Abstract
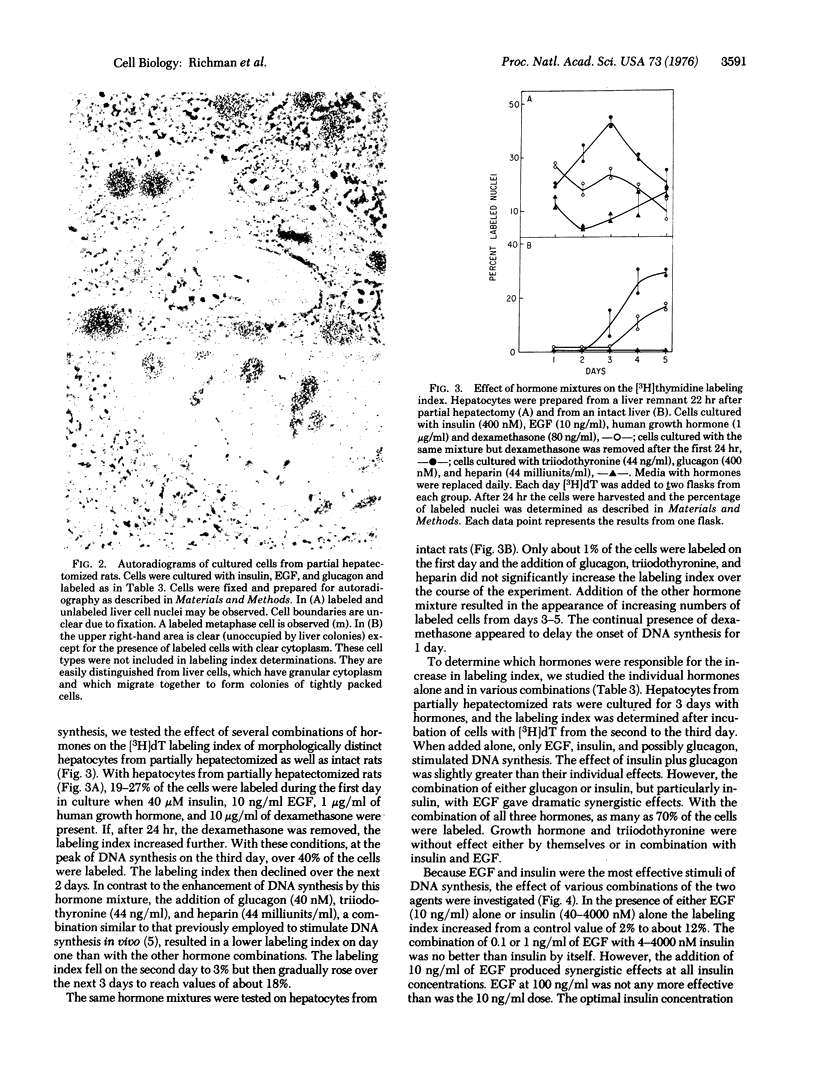
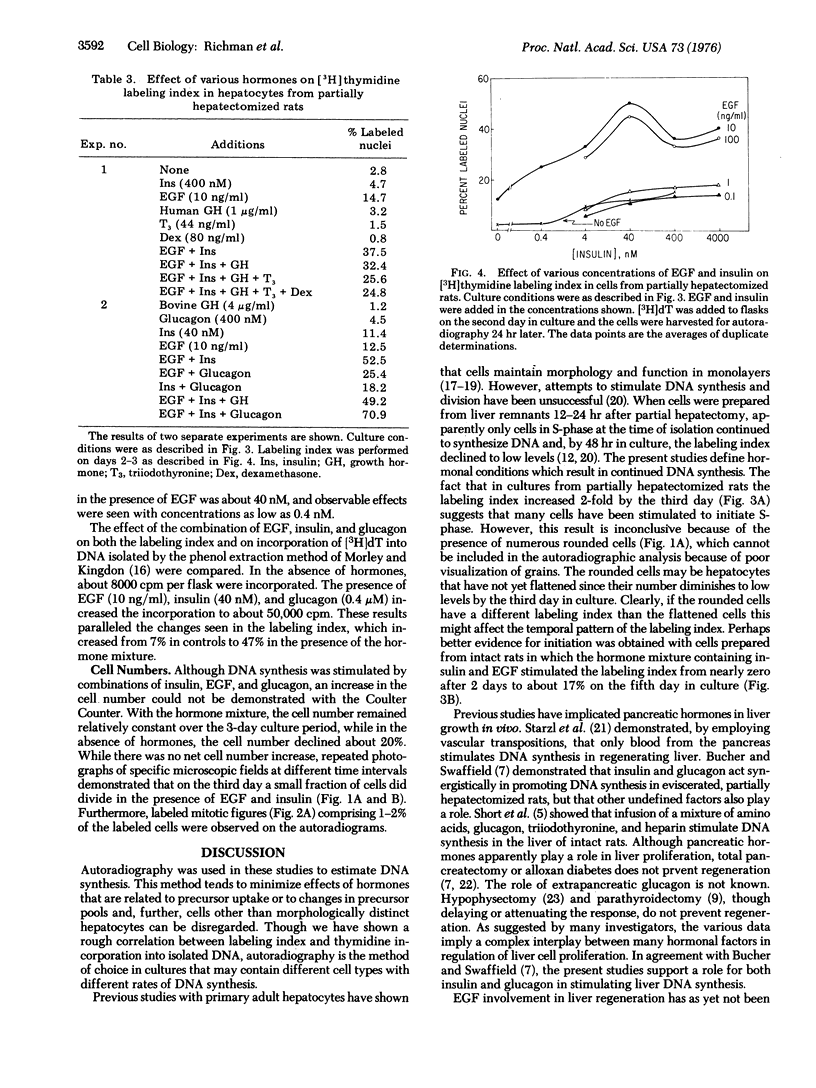
Adult rat hepatocytes have been previously isolated and maintained in monolayer culture, but attempts to stimulate DNA synthesis have been unsuccessful. Hormonal conditions are now described which induce DNA synthesis in cultured hepatocytes from partially hepatectomized rats. DNA synthesis was determined autoradiographically by the incorporation of [3H]thymidine into nuclei of morphologically distinct hepatocytes. Insulin (4-4000 nM) or epidermal growth factor (10 ng/ml) alone caused significant increases in the labeling index. The two hormones together acted synergistically to produce labeling indices of 35-50% on the third day of culture, compared with 2-7% in control cultures. The addition of glucagon (400 nM) further increased the labeling indes. Dexamethasone (80 ng/ml) inhibited DNA synthesis but, under certain conditions, enhanced cell attachment. Growth hormone and triiodothyronine had no significant effect on DNA synthesis. The mixture of epidermal growth factor, insulin, and glucagon also stimulated incorporation of [3H]thymidine into phenol-extracted DNA. Although DNA synthesis was stimulated, cell division occurred infrequently. These data suggest a prominent role for epidermal growth factor in promoting hepatic DNA synthesis by acting in concert with insulin and glucagon.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUCHER N. L. REGENERATION OF MAMMALIAN LIVER. Int Rev Cytol. 1963;15:245–300. doi: 10.1016/s0074-7696(08)61119-5. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L. E., Meyer U. A. Parenchymal cells from adult rat liver in nonproliferating monolayer culture. I. Functional studies. J Cell Biol. 1973 Dec;59(3):722–734. doi: 10.1083/jcb.59.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher M. L., Swaffield M. N. Regulation of hepatic regeneration in rats by synergistic action of insulin and glucagon. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1157–1160. doi: 10.1073/pnas.72.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus T. H., Pilkis S. J., Park C. R. Stimulation by glucagon of the incorporation of U-14C-labeled substrates into glucose by isolated hepatocytes from fed rats. Biochim Biophys Acta. 1975 Sep 8;404(1):110–123. doi: 10.1016/0304-4165(75)90152-x. [DOI] [PubMed] [Google Scholar]
- Cohen S., Carpenter G., Lembach K. J. Interaction of epidermal growth factor (EGF) with cultured fibroblasts. Adv Metab Disord. 1975;8:265–284. doi: 10.1016/b978-0-12-027308-9.50024-x. [DOI] [PubMed] [Google Scholar]
- GRISHAM J. W. A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver; autoradiography with thymidine-H3. Cancer Res. 1962 Aug;22:842–849. [PubMed] [Google Scholar]
- HARKNESS R. D. Regeneration of liver. Br Med Bull. 1957 May;13(2):87–93. doi: 10.1093/oxfordjournals.bmb.a069601. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Insulin and epidermal growth factor. Human fibroblast receptors related to deoxyribonucleic acid synthesis and amino acid uptake. J Biol Chem. 1975 May 25;250(10):3845–3853. [PubMed] [Google Scholar]
- Leffert H. L. Growth control of differentiated fetal rat hepatocytes in primary monolayer culture. V. Occurrence in dialyzed fetal bovine serum of macromolecules having both positive and negative growth regulatory functions. J Cell Biol. 1974 Sep;62(3):767–779. doi: 10.1083/jcb.62.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffert H. L., Paul D. Studies on primary cultures of differentiated fetal liver cells. J Cell Biol. 1972 Mar;52(3):559–568. doi: 10.1083/jcb.52.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman I. On the control of mammalian deoxyribonucleic acid synthesis. In Vitro. 1970 Jul-Aug;6(1):46–57. doi: 10.1007/BF02616133. [DOI] [PubMed] [Google Scholar]
- Lin R. C., Snodgrass P. J. Primary culture of normal adult rat liver cells which maintain stable urea cycle enzymes. Biochem Biophys Res Commun. 1975 May 19;64(2):725–734. doi: 10.1016/0006-291x(75)90380-0. [DOI] [PubMed] [Google Scholar]
- Moolten F. L., Bucher N. L. Regeneration of rat liver: transfer of humoral agent by cross circulation. Science. 1967 Oct 13;158(3798):272–274. doi: 10.1126/science.158.3798.272. [DOI] [PubMed] [Google Scholar]
- Morley C. G., Kingdon H. S. Use of 3 H-thymidine for measurement of DNA synthesis in rat liver--a warning. Anal Biochem. 1972 Jan;45(1):298–305. doi: 10.1016/0003-2697(72)90030-9. [DOI] [PubMed] [Google Scholar]
- PITOT H. C., PERAINO C., MORSE P. A., Jr, POTTER V. R. HEPATOMAS IN TISSUE CULTURE COMPARED WITH ADAPTING LIVER IN VIVO. Natl Cancer Inst Monogr. 1964 Apr;13:229–245. [PubMed] [Google Scholar]
- Rixon R. H., Whitfield J. F. Parathyroid hormone: a possible initiator of liver regeneration. Proc Soc Exp Biol Med. 1972 Oct;141(1):93–97. doi: 10.3181/00379727-141-36723. [DOI] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J., Brown R. F., Husakova A., Gilbertson J. R., Zemel R., Lieberman I. Induction of deoxyribonucleic acid synthesis in the liver of the intact animal. J Biol Chem. 1972 Mar 25;247(6):1757–1766. [PubMed] [Google Scholar]
- Starzl T. E., Francavilla A., Halgrimson C. G., Francavilla F. R., Porter K. A., Brown T. H., Putnam C. W. The origin, hormonal nature, and action of hepatotrophic substances in portal venous blood. Surg Gynecol Obstet. 1973 Aug;137(2):179–199. [PMC free article] [PubMed] [Google Scholar]
- Starzl T. E., Watanabe K., Porter K. A., Putnam C. W. Effects of insulin, glucagon, and insuling/glucagon infusions on liver morphology and cell division after complete portacaval shunt in dogs. Lancet. 1976 Apr 17;1(7964):821–825. doi: 10.1016/s0140-6736(76)90477-3. [DOI] [PubMed] [Google Scholar]
- Younger L. R., King J., Steiner D. F. Hepatic proliferative response to insulin in severe alloxan diabetes. Cancer Res. 1966 Jul;26(7):1408–1414. [PubMed] [Google Scholar]