Abstract
Purpose
To measure flow rate of balanced salt solution and IOP during simulated vitrectomy using two sets of high-speed dual-pneumatic probes.
Methods
A closed-model eye system measured IOP and flow rate of a balanced salt solution through infusion cannula. The Constellation Vision System was tested with two sets of high-speed dual-pneumatic probes (UltraVit 23-gauge and enhanced 25+-gauge 5000-cpm probes; UltraVit 23-gauge and enhanced 25+-gauge 7500-cpm probes; n = 6 each) under different vacuum levels and cut rates in three duty cycle modes.
Results
In both probe sets, flow rates were dependent on cut rate with the biased open and biased closed duty cycles. Flow rates were highest with the biased open duty cycle, lower with the 50/50 duty cycle, and lowest with the biased closed duty cycle. IOP, as expected, was inversely associated with flow rate using both probe sets.
Conclusions
The 7500-cpm probes offer greater control and customization compared with 5000-cpm probes under certain experimental conditions. At maximum cut rates, performance of 7500-cpm probes was similar to that of 5000-cpm probes, suggesting that 7500-cpm probes may be used without sacrifice of flow rate and IOP stability.
Translational Relevance
Customization of vitrectomy parameters allows greater surgeon control during vitrectomy and may expand the usefulness of vitrectomy probes.
Keywords: 5000 cpm, 7500 cpm, 23-gauge vitrectomy, 25-gauge vitrectomy, aspiration, Constellation Vision System, cut rate, duty cycle, flow rate, intraocular pressure, vitrectomy
Introduction
Vitrectomy surgery has evolved such that smaller-gauge probes and higher cutting speeds are used to reduce operative time1–4 and postoperative discomfort.1,3 Modifications such as reduced probe diameter, increased cut rate, and improved control of the length of time the cutting port is open relative to one complete cutting cycle (duty cycle)5 have provided enhanced control of flow rate and intraocular (IOP). Control of these parameters may reduce surgical complications such as retinal breaks6 and may expand instrument utility.7 However, flow rate and IOP measurements are not always evident.
Flow of Newtonian fluids such as balanced salt solution (BSS) through the eye can be described by Poiseuille's modified equation,8 as shown in Equation 1:
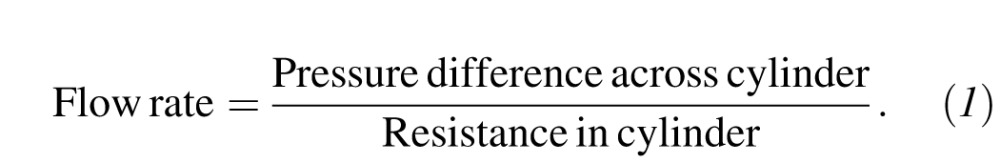 |
The pressure difference reflects both the applied pressure at the infusion bottle and the back pressure in the eye. Flow rate out of the eye through the vitrectomy probe depends on the pressure difference across the probe (affected by the applied vacuum level and the IOP) and the resistance inside the probe, which is affected by the viscosity of the aspirated liquid (η), the length of the probe needle (L), and the internal radius of the probe needle (r).9 These relationships are shown in Equation 2, which is a more detailed version of Equation 1:
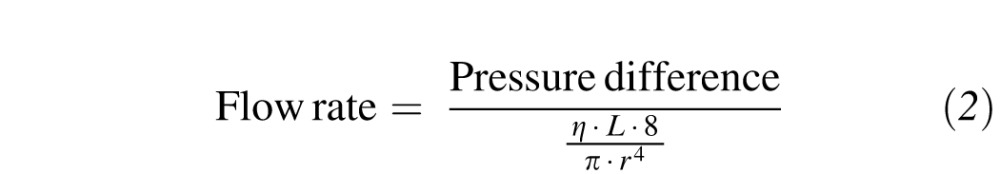 |
When the cutter is activated, Equation 2 must be modulated to account for the nonconstant flow out of the eye and the duty cycle. Because IOP depends on the fluid flow rate into the eye through the infusion line and out of the eye through the vitrectomy probe, alterations in flow rate affect IOP.
A surgeon can affect fluid flow rate by selecting the vitrectomy probe gauge, duty cycle, infusion pressure, and vacuum settings, and by cutting the vitreous body to lower its viscosity.5,10 In saline solutions, larger-gauge probes provide higher flow rates but greater potential for complication, whereas smaller-gauge probes reduce flow rate and surgical invasiveness.5,11–14 Fluid flow rate may be enhanced by increasing port open time (i.e., biased open duty cycle) and reduced by decreasing port open time (i.e., biased closed duty cycle) (Chu TG, Buboltz DC. IOVS. 2010;51:ARVO Abstract 3609). Similarly, increased vacuum provides increased flow rates with aspiration of saline solutions.14 However, modulation of the flow rate of viscous non-Newtonian fluids through various probe sizes, duty cycles, and aspiration vacuums is different from that of saline solutions because of the increased viscosity and semisolid properties of the material. The viscosity of the vitreous may be reduced by cutting the vitreous into smaller pieces that are more easily aspirated and thus allow better flow through the vitrectomy probes. Therefore, high cut rates may allow increased flow in viscous fluid10 and porcine vitreous15 to a certain point and may reduce the problematic risk factor of retinal breaks caused by removal of vitreous that is adherent to retinal tissue.16 Given the importance of the balance between flow rate and IOP during vitrectomy, the current study evaluated the effect of cut rate, duty cycle, and aspiration vacuum on fluid flow rate and IOP using high-speed dual-pneumatic 7500-cpm probes and compared their performance with 5000-cpm probes.
Methods
All infusion and probe tubing sets were assembled according to manufacturer's instructions. Infusion pressure was 30 mm Hg for all testing. The infusion liquid was BSS. The Constellation Vision System (Alcon Laboratories, Inc., Fort Worth, TX) was used to test two sets of probes: UltraVit (Alcon Laboratories, Inc.) 5000-cpm probes (23- and enhanced 25+-gauge; n = 6 for each gauge) and UltraVit 7500-cpm probes (23- and enhanced 25+-gauge; n = 6 for each gauge). Flow rates were investigated with three duty cycle settings at cut rates from 500 to 7500 cpm. In the instrument interface and user manual, the three duty cycle settings were labeled as follows: biased open (maximum port open), 50/50 (50% port open), and biased closed (minimum port open). Testing was performed with the “IOP controlled infusion” option turned off. Vacuum settings ranged from 250 to 650 mm Hg.
Model eyes were constructed from two clear acrylic domes with 28.6-mm diameters (Kit Kraft, Studio City, CA) glued together with Loctite 4014 adhesive (Henkel Corporation, Rocky Hill, CT). One model eye was constructed for each probe gauge. At the top of the globe, along the glued seam, two holes were drilled 16.3 mm apart, in alignment with the center point. This configuration was intended to replicate the location of the pars plana, where trocar incisions are usually placed during surgery. The diameter of the drilled holes depended on the probe diameter to be tested: 0.58 mm for the enhanced 25-gauge probe and 0.74 mm for the 23-gauge probe. Trocar cannulas (Alcon Laboratories, Inc.) of appropriate sizes were glued into the enhanced 25- and 23-gauge holes using Loctite adhesive. At the bottom of the globe, one center hole with a 6.35-mm diameter was drilled.
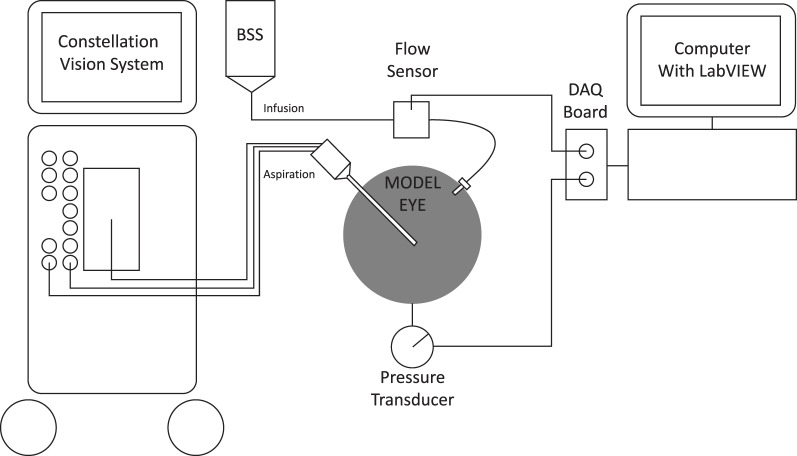
A pressure transducer (model 40PC015G; Honeywell International Inc., Columbus, OH) was attached to the bottom hole of the model eye to record IOP (Fig. 1). A silicone sleeve was sandwiched between the globe and the transducer to prevent fluid leakage. The transducer was calibrated with a Druck pressure calibrator (model DPI 610 100; General Electric Sensing, Billerica, MA). A vitrectomy probe was inserted into one trocar cannula atop the model eye. An infusion cannula was inserted into the second trocar cannula atop the model eye. A fluid flow meter (model TS410;Transonic Systems, Inc., Ithaca, NY) was attached to the infusion cannula, in line with the infusion flow. The flow meter and the pressure transducers were connected to a LabVIEW Data Acquisition board (National Instruments, Austin, TX), which relayed data to a computer. Flow and IOP data from the sensor measurements were exported as a Microsoft Excel file (Microsoft Corporation, Redmond, WA). All results are presented as mean ± SD.
Figure 1. .
Instrument setup. BSS, balanced salt solution; DAQ, data acquisition.
Results
Enhanced 25-Gauge Probes
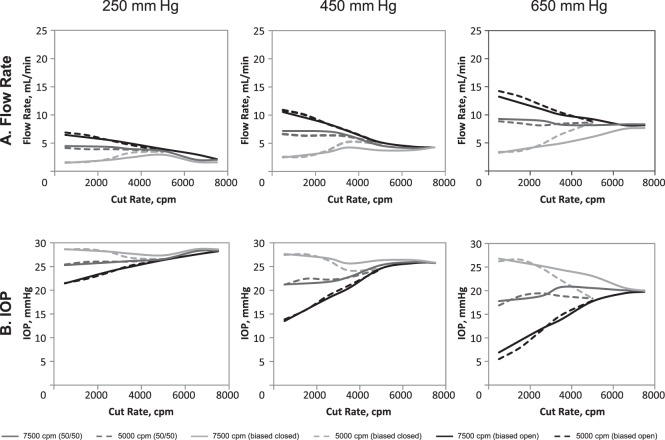
Flow rate and IOP measurements for enhanced 25-gauge 5000-cpm and 7500-cpm probes are shown in Figure 2.
Figure 2. .
Flow rate (A) and IOP (B) comparison for the Constellation Vision System with enhanced 25-gauge 5000- and 7500-cpm probes. Each data point represents the average of six measurements. IOP, intraocular pressure.
For 5000-cpm probes, overall flow rates were highest in the biased open duty cycle mode, lower in the 50/50 duty cycle mode, and lowest in the biased closed duty cycle mode (Fig. 2A). At 500 cpm, flow rate was greatest in biased open duty cycle mode, lower in 50/50 duty cycle mode, and lowest in biased closed duty cycle mode. With increasing cut rate, flow rate decreased with biased open, remained relatively constant with 50/50, and increased with biased closed duty cycles. Maximum flow rates (at 650-mm Hg vacuum) were 14.2 ± 0.5 mL/min in biased open and 8.8 ± 0.4 mL/min in 50/50 duty cycle mode; maximum flow rate was observed under the lowest cut rate (i.e., 500 cpm) with both duty cycle modes. In biased closed duty cycle mode, maximum flow rate was 8.6 ± 0.8 mL/min and occurred at the highest cut rate (5000 cpm). IOP tended to increase in biased open duty cycle mode, remain stable in 50/50 duty cycle mode, and slightly decrease in biased closed duty cycle mode with increasing cut rate (Fig. 2B).
For 7500-cpm probes, flow rates at 500 cpm and 650 mm Hg were greater in biased open and 50/50 duty cycle modes than biased closed duty cycle mode (Fig. 2A). Flow rates tended to decrease with increased cut rate in biased open duty cycle and remain constant with 50/50 duty cycle mode. In contrast, flow rate increased with increasing cut rate in biased closed duty cycle mode. Maximum flow rates were observed at 500 cpm for biased open and 50/50 duty cycle modes and at 7500 cpm in biased closed duty cycle mode. Maximum flow rates (at 650 mm Hg) were 13.2 ± 3.3 mL/min in biased open duty cycle mode, 9.3 ± 2.0 mL/min in 50/50 duty cycle mode, and 7.7 ± 2.4 mL/min in biased closed duty cycle mode. Flow rates with maximal vacuum (i.e., 650 mm Hg) and highest cut rate (7500 cpm) were similar among all duty cycle modes (8.1 ± 2.1 mL/min for biased open, 8.3 ± 1.8 mL/min for 50/50, 7.7 ± 2.4 mL/min for biased closed). IOP tended to increase with increasing cut rate and reduced flow rate in biased open duty cycle (Fig. 2B). Overall, an inverse relationship between IOP and flow rate was observed. Minimal variations in IOP were observed with increasing cut rate in 50/50 duty cycle mode, but IOP tended to be inversely related to flow and increased with increasing cut rate in biased closed duty cycle.
When 5000-cpm and 7500-cpm probes were compared, flow rate response to modulation of duty cycle was similar, with flow decreasing with biased open duty cycle and increasing with biased closed and 50/50 duty cycle (Fig. 2A). At maximum vacuum (i.e., 650 mm Hg), the flow rate through the 7500-cpm probes in the biased open duty cycle mode decreased from 13.2 ± 3.3 mL/min at 500 cpm to 8.1 ± 2.1 mL/min at maximum cut rate, but increased from 3.2 ± 0.8 mL/min to 7.7 ± 2.4 mL/min at 500 cpm and 7500 cpm, respectively, with biased closed duty cycle. Similarly, flow rate through the 5000-cpm probes with biased open duty cycle decreased from 14.2 ± 0.5 mL/min at 500 cpm to 8.8 ± 0.7 mL/min at maximum cut rate, whereas flow increased from 3.4 ± 0.4 mL/min to 8.6 ± 0.8 mL/min at maximum cut rate with biased closed duty cycle. Maximum flow rate at maximum cut rate for 5000-cpm and 7500-cpm probes were similar for all duty cycles.
The IOP was inversely related to flow rate with the biased open and biased closed duty cycles in both probe sets (Fig. 2B). At 650 mm Hg, flow rate with 7500-cpm vitrectomy probes increased from 6.9 ± 1.2 mm Hg at 500 cpm to 19.8 ± 0.8 mm Hg with biased open duty cycle and decreased from 26.8 ± 1.0 mm Hg at 500 cpm to 20.0 ± 0.9 mm Hg at maximum cut rate in the biased closed duty cycle. At the same aspiration vacuum setting, using 5000-cpm vitrectomy probes, flow rate increased with the biased open duty cycle (5.5 ± 1.2 mL/min at 500 cpm to 17.9 ± 1.5 mL/min at maximum cut rate) and decreased with biased closed duty cycle (from 26.2 ± 0.4 mL/min at 500 cpm to 18.4 ± 1.3 mL/min at maximum cut rate).
23-Gauge Probes
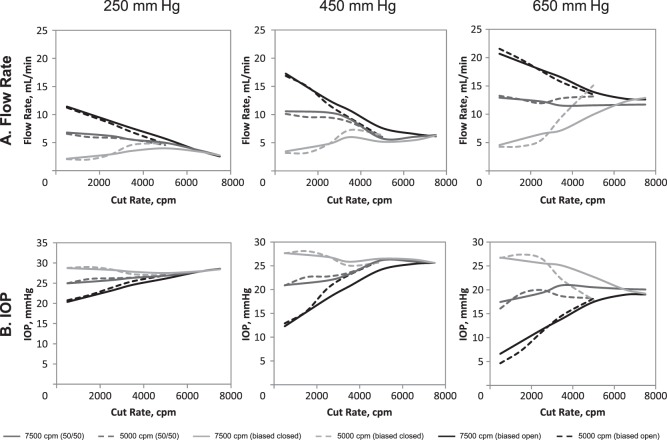
Flow rate and IOP measurements for 23-gauge 5000-cpm and 7500-cpm probes are shown in Figure 3.
Figure 3. .
Flow rate (A) and IOP (B) comparison for the Constellation Vision System with 23-gauge 5000- and 7500-cpm probes. Each data point represents the average of six measurements. IOP, intraocular pressure.
With 5000-cpm probes, flow rates followed trends similar to those observed with enhanced 25-gauge probes; flow rates were greatest in the biased open duty cycle mode, lower in the 50/50 duty cycle mode, and lowest in the biased closed duty cycle mode (Fig. 3A). With increasing cut rate, flow rate decreased in biased open, remained relatively stable in 50/50, and increased in biased closed duty cycle modes. Maximum flow rate was observed at 500 cpm in biased open (21.6 ± 1.3 mL/min at 650 mm Hg) and 50/50 (13.3 ± 0.5 mL/min at 650 mm Hg) duty cycle modes. In contrast, maximum flow rate with biased closed duty cycle (15.1 ± 5.4 mL/min) was observed at the 5000-cpm cut rate. IOP was relatively stable with increasing cut rate under 250- and 450-mm Hg aspiration vacuum, but decreased with increased cut rate under higher aspiration vacuum level (i.e., 650 mm Hg; Fig. 3B).
With 7500-cpm probes, flow rates at 500 cpm (650-mm Hg vacuum) were greater for biased open and 50/50 than for biased closed duty cycle modes (Fig. 3A). With increasing cut rate, flow rate decreased in biased open duty cycle and remained relatively stable in 50/50 duty cycle mode. In contrast, flow rate increased with increasing cut rate in biased closed duty cycle mode. Maximum flow rates (at 650 mm Hg) were 20.7 ± 1.9 mL/min with biased open duty cycle, 12.9 ± 0.6 mL/min with 50/50 duty cycle mode, and 12.9 ± 1.1 mL/min with biased closed duty cycle mode. Maximum flow rate occurred at 500 cpm with biased open and 50/50 duty cycle modes and at 7500 cpm in biased closed duty cycle mode. Flow rate was similar at the highest vacuum (i.e., 650 mm Hg) and highest cut rate (i.e., 7500 cpm) among all duty cycles (12.6 ± 1.2 mL/min for biased open, 11.7 ± 1.3 mL/min with 50/50, 12.9 ± 1.1 mL/min with biased closed). In biased open duty cycle, increased cut rate (and decreased flow) was associated with increased IOP, although the overall IOP remained similar to that observed with other duty cycles (Fig. 3B). IOP remained relatively stable with increased cut rate and flow rate in a 50/50 duty cycle mode. In biased closed duty cycle mode, flow rate tended to increase with increased cut rate, although minimal reductions in IOP were observed with the lower cut rates. In general, alterations in IOP were inversely related to flow rate.
When compared, trends for flow rate and IOP with the 7500- and 5000-cpm vitrectomy probes were similar (Fig. 3). With the 7500-cpm vitrectomy probes, flow rate in biased open duty cycle and 450-mm Hg vacuum decreased with increasing cut rates (Fig. 3A). For example, at 450 mm Hg, the flow rate was 17.2 ± 1.4 mL/min with 500 cpm compared with 6.1 ± 0.3 mL/min at maximum cut rate. However, when the duty cycle was altered to biased closed, the flow rate increased with increased cut rate (e.g., from 3.5 ± 0.5 mL/min at 500 cpm to 6.3 ± 0.4 mL/min with 7500 cpm). With 5000-cpm probes at 450-mm Hg vacuum, flow rate decreased with increased cut rate with biased open duty cycle (i.e., 16.9 ± 1.2 mL/min with 500 cpm versus 6.0 ± 0.9 mL/min with 5000 cpm) and increased with increased cut rate with closed biased duty cycle (i.e., 3.2 ± 0.4 mL/min at 500 cpm versus 6.4 ± 0.9 mL/min at 5000 cpm). With the biased closed duty cycle, maximum flow rate at maximum cut rate was similar to the 5000-cpm and 7500-cpm probes (i.e., 6.4 ± 0.9 mL/min with 5000-cpm probes and 6.3 ± 0.4 mL/min with 7500-cpm probes).
The IOP with 23-gauge probes demonstrated trends similar to those seen with enhanced 25-gauge probes in both sets of vitrectomy probes (Fig. 3B). With both probe types, a positive association between IOP and cut rate was observed with biased open duty cycle, whereas an inverse relationship was demonstrated with biased closed duty cycle (Fig. 3B). At 450-mm Hg aspiration vacuum setting, IOP was 12.9 ± 1.0 mm Hg at the lowest cut rate and 26.2 ± 0.4 mm Hg at maximum cut rate with 5000-cpm probes and biased open duty cycle. Similarly, IOP increased with increasing cut rate with biased open duty cycle with 7500-cpm probes (12.3 ± 0.5 mm Hg at 500 cpm versus 25.6 ± 0.3 mm Hg at maximum cut rate). In contrast, a slight decrease in IOP was observed under the same conditions with biased closed duty cycle in 7500-cpm vitrectomy probes (27.7 ± 0.4 mm Hg with 500 cpm versus 25.6 ± 0.4 mm Hg at maximum cut rate) and 5000-cpm probes (27.7 ± 0.4 mm Hg at 500 cpm versus 25.8 ± 0.8 mm Hg at maximum cut rate). Because of the relationship between cut rate, flow rate, and duty cycle, IOP was inversely associated with flow rate with both duty cycles (i.e., with biased open duty cycle, flow rate decreased and IOP increased with increasing cut rate; with biased closed duty cycle, flow rate increased and IOP slightly decreased with increased cut rate).
Discussion
In this study, flow rate and IOP were assessed using 5000- and 7500-cpm vitrectomy probes under different vitrectomy system parameters. In general, trends were similar between the 5000- and 7500-cpm cutters. For each gauge size within both probe sets, aqueous flow rate decreased with increased cut rate under the biased open duty cycle (as previously demonstrated with 5000-cpm probes17), but increased with increased cut rate with the biased closed duty cycle. The 50/50 duty cycle did not greatly alter flow rate. Flow rate at 450 mm Hg at maximum cut speed (i.e., either 5000 or 7500 cpm) was similar (23-gauge probes: range, 6.0–6.4 mL/min for 5000-cpm probes and 6.1–6.4 mL/min for 7500-cpm probes; enhanced 25-gauge probes: range 4.9–5.0 mL/min for 5000-cpm probes and 4.3 mL/min for 7500-cpm probes) for each gauge size under all duty cycles, suggesting that fluid flow performance in 7500-cpm probes is maintained with increasing cut rates.
Because dual-pneumatic probes allow modulation of duty cycle, surgeons can not only use cut rate, vacuum, and gauge selection to control flow, but also can use an additional option: duty cycle. Increasing cut rate has advantages such as reducing retinal traction17; however, high cut rates may be less than optimal because of associated reductions in flow rate. Through modulation of duty cycle (not cut rate), the range of BSS flow rates achievable at 5000-cpm cut rate with the 7500-cpm probes was greater than that observed with 5000-cpm probes at the same cut rate. For example, with enhanced 25-gauge probes and 7500-cpm probes at 5000 cpm and 650 mm Hg, flow rate ranged from 6.08 mL/min (with biased closed duty cycle) to 9.22 mL/min (with biased open duty cycle). In comparison, under the same conditions, flow rate through 5000-cpm probes ranged from 8.59 mL/min (with biased closed duty cycle) to 8.81 mL/min (with biased open duty cycle). Thus, 7500-cpm probes, through modulation of duty cycle, may provide surgeons with greater flow rate control while retaining the benefits of high cut rates. This may be useful for procedures that require high flow but would benefit from use of a high cut rate.
The IOP was similar among 23- and enhanced 25-gauge 5000-cpm and 7500-cpm probes under some experimental conditions. Under low aspiration vacuum, alterations in IOP as a function of cut rate were almost identical with 5000- and 7500-cpm probe sets. However, greater deviations were observed when higher vacuums were applied. IOP was inversely related to flow rate with all gauge sizes in both the 5000- and 7500-cpm probe sets with biased closed duty cycle and increased with increased cut rate in biased open duty cycle; 50/50 duty cycle produced little change. Changes in IOP with duty cycle at 5000 cpm and 650 mm Hg were minimal with 5000-cpm probes (IOP values with enhanced 25 gauge, 20.0–20.4 mm Hg; with 23 gauge, 21.5–22.3 mm Hg) and 7500-cpm probes (IOP values with enhanced 25 gauge, 17.7–23.1 mm Hg; with 23 gauge, 24.2–26.5 mm Hg). However, mean IOP was higher with 7500-cpm probes than 5000-cpm probes under the same conditions (5000 cpm, biased closed duty cycle).
In this study, viscosity was constant, so cut rate affected flow only by its effect on duty cycle, not by its effect on viscosity. Flow rates of non-Newtonian fluids such as vitreous humor, which model conditions similar to the beginning of a vitrectomy, would be useful for comparison with these BSS flow results. Testing vitreous body would also elucidate the viscosity-altering effects of the different cut rates on the resultant flow rates.
Conclusion
Under some experimental conditions, UltraVit 7500-cpm probes provide greater control of fluid flow and IOP than do 5000-cpm probes. At the maximum cut rate, fluid flow rate and IOP were similar for both high-speed probes, most likely because the short port open and port closed times did not allow for a difference between duty cycles. These results suggest that surgeons can take advantage of the benefits of the 7500-cpm cutters without sacrificing desirable flow rate and IOP.
Acknowledgments
This paper was presented in part at the 2010 Association for Research in Vision and Ophthalmology Congress, Fort Lauderdale, Florida, United States, May 2010, and at the 2012 Association for Research in Vision and Ophthalmology Congress, Fort Lauderdale, Florida, United States, May 2012.
Supported by Alcon Research, Ltd. Alcon assisted with the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Medical writing support was provided by Jillian R. Gee, PhD, CMPP, of Complete Healthcare Communications, Inc. (Chadds Ford, Pennsylvania, USA), and was funded by Alcon.
DCB conceived of the study and participated in its design and coordination but passed away prior to manuscript approval and submission. DJKA performed and/or supervised the measurements, revised drafts of the manuscript, and approved the final manuscript. DJKA is an employee of Alcon Research, Ltd. DCB was an Alcon Research, Ltd. employee at the time of his passing.
Disclosure: D.J.K. Abulon, Alcon Research, Ltd. (E); D.C. Buboltz, Alcon Research, Ltd. (E)
Footnotes
Deceased
References
- 1.Yanyali A, Celik E, Horozoglu F, Oner S, Nohutcu AF. 25-Gauge transconjunctival sutureless pars plana vitrectomy. Eur J Ophthalmol. 2006;16::141–147. doi: 10.1177/112067210601600123. [DOI] [PubMed] [Google Scholar]
- 2.Fujii GY, De Juan E, Jr, Humayun MS, et al. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology. 2002;(109):1807–1812. doi: 10.1016/s0161-6420(02)01179-x. discussion 1813. [DOI] [PubMed] [Google Scholar]
- 3.Recchia FM, Scott IU, Brown GC, et al. Small-gauge pars plana vitrectomy: a report by the American Academy of Ophthalmology. Ophthalmology. 2010;117::1851–1857. doi: 10.1016/j.ophtha.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Romero P, Salvat M, Almena M, Baget M, Mendez I. Experience with 25-gauge transconjunctival vitrectomy compared to a 20-gauge system. J Fr Ophtalmol. 2006;(29):1025–1032. doi: 10.1016/s0181-5512(06)73891-8. Analysis of 132 cases [in French] [DOI] [PubMed] [Google Scholar]
- 5.Thompson JT. Advantages and limitations of small gauge vitrectomy. Surv Ophthalmol. 2011;56::162–172. doi: 10.1016/j.survophthal.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Tarantola RM, Tsui JY, Graff JM, et al. Intraoperative sclerotomy-related retinal breaks during 23-gauge pars plana vitrectomy. Retina. 2013;33::136–142. doi: 10.1097/IAE.0b013e31825e1d62. [DOI] [PubMed] [Google Scholar]
- 7.Dugel PU. Efficiency in vitreoretinal surgery = safety. Retina Today. 2010;((September)):5–6. insert. [Google Scholar]
- 8.Downey JM. Hemodynamics. In: Johnson LR, editor. Essential Medical Physiology. 3rd ed. San Diego, CA: Elsevier;; 2003. pp. 157–174. In. ed. [Google Scholar]
- 9.Dugel PU, Buboltz D. New parameters in vitreoretinal surgery. Retina Today. 2011;((October)):83–88. [Google Scholar]
- 10.Hubschman JP, Gupta A, Bourla DH, et al. 20-, 23-, and 25-gauge vitreous cutters: performance and characteristics evaluation. Retina. 2008;28::249–257. doi: 10.1097/IAE.0b013e31815ec2b3. [DOI] [PubMed] [Google Scholar]
- 11.DeBoer C, Fang S, Lima LH, et al. Port geometry and its influence on vitrectomy. Retina. 2008;28::1061–1067. doi: 10.1097/IAE.0b013e3181840b64. [DOI] [PubMed] [Google Scholar]
- 12.Fang SY, DeBoer CM, Humayun MS. Performance analysis of new-generation vitreous cutters. Graefes Arch Clin Exp Ophthalmol. 2008;246::61–67. doi: 10.1007/s00417-007-0672-8. [DOI] [PubMed] [Google Scholar]
- 13.Nagpal M, Wartikar S, Nagpal K. Comparison of clinical outcomes and wound dynamics of sclerotomy ports of 20, 25, and 23 gauge vitrectomy. Retina. 2009;29::225–231. doi: 10.1097/IAE.0b013e3181934908. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro RM, Teixeira AG, Diniz B, et al. Performance analysis of ultrahigh-speed vitreous cutter system. Retina. 2013;33::928–932. doi: 10.1097/IAE.0b013e31826f069e. [DOI] [PubMed] [Google Scholar]
- 15.Magalhaes O, Jr, Chong L, DeBoer C, et al. Vitreous dynamics: vitreous flow analysis in 20-, 23-, and 25-gauge cutters. Retina. 2008;28::236–241. doi: 10.1097/IAE.0b013e318158e9e0. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira A, Chong LP, Matsuoka N, et al. Vitreoretinal traction created by conventional cutters during vitrectomy. Ophthalmology. 2010;117:1387–1392. doi: 10.1016/j.ophtha.2009.11.004. e2. [DOI] [PubMed] [Google Scholar]
- 17.Diniz B, Ribeiro RM, Fernandes RB, et al. Fluidics in a dual pneumatic ultra high-speed vitreous cutter system. Ophthalmologica. 2013;229::15–20. doi: 10.1159/000343073. [DOI] [PMC free article] [PubMed] [Google Scholar]