Abstract
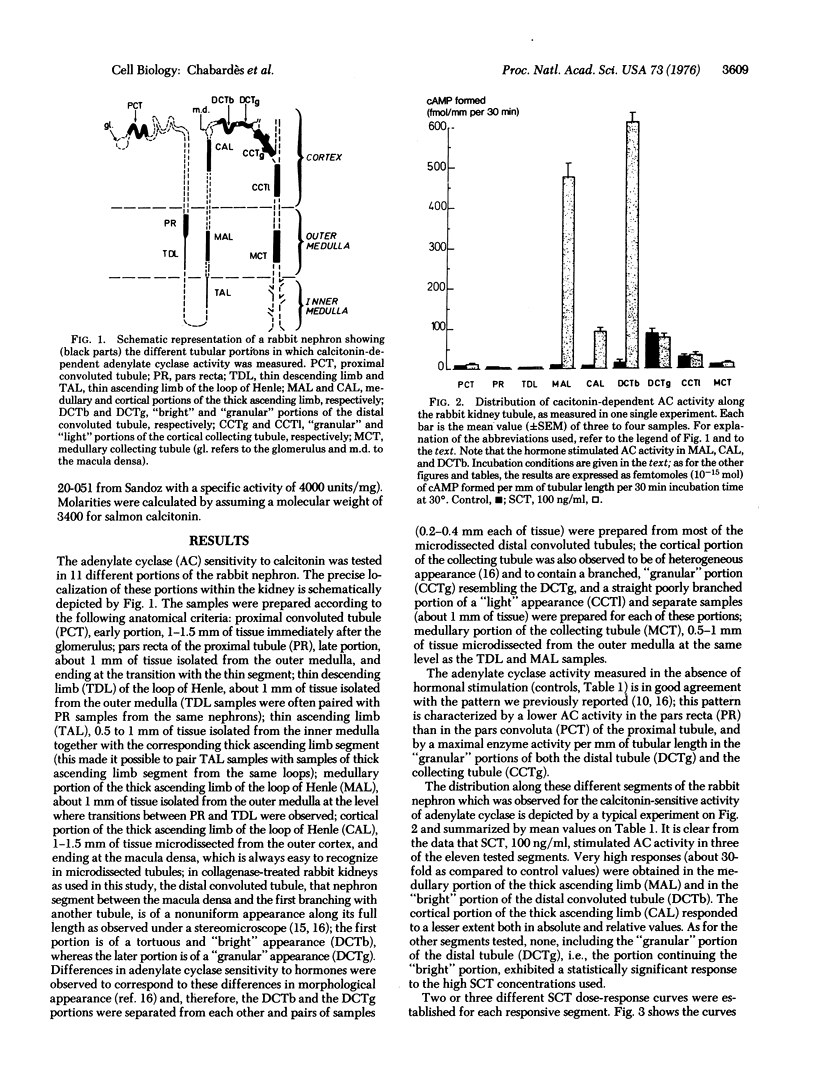
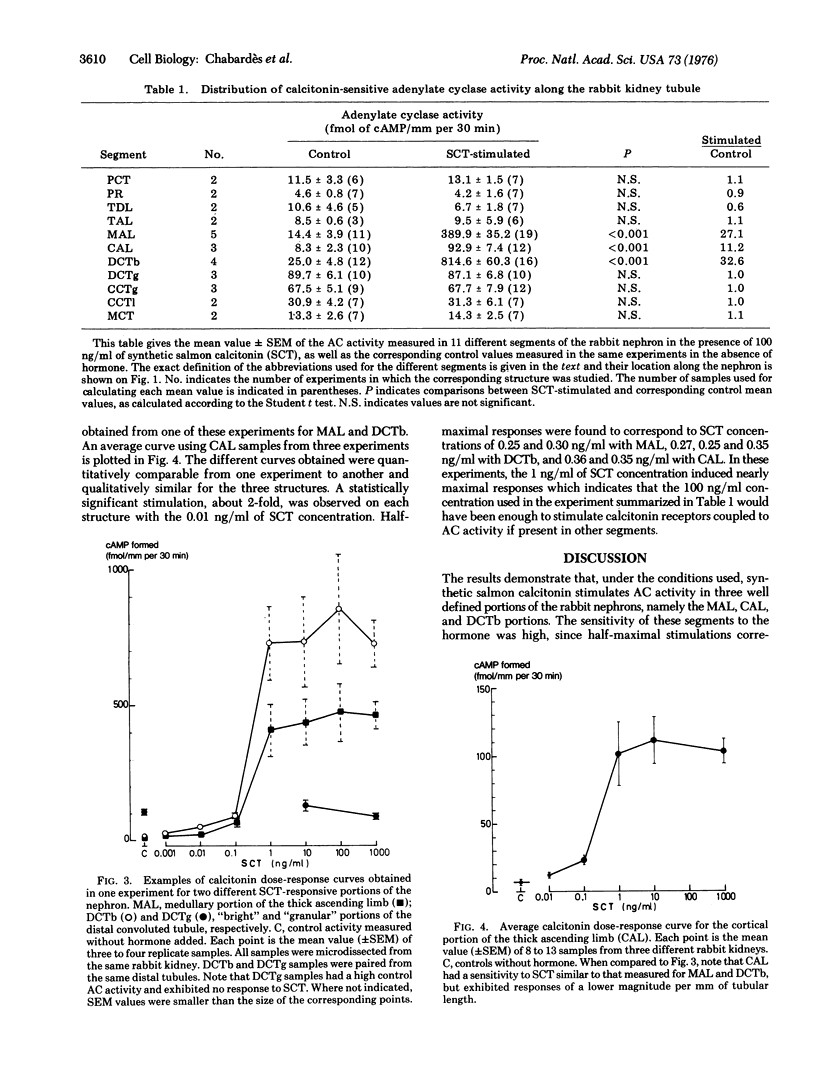
The adenylate cyclase [ATP pyrophosphatelyase (cyclizing), EC 4.6.1.1] sensitivity to salmon calcitonin in 11 different segments of the rabbit nephron was investigated using a micromethod for enzyme activity measurements in samples, each containing a single piece of tubule. The required segments were isolated by microdissection from collagenase-treated rabbit kidneys. The results were expressed as femtomoles of adenosine 3':5'-cyclic monophosphate formed per mm of tubular length per 30 min of incubation time. In the presence of 0.1 mug/ml of synthetic salmon calcitonin, it was found that eight segments exhibited no hormonal sensitivity whereas maximal responses were induced in three segments, the "bright" portion of the distal convoluted tubule, the cortical and the medullary portions of the thick ascending limb of the loop of Henle (stimulated over control activity ratios were 32, 11, and27). The very high sensitivity to calcitonin of the adenylate cyclase contained in these three segments (0.01 ng/ml of salmon calcitonin inducing a 2-fold stimulation; half-miximal stimulation corresponding to about 0.3 ng/ml of salmon calcitonin) suggests that the distal convoluted tubule, as well as th cortical and medullary portions of the thick ascending limb of the loop of Henle represent physiological target structures of calcitonin action within the kidney.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardaillou R. Kidney and calcitonin. Nephron. 1975;15(3-5):250–260. doi: 10.1159/000180515. [DOI] [PubMed] [Google Scholar]
- Aurbach G. D., Heath D. A. Parathyroid hormone and calcitonin regulation of renal function. Kidney Int. 1974 Nov;6(5):331–345. doi: 10.1038/ki.1974.118. [DOI] [PubMed] [Google Scholar]
- Bockaert J., Roy C., Rajerison R., Jard S. Specific binding of (3H) lysine-vasopressin to pig kidney plasma membranes. Relationship of receptor occupancy to adenylate cyclase activation. J Biol Chem. 1973 Sep 10;248(17):5922–5931. [PubMed] [Google Scholar]
- Brewer H. B., Jr, Schlueter R. J., Aldred J. P. Isolation and characterization of bovine thyrocalcitonin. J Biol Chem. 1970 Sep 10;245(17):4232–4240. [PubMed] [Google Scholar]
- Chabardès D., Imbert-Teboul M., Montégut M., Clique A., Morel F. Catecholamine sensitive adenylate cyclase activity in different segments of the rabbit nephron. Pflugers Arch. 1975 Dec 19;361(1):9–15. doi: 10.1007/BF00587334. [DOI] [PubMed] [Google Scholar]
- Chabardès D., Imbert M., Clique A., Montégut M., Morel F. PTH sensitive adenyl cyclase activity in different segments of the rabbit nephron. Pflugers Arch. 1975;354(3):229–239. doi: 10.1007/BF00584646. [DOI] [PubMed] [Google Scholar]
- Chase L. R. Selective proteolysis of the receptor for parathyroid hormone in renal cortex. Endocrinology. 1975 Jan;96(1):70–76. doi: 10.1210/endo-96-1-70. [DOI] [PubMed] [Google Scholar]
- Heersche J. N., Marcus R., Aurbach G. D. Calcitonin and the formation of 3',5'-AMP in bone and kidney. Endocrinology. 1974 Jan;94(1):241–247. doi: 10.1210/endo-94-1-241. [DOI] [PubMed] [Google Scholar]
- Hirsch P. F., Munson P. L. Thyrocalcitonin. Physiol Rev. 1969 Jul;49(3):548–622. doi: 10.1152/physrev.1969.49.3.548. [DOI] [PubMed] [Google Scholar]
- Imbert M., Chabardes D., Montegut M., Clique A., Morel F. Présence d'une adenyl-cyclase stimulée par la vasopressine dans la branche ascendante des anses des néphrons du rein de Lapin. C R Acad Sci Hebd Seances Acad Sci D. 1975 May 12;280(18):2129–2132. [PubMed] [Google Scholar]
- Imbert M., Chabardès D., Montegut M., Clique A., Morel F. Vasopressin dependent adenylate cyclase in single segments of rabbit kidney tubule. Pflugers Arch. 1975 Jun 26;357(3-4):173–186. doi: 10.1007/BF00585973. [DOI] [PubMed] [Google Scholar]
- Imbert M., Chabardès D., Montégut M., Clique A., Morel F. Adenylate cyclase activity along the rabbit nephron as measured in single isolated segments. Pflugers Arch. 1975;354(3):213–228. doi: 10.1007/BF00584645. [DOI] [PubMed] [Google Scholar]
- Kurokawa K., Nagata N., Sasaki M., Nakane K. Effects of calcitonin in the concentration of cyclic adenosine 3',5'-monophosphate in rat kidney in vivo and in vitro. Endocrinology. 1974 Jun;94(6):1514–1518. doi: 10.1210/endo-94-6-1514. [DOI] [PubMed] [Google Scholar]
- Loreau N., Lepreux C., Ardaillou R. Calcitonin-sensitive adenylate cyclase in rat renal tubular membranes. Biochem J. 1975 Sep;150(3):305–314. doi: 10.1042/bj1500305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus R., Aurbach G. D. Bioassay of parathyroid hormone in vitro with a stable preparation of adenyl cyclase from rat kidney. Endocrinology. 1969 Nov;85(5):801–810. doi: 10.1210/endo-85-5-801. [DOI] [PubMed] [Google Scholar]
- Marx S. J., Aurbach G. D. Renal receptors for calcitonin: coordinate occurrence with calcitonin-activated adenylate cyclase. Endocrinology. 1975 Aug;97(2):448–453. doi: 10.1210/endo-97-2-448. [DOI] [PubMed] [Google Scholar]
- Marx S. J., Fedak S. A., Aurbach G. D. Preparation and characterization of a hormone-responsive renal plasma membrane fraction. J Biol Chem. 1972 Nov 10;247(21):6913–6918. [PubMed] [Google Scholar]
- Marx S. J., Woodward C. J., Aurbach G. D. Calcitonin receptors of kidney and bone. Science. 1972 Dec 1;178(4064):999–1001. doi: 10.1126/science.178.4064.999. [DOI] [PubMed] [Google Scholar]
- Marx S. J., Woodward C., Aurbach G. D., Glossmann H., Keutmann H. T. Renal receptors for calcitonin. Binding and degradation of hormone. J Biol Chem. 1973 Jul 10;248(13):4797–4802. [PubMed] [Google Scholar]
- Melson G. L., Chase L. R., Aurbach G. D. Parathyroid hormone-sensitive adenyl cyclase in isolated renal tubules. Endocrinology. 1970 Mar;86(3):511–518. doi: 10.1210/endo-86-3-511. [DOI] [PubMed] [Google Scholar]
- Morel F., Chabardès D., Imbert M. Functional segmentation of the rabbit distal tubule by microdetermination of hormone-dependent adenylate cyclase activity. Kidney Int. 1976 Mar;9(3):264–277. doi: 10.1038/ki.1976.29. [DOI] [PubMed] [Google Scholar]
- Murad F., Brewer H. B., Jr, Vaughan M. Effect of thyrocalcitonin on adenosine 3':5'-cyclic phosphate formation by rat kidney and bone. Proc Natl Acad Sci U S A. 1970 Feb;65(2):446–453. doi: 10.1073/pnas.65.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]


