Schäfer et al., 1994) and then a Tc cassette was introduced into the HindIII restriction site in the new plasmid. This allowed the expression of the gene downstream mlr6335 (mlr8762 gene) under the lac promoter activity of pK18mob. The resulting plasmid was introduced by biparental mating both into the MAFF303099 pMP2112 and y4yS pMP2112 strains. The single homologous recombination event was selected as described above.
Cell fractionation
Bacterial protein extraction involved centrifuging 1 ml of the bacterial cultures and resuspending the resulting pellets in SDS-PAGE sample buffer (50 mM Tris-HCl pH 6.8, 2% SDS, 0.1% Bromophenol Blue, and 10% glycerol) with the addition of 2% β-MSH and 0.8 M Urea. Bacterial membranes were isolated by cellular lysis using osmotic shock. Briefly, cells were harvested by centrifugation. Bacterial pellet was resuspended in lysis buffer (50 mM Tris-HCl pH 8.0, 5 mM EDTA, 12% Sucrose, 2 mM of phenylmethylsulfonyl fluoride, 0.02 mg/ml of lisozyme, and protease inhibitor cocktail from Sigma). After overnight incubation at 4°C, eight volumes of cold water were vigorously added to the suspension. After the addition of 10 μg/ml of DNAse and MgCl2 (final concentration 10 mM), the suspension was incubated 1 h at 4°C and then centrifugated at 3000 × g. Supernatant was subjected to ultracentrifugation by 4 h at 164,000 × g. The soluble fraction containing cytoplasmic and, presumably, periplasmic proteins, was precipitated with 10% of TCA. The pellet comprising bacterial membranes was resuspended in 3% ZW-3-14 with 250 mM NaCl to increase membrane proteins solubilization (Guilvout et al., 2006) and incubated at room temperature for 1 h. Then phenol treatment was made to dissociate possible multimers as was previously described (Guilvout et al., 2006). Outer and inner membranes were fractionated by differential detergent solubilization of total membranes as previously described (Koster et al., 1997) using 2% Sarkosyl. The Sarkosyl-soluble fraction contained the inner membrane proteins, whereas the pellet contained the OM proteins. Pellets were resuspended in SDS-PAGE sample buffer and heated at 65°C or at 100°C for 5 min. In the latter, β-MSH and Urea were added to the cracking buffer.
Inner and outer membrane protein-containing fractions were separated also by equilibrium density gradient centrifugation according Osborn et al. (1972). Fraction aliquots were analyzed to determine protein content (Bio-Rad protein assay) and NADH oxidase activity (as described by Osborn et al., 1972). For immunoblot analysis, equivalent volumes of each fraction were precipitated with 10% TCA and heated in SDS-PAGE sample buffer at 100°C.
Isolation of extracellular proteins
Supernatant protein extractions were carried out by direct trichloroacetic acid precipitation as previously described (Sánchez et al., 2009).
Analysis of proteins by gel electrophoresis
Proteins were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then stained using silver nitrate. For immunoblotting, the anti-NGR234 strain NopA, the anti-NGR234 strain NopX (Marie et al., 2004), the anti-Brucella Omp19 or a commercially available anti-FLAG M2 monoclonal antibody (Sigma) were used. SuperSignal West Femto reagent (Thermo Scientific) was used as a substrate for horseradish peroxidase to detect the proteins encoded by the chromosome-integrated translational fusions (Y4yS-3xFLAG and RhcC2-3xFLAG). When indicated, detection of mouse anti-FLAG and rabbit anti-Omp19 antibodies was made with fluorescent antibodies anti-mouse and anti-rabbit and subsequent revealing analysis in the Li-Cor, Odyssey equipment.
Competitive analysis
For competitive analysis, the indicated strains were mixed together in equal amount and used to inoculate Lotus plants as previously described (D'Antuono et al., 2005). The proportion of nodules occupied by each strain was determined as previously described (Sánchez et al., 2009). The strain that occupies the higher proportion of nodules is the strain that presents higher competitiveness. Statistical analyses were carried out by ANOVA and the Chi-square test.
Bioinformatic analysis
The amino acid sequences of TPR proteins were aligned using MUSCLE v(3.8.31) (Edgar, 2004). Phylogenetic trees were recovered using the maximum likelihood optimality criterion and the JTT matrix-based model (Jones et al., 1992). A bootstrap consensus tree inferred from 1000 replicates was taken to represent the evolutionary history of the taxa analyzed (Felsenstein, 1985). Branches corresponding to partitions reproduced in less than 45% bootstrap replicates were collapsed. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) was shown next to the branches (Felsenstein, 1985). Initial trees for the heuristic search were obtained automatically by applying the Neighbor-Joining and BioNJ algorithms to a matrix of pairwise distances estimated using a JTT model, and then selecting the topology with superior log likelihood value. All positions containing gaps and missing data were eliminated. The final dataset had a total of 106 positions. Evolutionary analyses were conducted in MEGA6 (Tamura et al., 2013).
The mirror tree online server (Ochoa and Pazos, 2010) was used to assess the co-evolutionary relationship between M.loti Mlr8765 and Mlr6335. Two homologous groups were created for each reference protein, the first one containing high scoring BLAST hits retrieved from NCBI NR database with a coverage >60% (see Supplementary text 1 and Supplementary text 2), and the second one, containing the first group plus seven distant elements of TPR secretins/pilotins pairs that are known to interact as a part of the secretion system (see Supplementary text 3 and Supplementary text 4). The four groups were aligned using ClustalX v2.1, with the standard settings and submitted to the web server where phylogenetic trees are obtained from these alignments with the neighbor-joining (NJ) algorithm implemented in ClustalW (Chenna et al., 2003) using bootstrap (100 repeats) and excluding gaps for the calculation. The distance matrices are obtained by summing the branch lengths that separate each pair of proteins in the tree. Instead of calculating the complete matrices the tree similarity between the two families is calculated as the correlation between their distance matrices according to the standard equation (Pazos and Valencia, 2001):
Where n is the number of elements of the matrices, that is, n = (N2-N)/2 being N the number of common organisms, Ri are the elements of the first distance matrix (the distance among all the proteins in the first multiple sequence alignment), Si is the corresponding value for the second matrix and R and S, are the averages of Ri and Si respectively.
Results and discussion
The M. loti y4yS mutant strain exhibits the same nodulation phenotype as the T3SS mutant strain rhcN
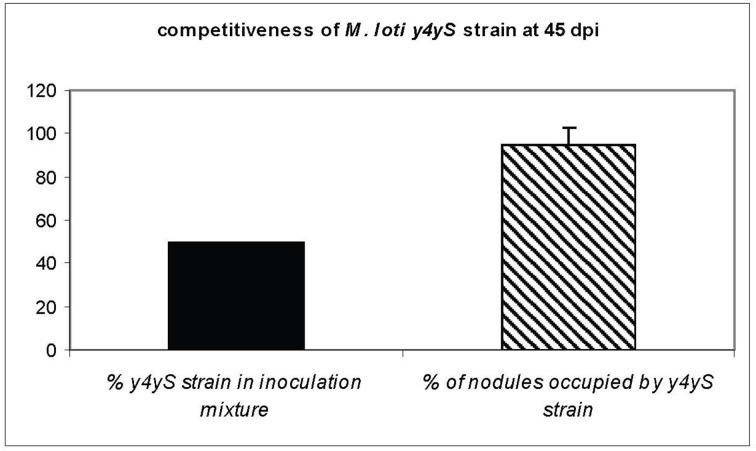
A MAFF303099 y4yS mutant was generated by the integration of a non-polar Gm-resistance cassette into the gene. Previously, we described that the M. loti rhcN mutant strain is more competitive than the wild-type strain in co-inoculation assays on Lotus tenuis cv. Esmeralda (Sánchez et al., 2012). M. loti RhcN protein shows homology to T3SS ATPases and is required for M. loti T3SS functionality (Sánchez et al., 2009). On the other hand, mutants in one, two or three of the genes coding for the putative effectors secreted by this system showed decreased competitiveness than the wild-type strain (Sánchez et al., 2009). Taking into account that this assay allows us to infer the effect of T3SS mutation on nodulation phenotype, we compared the competitiveness of the y4yS mutant strain with that of the wild-type strain on L. tenuis cv. Esmeralda. At 45 days post inoculation (dpi) with a rhizobial mixture (1:1) of the wild-type and the y4yS mutant strains, 95.5% of the nodules were occupied only by the mutant strain and the remaining 4.5% only by the wild-type strain. No mixed nodules were observed (Figure 2). The wild-type strain inoculated alone showed a normal nodulation phenotype (data not shown). The fact that the nodulation phenotype of the y4yS mutant on Lotus tenuis cv. Esmeralda resembles the phenotype observed when the mutation affects system functionality suggests that the protein codified by y4yS (hereafter Y4yS) is also required for M. loti T3SS functionality. Analysis of the in vitro mutant growth rate showed no differences with the wild-type strain (data not shown).
Figure 2.
Competition assay on Lotus tenuis cv. Esmeralda. Plants were co-inoculated with an equal mixture of the wild-type and y4yS mutant strains. The percentage of nodules occupied by the y4yS strain at 45 days post-inoculation (dpi) is shown.
Type III secretion is abolished in the y4yS mutant strain
We have previously demonstrated M. loti T3SS functionality by analyzing protein secretion to the culture supernatant (Sánchez et al., 2009). Among the proteins secreted by rhizobial T3SS are pili components such as NopX and NopA (Saad et al., 2008; Sánchez et al., 2009). It has been described that while a mutation in NopX does not affect NopA secretion in Rhizobium sp. strain NGR234, a mutation in NopA abolishes NopX secretion (Deakin et al., 2005). To discriminate between Y4yS being the NopX chaperone (putative class II chaperone) or the NopA chaperone (putative class V chaperone case), we decided to determine the effect of a mutation in y4yS on the secretion of the proteins. As was previously described (Sánchez et al., 2009, 2012), all the strains used (here and thereafter) contain the plasmid pMP2112 (López-Lara et al., 1995), which constitutively expresses nodD of Rhizobium leguminosarum and allows the in vitro induction of M. loti T3SS with naringenin.
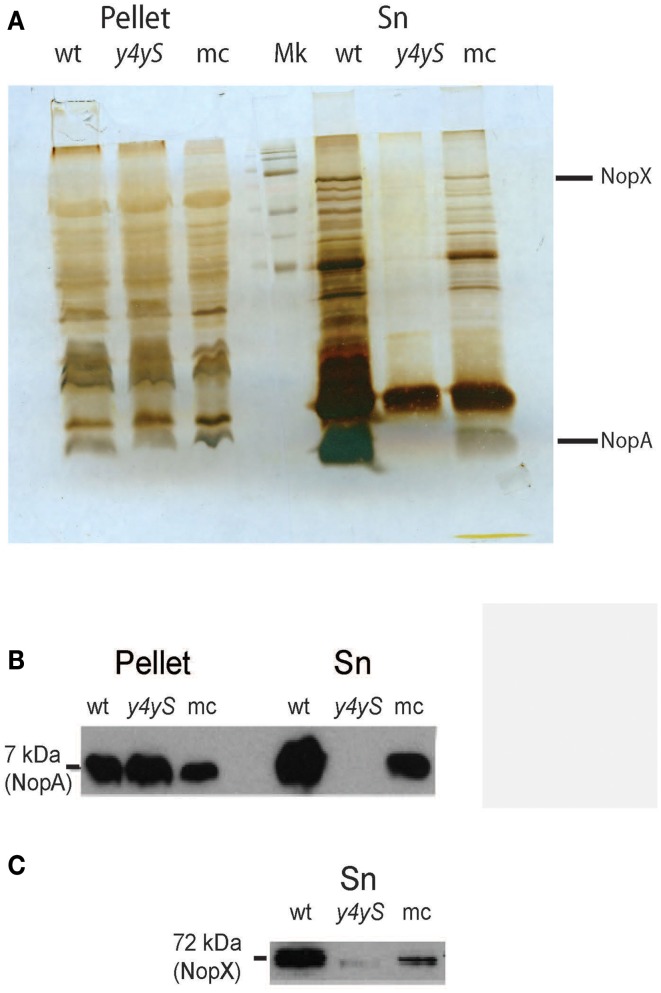
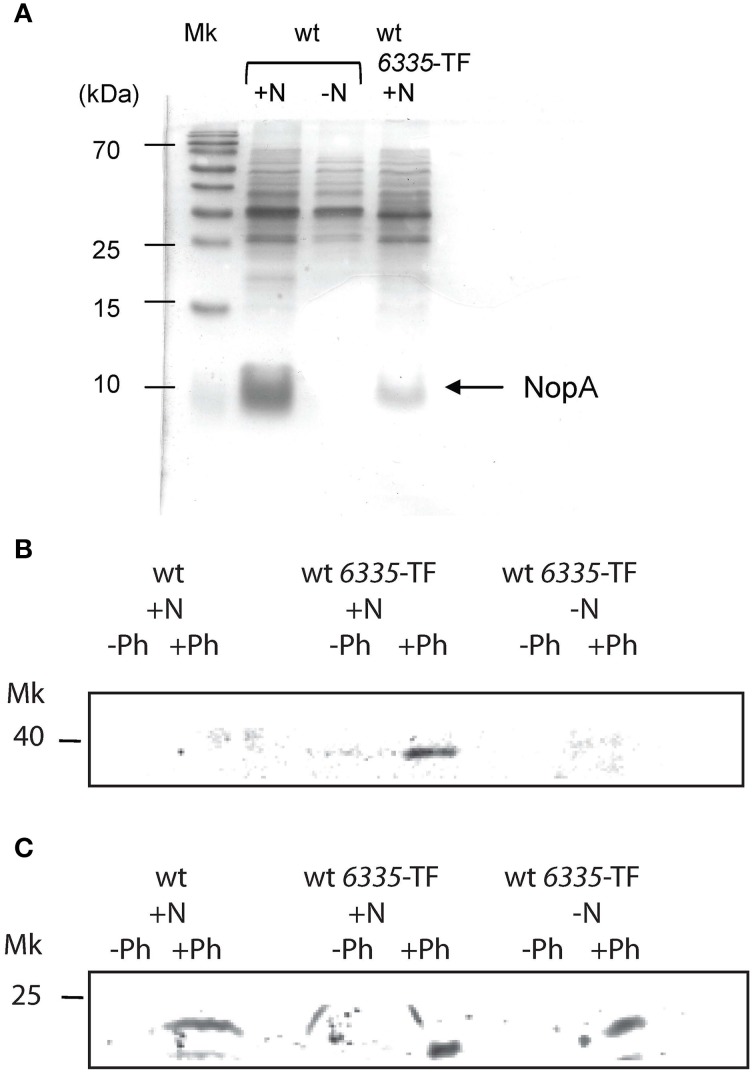
The silver stained gel showed that protein secretion to the culture supernatant in the T3SS inducing conditions was negatively affected in the y4yS mutant strain (Figure 3A). A Western blot analysis using anti-NopX and anti-NopA confirmed that the secretion of both NopX and NopA, proteins normally secreted by T3SS, was inhibited in the mutant strain (Figures 3B,C). The secretion defect was reversed by mutant complementation with a gene copy into a plasmid of moderate copy number (Figure 3). Therefore, we conclude from this experiment that Y4yS is not a NopX chaperone because not only NopX secretion was inhibited but also NopA secretion was negatively affected. We cannot exclude that Y4yS is the NopA chaperone.
Figure 3.
Analysis of T3SS secreted proteins in the wild-type, y4yS mutant, and y4yS mutant complemented strains. Supernatant (Sn) and intracellular proteins (pellet) were isolated from MAFF303099 (wt), its y4yS mutant (y4yS), and the mutant complemented with a plasmid of moderate copy number containing the full-length y4yS gene under the lac promoter (mc). Bacteria were grown in T3SS inducing conditions. All bacteria contain plasmid pMP2112. Proteins were separated by 15% SDS-PAGE, stained with silver nitrate (A) or transferred to membranes and probed with anti-NopA antibody (B) or anti-NopX antibody (C).
Very much as in the wild-type, NopA was detected in the mutant pellet. This indicates that the defect in protein secretion was not a consequence of a defect in NopA expression and discards a negative T3SS transcriptional regulator role for the protein coded by mlr8765. NopX could not be detected in wild-type or mutant pellets (data not shown). T3SS secreted proteins of some rhizobia could be detected in the culture supernatant but not in the bacterial pellet even in the case of mutants that are affected in secretion (Deakin et al., 2005; Krishnan et al., 2007). It was speculated that the accumulation of Nops inside the cell could be deleterious to the rhizobial cells and thus subjected to rapid degradation. This could be also true for NopX in M. loti.
Y4yS is localized in bacterial membranes
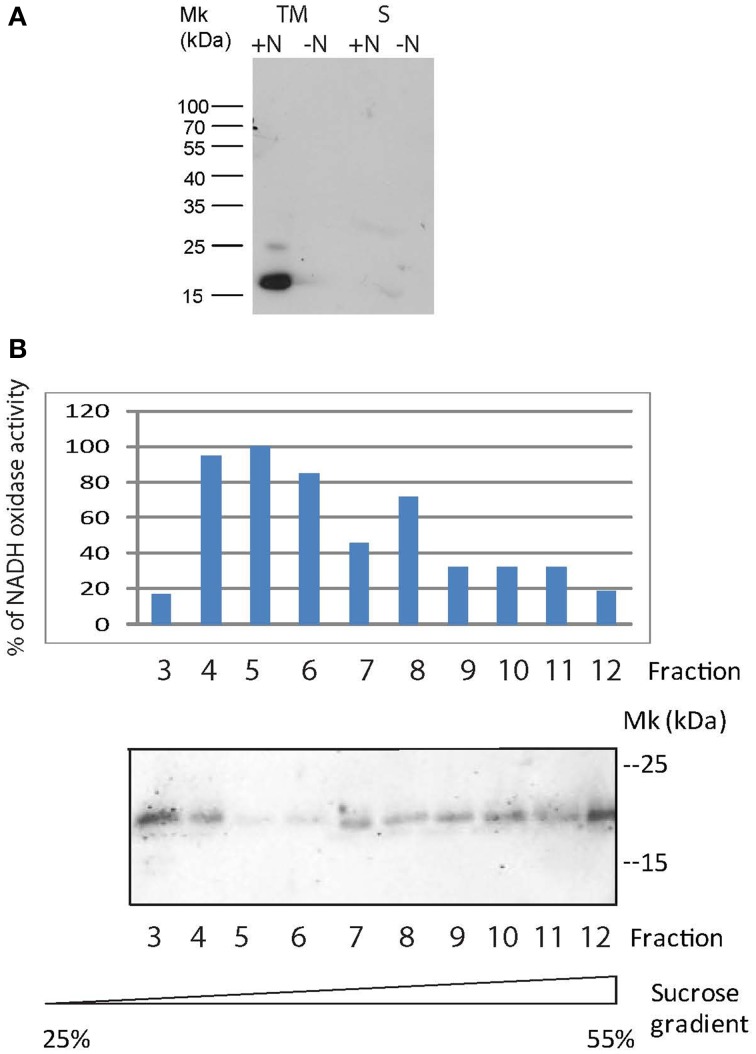
We determined the cellular localization of Y4yS. Previously, the protein fused to the triple (3x) copy of the FLAG peptide was introduced into the M. loti y4yS mutant strain, cloned into a plasmid of moderate copy number under a constitutively active promoter in rhizobia. A Western blot analysis of total bacterial extract showed the presence of a band between 15 and 25 kDa, in agreement to the theoretical molecular weight of the protein (16 kDa) (Supplementary Figure 1). Localization analysis detected the fused protein both in the membrane and cytoplasm fractions although higher levels were detected in membranes (data not shown). The possibility of over expression artifacts led us to integrate the tagged protein into the M. loti genome via a single recombination event in order to have only one copy of the fused construct in the cell. Once chromosome integrated, the fused proteins were expressed from the corresponding chromosomal promoter. Western blot analysis of the various cellular fractions showed that Y4yS was localized exclusively in bacterial membranes (Figure 4A).
Figure 4.
Expression and localization of the 3x FLAG Y4yS fused protein. (A) Total membranes (TM) and fractions corresponding to the cytoplasm and periplasm (S) of the MAFF303099 strain with sequence encoding the 3xFLAG Y4yS fused protein integrated into the bacterial chromosome. The two bacteria contain plasmid pMP2112. Proteins were separated by 10 % SDS-PAGE and then immuno-blotted and probed with an anti-FLAG antibody. Positions of size markers loaded onto the gels are labeled (in kDa). ±N indicate bacterial culture in the presence or absence of naringenin. (B) Subcellular localization of Y4yS-FLAG expressed from a pBBR1MCS-4 plasmid into an y4yS mutant strain was determined by sucrose density gradient centrifugation. Inner and outer membranes were fractionated as described in Materials and Methods. Fractions were collected as 1-ml aliquots from the top of a discontinuous sucrose gradient. Fractions enriched in the inner membranes were identified by monitoring NADH oxidase activity. Enzyme activity was expressed as percentage of maximal activity. Y4yS-FLAG was detected by immunobloting with anti-FLAG (from mouse) and fluorescent anti-mouse antibodies. Bacteria contain plasmid pMP2112.
Western blot results also indicate that Y4yS expression occurred in naringenin-induced culture, which is the condition of induction of M. loti T3SS expression (Sánchez et al., 2009) (Figure 4A). This confirms that the y4yS gene forms part of an operon of co-transcribed ORFs nopC-nopA-rhcD-rhcV-y4yS under a promoter region with a tts box localized upstream nopC (Tampakaki, 2014).
We then determined the inner or outer membrane localization of the Y4yS-FLAG protein. Attempts to detect the chromosome-encoded fusion protein were unsuccessful probably because of the low protein levels in the cell. We decided to make this determination with bacteria expressing the fused protein from the pBBR1MCS-4 plasmid. Inner and outer membranes were separated by density gradient centrifugation. Results showed that the Y4yS protein is localized both in outer and inner membranes (Figure 4B). Attempts to detect the Omp19 protein by Western blot (an OM porin used as OM marker) were unsuccessful probably because of sample dilution.
Y4yS presents sequence features of T4SS pilotins and TadD protein
Since T3SS chaperones are generally cytoplasmic proteins, Y4yS membrane localization argues against a chaperone role for this protein (Francis, 2010). However, this role cannot be completely excluded. Two class V chaperones for needle components in Escherichia coli, EscG and EscE, were described to be in the inner membrane (Sal-Man et al., 2013). Nevertheless, these proteins do not present TPR domains. TPR proteins have been described in T4SS of Pseudomonas (PilF), Yersinia (PilF) and Neisseria (PilW) and in the Tad system of A. actinomycetemcomitans (TadD), where they function as pilotins and docking proteins required for the formation of the secretin complex at the OM (Clock et al., 2008; Koo et al., 2012). These four TPR proteins are localized in bacterial membranes and, in addition to the TPR domain, they present a lipidation site at their N-terminus characterized by a specific consensus motif, the lipobox (V/L)XXC. This motif is characteristic of the processing site of lipoproteins (Wu and Tokunaga, 1986). In silico analysis of Y4yS indicates that the protein contains a predicted site for N-terminal cleavage by peptidase II in accordance with its membrane localization and that it presents the characteristic sequence for lipidation LGCC (Figure 1). δ-Blast homology searches to the Y4yS amino acid sequence showed homology, although poor, to PilW (E-value 5 × 10−4), PilF (E-value 4 × 10−4), and Aggregatibacter TadD (E-value 2 × 10−4). Since homology could be due to the presence of the TPR domain in these or other proteins, we decided to apply phylogenetic analysis to several bacterial TPR proteins that were described to be involved in secretion systems. This included TadD from A. actinomycetemcomitans (Clock et al., 2008), pilotins such as Pseudomonas PilF, Neisseria PilW, and Myxococcus tgl (Koo et al., 2012), T3SS chaperones with TPR domains such as LcrH, PcrH, IpgC, YscG, PscG, and SicA (Francis, 2010; Cerveny et al., 2013), and other unknown TPR proteins that are part of the secretion system gene cluster, the B. japonicum bll1801 gene and the Pseudomonas PSPPH2519 and PSPPH2523 genes (Gazi et al., 2012). The analysis also included the Mlr8765 ortholog in S. fredii. The protein encoded by M. loti mlr8765 was phylogenetically related to few of these proteins, and among them, the most closely related was the TadD protein from A. actinomycetemcomitans (Figure 5A). The protein alignment is shown in Supplementary Figure 2. It has been recently described that two genes code for proteins with homology to secretins in the Rhizobiales-T3SS family (also referred as Rhc-T3SS), which includes rhizobia and some strains of Pseudomonas syringae T3SS (Abby and Rocha, 2012). In M. loti MAFF3030999, the two genes are mlr6335 and mlr6338. mlr6335 codes for RhcC2, which shows homology with the secretins of the Tad (tight adherence) macromolecular transport system present in bacteria such as Caulobacter and Aggregatibacter (CpaC and RcpA respectively) (Abby and Rocha, 2012). mlr6338 codes for a protein that presents homology with the N-terminal part of T3SS secretins (Clock et al., 2008; Abby and Rocha, 2012). A phylogenetic analysis of the T3SS secretins together with secretins from T2SS, type IV pilus, Tad system and filamentous phages showed that rhizobial secretin RhcC2 groups together with secretins from the Tad loci (RcpA) (Abby and Rocha, 2012; Clock et al., 2008). The same study concludes that rhizobia originally had a non-flagellar-T3SS-like secretin, RhcC1, and secondarily acquired the secretin RhcC2 from a Tad locus through a partial homologous gene replacement (Abby and Rocha, 2012). In Aggregatibacter the tadD gene is downstream the tadC gene. M. loti has a cluster of Tad gene homologs (mlr5593 to mlr5604). M. loti Tad secretin coded by mlr5597 gene presents a 32% homology with M. loti T3SS secretin RhcC2. A gene localized downstream the M. loti tadC gene (mlr5604) and in opposite direction to the Tad cluster (mll5605) encodes an unknown protein with 32% homology with M. loti Y4yS. The above-described data raised the possibility that Y4yS, which shares sequence features and is evolutionarily related to TadD, might be a protein required for the complex formation of RhcC2 (evolutionarily related to the Tad secretin) in M. loti MAFF303099 T3SS. The Rhc-T3SS family is subdivided into three subgroups according to the organization of the T3SS core genes (Gazi et al., 2012). T3SS core genes of subgroup I, represented by Rhizobium sp. NGR234, B. japonicum USDA 110, S. fredii, and M. loti MAFF303099, are organized in three segments (Gazi et al., 2012; Tampakaki, 2014). The second fragment in members of this subgroup harbors the genes rhcD, rhcV and y4yS. Since members of Subgroup I have both RhcC2 and Y4yS, our hypothesis could be extended to the four above-mentioned strains. To analyze the existence of an evolutionary relationship between Y4yS to RhcC2, despite being coded in separate segments of the Rhc-T3SS cluster, a comparison between the phylogenetic trees of Y4yS homologs and the respective secretin RhcC2 homologs was made using the Mirror tree online server (Ochoa and Pazos, 2010). Two homologous groups were created for each reference protein as was described in Materials and Methods. Mlr8765 and Mlr6335 showed a high correlation coefficient in both pairs of homologous protein groups with a P-value <1e-6 (Figure 5B). Interestingly, the larger group, which included distant homologs, presented a higher correlation score that the smaller and more closed related group. This result suggests that the TPR secretin/pilotin or docking protein and the rhizobial T3SS secretin/Y4yS homolog proteins are coevolving and argues in favor of the existence of a physical interaction between RhcC2 and Y4yS proteins.
Figure 5.
(A) Phylogenetic analysis of bacterial TPR proteins involved in secretion systems. Bacterial TPR proteins described in the text were subjected to a phylogenetic analysis as indicated in Materials and Methods. A cladogram could be constructed only with the proteins indicated next to each branch arm. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test are shown next to the branches. (B) Inter-protein distances of two phylogenetic trees represented in a scatterplot showing the conserved correlation between the Left Family Distances corresponding to Mlr8765 homologs and the Right Family Distances corresponding to Mlr6335 homologs. (a) Smaller homolog protein set composed by high identity blast hits. (b) Larger homolog protein set that includes TPR secretins and their respective pilotins or docking proteins to the set of high identity blast hits.
The M. loti y4yS mutant strain presents lower RhcC2 protein levels in the bacterial membranes
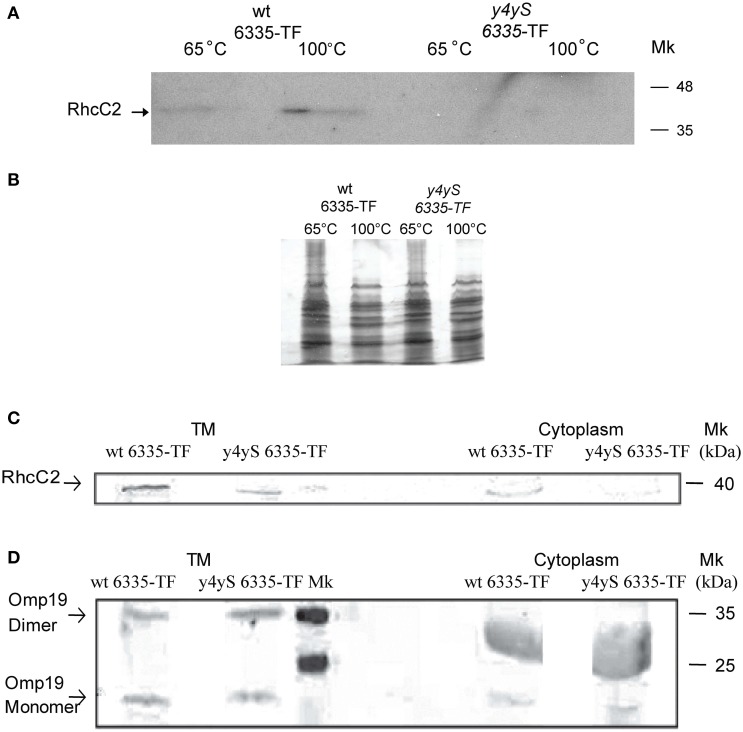
To analyze the involvement of Y4yS in the formation of the M. loti RhcC2 complex, we integrated the mlr6335 gene that codes for RhcC2 fused to the triple (3x) copy of the FLAG peptide into the chromosome of the M. loti MAFF303099 and y4yS mutant strains. Sequences coding for Flag-tagged proteins were integrated into the chromosome by a single homologous recombination event. Following chromosomal integration, the fused proteins were expressed from the corresponding chromosomal promoter. NopA secretion in inducing conditions was analyzed for the wild-type strain with the integrated fused protein to rule out the possibility of abolishing type III secretion due to the C-terminal modification of RhcC2 or by defects in the gene expressed downstream, mlr8762. Figure 6A shows that the isolated strain, although in lower levels than the wild-type strain, still secretes NopA. Western blot analysis using anti-FLAG antibody indicates that a protein of about 35–40 kDa (40 kDa was the expected molecular weight) was detected only in the total membrane fraction of the wild-type strain with the tagged RhcC2 induced with naringenin but not in the total membrane fraction of the wild type strain without tagging nor in the wild type strain with the tagged protein but without induction with naringenin (Figure 6B). The detection of RhcC2 in the total membrane fraction required phenol treatment. Results confirm that RhcC2 protein is expressed under naringenin induction. Consequently, its expression depends on the activity of the upstream promoter with the tts box. Detection using anti-Omp19 antibodies shows similar protein levels in the three preparations (Figure 6C). As the secretin complex has been described to have OM localization, we isolated the OM of the wild-type and mutant strain with the tagged RhcC2 protein. Since the secretin complex of the Tad system is resistant to detergent at 65°C but sensitive to boiling (Clock et al., 2008), we analyzed the presence of the RhcC2-3x-FLAG protein in OM of the wild-type and y4yS mutant strains by SDS-PAGE electrophoresis after resuspending and heating the samples at 65°C and 100°C in the SDS-PAGE sample buffer. The anti-FLAG antibody detected a monomer of about 40 kDa only in the wild-type OM (Figure 7A). Under silver staining the samples revealed a similar pattern and amount of proteins (Figure 7B). A slight increase in 40 kDa protein levels was observed when the sample was boiled in the SDS-PAGE sample buffer at 100°C instead of at 65°C (Figure 7A). Unfortunately, all attempts to detect the high molecular polymers corresponding to secretin oligomers were unsuccessful. In some systems, it is difficult to observe the secretin complex with Western blots due to problems of complex solubility and efficient transfer of high-molecular-weight species to nitrocellulose (Burghout et al., 2004; Clock et al., 2008). The absence of RhcC2 in mutant OM indicates that the mutation in Y4yS affects the production, stability or localization of RhcC2. The T3SS protein expression is not affected in the y4yS mutant so a deficiency in transcription is quite unlikely. In some T3SS systems, secretin is localized in the inner membrane in the absence of pilotin (Koster et al., 1997; Koo et al., 2008), whereas in the Tad system, no endogenous secretin is localized in the whole cell extract in the absence of pilotin and in physiological conditions (Clock et al., 2008). To address this problem we determined total RhcC2 protein levels in the cell. Figures 7C,D show the Western blot results on total membrane and cytoplasmic fractions, of wild type and y4yS mutant strains containing the tagged RhcC2 protein. Results indicate that the flagged RhcC2 protein is localized in membranes and that the y4yS mutant exhibits lower levels of this protein in total bacterial extract (membranes and cytoplasm), resembling the results observed in the Tad system (Clock et al., 2008). Omp19, the OM marker, can be detected as a monomer and/or a dimer (unpublished results). Figure 7D shows that total Omp19 taken as the sum of monomer and dimer is the same in the two samples that were compared.
Figure 6.
Detection of the 3xFLAG RhcC2 fused protein in total membrane fraction. (A) Silver staining of supernatant proteins of MAFF303099 (wt) and MAFF303099 with sequence encoding the 3xFLAG RhcC2 fused protein integrated into the bacterial chromosome (wt 6335-TF). Total membranes of wt and wt 6335-TF strains were separated by 12.5% SDS-PAGE and immune-bloted and probed with anti-FLAG antibody (from mouse) (B), and with anti-Omp19 antibody (from rabbit) (C). For detection fluorescent anti-mouse and anti-rabbit antibodies were used. All bacteria contain plasmid pMP2112. ± N: with or without induction with naringenin, ± Ph: with or without phenol treatment.
Figure 7.
Detection of the 3x FLAG RhcC2 fused protein. (A) OMs of wt 6335-TF and y4yS mutant strain with sequence encoding the 3xFLAG RhcC2 fused protein integrated into the bacterial chromosome (y4yS 6335-TF) heated at 65°C or 100°C were separated by 15% SDS-PAGE and then immuno-bloted and probed with an anti-FLAG antibody, (B) Silver staining of samples described in A separated by 7.5% SDS-PAGE. Total membranes (TM) and cytoplasmic fractions of wt 6335-TF and y4yS 6335-TF strains heated at 100°C, separated by 12.5% SDS-PAGE and then immuno-bloted and probed with anti-FLAG (C) and anti-Omp19 (D) antibodies and revealed with fluorescent antibodies. All bacteria contain plasmid pMP2112 and all bacterial cultures were made in the presence of naringenin. Positions of RhcC2, and of monomer and dimer of Omp19 are indicated. Positions of size markers loaded onto the gels are labeled (in kDa). Anti-Omp19 antibodies nonspecifically probe a great band both in wt and mutant cytoplasmic fractions between markers of 25 and 35 kDa.
Conclusion
In the present study we determined that a M. loti y4yS mutant strain shows higher competitiveness for nodulation on Lotus tenuis cv. Esmeralda than the wild type strain, as it was previously observed for a mutant affected in the T3SS functionality. The product encoded by y4yS is a membrane protein. Its absence affects secretion through T3SS. The inability of the y4yS mutant to secrete NopA and NopX proteins may be due to a role contributing to the structure or secretion regulation of T3SS. Secretion analyses alone could not determine if Y4yS is a pili chaperone, a secretion regulator or a protein involved in T3SS structure assembly.
A number of observations led us to examine the effect of the y4yS mutation on RhcC2 levels in the cell: (1) Y4yS shared characteristics with membrane proteins involved in secretin complex formation such as the lipobox sequence and the TPR domain, (2) Y4yS shared certain homology with the Tad docking protein, (3) Y4yS also showed closer evolutionary relationship with TadD than with class V chaperones and other TPR proteins, (4) the fact that Mesorhizobium loti secretin RhcC2 originated from the Tad locus, and (5) our discovery of a coevolutionary relationship between the TPR secretin/pilotin or docking protein and RhcC2/Y4yS proteins. We found that the absence of Y4yS negatively affects RhcC2 levels in the cell. Future analyses will determine if this results from an effect on production or stability of RhcC2. Y4yS was localized in OM (in addition to its inner membrane localization) and y4yS mutation affects RhcC2 levels in membranes. Since some secretin proteins require an OM lipoprotein (pilotin or docking protein) for stabilization or membrane insertion, we here propose that Y4yS may have this role for the RhcC2 secretin of M. loti and be a membrane protein relevant for the structure assembly of M. loti MAFF303099 T3SS. For the Tad system in Aggregatibacter, where secretin is not observed in a TadD mutant strain, the loss of stabilizing physical interactions between these two transport system components may account for the abundance defect observed.
Since T3SS pilotins have not been shown to harbor TPR domains, our results could represent the first report of a pilotin-like protein with TPR domains in T3SS complexes. M. loti secretin RhcC2 and Y4yS have homologs in Rhizobium sp. NGR234, B. japonicum USDA 110, and S. fredii. Thus, the present results may be extensive to these three strains of rhizobia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The project was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica of Argentina (ANPCyT) (PICT-2007-650, and PICT-2011-1212). We acknowledge Dr. William Deakin for anti-NGR234 NopA and NopX antibodies and Dr. Juliana Cassattaro for anti-Brucella Omp19.
Supplementary material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fpls.2015.00012/abstract
Intracellular (pellet) proteins were isolated from the y4yS mutant containing plasmid pBBR1MCS-4 with the 3xFLAG fused Y4yS protein expressed under the lac promoter (constitutive in rhizobia), Proteins were separated by 10% SDS-PAGE and then immuno-blotted and probed with an anti-FLAG antibody. Positions of size markers loaded onto the gels are labeled (in kDa). ± N indicate bacterial culture in the presence or absence of naringenin. Bacteria contain plasmid pMP2112.
Alignment between the aminoacid sequences of TPR proteins using MUSCLE v(3.8.31) (Edgar, 2004).
References
- Abby S. S., Rocha E. P. C. (2012). The non-flagellar type III secretion system evolved from the bacterial flagellum and diversified into host-cell adapted systems. PLoS Genet. 8:e1002983. 10.1371/journal.pgen.1002983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano J. R., Collmer A. (2004). Type III secretion system effector proteins: double agents I bacterial disease and plant defense. Annu. Rev. Phytopathol. 42, 385–414. 10.1146/annurev.phyto.42.040103.110731 [DOI] [PubMed] [Google Scholar]
- Bartsev A. V., Deakin W. J., Boukli N. M., McAlvin C. B., Stacey G., Malnoe P., et al. (2004). NopL, an effector protein of Rhizobium sp. NGR234, thwarts activation of plant defense reactions. Plant Physiol. 134, 871–879. 10.1104/pp.103.031740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos M. P., Tommassen J. (2004). Biogenesis of the Gram-negative bacterial outer membrane. Curr. Opin. Microbiol. 7, 610–616. 10.1016/j.mib.2004.10.011 [DOI] [PubMed] [Google Scholar]
- Burghout P., Beckers F., de Wit E., van Boxtel R., Cornelis G. R., Tommassen J., et al. (2004). Role of the pilot protein in the biogenesis of the YscC secretin in Yersinia enterocolitica. J. Bacteriol. 186, 5366–5375. 10.1128/JB.186.16.5366-5375.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerveny L., Straskova A., Dankova V., Hartlova A., Ceckova M., Staud F., et al. (2013). Tetratricopeptide repeat motifs in the world of bacterial pathogens: role in virulence mechanisms. Infect. Immun. 81, 629–635. 10.1128/IAI.01035-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenna R., Sugawara H., Koike T., Lopez R., Gibson T. J., Higgins D. G., et al. (2003). Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31, 3497–3500. 10.1093/nar/gkg500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clock S. A., Planet P. J., Perez B. A., Figurski D. H. (2008). Outer membrane components of the Tad (tight adherence) secreton of Aggregatibacter actinomycetemcomitans. J. Bacteriol. 190, 980–990. 10.1128/JB.01347-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin S., Guilvout I., Nickerson N. N., Pugsley A. P. (2011). Sorting of an integral outer membrane protein via the lipoprotein-specific Lol pathway and a dedicated lipoprotein pilotin. Mol. Microbiol. 80, 655–665. 10.1111/j.1365-2958.2011.07596.x [DOI] [PubMed] [Google Scholar]
- Cornelis G. R. (2002). Yersinia type III secretion: send in the effectors. J. Cell. Biol. 158, 401–408. 10.1083/jcb.200205077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea L. D., Regan L. (2003). TPR proteins: the versatile helix. Trends Biochem. Sci. 28, 655–662. 10.1016/j.tibs.2003.10.007 [DOI] [PubMed] [Google Scholar]
- D'Antuono A. L., Casabuono A., Couto A., Ugalde R. A., Lepek V. C. (2005). Nodule development induced by Mesorhizobium loti mutant strains affected in polysaccharide synthesis. Mol. Plant Microbe Interact. 18, 446–457. 10.1094/MPMI-18-0446 [DOI] [PubMed] [Google Scholar]
- Dai W. J., Zeng Y., Xie Z. P., Staehelin C. (2008). Symbiosis-promoting and deleterious effects of NopT, a novel type 3 effector of Rhizobium sp. Strain NGR234. J. Bacteriol. 190, 5101–5110. 10.1128/JB.00306-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin W. J., Marie C., Saad M. M., Krishnan H. B., Broughton W. J. (2005). NopA is associated with cell surface appendages produced by the type III secretion system of Rhizobium sp. Strain NGR234. Mol. Plant Microbe Interact. 18, 499–507. 10.1094/MPMI-18-0499 [DOI] [PubMed] [Google Scholar]
- de Lorenzo V., Timmis K. N. (1994). Analysis and construction of stable phenotypes in Gram-negative bacteria with Tn5- and Tn10-derived mini-transposons. Methods Enzymol. 235, 386–405. 10.1016/0076-6879(94)35157-0 [DOI] [PubMed] [Google Scholar]
- Douglas C. J., Staneloni R. J., Rubin R. A., Nester E. W. (1985). Identification and genetic analysis of an Agrobacterium tumefaciens chromosomal virulence region. J. Bacteriol. 161, 850–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edqvist P. J., Broms J. E., Betts H. J., Forsberg A., Pallen M. J., Francis M. S. (2006). Tetratricopeptide repeats in the type III secretion chaperone, LcrH: their role in substrate binding and secretion. Mol. Microbiol. 59, 31–44. 10.1111/j.1365-2958.2005.04923.x [DOI] [PubMed] [Google Scholar]
- Felsenstein J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791 10.2307/2408678 [DOI] [PubMed] [Google Scholar]
- Francis M. S. (2010). Type III secretion chaperones: a molecular toolkit for all occasions, in Handbook of Molecular Chaperones, eds Durante P., Colucci L. (Umeå: Nova Sciece Publishers, Inc; ), 79–148. [Google Scholar]
- Galán J. E. (2001). Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell Dev. Biol. 17, 53–86. 10.1146/annurev.cellbio.17.1.53 [DOI] [PubMed] [Google Scholar]
- Gazi A. D., Sarris P. F., Fadouloglou V. E., Charova S. N., Mathioudakis N., Panopoulos N. J., et al. (2012). Phylognetic analysis of a gene cluster encoding an additional, rhizobial-like type III secretion system that is narrowly distributed among Pseudomonas syringae strains. BMC microbiol. 12:188. 10.1186/1471-2180-12-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilvout I., Chami M., Engel A., Pugsley A. P., Bayan N. (2006). Bacterial outer membrane secretin PulD assembles and inserts into the inner membrane in the absence of its pilotin. EMBO J. 25, 5241–5249. 10.1038/sj.emboj.7601402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubber A., Vergunst A. C., Sullivan J. T., Hooykaas P. J. J., Ronson C. W. (2004). Symbiotic phenotypes and translocated effector proteins of the Mesorhizobium loti strain R7A VirB/D4 type IV secretion system. Mol. Microbiol. 54, 561–574. 10.1111/j.1365-2958.2004.04292.x [DOI] [PubMed] [Google Scholar]
- Jones D. T., Taylor W. R., Thornton J. M. (1992). The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 8, 275–282. [DOI] [PubMed] [Google Scholar]
- Kambara K., Ardissone S., Kobayashi H., Saad M. M., Schumpp O., Broughton W. J., et al. (2009). Rhizobia utilize pathogen-like effector proteins during symbiosis. Mol. Microbiol. 71, 92–106. 10.1111/j.1365-2958.2008.06507.x [DOI] [PubMed] [Google Scholar]
- Kaneko T., Nakamura Y., Sato S., Asamizu E., Kato T., Sasamoto S., et al. (2000a). Complete genome structure of the nitrogen-fixing Symbiotic bacterium Mesorhizobium loti. DNA Res. 7, 331–338 10.1093/dnares/7.6.331 [DOI] [PubMed] [Google Scholar]
- Kaneko T., Nakamura Y., Sato S., Asamizu E., Kato T., Sasamoto S., et al. (2000b). Complete genome structure of the nitrogen-fixing Symbiotic bacterium Mesorhizobium loti (Suppplement). DNA Res. 7, 381–406. 10.1093/dnares/7.6.381 [DOI] [PubMed] [Google Scholar]
- Kimbrel J. A., Thomas W. J., Jiang Y., Creason A. L., Thireault C. A., Sachs J. L., et al. (2013). Mutualistic co-evolution of type III effector genes in Sinorhizobium fredii and Bradyrhizobium japonicum. PLoS Pathog. 9:e1003204. 10.1371/journal.ppat.1003204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo J., Burrows L. L., Howell P. L. (2012). Decoding the roles of pilotins and accessory proteins in secretin escort services. FEMS Microbiol. Lett. 328, 1–12. 10.1111/j.1574-6968.2011.02464.x [DOI] [PubMed] [Google Scholar]
- Koo J., Tammamm S., Ku S. Y., Sampaleanu L. M., Burrows L. L., Howell P. L. (2008). PilF is an outer membrane lipoprotein required for multimerization and localization of the Pseudomonas aeruginosa Type IV pilus secretin. J. Bacteriol. 190, 6961–6969. 10.1128/JB.00996-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkov K. V., Gonen T., Hol W. G. J. (2011). Secretins: dynamic channels for protein transport across membranes. Trends Biochem. Sci. 36, 433–443. 10.1016/j.tibs.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster M., Bitter W., de Cock H., Allaoui A., Cornelis G. R., Tommassen J. (1997). The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol. Microbiol. 26, 789–797. 10.1046/j.1365-2958.1997.6141981.x [DOI] [PubMed] [Google Scholar]
- Kovach M. E., Elzer P. H., Hill D. S., Robertson G. T., Farris M. A., Martin Roop I. I. R., et al. (1995). Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166, 175–176. 10.1016/0378-1119(95)00584-1 [DOI] [PubMed] [Google Scholar]
- Krause A., Doerfel A., Gottfert M. (2002). Mutational and transcriptional analysis of the type III secretion system of Bradyrhizobium japonicum. Mol. Plant Microbe Interact. 15, 1228–1235. 10.1094/MPMI.2002.15.12.1228 [DOI] [PubMed] [Google Scholar]
- Krishnan H. B., Kim W. S., Sun-Hyung J. (2007). Calcium regulates the production of nodulation outer proteins (Nops) and precludes pili formation by Sinorhizobium fredii USDA257, a soybean symbiont. FEMS Microbiol. Lett. 271, 59–64. 10.1111/j.1574-6968.2007.00698.x [DOI] [PubMed] [Google Scholar]
- López-Lara I. M., van den Berg J. D. J., Thomas-Oates J. E., Glushka J., Lugtenberg B. J. J., Spaink H. P. (1995). Structural identification of the lipo-chitin oligosaccharide nodulation signals of Rhizobium loti. Mol. Microbiol. 15, 627–638. 10.1111/j.1365-2958.1995.tb02372.x [DOI] [PubMed] [Google Scholar]
- Marie C., Broughton W. J., Deakin W. J. (2001). Rhizobium type III secretion systems: legume charmers or alarmers? Curr. Opin. Plant Biol. 4, 336–342. 10.1016/S1369-5266(00)00182-5 [DOI] [PubMed] [Google Scholar]
- Marie C., Deakin W. J., Ojanen-Reuhs T., Diallo E., Reuhs B., Broughton W. J., et al. (2004). TtsI, a key regulator of Rhizobium species NGR234 is required for type III-dependent protein secretion and synthesis of rhamnose-rich polysaccharides. Mol. Plant Microbe Interact. 17, 958–966. 10.1094/MPMI.2004.17.9.958 [DOI] [PubMed] [Google Scholar]
- Murakami M. T., Sforça M. L., Neves J. L., Paiva J. H., Domingues M. N., Pereira A. L., et al. (2010). The repeat domain of the type III effector protein PthA shows a TPR-like structure and undergoes conformational changes upon DNA interaction. Proteins 78, 3386–3395. 10.1002/prot.22846 [DOI] [PubMed] [Google Scholar]
- Ochoa D., Pazos F. (2010). Studying the co-evolution of protein families with the Mirror tree web server. Bioinformatics 26, 1370–1371. 10.1093/bioinformatics/btq137 [DOI] [PubMed] [Google Scholar]
- Okazaki S., Okabe S., Higashi M., Shimoda Y., Sato S., Tabata S., et al. (2010). Identification and functional analysis of type III effector proteins in Mesorhizobium loti. Mol.Plant Microbe Interact. 23, 223–234. 10.1094/MPMI-23-2-0223 [DOI] [PubMed] [Google Scholar]
- Okuda S., Tokuda H. (2011). Lipoprotein sorting in bacteria. Annu. Rev. Microbiol. 65, 239–259. 10.1146/annurev-micro-090110-102859 [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. (1972). Mechanism of assembly of the outer membrane of Salmonella typhimurium. J. Biol. Chem. 274, 3962–3972. [PubMed] [Google Scholar]
- Pallen M. J., Francis M. S., Fütterer K. (2003). Tetratricopeptide-like repeats in type-III-secretion chaperones and regulators. FEMS Microbiol. Lett. 223, 53–60. 10.1016/S0378-1097(03)00344-6 [DOI] [PubMed] [Google Scholar]
- Pazos F., Valencia A. (2001). Similarity of phylogenetic trees as indicator of protein-protein interaction. Protein Eng. 14, 609–614. 10.1093/protein/14.9.609 [DOI] [PubMed] [Google Scholar]
- Rodrigues J. A., López-Baena F. J., Ollero F. J., Vinardell J. M., Espuny M. del R., Bellogin R. A., et al. (2007). NopM and NopD are rhizobial nodulation outer proteins: identification using LC-MALDI and LC-ESI with a monolithic capillary column. J. Proteome Res. 6, 1029–1037. 10.1021/pr060519f [DOI] [PubMed] [Google Scholar]
- Saad M. M., Staehelin C., Broughton W. J., Deakin W. J. (2008). Protein-protein interactions within type III secretion system-dependent pili of Rhizobium sp. Strain NGR234. J. Bacteriol. 190, 750–754. 10.1128/JB.01116-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez C., Iannino F., Deakin W. J., Ugalde R. A., Lepek V. C. (2009). Characterization of the Mesorhizobium loti MAFF303099 type three protein secretion system. Mol. Plant Microbe Interact. 22, 519–528. 10.1094/MPMI-22-5-0519 [DOI] [PubMed] [Google Scholar]
- Sánchez C., Mercante V., Babuin M. F., Lepek V. C. (2012). Dual effect of Mesorhizobium loti T3SS functionality on the symbiotic process. FEMS Microbiol. Lett. 330, 148–156. 10.1111/j.1574-6968.2012.02545.x [DOI] [PubMed] [Google Scholar]
- Sal-Man N., Setiaputra D., Scholz R., Deng W., Yu A. C., Strynadka N. C., et al. (2013). EscE and EscG are cochaperones for the type III needle protein EscF of enteropathogenic Escherichia coli. J. Bacteriol. 195, 2481–2489. 10.1128/JB.00118-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer A., Tauch A., Jäger W., Kaninowski J., Thierbach G., Pühler A. (1994). Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145, 69–73. 10.1016/0378-1119(94)90324-7 [DOI] [PubMed] [Google Scholar]
- Schechter L. M., Guenther J., Olcay E. A., Jang S., Krishnan H. B. (2010). Translocation of NopP by Sinorhizobium fredii USDA257 into Vigna unguiculata root nodules. Appl. Environ. Microbiol. 76, 3758–3761. 10.1128/AEM.03122-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerker J. M., Shapiro L. (2000). Identification and cell cycle control of a novel pilus system in Caulobacter crescentus. EMBO J. 19, 3223–3234. 10.1093/emboj/19.13.3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorpil P., Saad M. M., Boukli N. M., Kobayashi H., Ares-Orpel F., Broughton W. J., et al. (2005). NopP, a phosphorylated effector of Rhizobium sp. Strain NGR234, is a major determinant of nodulation of the tropical legumes Flemingia congesta and Tephrosia vogelii. Mol. Microbiol. 57, 1304–1317. 10.1111/j.1365-2958.2005.04768.x [DOI] [PubMed] [Google Scholar]
- Spano S., Ugalde J. E., Galán J. E. (2008). Delivery of a Salmonella Typhi exotoxin from a host intracellular compartment. Cell Host Microbe 3, 30–38. 10.1016/j.chom.2007.11.001 [DOI] [PubMed] [Google Scholar]
- Sun P., Tropea J. E., Austin B. P., Cherry S., Waugh D. S. (2008). Structural characterization of the Yersinia pestis type III secretion system needle protein YscF in complex with its heterodimeric chaperone YscE/YscG. J. Mol. Biol. 377, 819–830. 10.1016/j.jmb.2007.12.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampakaki A. P. (2014). Commonalities and differences of T3SSs in rhizobia and plant pathogenic bacteria. Front. Plant Sci. 5:114. 10.3389/fpls.2014.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugalde J. E., Czibener C., Feldman M. F., Ugalde R. A. (2000). Identification and characterization of the Brucella abortus phosphoglucomutase gene: role of lipopolysaccharide in virulence and intracellular multiplication. Infect. Immun. 68, 5716–5723. 10.1128/IAI.68.10.5716-5723.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viarre V., Cascales E., Ball G., Michel G. P., Filloux A., Voulhoux R. (2009). HxcQ liposecretin is self-piloted to the outer membrane by its N-terminal lipid anchor. J. Biol. Chem. 284, 33815–33823. 10.1074/jbc.M109.065938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viprey V., Del Greco A., Golinowski W., Broughton W. J., Perret X. (1998). Symbiotic implications of type III protein secretion machinery in Rhizobium. Mol. Microbiol. 28, 1381–1389. 10.1046/j.1365-2958.1998.00920.x [DOI] [PubMed] [Google Scholar]
- Wassem R., Kobayashi H., Kambara K., Le Quéré A., Walker G. C., Broughton W. J., et al. (2008). TtsI regulates symbiotic genes in Rhizobium species NGR234 by binding to tts boxes. Mol. Microbiol. 68, 736–748. 10.1111/j.1365-2958.2008.06187.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehbi H., Portillo E., Harvey H., Shimkoff A. E., Scheurwater E. M., Howell P. L., et al. (2011). The peptidoglycan-binding protein FimV promotes assembly of the Pseudomonas aeruginosa type IV pilus secretin. J. Bacteriol. 193, 540–550. 10.1128/JB.01048-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel M., Friedrich L., Gottfert M., Zehner S. (2010). The type III-secreted protein NopE1 affects symbiosis and exhibits a calcium-dependent autocleavage activity. Mol. Plant Microbe Interact. 23, 124–129. 10.1094/MPMI-23-1-0124 [DOI] [PubMed] [Google Scholar]
- Woodcock D. H., Crowther P. J., Doherty J., Jefferson S., DeCruz E., Noyer-Weidner S., et al. (1989). Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 17, 3469–3478. 10.1093/nar/17.9.3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M. (1986). Biogenesis of lipproteins in bacteria. Curr. Top. Microbiol. Immunol. 125, 127–157. 10.1007/978-3-642-71251-7_9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intracellular (pellet) proteins were isolated from the y4yS mutant containing plasmid pBBR1MCS-4 with the 3xFLAG fused Y4yS protein expressed under the lac promoter (constitutive in rhizobia), Proteins were separated by 10% SDS-PAGE and then immuno-blotted and probed with an anti-FLAG antibody. Positions of size markers loaded onto the gels are labeled (in kDa). ± N indicate bacterial culture in the presence or absence of naringenin. Bacteria contain plasmid pMP2112.
Alignment between the aminoacid sequences of TPR proteins using MUSCLE v(3.8.31) (Edgar, 2004).