Abstract
Purpose:
To determine if potentially viable alternatives to the clinical use of intravitreal triamcinolone acetonide should be considered based on a comparative assessment of the in vitro effects of five commercially available corticosteroids. We hypothesized that dexamethasone, betamethasone, methylprednisolone, loteprednol etabonate, and fluocinolone acetonide, at clinically relevant doses, may show different levels of in vitro cytotoxicity to retinal cells.
Methods:
Cultures of human retinal pigment epithelial cells (ARPE-19) and rat embryonal neurosensory precursor retinal cells (R28) were treated with dexamethasone, betamethasone, methylprednisolone, loteprednol, or fluocinolone acetonide. Cell viability as a measure of cell death was determined by trypan blue dye exclusion assay. The mechanical effect of drug crystals was evaluated by solubilizing the steroid formulations. Mitochondrial dehydrogenase and membrane potential were assessed to measure cell damage.
Results:
Betamethasone, loteprednol, and methylprednisolone, in commercially available forms, caused significant cytotoxic changes to retinal cells in vitro at clinically relevant doses. This effect was less pronounced with solubilized betamethasone. Dexamethasone at concentrations up to 5 times the clinical dose of free drug injections and 1000 times greater than a drug implant did not cause decreased cell viability. Fluocinolone acetonide at doses 1000 times higher than observed with drug delivery systems showed no cytotoxic effect.
Conclusions:
Betamethasone, loteprednol, and methylprednisolone exhibited cytotoxicity at clinically relevant doses and do not appear to be good therapeutic options for intravitreal use. In comparison, dexamethasone and fluocinolone acetonide, which exhibited fewer cytotoxic effects than other steroids, may be potentially viable alternatives to triamcinolone acetonide for clinical use.
INTRODUCTION
Corticosteroids, a subclass of adrenal cortex–derived steroids, include both the glucocorticoids and mineralocorticoids. These hormones participate in various physiologic functions and pharmacologic effects both outside and within the eye. This class of drugs has been used to treat ocular pathologies via a variety of different routes, including oral, intravenous, topical, periocular, and intravitreal. They prevent leukocyte migration, reduce fibrin deposition, stabilize the endothelial cell tight junctions, and inhibit the synthesis of vascular endothelial growth factor (VEGF), prostaglandins, and proinflammatory cytokines.1
The systemic administration of corticosteroids generates many adverse events, such as osteoporosis, adrenal suppression, exacerbation of diabetes, and a cushingoid state.2–4 Topical or peribulbar administration delivers suboptimal vitreous drug levels and is associated with relatively high systemic corticosteroid concentrations, which can cause systemic side effects.5–8 Alternatively, direct intravitreal corticosteroid administration conveniently bypasses the blood-ocular barrier, leading to high concentration with little or no systemic adverse events.9 As a consequence, over the past decade, steroids are more frequently being delivered directly to the posterior segment of the eye to treat a wide range of retinal disorders.10–15
Clinically, the most commonly used intravitreal steroid is triamcinolone acetonide because of its durability and clinical efficacy associated with the stability of its depot formulation. However, triamcinolone acetonide has been reported by us and others to have direct cytotoxicity on retinal cells in culture. By comparison, Citirik and coworkers,16 using a rat model, showed that intravitreal injections of low-dose betamethasone (0.075 mg) and dexamethasone (0.1 mg) did not cause increased oxidative damage, whereas methylprednisolone and higher doses of betamethasone (0.15 mg) and dexamethasone (0.2 mg) were relatively toxic. In rat retinal degeneration models, it was demonstrated that intravitreal fluocinolone acetonide had neuroprotective effects with reduced neuroinflammation.17,18 However, these steroids have not been tested side-by-side in retinal cell models in vitro. Based upon these previous studies, we hypothesized that there would be different levels of cytotoxicity to retinal cells exposed to commonly used steroids (dexamethasone, betamethasone, methylprednisolone, loteprednol etabonate, and fluocinolone acetonide), with some steroids showing minimal to no toxic effects and others demonstrating significant damage to the cells. Evidence of lower in vitro cytotoxicity in alternative corticosteroids may lead us to consider viable alternatives to triamcinolone acetonide for use in clinical practice.
MECHANISMS OF ACTION OF INTRAOCULAR STEROIDS
In addition to its anti-inflammatory properties, the glucocorticoid steroids improve vascular permeability and have antifibrotic and antiangiogenic effects. When it comes to the treatment of macular edema, steroids improve blood-retinal barrier permeability through a series of different mechanisms, including the stabilization of lipid membranes, down-regulation of eucosanoid production, inhibition of macrophage migration,19–22 and increase in the expression of retinal endothelial junction proteins.23
Intravitreal or periocular injections of triamcinolone acetonide have been shown to decrease vascular leakage,24 reduce VEGF expression by retinal pigment epithelial cells after oxidative stress,25 down-regulate the expression of genes related to VEGF production in vascular smooth muscle cells,26 and reduce the intravitreal concentration of VEGF in patients with proliferative diabetic retinopathy.27 Moreover, triamcinolone acetonide down-regulates the expression of adhesion molecules involved in the inflammatory response28 and inhibits the leukocyte-endothelial interaction in diabetic rats.29
All the effects described above are regulated by genomic or posttranslational interactions and are typically observed hours after treatment. However, some clinical data show macular edema improvement within 1 hour of intravitreal injection,30 suggesting that at least part of the triamcinolone acetonide effects occur through nongenomic signaling, such as by activation of intracellular cascades caused by direct interaction of the drug to cellular membranes without the direct involvement of glucocorticoid receptors.30
HISTORICAL BACKGROUND ON INTRAVITREAL STEROID USE
The first intravitreal injection of steroids dates back to 1974, when Graham and Peyman31 injected intravitreal dexamethasone in an experimental model of endophthalmitis. Subsequently, Peyman and coworkers32–34 published case reports where dexamethasone was used as an adjunct for the treatment of bacterial endophthalmitis with gentamicin.
Late in the 1970s, Machemer and Tano35,36 showed that a single intravitreal injection of dexamethasone inhibited fibroblast growth and reduced the incidence of retinal detachment in a rabbit animal model. Similar experiments were conducted using triamcinolone acetonide, with equivalent results.37
In 1985, Ishibashi and coworkers38 tested the effects of steroids on a primate model of laser-induced subretinal neovascular membranes. Eyes treated with either dexamethasone or a combination of dexamethasone and triamcinolone acetonide developed subretinal neovascularization less frequently when compared to controls submitted to laser photocoagulation without pretreatment with steroids, and the investigators presumed the results were related to an antiangiogenic effect of the steroids.
While a limited number of case reports from the 1970s described the use of dexamethasone as an adjunct in the clinical treatment of infectious endophthalmitis, the expanded clinical use of steroids to treat a wide variety of ocular conditions is much more recent. In 1995, Penfold and coworkers39 published a pilot study on the use of intravitreal triamcinolone acetonide in cases of exudative age-related macular degeneration. Thirty eyes of 28 patients were evaluated, and after 18 months of follow-up, no serious adverse events were reported. Visual acuity was significantly better, and less exudation was noted in the treatment arm when compared to control untreated eyes. In a similar study reported in 2000,40 a single intravitreal injection of 4 mg of triamcinolone acetonide was administered in cases of exudative age-related macular degeneration, and better visual acuity was observed in the treatment arm after 3 and 6 months when compared to untreated controls. However, an increase in intraocular pressure (IOP) was noted in 25% of the treated patients, which was successfully managed with topical therapy.
By the late 1990s, the use of intravitreal steroids had spread to treat a wide variety of ocular conditions, such as noninfectious uveitis,41–45 macular edema related to Irvine-Gass syndrome,46 venous occlusion,47 diabetes,48,49 radiation retinopathy,50,51 and as an adjunct to photodynamic therapy in cases of exudative age-related macular degeneration.52
CURRENTLY USED INTRAOCULAR STEROIDS
The endogenous corticosteroids cortisol, cortisone, and corticosterone induce glucocorticoid as well as mineralocorticoid receptor activity, as does the synthetic steroid prednisone.53 In contrast, synthetic steroids such as dexamethasone, triamcinolone acetonide, and fluocinolone acetonide are potent and selective glucocorticoid receptor agonists with limited mineralocorticoid receptor activity.54 Despite the large number of synthetic glucocorticoids available, only the three steroids mentioned above are currently approved for intraocular use in humans.
There are two main structural elements that differentiate those three compounds: fluocinolone acetonide and triamcinolone acetonide have a stable C16–C17 acetonide group, whereas dexamethasone has a methyl group at the C16 position and a hydroxyl group at the C17 position. Fluocinolone acetonide also has a fluorine at the C6 position.55
Triamcinolone acetonide is five times more potent than the endogenous hydrocortisone when it comes to anti-inflammatory action, but is five times less potent than dexamethasone or fluocinolone acetonide.56 Dexamethasone, the most soluble glucocorticoid, has the highest maximum vitreous concentration, reaching a level of 3.0 mg/mL after injection of free drug solution, while triamcinolone acetonide depot intravitreal injection reaches a maximum vitreous concentration of 1.2 mg/mL.57 Consequently, the posterior segment injection of dexamethasone generates approximately 12.5 times higher anti-inflammatory potency when compared to triamcinolone acetonide, which generates a 4.8 times higher potency when compared to fluocinolone acetonide. Extended delivery of those drugs is dependent on either a drug-delivery device (as with dexamethasone or fluocinolone acetonide), or the natural dissolution of the glucocorticoid crystals (as with triamcinolone acetonide). No data is available on the half-life of intravitreal injections of fluocinolone acetonide, as there is no commercially available solution of fluocinolone acetonide. However, fluocinolone acetonide is used clinically in an extended drug delivery implant that has been shown to produce a steady-state intravitreal level of 0.15 μg/mL.58 The intraocular half-life of dexamethasone is very short (3.5 hours in rabbit eyes59 and 5.5 hours in humans60), so its clinical use in situations such as chronic retinal diseases or intraocular inflammation requires the use of slow-releasing intraocular drug delivery devices.
The dexamethasone drug delivery device (Ozurdex; Allergan Inc, Irvine, California) is approved in the United States for the treatment of macular edema secondary to branch or central retinal vein occlusion and for the treatment of posterior noninfectious uveitis, and is under review by the US Food and Drug Administration (FDA) for approval for the treatment of diabetic macular edema.61 The active drug is dispersed through a biodegradable copolymer of lactic and glycolic acid, forming a matrix structure (Novadur; Allergan).61 In the early phase 1 and 2 clinical trials, the dexamethasone drug delivery system was surgically implanted into the vitreous cavity through a pars plana incision.62–64 Subsequently, a single-use 22-gauge applicator was developed, allowing the injection of the matrix through a self-sealing biplanar incision.65 The pharmacokinetic profile indicates a high concentration of the drug for 60 days after injection, followed by a period with lower detectable levels of the drug up to day 210. In a monkey model, the maximum vitreous concentration was recorded at day 60 (213 ± 49 ng/mL) and the drug was present up to day 180.66
There are currently two different fluocinolone acetonide implants developed for clinical use. Retisert (Bausch and Lomb, Rochester, New York) is a slow-releasing fluocinolone acetonide device approved by the FDA in 2005 for the treatment of chronic noninfectious uveitis affecting the posterior segment of the eye.67 The 0.59-mg implant is surgically implanted and sutured to the sclera wall and releases the steroid at an initial rate of 0.6 μg/day, reaching a steady-state drug level after 1 month, with a release rate of 0.3 to 0.4 μg/day for about 30 months. Its main side effects are the high incidence of cataracts (90%+) among phakic patients and increase in IOP (>30%), which frequently requires surgical treatment due to its severity.68,69 Iluvien (Alimera Sciences, Alpharetta, Georgia) is an injectable sustained-release nondegradable implant of fluocinolone acetonide that contains roughly half the amount of fluocinolone acetonide as the Retisert implant and releases 0.2 μg/day of fluocinolone acetonide for over 2 years. It was recently approved in some European countries for the treatment of diabetic macular edema, based on the results of two large pivotal multicenter clinical trials. The FDA in the United States did not initially approve it for clinical use, though at the time of this writing, the FDA has indicated a willingness to approve it for a subset of patients with chronic diabetic macular edema, and the company is negotiating a potential limited label with the FDA.70
Different than dexamethasone, triamcinolone acetonide is only slightly soluble in water and has been shown to remain in the vitreous cavity five times longer than hydrocortisone.56 One of the major advantages of triamcinolone acetonide over dexamethasone is the sustained release of minute amounts from its crystalline form, which provides up to 9 months of antiproliferative, antiangiogenesis, and antipermeability effects in a pigmented rabbit animal model.71 Edelman and coworkers72 also showed that intravitreal triamcinolone acetonide suppresses VEGF-related retinopathy for 45 days, compared to 3 days of suppression obtained after intravitreal dexamethasone injection, which also shows a longer action of triamcinolone acetonide intravitreal injections compared to dexamethasone.
Recently, the FDA approved two different preservative-free formulations for intraocular use. Triesence (Alcon Labs, Fort Worth, Texas) was approved by the FDA in 2007 for intraoperative use to stain vitreous membranes and to treat ocular inflammatory conditions.73 The aqueous suspension provides 40 mg/mL of triamcinolone acetonide, with sodium chloride for isotonicity, 0.5% carboxymethylcellulose sodium, and 0.015% polysorbate 80.74
Trivaris (Allergan) was approved by the FDA for intraocular use in 2008. Each syringe of the sterile aqueous gel suspension of Trivaris contains 8 mg of triamcinolone acetonide in 0.1 mL (80 mg/mL) in a hydrogel Hyladur vehicle that is preservative-free.75 While it is not commercially available or marketed by its proprietary pharmaceutical company, it has been used in clinical trials such as the National Institutes of Health (NIH)–sponsored Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study for subjects with macular edema due to retinal vein occlusion,76 and various Diabetic Retinopathy Clinical Research Network (DRCRnet) randomized trials including protocol B, which compared laser to intravitreal triamcinolone acetonide for diabetic macular edema, and protocol I, which compared ranibizumab with immediate or deferred laser to laser alone to triamcinolone acetonide plus laser for diabetic macular edema.77
In an experimental pigmented rabbit model, both preservative-free FDA-approved triamcinolone acetonide formulations (Triesence and Trivaris) had a shorter anti-VEGF action and a shorter permanence in the vitreous cavity when compared to Kenalog. This difference in pharmacokinetics was potentially related to the size of the triamcinolone acetonide crystals in each of those triamcinolone acetonide formulations.71
OCULAR SIDE EFFECTS
The most common adverse effects related to intravitreal steroid injection are cataract formation and increase in IOP.78–81 Bakri and Beer80 reported an increase in IOP of at least 5 mm Hg in 49% of eyes 12 weeks after intravitreal triamcinolone acetonide injection; 28% of the patients had an increase in IOP of more than 10 mm Hg. Jonas and coworkers79 observed an increase in IOP in 50% of eyes 4 to 8 weeks after intravitreal triamcinolone acetonide injection. In most of the cases, IOP returned to normal after 6 months of follow-up. In the SCORE trial, increases in IOP of more than 10 mm Hg from baseline were observed in 8.9% of eyes at month 12, and 3.5% had an IOP greater than 35 mm Hg at 12 months.76,81 Wingate and Beaumont78 observed an IOP increase of 5 mm Hg or greater in 32% of eyes injected with triamcinolone acetonide after 1 to 2 months. Most series show adequate IOP control with topical drugs after triamcinolone acetonide injection. However, some cases need trabeculectomy or even vitrectomy with triamcinolone acetonide removal in order to achieve IOP control.82
In clinical testing of the Retisert fluocinolone acetonide implant, data pooled from three multicenter, randomized clinical trials showed that after 3 years of follow-up, 71% of implanted eyes had an increase in IOP of at least 10 mm Hg, and 55.1%, 24.7%, and 6.2% of the eyes implanted reached an IOP of 30 mm Hg or more, 40 mm Hg or more, or 50 mm Hg or more, respectively, by year 3. IOP-lowering medication was used in 74.8% of eyes, and IOP-lowering surgeries were needed in 36.6% of implanted eyes.69 In the Fluocinolone Acetonide in Macular Edema (FAME) study evaluating the Iluvien fluocinolone acetonide implant, which contains roughly one-half the amount of fluocinolone acetonide as the Retisert fluocinolone acetonide implant, the proportion of Iluvien-treated subjects requiring treatment with IOP-lowering medication was 38% compared to 14% in the sham-treated group. This proportion increased to 47% in those subjects with greater than median IOP at baseline (≥15 mm Hg).70 Incisional glaucoma surgery was performed in 8.1% of eyes in the high-dose group (0.5 μg/day), 3.7% in the low-dose group (0.2 μg/day), and 0.5% in the sham group after 24 months of follow-up.70
Ocular hypertension was reported in significantly more eyes receiving the dexamethasone implant than in controls in the Global Evaluation of Implantable Dexamethasone in Retinal Vein Occlusion With Macular Edema (GENEVA) multicenter study comparing Ozurdex vs sham injections for macular edema caused by central or branch retinal vein occlusion. Changes in IOP in the dexamethasone-implanted eyes peaked at day 60 and were no different from sham by day 180. Most eyes with increases in IOP were successfully managed with IOP-lowering medication, but a limited number of eyes required surgical procedures to control IOP.83 After 12 months of follow-up, only 0.9% (3/324) of eyes treated with two injections of Ozurdex had an increase in IOP from baseline greater than 10 mm Hg, and no eyes had an IOP greater than 35 mm Hg at that time point.84 In patients with diabetic macular edema injected with Ozurdex, an IOP of 25 mm Hg or higher was seen in 7.5% of eyes in the 700-μg Ozurdex group and in 12.7% of eyes in the 350-μg dexamethasone drug delivery system group 90 days after injection. All eyes were successfully managed with observation or IOP-lowering medication.62
Cataract progression is another common adverse event after injections of intravitreal steroids, though this finding is typically seen in the second year of treatment rather than in the first year. The DRCRnet reported that 83% of phakic eyes that received intravitreal triamcinolone acetonide for diabetic macular edema needed cataract surgery after 3 years of follow-up, vs 31% of patients in the laser-treatment arm.77 In the SCORE trial, after a more limited 12-month follow-up, cataract extraction was required in 5.3% of phakic eyes with central retinal vein occlusion and 3.6% of phakic eyes with branch retinal vein occlusion.76,81
Nearly all phakic patients receiving the Retisert implant developed cataract within 3 years after implantation.68 In the FAME study, where patients received one two doses of the injectable fluocinolone acetonide implant, the incidence of cataract surgery among all phakic subjects was 74.9% in the low-dose group (0.2 μg/day), 84.5% in the high-dose group (0.5 μg/day), and 23.1% in the sham group, after a follow-up of 24 months.70
In the GENEVA study, where patients with macular edema due to branch or central retinal vein occlusion were treated with Ozurdex, cataract extraction was performed in 1.3% of phakic eyes after a 12-month follow-up.84 However, more cataract progression occurred in eyes that received more than one Ozurdex injection during that time period.84 No significant difference in the number of reports of cataract was noted after Ozurdex injection in patients with diabetic macular edema after a 6-month follow-up in a phase 2 clinical trial.62 However, as noted above from eyes treated with steroids in clinical trials lasting 2 or more years, cataract progression with steroids is more commonly seen after 18 to 24 months of treatment, so that trials reporting more short-term data are not as reliable with regard to long-term cataract incidence.
Bacterial endophthalmitis after intravitreal steroids is a less common complication, being reported in 0.38% to 1.70% of intravitreal triamcinolone acetonide injections. Known risk factors include diabetes mellitus, multiple injections using the same triamcinolone acetonide vial, filtering blebs, and blepharitis.85 There is also a known risk of sterile endophthalmitis following injections of intravitreal triamcinolone acetonide, with a prevalence ranging from 0.1% to 23.8%.86–91 The clinical characteristics of sterile endophthalmitis may overlap with bacterial endophthalmitis. Usually, the sterile cases have better initial visual acuity, less pain, less chemosis, and better visual prognosis. Evidence suggests that a toxic sterile reaction triggered by the preservative benzyl alcohol is at least partially related to the genesis of the exacerbated intraocular inflammation, as the prevalence of sterile endophthalmitis decreases (but is not totally prevented) by the intravitreal use of preservative-free triamcinolone acetonide formulations.92,93 Additionally, in patients with sterile endophthalmitis, it seems that inflammatory reactions are less severe when preservative-free triamcinolone acetonide is used instead of Kenalog.93
Less common side effects related to intravitreal triamcinolone acetonide injection include the reactivation of posterior placoid chorioretinopathy,94 exacerbation of central serous chorioretinopathy,95 or rupture of filtering blebs.96 A crystalline retinal maculopathy related to intravitreal triamcinolone acetonide injections, characterized by ring-shaped iridescent spots around the macula, has been described but does not seem to affect visual acuity.97
CLINICAL TRIALS INVOLVING INTRAVITREAL STEROID USE FOR THE TREATMENT OF MACULAR EDEMA
Because of the mechanisms of action and potential improvement in macular thickness and visual acuity observed clinically with intravitreal steroids, a number of clinical trials assessing the risks and benefits of intravitreal steroids have been performed. The DRCRnet protocol B clinical trial compared 378 eyes with diabetic macular edema receiving 1 or 4 mg of preservative-free triamcinolone acetonide (Trivaris) or focal/grid photocoagulation. After 3 years of follow-up, visual acuity outcomes slightly favored the laser group compared to the two groups receiving preservative-free triamcinolone acetonide, so there was no long-term benefit of intravitreal triamcinolone relative to focal/grid photocoagulation for patients with diabetic macular edema. Additionally, the side effect profile favored laser over steroid use due to cataract and glaucoma formation in the triamcinolone-treated groups.77
In the SCORE study, grid photocoagulation or observation was compared to repeated injections of 1 or 4 mg of preservative-free triamcinolone acetonide (Trivaris). All treatments were equally effective in improving visual acuity in patients with macular edema due to branch retinal vein occlusion: a 15-letter gain from baseline to month 12 was observed in 29% of patients after laser photocoagulation and in 26% and 27% of patients treated with 1 or 4 mg triamcinolone acetonide, respectively. However, due to cataract formation and glaucoma in the steroid arms, laser was deemed to be the preferred treatment.81
In contrast, intravitreal triamcinolone acetonide was determined to be superior to observation for macular edema in patients with central retinal vein occlusion. Treatment with 1 or 4 mg of Trivaris produced a 15-letter gain in 27% and 26%, respectively, of the patients after 12 months of follow-up.76 There were no statistically significant differences in visual acuity between both steroid treatment arms, but the adverse rates of elevated IOP and cataract were higher in patients receiving 4 mg triamcinolone acetonide. Therefore, the SCORE investigators recommended 1 mg intravitreal triamcinolone acetonide for the treatment of macular edema due to central retinal vein occlusion.76
The fluocinolone acetonide implant (Retisert), approved by the FDA to treat chronic, noninfectious posterior uveitis, has been investigated as a treatment for diabetic macular edema. Multiple randomized clinical trials have demonstrated medium-term anatomic and visual improvement, but the device has not been approved in diabetics due to the previously described ocular side effects. The device contains 0.59 mg of fluocinolone acetonide and releases drug for up to 3 years. The implant is sutured to the sclera wall in an operating room setting.98 Another steroid drug implant containing fluocinolone acetonide has also been tested in patients with diabetic macular edema. The Iluvien implant (Alimera Sciences) contains roughly half the amount of fluocinolone acetonide as the Retisert implant and is injected into the eye with a 25-gauge injector system in an office setting. Two forms of the implant were tested—a low-dose device that releases 0.2 μg fluocinolone acetonide per day for up to 3 years, and a high-dose device that releases 0.5 μg fluocinolone acetonide per day for up to 2 years. The FAME study tested subjects with persistent diabetic macular edema despite at least one macular laser treatment, and randomized those patients to sham injection (n=122), low-dose fluocinolone acetonide insert injection (0.2 μg/day, n=375), or high-dose fluocinolone acetonide insert injection (0.5 μg/day, n=393). After 2 years of follow-up, the percentage of patients with at least a 15-letter improvement from baseline was 28.7% in the low-dose group and 28.6% and high-dose group, compared with 16.2% in the sham group (P=.002). The mean improvement in best-corrected visual acuity between baseline and month 24 was 4.4 in the low-dose group and 5.4 letters in the high-dose group, compared with 1.7 letters in the sham-injected group.70
The Ozurdex GENEVA Study Group tested the dexamethasone drug delivery system in patients with macular edema due to branch or central retinal vein occlusion. A total of 1267 patients were enrolled to receive either the 350-μg or 700-μg dexamethasone implant or sham injections. The proportion of eyes achieving at least a 15-letter improvement from baseline best-corrected visual acuity was significantly greater in both dexamethasone implant treatment groups than in the sham group from days 30 to 90, but not on day 180, with the peak response at day 60. After 6 months of follow-up, mean increase in visual acuity was greater in both dexamethasone groups when compared to sham, and no difference at any time point was observed between the 350-μg or 700-μg dexamethasone implants. The mean decrease in central retinal thickness was significantly greater with either dexamethasone implant than with sham after 90 days, but not after 180 days of follow-up.83 Reinjection of the dexamethasone implant in patients who met re-treatment criteria was safe and well tolerated over 12 months. The 12-month extension study included 327 patients who were initially treated with sham and did not receive a first dexamethasone implant over the first 6 months of the study. In this group, the mean improvement in best-corrected visual acuity did not seem to be as robust as in patients who received the implant in the beginning of the study.84 A subanalysis of the GENEVA trial confirmed this finding and concluded that each 1-month increase in duration of macular edema prior to treatment was associated with approximately a 10% lower likelihood of achieving a better corrected visual acuity improvement of 15 letters or more after 6 or 12 months of treatment, as well as a significantly less likelihood of achieving a reduction in central retinal thickness of 200 μm or more 6 months after treatment.99
In a phase 2 clinical trial assessing the dexamethasone implant, 315 patients with persistent macular edema were randomized to observation or treatment with either the 350-μg or 700-μg dexamethasone implants. A statistically significant difference in the proportion of eyes achieving at least a 10-letter improvement in visual acuity was observed between the 700-μg dexamethasone implant and the observation group at days 60 (26% vs 9%) and 90 (33% vs 12%), but the difference was no longer statistically significant at day 180. A statistically significant 10-letter improvement compared to the observation arm was also noted with the 350-μg implant group after day 60, but not after days 90 or 180. Moreover, there was a statistically significant difference in both central retinal thickness and fluorescein leakage in eyes that received the high-dose implant compared with the observation group.63 A subanalysis of this phase 2 study concluded that the 700-μg dexamethasone implant produced statistically significant improvements in visual acuity that were independent from the pattern of persistent diabetic macular edema initially presented (focal, cystoid, diffuse, or combined).100
The CHAMPLAIN Study101 was a prospective, multicenter, open-label, 26-week study that recruited 56 patients with persistent diabetic macular edema and a history of previous pars plana vitrectomy. The study eye received a single intravitreal injection of the 700-μg dexamethasone. Improvement in central retinal thickness was seen as early as 1 week after the injection. The mean increase in visual acuity was 6.0 letters at week 8 and 3.0 letters at week 26 (P=.0046). At week 8, 30.4% of patients had gained more than 10 letters of visual acuity, and that number dropped to 21% after week 26.
IN VITRO TOXICITY OF GLUCOCORTICOID STEROIDS
Numerous studies have assessed the effects of steroids on ocular cells in culture, with the preponderance of those studies evaluating the effects of triamcinolone acetonide on retinal cells. Kenalog (Bristol-Meyers Squibb, Princeton, New Jersey), initially developed for intra-articular or intramuscular applications, is still used for intravitreal injection despite not being recommended for intraocular use by its manufacturer. Its vehicle includes 0.99% benzyl alcohol as a preservative and bactericidal agent. The delivery of the drug through a 30-gauge pars plana injection makes it a very convenient in-office procedure. However, the ocular safety of Kenalog has long been a concern,102 which has resulted in a “black box” warning regarding its ocular side effect profile on its package insert. Previous studies have shown that triamcinolone acetonide is toxic to retinal cells in vitro. Yeung and coworkers103 incubated immortalized retinal pigment epithelial cells (ARPE-19) and human glial cells with Kenalog, and concentrations as low as 0.01 mg/mL caused a decrease in cell viability and an increase in caspase-3 expression after 24-hour exposure. The exposure of retinal cells to benzyl alcohol, the preservative in Kenalog, at clinically relevant concentrations up to 0.025% did not cause any reduction in cell viability. Shaikh and coworkers104 incubated ARPE-19 cells with Kenalog or a preservative-free formulation from a compounding pharmacy (Leiter’s Pharmacy, San Jose, California) for 5 days, and assessed cell viability using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. A reduction in cell viability was noted with both formulations tested at the concentration of 1.0 mg/mL. Narayanan and coworkers105 exposed ARPE-19 and R28 (rat embryonic precursor neurosensory retinal cells) to Kenalog and tested cell viability with the trypan-blue dye exclusion assay and mitochondrial damage via the mitochondrial dehydrogenase activity after 2, 6, or 24 hours of exposure. Concentrations higher than 200 μg/mL caused a reduction in cell viability in both cell lines tested, and a decrease in mitochondrial dehydrogenase levels, indicative of a diminished number of metabolically active cells, whereas treatment with the triamcinolone acetonide–free vehicle did not cause a reduction in cell viability. Oh and coworkers106 incubated ARPE-19 cells and cultured choroidal fibroblasts with a triamcinolone acetonide formulation containing preservative (Triamcinolone; Dong Kwang Pharmaceutical Co Ltd, Seoul, Korea) and observed that 0.01 mg/mL triamcinolone acetonide caused a reduction of cell viability in both cell lines after a 30-minute exposure. Chung and coworkers107 treated primary rat retinal cells in culture for 12 to 24 hours with either Kenalog or dexamethasone and assessed the number of dead cells by measuring cellular DNA contents using DNA-binding fluorescent dyes. Concentrations ranging from 100 to 800 μg/mL of triamcinolone acetonide caused a significant reduction of retinal cells in culture. Triamcinolone acetonide–induced cytotoxicity was mediated by oxidative stress, p38 kinase, c-Jun n-terminal kinase, caspase-1, and caspase-3. Zacharias and coworkers108 exposed ARPE-19 and R28 cells to Triesence. The drug in its crystalline form reduced cell viability in both cell lines. The toxicity was diminished when the drug was solubilized in dimethyl sulfoxide (DMSO), but even then an up-regulation in caspase activity was noted, suggestive of apoptosis.
Triamcinolone acetonide exposure reduces cell viability to other eye-related cell lines in vitro, such as the human lens epithelial (HLE-B3) cells109 or human trabecular meshwork (HTM) cells.110 Concentrations ranging from 200 to 1000 μg/mL caused a decrease in cell viability and an increase in caspase activity in human lens epithelial cells, indicative of apoptosis after 24-hour exposure.109 Exposure of HTM cells to triamcinolone acetonide in crystalline or solubilized forms caused a decrease in cell viability without caspase-3/7 up-regulation, suggestive of necrosis.110
Despite the general agreement in the literature regarding the observation of in vitro retinal cell toxicity after triamcinolone acetonide exposure, its mechanism is still to be determined. Some evidence indicates that it is at least partially related to the preservative benzyl alcohol.111–113 Due to the potential retinal toxicity related to the preservative, many retinal specialists shifted to preservative-free triamcinolone acetonide formulations in clinical use. However, despite the lack of preservative in its composition, a significant decrease in cell viability of retinal cells is noted in vitro after exposure to triamcinolone acetonide, which is avoided in vitro by the solubilization of the drug.108 Therefore, the toxicity observed in vitro is at least partially related to direct contact of the triamcinolone acetonide crystals on the retinal cells.114–116
Although there is significant evidence showing the in vitro toxicity of triamcinolone acetonide, electroretinographic (ERG) data in animals is inconclusive, and most studies show no in vivo toxicity.111,112,117–120 This in vivo: in vitro toxicity dissociation may be related to intraocular protective barriers that prevent the direct contact of triamcinolone acetonide crystals on retinal cells, such as the vitreous gel or the internal limiting membrane (ILM).114,115 Therefore, patients submitted to vitrectomy with or without ILM removal, or patients submitted to inadvertent subretinal triamcinolone acetonide injection, may theoretically be more prone to in vivo retinal toxicity, similar to what is seen in vitro.108,114,115,121
Given the general concerns regarding the potential of in vivo triamcinolone acetonide toxicity to the retina, despite the general benefit seen when used clinically, we propose that other glucocorticoid steroid options should be tested in vitro in order to determine whether an alternate, safer option may exist among clinically available steroid formulations. The literature assessing comparative cytotoxicity of the various steroid formulations in vitro is somewhat limited. The current study proposes to assess the range of commercially available steroids at clinically relevant doses on retinal cells in culture in order to determine whether their cytotoxic profile is favorable in comparison to that of triamcinolone acetonide, as a precursor to subsequent clinical testing.
OBJECTIVE
The objective of this study was to determine whether viable alternatives to triamcinolone acetonide, the steroid most commonly used for retinal pathologies, can be considered among commercially available steroid formulations based on their relative in vitro retinal cytotoxicities. This series of experiments utilizes standard in vitro cell cultures of retinal cell lines comprising neurosensory retina and retinal pigment epithelium cells as a model for the mammalian retina. Additionally, standard in vitro assays that assess cell injury (mitochondrial membrane potential and mitochondrial dehydrogenase assays) and cell death (trypan blue dye exclusion cell viability assay) were employed after retinal cells were exposed to the various commercially available steroid preparations in order to quantify and compare the degree of cytotoxicity induced by each steroid formulation. These differential cytotoxicity studies on retinal cells in culture may help guide clinicians in determining whether safer and more effective alternative steroid formulations can be considered for clinical testing based on the relative degree of toxicity observed in vitro, particularly in comparison to the steroid most commonly used for retinal conditions, triamcinolone acetonide.
Therefore, we hypothesized that dexamethasone, betamethasone, methylprednisolone, loteprednol etabonate, and fluocinolone acetonide, at clinically relevant doses, may show different levels of in vitro cytotoxicity to retinal cells when compared to triamcinolone acetonide.
MATERIALS AND METHODS
CELL CULTURE RATIONALE, TECHNIQUE, AND DRUG EXPOSURE CALCULATION
The current set of studies presented here utilizes two cell lines representing the two key constituents of the mammalian retina: neurosensory retinal cells and retinal pigment epithelial cells. Both cell lines utilized in these experiments are commonly used in laboratory settings, and there is extensive literature validating their use. Both cell lines used here are immortalized cell lines and are available from either commercial or university laboratories. The advantage of utilizing immortalized cell lines is that their characteristics are highly reproducible so that it is possible for other laboratories to confirm or disprove published findings in a reliable manner. Primary cell lines are frequently used as well in this field, as primary cell cultures produce cells that retain more of the features of the original cell type. However, primary cell cultures can be difficult to produce and may be somewhat variable based on the source of the original cell (ie, age/race/disease state of the donor), so it may be difficult to standardize techniques and reproduce findings from other laboratories. The field of predictive toxicology is highly dependent on the utilization of in vitro methods as a basis for determining whether the experimental findings of relative safety or toxicity can justify subsequent in vivo investigation either in animals or in humans. The cell culture media were also carefully chosen in the context of their use in testing glucocorticoid cytotoxicity. According to the manufacturer, Fetal Bovine Serum remains the most widely used undefined supplement in eukaryotic, especially mammalian, cell culture media because it provides a wide array of functions in cell culture. Fetal Bovine Serum delivers nutrients, growth, and attachment factors and protects cells from oxidative damage and apoptosis by mechanisms that are difficult to reproduce in serum-free media systems. Charcoal Stripped Fetal Bovine Serum is sometimes used as an alternate cell culture medium, primarily for experiments requiring decreased levels of various steroid hormones.122 In our study, the influence of other steroid hormones, such as T3, T4, and progesterone, are likely negligible, which led to the preference of regular Fetal Bovine Serum here and in numerous other relevant studies from both our laboratory and others.103–110,114,115,123
An ARPE-19 cell line was obtained from ATCC (Manassas, Virginia). ARPE-19 cells were grown in the standard cell culture medium utilized for ARPE-19 cells, consisting of a 1:1 mixture (vol/vol) of Dulbecco’s Modified Eagle’s Medium with Ham’s F12 nutrient mixture (DMEM/F12; Gibco, Carlsbad, California), nonessential amino acids 10 mM 1X, 0.37% sodium bicarbonate, 0.058% L-glutamine, 10% fetal bovine serum, and antibiotics (penicillin G 100 U/mL, streptomycin sulfate 0.1 mg/mL, gentamicin 10 μg/mL, Fungizone [amphotericin B] 2.5 μg/mL).
R28 cells were derived from postnatal day 6 rat retina in the laboratory of Gail M. Seigel at the University of Buffalo, State University of New York.124 R28 cells express genes characteristic of neurons125 as well as functional neuronal properties.126 R28 cells were cultured in the standard cell culture medium utilized for R28 cells as recommended by their originating laboratory at the University of Buffalo: Dulbecco’s Modified Eagle Medium, high glucose (DMEM high glucose; Gibco) with 10% fetal bovine serum, 1X minimum essential medium (MEM), nonessential amino acids 10 mM 1X, 0.37% sodium bicarbonate, and 10 μg/mL gentamicin.
The cells were plated onto 6-well tissue culture plates for the cell viability assay, 16-well plates for the mitochondrial membrane potential JC-1 assay, or 96-well enzyme-linked immunosorben assay (ELISA) plates for the mitochondrial dehydrogenase WST-1 assay and incubated at 37°C in 5% CO2 to reach 70% to 80% confluence, then were placed in a serum-free medium to create a sessile nonproliferative environment before being exposed to the drug.
Clinically relevant drug concentrations were calculated in each experiment by assuming that the volume of commercially available steroid, if it were to be injected into a human eye, would be equally distributed throughout the 4-mL human vitreous. With this assumption, concentrations of drug exposed to retinal cells in each experiment were based on actual therapeutic amounts injected intravitreally in a clinical setting. In the case of topical steroid formulations such as loteprednol etabonate, which have not been injected into the vitreous previously, we assumed that the clinically relevant amount would be 0.1 mL of drug solution from the commercially available eyedrops preparation, since 0.1 mL is considered the largest volume that can physically be safely injected into the human vitreous at one time. For example, triamcinolone acetonide is typically injected as 4 mg of drug in a 0.1-mL injected volume taken from the commercially available bottle. Assuming equal distribution of the 4 mg of injected triamcinolone acetonide throughout the 4-mL vitreous, then a clinically equivalent dose of triamcinolone acetonide would be 1 mg/mL. Cells were exposed to a range of drug concentrations, typically ranging from one-tenth the clinically equivalent dose up to as much as 10 times that amount, depending on solubility and crystalline characteristics of each steroid formulation.
EXPOSURE TO DEXAMETHASONE SODIUM PHOSPHATE
Cells were treated with one of four different clinically relevant concentrations of dexamethasone sodium phosphate (DEX-S; American Regent Laboratories Inc, Shirley, New York): 0.125 mg/mL, 0.25 mg/mL, 0.50 mg/mL, or 1 mg/mL for 2, 6, or 24 hours. ARPE-19 and R28 cells were also treated with the corresponding concentrations of the preservative benzyl alcohol (Fisher Scientific, Fair Lawn, New Jersey) alone: 0.03125%, 0.0625%, 0.125%, or 0.25%. The clinically relevant dose assumed that in clinical settings, 0.4 mg of dexamethasone is frequently injected into the vitreous, and assuming equal distribution of drug throughout the 4-mL human vitreous, then 0.1 mg/mL would be the clinically equivalent dose. The doses tested here ranged from 1.25× the clinical dose to 10× the clinical dose.
In order to access the contribution of the preservative benzyl alcohol in our results, dexamethasone powder (DEX-P; Alexis Biochemicals Corporation, San Diego, California) was solubilized in DMSO, and subsequently both cell lines were exposed to 1 mg/mL dexamethasone powder for 24 hours.
Before performing the dye exclusion assay, pictures of cells were taken using 20× magnification phase contrast microscopy in order to verify the condition of the cultures.
EXPOSURE TO COMMERCIAL BETAMETHASONE (BETA-C)
Celestone Soluspan comes in a suspension of 6 mg/mL, which contains 3 mg betamethasone sodium acetate and 3 mg betamethasone sodium phosphate. Its preservatives include 7.1 mg dibasic sodium phosphate, 3.4 mg monobasic sodium phosphate, 0.1 mg edentate disodium, and 0.2 mg benzalkonium chloride. The clinically equivalent dose concentration would therefore assume that 0.1 mL of the 6 mg/mL steroid suspension containing a total of 600 μg of drug would be injected into the 4 mL human vitreous, and that this amount of drug would distribute equally throughout the vitreous, creating a concentration of 150 μg/mL as the clinically equivalent dose. A range of concentrations above and below this clinically equivalent dose of commercial betamethasone (Celestone Soluspan; Schering Corporation, Kenilworth, New Jersey) or preservative benzalkonium chloride alone (Alfa Aesar, Ward Hill, Massachusetts) were added to the culture media for 2, 6, or 24 hours. Cells were exposed to four different concentrations of Celestone Soluspan: 30, 45, 90, or 180 μg/mL, and corresponding preservative benzalkonium chloride alone concentrations of 1, 1.5, 3, or 6 μg/mL. The cell viability was measured at 2, 6, or 24 hours.
EXPOSURE TO SOLUBILIZED BETAMETHASONE (BETA-S)
Cells were treated for 24 hours with 90, 180, 360, or 720 μg/mL concentration of betamethasone resuspended in DMSO, after removal by centrifugation of the supernatant. The reference clinically equivalent dose per above is 150 μg/mL, so that the solubilized drug was tested at levels ranging from 0.6 times the clinically equivalent dose up to 4.8 times that dose. Briefly, the commercial preparation was centrifuged at 5000 rpm for 1 minute, the supernatant containing the vehicle was pipetted out, and the pellet of betamethasone was resuspended in an equivalent amount of DMSO to achieve the same concentrations of betamethasone as in the commercial suspension (6 mg/mL). The solution was then added to the serum-free culture media, and serial dilutions were prepared to obtain the desired concentrations. Control groups consisted of ARPE-19 or R28 cells that were incubated in serum-free medium for 24 hours, then treated with one of the corresponding four concentrations of DMSO.
EXPOSURE TO METHYLPREDNISOLONE (MPA)
Methylprednisolone acetate (MPA; Pharmacia & Upjohn Company, Kalamazoo, Michigan) comes in a 40 mg/mL suspension. Each milliliter contains 29.1 mg of polyethylene glycol, 1.94 mg of polysorbate, 1.42 mg of monobasic sodium phosphate, and 9.16 mg of benzyl alcohol, and sodium chloride is added to adjust tonicity. Assuming a maximum volume of injection of 0.1 mL of this 40 mg/mL drug solution into the 4-mL human vitreous and equal distribution of drug throughout the human vitreous, the clinically equivalent concentration is 1000 μg/mL. The cells were treated with one of four different concentrations of the drug, ranging from 1/40th to one-half the clinical dose: 25, 50, 100, or 500 μg/mL, or to one of the corresponding four concentrations of the vehicle alone, isolated after the drug was centrifuged: 18.75, 37.5, 75, and 375 μg/mL. Cell viability was measured after 2, 6, or 24 hours of exposure using the trypan blue dye exclusion cell viability assay. In order to test the effects of the direct contact of the crystals to the cells, we used the same methods described for bethamethasone: the drug was centrifuged and the pellet was resuspended in DMSO. The solution was then added to the serum-free culture media, and serial dilutions were prepared to obtain all the desired concentrations. ARPE-19 and R28 cells were incubated in serum-free medium for 24 hours, then treated with solubilized methylprednisolone at the concentrations of 50, 100, 500, or 1000 μg/mL, for 24 hours, ranging from 1/20th to 1× the clinical dose. Control cells were cells treated with the corresponding four concentrations of DMSO used to solubilize the drug and untreated cells.
EXPOSURE TO LOTEPREDNOL (LE)
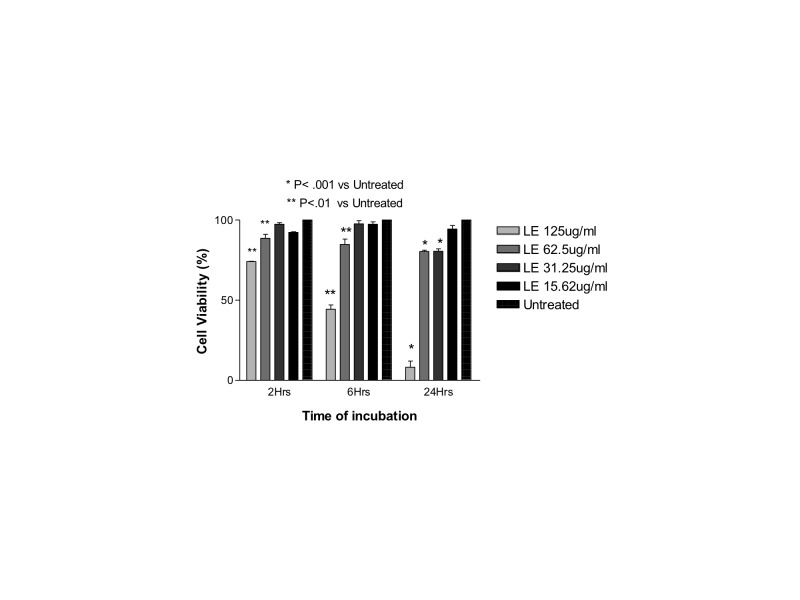
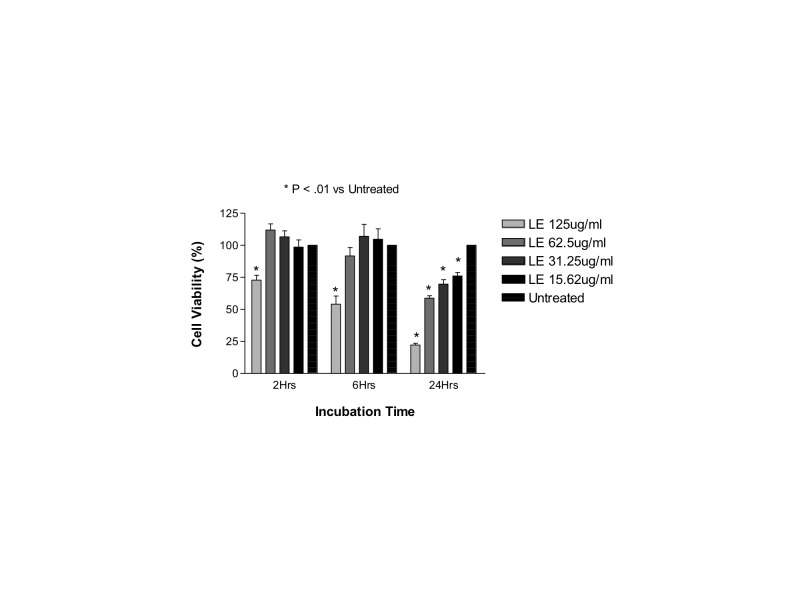
Both cells line were treated with 125 μg/mL, 62.5 μg/mL, 31.25 μg/mL, or 15.62 μg/mL of loteprednol etabonate 0.5% suspension (Lotemax; Schering-Plough; Kenilworth, New Jersey) for 2, 6, or 24 hours. These concentrations were prepared using serial dilutions of the whole drug in the respective serum-free culture medium. The solution of the drug mixed with media was then directly added to the cells. Untreated ARPE-19 and R28 cells incubated in serum-free medium for 24 hours served as controls. The clinically equivalent dose was calculated by assuming that a maximum volume of 0.1 mL of the 0.5% commercial suspension would be injected into the eye. By definition, a 0.5% drug solution contains 0.5 g/100mL of drug, or 5 mg/mL, and an injection of 0.1 mL of this solution would contain 500 μg of drug. Assuming equal distribution throughout the 4-mL human vitreous would mean that the theoretical clinically equivalent dose would be 125 μg/mL. The doses tested ranged from the clinical dose to one-eighth the clinical dose.
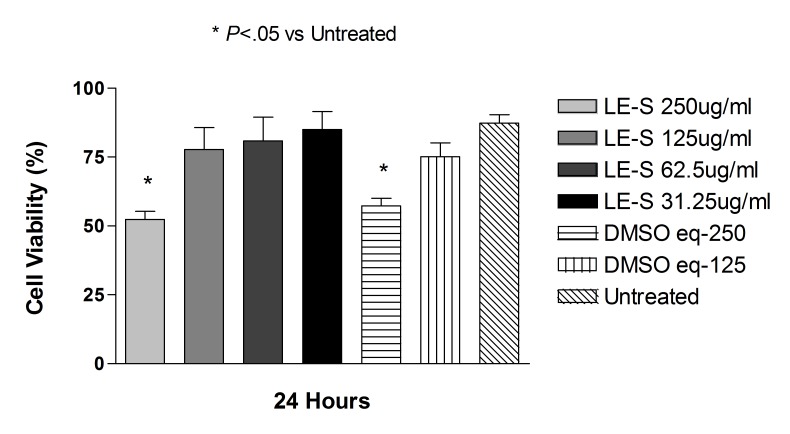
The drug was also solubilized in DMSO according to the protocol described above, and cell viability was measured after 24-hour exposure to concentrations of 250, 125, 62.5, or 31.25 μg/mL. ARPR-19 or R28 cells treated with the same concentration of DMSO used to solubilize loteprednol etabonate at concentrations of 250 or 125 μg/mL, as well as untreated cells, served as controls.
EXPOSURE TO FLUOCINOLONE ACETONIDE
Fluocinolone acetonide is commercially available as a powder (Spectrum Chemical Mfg Corp, Gardena, California). For purposes of these experiments, it was solubilized in ethanol. Both cell lines (ARPE-19 and R28) were treated with the following concentrations of the drug dissolved in ethanol: 1, 10, 150, 500, or1000 μg/mL. Since no commercial solution of fluocinolone acetonide exists, clinically equivalent dosing was based on the triamcinolone acetonide dose of 1000 μg/mL. Cell viability was measured using the trypan blue dye exclusion assay at 2, 6, or 24 hours. Cells incubated with the same amount of ethanol used to solubilize the powder served as controls, and their cell viability was obtained after 24 hours. Data was normalized to the untreated controls, which were considered as 100%.
DYE EXCLUSION ASSAY
In order to determine cell viability after exposure to the various steroid formulations, trypan blue dye exclusion assays were performed. The trypan blue dye exclusion cell viability assay is a commonly used in vitro assay that determines the degree of frank cell death. It evaluates the percentage of viable cells compared to the total amount of collected cells by determining the ratio of healthy unstained cells vs damaged/dead cells that stain blue.105 Cells were harvested from the 6-well plates by treatment with 0.2% trypsin-EDTA and were then incubated at 37°C for 5 minutes. The cells were centrifuged at 1000 revolutions per minute (rpm) for 5 minutes and then resuspended in 1 mL of culture medium. Automated cell viability analysis was done using ViCell analyzer (Beckman Coulter Inc, Brea, California), in which the resuspended cell suspension is incubated with trypan blue dye. The dead cells take up dye; living cells do not, as the dye binds to the intracellular proteins of leaky cells. The analyzer performs an automated trypan blue dye exclusion assay and gives the percentage of viable cells. The cell viability assay was performed for all different steroids tested.
MITOCHONDRIAL DEHYDROGENASE ASSAY
In addition to assessing frank cell death via the dye exclusion assay above, one of two cell injury assays was performed on each steroid, as significant toxicity that falls short of cell death would not be registered by the dye exclusion assay. Mitochondria have a key role on apoptosis regulation, as they take part in the production of oxygen free radicals and are susceptible to oxidative damage through lipid peroxidation, protein oxidation, and mitochondrial DNA mutation. Therefore, assays such as the JC-1 mitochondrial membrane potential assay or the mitochondrial dehydrogenase level assay, designed to test subtle mitochondrial changes, may reveal more subtle cellular changes than can be determined through the dye exclusion cell viability assay.105,108,127–129
In order to assess mitochondrial function, mitochondrial dehydrogenase (succinate-tetrazolium-reductase) activity was determined using the WST-1 (4-[3-(4-Iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate) colorimetric assay (Roche Diagnostics, Indianapolis, Indiana). Ten microliters of the formazan dye were added to each well and incubated for 2 hours at 37°C. Absorbance was measured at 490 nm on a multi-well spectrophotometer (PerkinElmer, Oak Brook, Illinois). The experiments were performed three times and in triplicate for each cell type and drug concentration. The mitochondrial dehydrogenase assay was conducted for the dexamethasone experiments
MITOCHONDRIAL MEMBRANE POTENTIAL (ΔΨM) MEASUREMENTS
As a further test of mitochondrial damage, the JC-1 mitochondrial membrane potential assay was performed. Detection of mitochondrial membrane potential (ΔΨm) values was performed using the JC-1 mitochondrial membrane potential detection kit (Biotium, Hayward, California). JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolyl-carbocyanine-iodide), a cationic dye that that accumulates as aggregates in the mitochondrial membranes of healthy cells, which result in a red fluorescence (590 nm). The damaged cells, which have diminished ΔΨm values, show a green fluorescence (529 nm). The ratio of red (live cells) and green (dead/damaged cells) fluorescence is measured in each sample. The fluorescent signal was measured with the scanning unit (FMBIO III; Hitachi, San Bruno, California) set to detect green (510 to 525 nm) and red (590 nm) emissions. Ratios of red to green fluorescence were calculated, and the data were analyzed by unpaired Student t test. The JC-1 mitochondrial permeability assay was utilized in the solubilized betamethasone, loteprednol, and methyl prednisolone experiments.
STATISTICAL ANALYSIS
Data were subjected to statistical analysis by analysis of variance (ANOVA) using GraphPad Prism 3.0 version statistics program (GraphPad Software Inc, San Diego, California). The Newman-Keuls multiple comparison test was done to compare the data within each experiment. P values less than .05 were considered statistically significant. Error bars in the graphs represent standard error of the mean with experiments performed in triplicate.
RESULTS
DEXAMETHASONE
Dexamethasone Solution
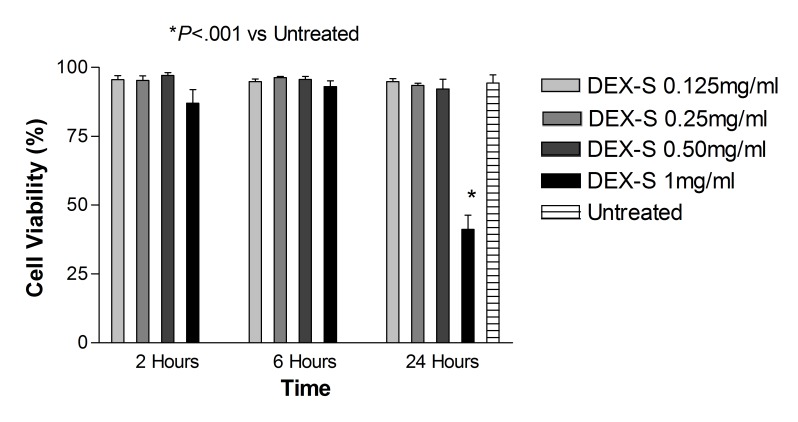
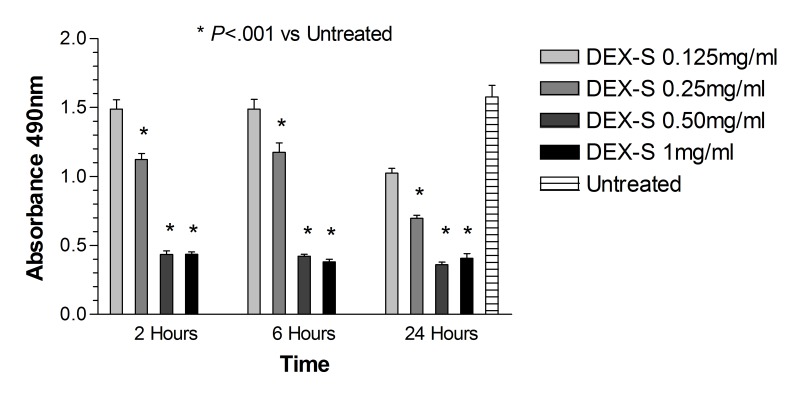
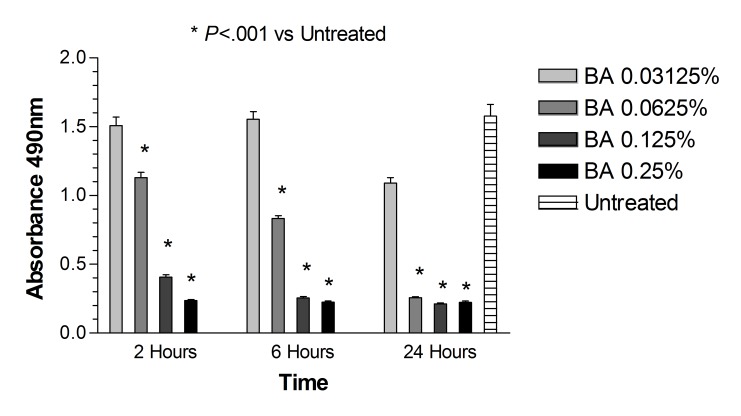
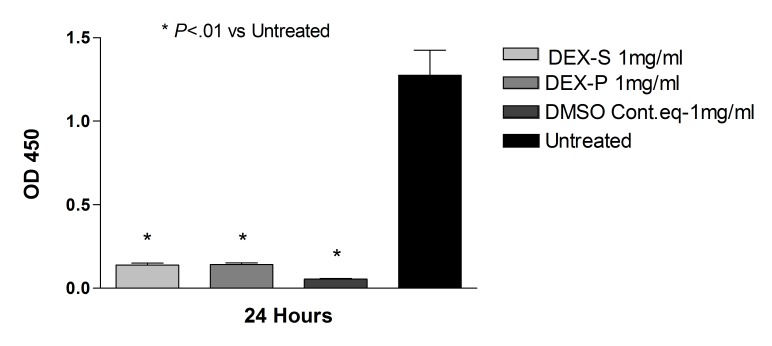
Dye exclusion assay: ARPE-19 cells. After 2, 6, and 24 hours of treatment with dexamethasone concentrations 0.125 mg/mL, 0.25 mg/mL, and 0.50 mg/mL (up to 5× the clinical dose), and after 2 and 6 hours of treatment with 1 mg/mL dexamethasone concentration (10× the clinical dose), cell viability was not significantly different (P>.05) when compared with untreated controls (Figure 1). The corresponding benzyl alcohol preservative concentrations alone also did not show a significant decrease in cell viability as compared with controls. The viability of ARPE-19 cells exposed for 24 hours to 1 mg/mL dexamethasone (41.19 ± 5.18%) and the corresponding concentration of preservative alone (64.56 ± 1.74%) was significantly decreased (P<.001) compared to that of untreated controls (93.17 ± 2.56%) (Figures 1 and 2).
FIGURE 1.
Percentage of cell viability for human retinal pigment epithelial cells exposed to commercially available dexamethasone solution (Dex-S). There was a significant decrease in cell viability after 24 hours for cells exposed to 1 mg/mL Dex-S when compared to untreated controls (P<.001).
FIGURE 2.
Percentage of cell viability for human retinal pigment epithelial cells exposed to the preservative in dexamethasone solution, benzyl alcohol (BA). After 24 hours, cells exposed to the highest concentration tested (equivalent to the amount contained in 1 mg/mL dexamethasone solution) showed a significant decrease in cell viability when compared to controls (P<.001).
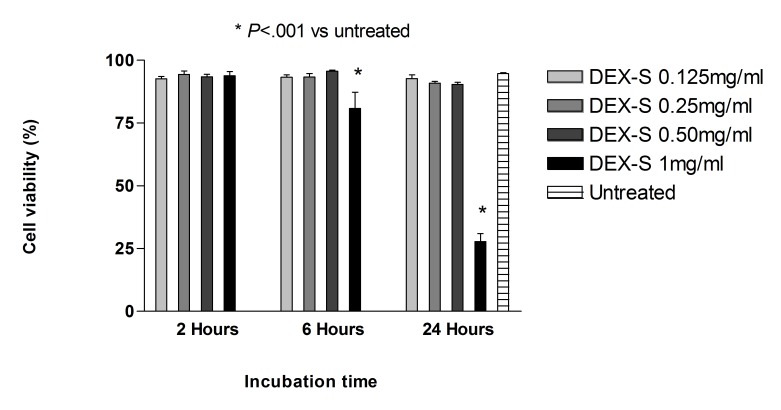
Dye exclusion assay: R28 cells. After 2, 6, and 24 hours of treatment with dexamethasone concentrations 0.125 mg/mL, 0.25 mg/mL, and 0.50 mg/mL, and 2 hours of treatment with 1 mg/mL dexamethasone concentration, cell viability was not significantly different (P>.05) when compared with untreated controls (Figure 3). The corresponding benzyl alcohol preservative concentrations also did not show a significant decrease in cell viability as compared with controls. Viability of R28 cells exposed for 24 hours to 1 mg/mL dexamethasone (25.42 ± 3.73%) and the corresponding concentration of preservative alone (38.75 ± 2.92%) was significantly decreased (<.001) compared to that of untreated controls (95.05 ± 0.15%) (Figures 3 and 4).
FIGURE 3.
Percentage of cell viability for rat embryonal neurosensory precursor retinal cells exposed to commercially available dexamethasone solution (Dex-S). There was a significant decrease in cell viability after 6 or 24 hours of exposure for cells treated with 1 mg/mL Dex-S when compared to untreated controls (P<.001).
FIGURE 4.
Percentage of cell viability for rat embryonal neurosensory precursor retinal cells exposed to the preservative benzyl alcohol (BA). After 24 hours, cells exposed to 1 mg/mL dexamethasone solution showed a significant decrease in cell viability when compared to controls (P<.001).
In addition, at 24 hours, cell viability with 1 mg/mL dexamethasone was significantly lower (P<.05) than that with the preservative alone.
The viability of R28 cells exposed for 6 hours to 1 mg/mL dexamethasone (74.55 ± 0.13%) was significantly decreased (P<.001) compared to that of the corresponding concentration of preservative alone (90.35 ± 0.69%) as well as that of untreated controls (95.05 ± 0.15%). However, at 6 hours, the viability with corresponding concentration of preservative alone was itself not significantly different when compared with untreated controls.
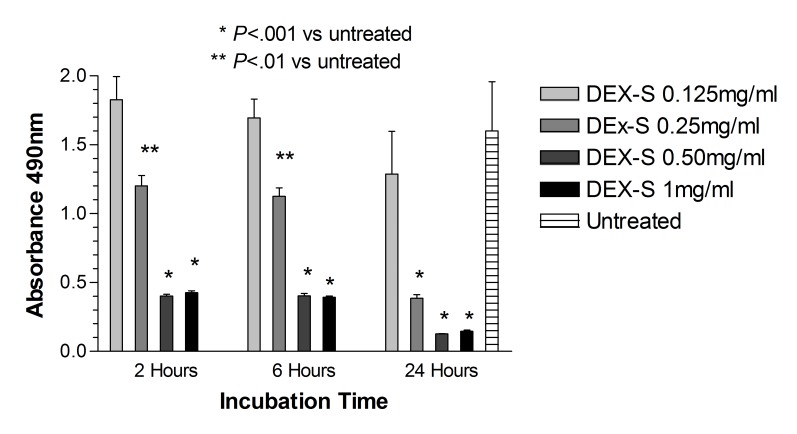
Mitochondrial dehydrogenase assay: ARPE-19 cells. As compared with untreated controls, there was a significant reduction (P<.001) in the mitochondrial dehydrogenase activity in ARPE-19 cells after 2 and 6 hours of treatment with dexamethasone concentrations 0.50 mg/mL and 1 mg/mL (5× and 10× the clinical dose), and the corresponding concentrations of preservative alone (Figure 5). Additionally, significant reduction in mitochondrial dehydrogenase activity was noted in cells treated with 0.25 mg/dL dexamethasone after 2 and 6 hours, but not with cells treated with the corresponding concentration of preservative alone at the same time points (P<.01). No significant toxicity was observed in cells treated with the 1.25× clinically equivalent dose of 0.125 mg/mL dexamethasone or the corresponding concentration of benzyl alcohol when compared to untreated cells. There was significantly lower (P<.001) mitochondrial dehydrogenase activity in the ARPE-19 cells after treatment with dexamethasone concentration 0.50 mg/mL and 0.25 mg/mL for 2 and 6 hours when compared with the corresponding concentration and duration of preservative alone (P<.001).
FIGURE 5.
Mitochondrial dehydrogenase (WST-1) assay for human retinal pigment epithelial cells exposed to dexamethasone solution (Dex-S). After 2, 6, or 24 hours, cells exposed to 0.25, 0.5, or 1 mg/mL had a significant reduction in mitochondrial activity when compared to untreated controls (P<.001 for 0.5 or 1.0 mg/mL; P<.01 for 0.25 mg/mL).
After 24 hours, mitochondrial dehydrogenase was significantly reduced (P<.001) in ARPE-19 cells treated with 0.25, 0.50, and 1 mg/mL dexamethasone (0.92 ± 0.09, 0.46 ± 0.04, 0.33 ± 0.01 absorbance at 490 nm) or corresponding preservative (0.38 ± 0.03, 0.21 ± 0.02, 0.19 ± 0.01) compared to untreated controls (1.57 ± 0.03; P<.001). There was no statistical difference when comparing cells treated with dexamethasone and the corresponding dose of benzyl alcohol, except at the 0.25 mg/mL dose, where cells treated with dexamethasone had a significantly higher absorbance (P<.001) (Figures 5 and 6).
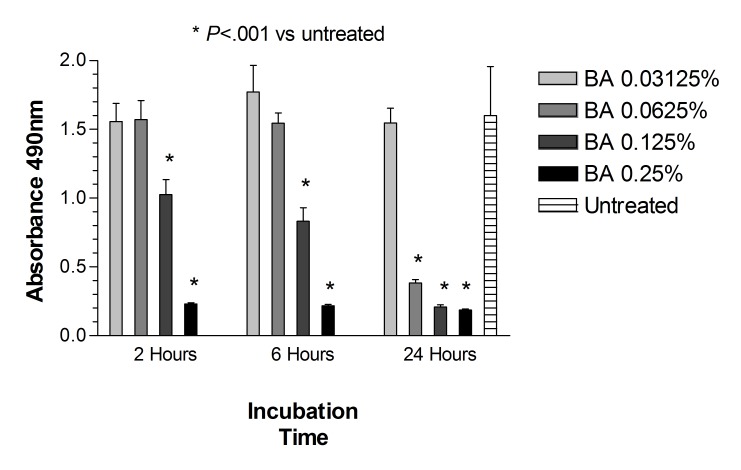
FIGURE 6.
Mitochondrial dehydrogenase (WST-1) assay for human retinal pigment epithelial cells exposed to benzyl alcohol (BA). After 2, 6, or 24 hours, cells exposed to BA at concentrations equivalent to 0.5 or 1.0 mg/mL had a significant reduction in mitochondrial activity when compared to untreated controls (P<.01 for 2 or 6 hours of exposure; P<.001 for 24 hours of exposure). Similar findings were observed after 24 hours of cell exposure to BA at equivalent concentration of 0.25 mg/mL (P<.001).
Mitochondrial dehydrogenase assay: R28 cells. There was a significant reduction (P<.001) in the mitochondrial dehydrogenase activity in R28 cells after 2 hours of treatment with dexamethasone concentrations of 0.25 mg/mL, 0.50 mg/mL, and 1 mg/mL, or the corresponding concentrations of preservative alone, compared to untreated controls (Figures 7 and 8). No clinically significant toxicity was observed in cells treated with 0.125 mg/mL dexamethasone or the respective preservative control. Additionally, cells treated with 1.0 mg/mL dexamethasone showed significantly less toxicity than the respective preservative control (P<.05).
FIGURE 7.
Mitochondrial dehydrogenase (WST-1) assay for human retinal pigment epithelial cells exposed to dexamethasone solution (Dex-S). After 2, 6, or 24 hours, cells exposed to 0.25, 0.5, or 1 mg/mL had a significant reduction in mitochondrial activity when compared to untreated controls.
FIGURE 8.
Mitochondrial dehydrogenase (WST-1) assay for rat embryonal neurosensory precursor retinal cells exposed to benzyl alcohol (BA). After 2, 6, or 24 hours, cells exposed to BA at concentrations equivalent to 0.25, 0.5, or 1.0 mg/mL had a significant reduction in mitochondrial activity when compared to untreated controls (P<.001).
After 6 hours, a significant reduction in mitochondrial dehydrogenase activity was observed in cells treated with dexamethasone concentrations of 0.25 mg/mL, 0.50 mg/mL, and 1 mg/mL, or the corresponding concentrations of benzyl alcohol alone. Cells treated with 1.25× the clinical dose of 0.125 mg/mL dexamethasone or the corresponding concentration of preservative alone did not show significant toxicity. Moreover, cells treated with 0.25 mg/mL dexamethasone showed significantly less toxicity than cells treated with the corresponding concentration of the preservative alone (P<.001) (Figures 7 and 8).
Mitochondrial dehydrogenase was significantly reduced in R28 cells treated with 0.25, 0.50, and 1 mg/mL dexamethasone (0.70 ± 0.02, 0.36 ± 0.02, 0.41 ± 0.03 absorbance at 490 nm for R28) or corresponding preservative (0.26 ± 0.01, 0.21 ± 0.01, 0.22 ± 0.01) after 24 hours compared to untreated controls (1.58 ± 0.09; P<.001). Additionally, R28 cells treated with 1.0 mg/mL or 0.25 mg/mL dexamethasone showed significantly less toxicity when compared to cells treated with the corresponding concentration of preservative alone (P<.05 and P<.001, respectively).
Dexamethasone Powder
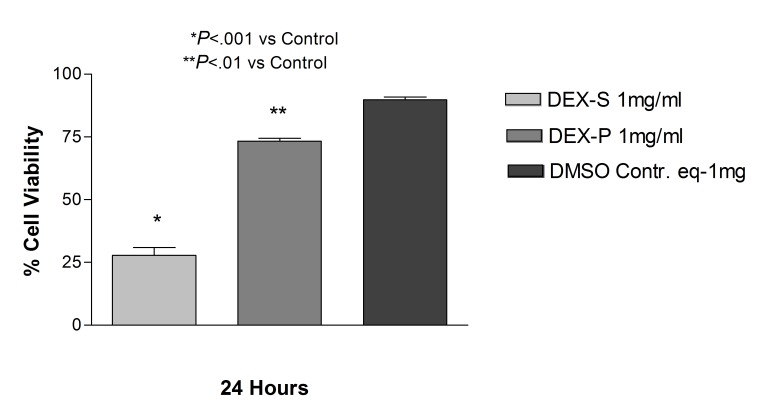
The observation of decreased cell viability of dexamethasone at the highest dose tested (1 mg/mL) prompted us to question whether it was a consequence of the preservative itself. Therefore we tested preservative-free dexamethasone powder at the 1 mg/mL dose in both ARPE-19 and R28 cell cultures. In the ARPE-19 cultures treated with 1 mg/mL dexamethasone, there was no statistical difference in cell viability between cells treated with dexamethasone powder and cells treated with the commercially available solution (32.4 ± 4.1 and 39.7 ± 4.9, respectively). Additionally, both showed a decrease in cell viability when compared to cells treated with the equivalent dose of DMSO used to solubilize the powder (90.2 ± 1.4; P<.001) (Figure 9). R28 cells exposed to 1 mg/mL dexamethasone powder for 24 hours showed a significant decrease in cell viability (73.3 ± 2.1; P<.01) when compared to untreated controls (89.8 ± 1.9), although less toxicity than cells treated with the commercially available dexamethasone (27.8 ± 5.5) at the same concentration and duration (Figure 10).
FIGURE 9.
Comparison between human retinal pigment epithelial cells treated with 1 mg/mL dexamethasone solution (Dex-S), 1.0 mg/mL dexamethasone powder (Dex-P), or DMSO controls. There was no significant difference in cell viability between cells treated with dexamethasone powder or the commercially available solution, and both showed a decrease in cell viability when compared to controls (P<.001).
FIGURE 10.
Comparison between rat embryonal neurosensory precursor retinal cells treated with 1.0 mg/mL dexamethasone solution (Dex-S), 1.0 mg/mL dexamethasone powder (Dex-P), or DMSO controls. Cells exposed to 1.0 mg/mL dexamethasone powder showed a significant decrease in cell viability when compared to controls, but less toxicity than cells treated with commercially available dexamethasone (P<.01).
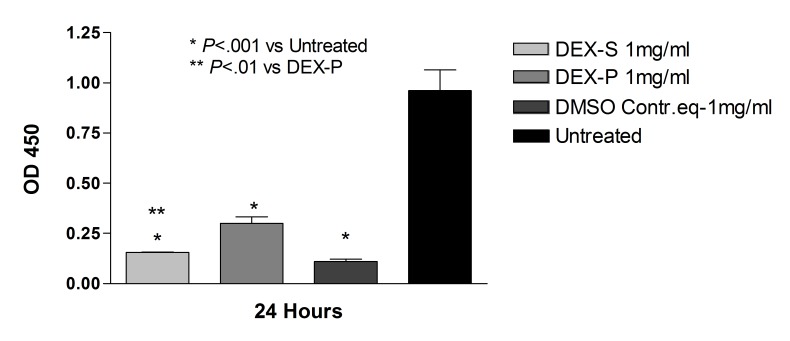
ARPE-19 cells treated with 1 mg/mL dexamethasone powder resuspended in DMSO showed significantly higher mitochondrial dehydrogenase activity compared to ARPE-19 cells treated with the commercially available dexamethasone solution (0.70 ± 0.2 and 0.33 ± 0.01, respectively; P<.01) (Figure 11). In the R28 cultures, there was no statistical difference in mitochondrial dehydrogenase activity in cells treated with dexamethasone solution or powder for 24 hours at 1.0 mg/mL concentration (Figure 12).
FIGURE 11.
Mitochondrial dehydrogenase (WST-1) assay for human retinal pigment epithelial cells after 24-hour exposure to dexamethasone solution (Dex-S), dexamethasone powder solubilized in DMSO (Dex-P), DMSO at an equivalent dose to solubilize the powder, and untreated controls. There was a reduction in mitochondrial dehydrogenase in all groups compared to untreated controls (P<.001). Cells treated with 1.0 mg/mL Dex-P had significantly higher mitochondrial dehydrogenase activity when compared to cells treated with Dex-S at the same concentration (P<.01).
FIGURE 12.
Mitochondrial dehydrogenase (WST-1) assay for rat embryonal neurosensory precursor retinal cells after 24-hour exposure to dexamethasone solution (Dex-S), dexamethasone powder solubilized in DMSO (Dex-P), DMSO at an equivalent dose used to solubilize the powder, and untreated controls. There was a reduction in mitochondrial dehydrogenase in all groups compared to untreated controls (P<.01). No difference was found between cells treated with Dex-S and Dex-P.
BETAMETHASONE
Commercial Betamethasone (Beta-C)
In both cell lines, betamethasone 180 μg/mL (1.2× the clinical dose) caused a significant decrease in cell viability at all time points. In ARPE-19 cells treated with 180 μg/mL, viability was 51.4 ± 2.8%, 51.0 ± 5.4%, and 18.1 ± 3.6% compared to control untreated ARPE-19 cells (95.0%, 93.0%, and 92.2% at 2, 6, and 24 hours, respectively; P<.001). In R28 cells treated with 180 μg/mL, viability was 68.1 ± 6.6%, 52.4 ± 5.6%, and 40.9 ± 1.6% compared to untreated R28 cells (89.3%, 86.0%, and 89.4% viability at 2, 6, and 24 hours, respectively; P<.001).
Betamethasone 90 μg/mL was toxic at 24 and 6 hours in both cell lines. ARPE-19 cells showed values of 69.9 ± 11.1% and 71.1 ± 3.9% at 24 and 6 hours, respectively, and mean cell viability in R28 cells was 57.8 ± 2.3% and 77.1 ± 5.3% (P<.001), respectively.
The lowest tested dose (30 μg/mL; 0.2× the clinical dose) was not toxic to either cell line, with mean cell viability of 91.2 ± 2.3%, 92.5 ± 2.1%, and 91.6 ± 1.0% for ARPE-19 cells and 88.8 ± 0.7%, 80.6 ± 2.0%, and 82.0 ± 2.6% for R28 cells at 2, 6, and 24 hours, respectively.
However, the next lowest dose (45 μg/mL) showed mild toxicity at 24 hours with cell viability of 80.8 ± 4.4% for ARPE-19 cells. The mean cell viability for R28 was reduced even further to 76.8 ± 4.5% at this dose.
Solubilized Betamethasone (Beta-S)
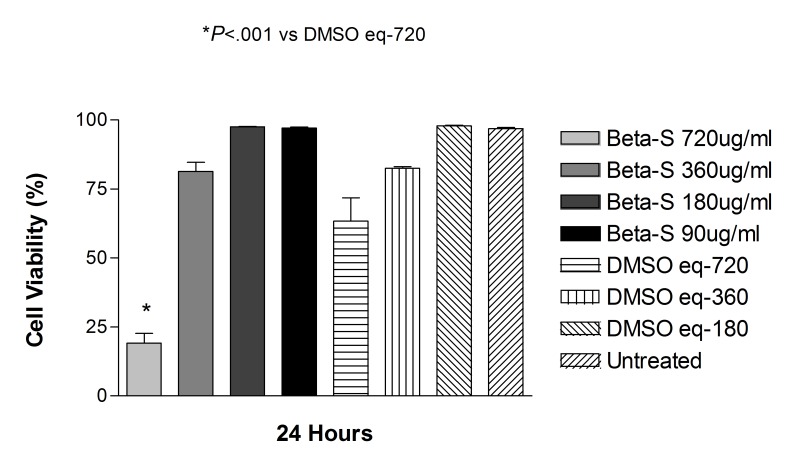
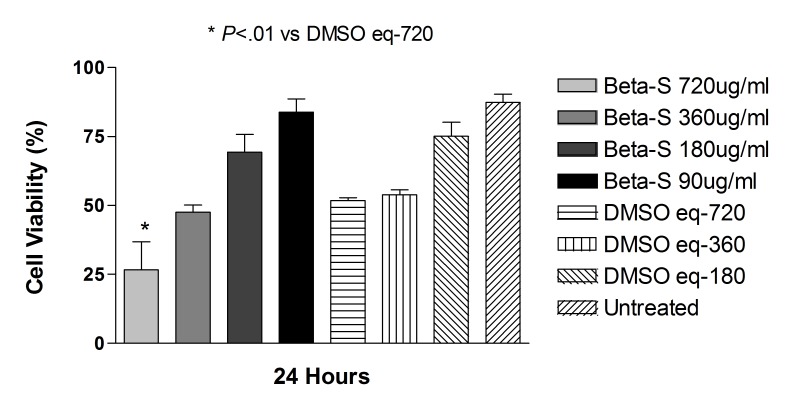
Betamethasone as a solution at doses of 90, 180, and 360 μg/mL did not significantly affect the cell viability at 24 hours compared with controls ARPE-19 and R28 cells (Figures 13 and 14).
FIGURE 13.
Cell viability of human retinal pigment epithelial cells exposed to betamethasone solubilized in DMSO (Beta-S). Only the highest concentration tested (720 μg/mL) showed a significant decrease in cell viability when compared to untreated cells or DMSO-treated controls (P<.001).
FIGURE 14.
Cell viability of rat embryonal neurosensory precursor retinal cells exposed to betamethasone solubilized in DMSO (Beta-S). Only the highest concentration tested (720 μg/mL) showed a significant decrease in cell viability when compared to untreated cells or DMSO-treated controls (P<.01).
Mean viability of ARPE-19 cells exposed to 360, 180, and 90 μg/mL betamethasone dissolved in DMSO for 24 hours was 81.3 ± 6.7%, 97.5 ± 0.4%, and 97.1.3 ± 0.7%, respectively, compared with DMSO with no drug-treated ARPE cells (P>.05 for all). In R28 cells, at the same above concentrations, cell viability was 47.5 ± 4.6%, 69.3 ± 11.3%, and 83.8 ± 8.4%, respectively, compared with DMSO-treated R28 cells (P>.05).
Betamethasone dissolved in DMSO significantly affected cell viability only at the highest corresponding dose of 720 μg/mL (Figures 13 and 14). Percentage of cell viability in ARPE-19 cells after 24 hours of treatment was 19.1 ± 7.2%, compared with control ARPE cells at 63.4 ± 16.9% for untreated controls (P<.001). At the same dose and time point, R28 cells showed cell viability of 26.6 ± 17.6%, compared with 51.8 ± 1.7% for untreated controls (P<.01).
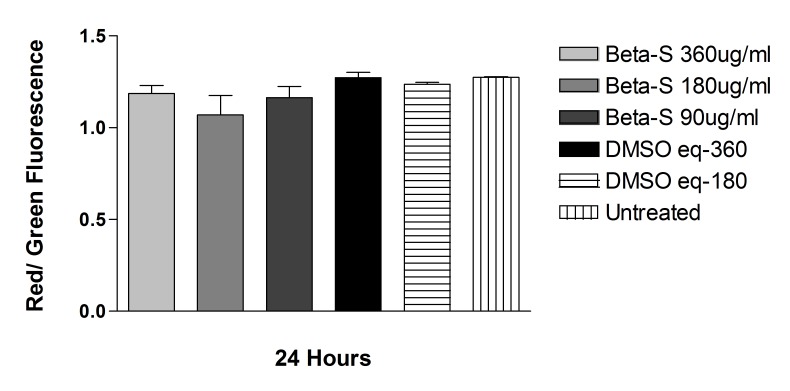
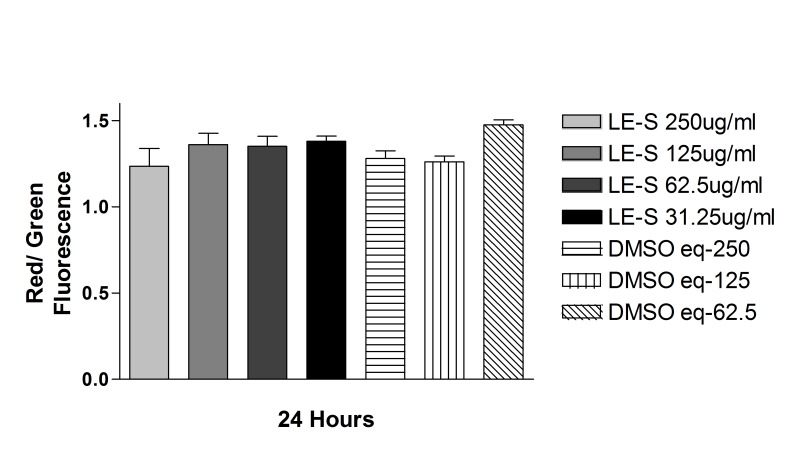
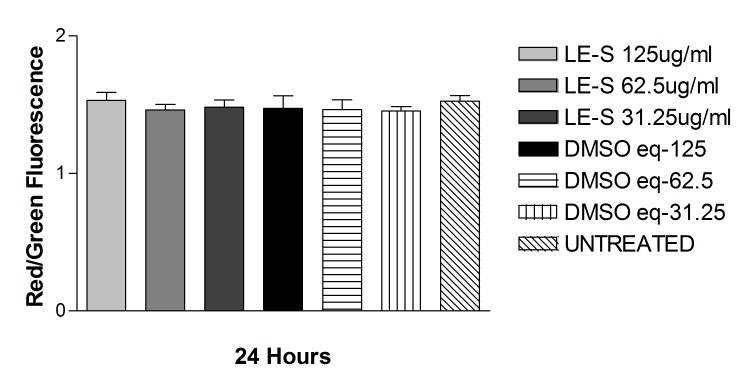
The JC-1 mitochondrial permeability assay did not show any statistically significant difference in either cell line for cells treated with solubilized betamethsone, its respective controls treated with the same concentration of DMSO used to solubilize the drug, or untreated controls, after 24-hour exposure. For ARPE-19 cells, the red/green ratio was, respectively: 1.09 ± 0.16, 1.07 ± 0.17, 1.16 ± 0.09, 1.29 ± 0.09, 1.23 ± 0.02, 1.26 ± 0.03, and 1.25 ± 0.03, for solubilized betamethasone 360, 180, or 90 μg/mL, its respective DMSO controls, or untreated cells. For R28 cells, the red/green ratio was 0.98 ± 0.10, 1.12 ± 0.11, 1.07 ± 0.12, and 1.19 ± 0.03, for solubilized betamethasone-S 180 or 90 μg/mL or its respective DMSO-treated controls (Figure 15 and 16).
FIGURE 15.
Mitochondrial membrane potential (ΔΨm) of human retinal pigment epithelial cells exposed to betamethasone solubilized in DMSO (Beta-S). No significant difference in mitochondrial potential was found between cells treated with solubilized betamethasone, DMSO-treated, or untreated controls.
FIGURE 16.
Mitochondrial membrane potential (ΔΨm) of rat embryonal neurosensory precursor retinal cells exposed to Beta-S. No significant difference in mitochondrial potential was found between cells treated with solubilized betamethasone, DMSO-treated, or untreated controls.
METHYLPREDNISOLONE
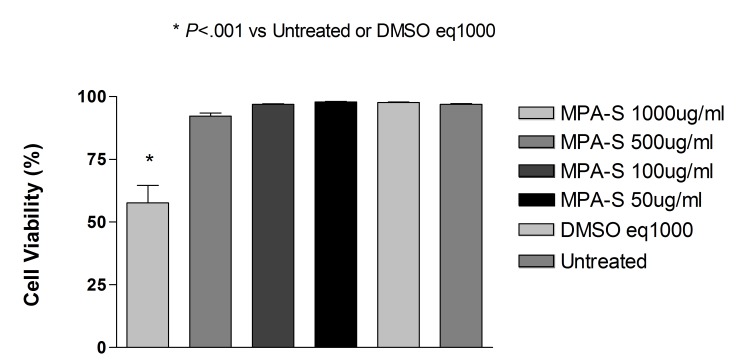
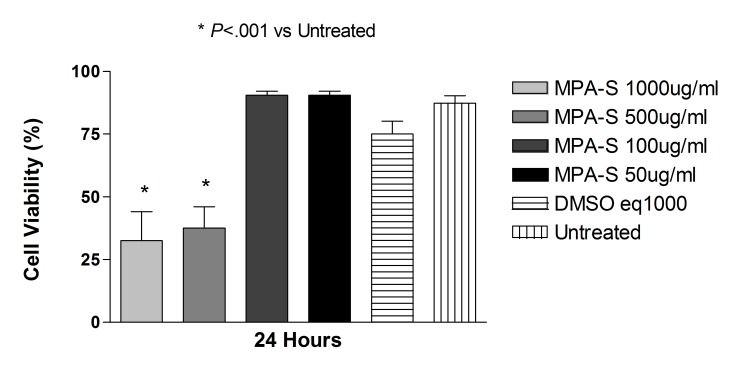
Commercially Available Methylprednisolone
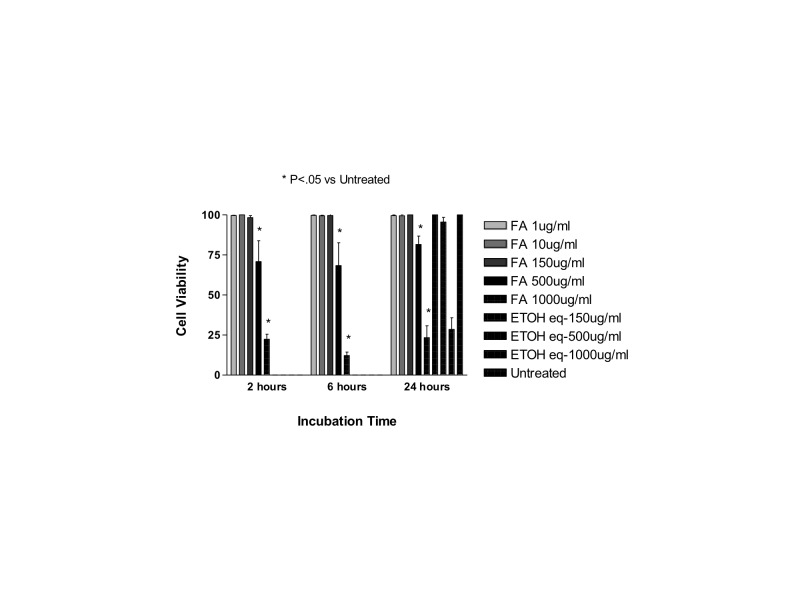
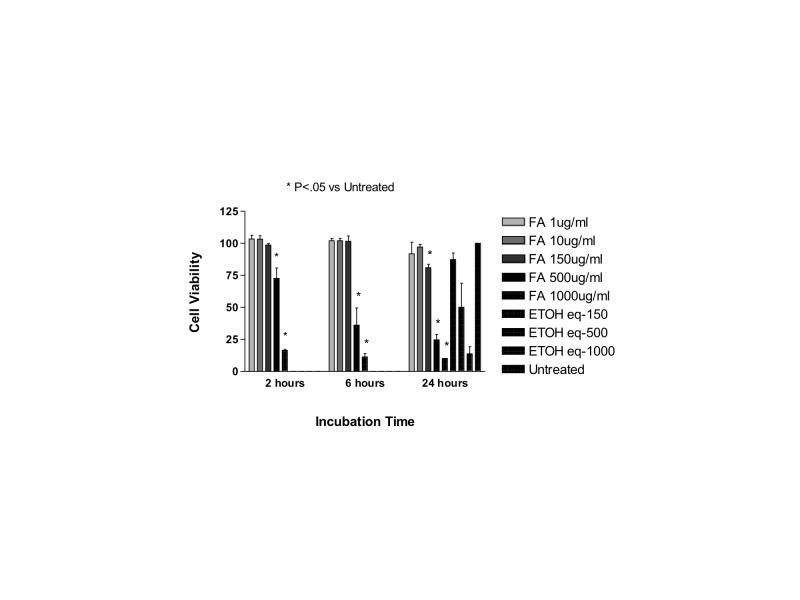
Exposure to methylprednisolone 50, 100, or 500 μg/mL (clinical dose is 1000 μg/mL) caused a reduction in cell viability in R28 cells after 2, 6, and 24 hours and in ARPE-19 cells after 6 and 24 hours. Equivalent amounts of the preservative used as controls did not cause a significant reduction in cell viability.
For ARPE-19 cells after 2 hours of exposure, the mean cell viability ± SD for MPA 500, 100, 50, and 25 μg/mL, respectively, was: 5.70 ± 0.75, 89.87 ± 3.03, 90.23 ± 2.63, and 93.03 ± 2.63. Cells exposed to the preservative at concentrations of 375, 75, 37.5, or 18.75 μg/mL showed mean cell viability ± SD of 91.93 ± 0.97, 91.83 ± 2.05, 91.77 ± 1.00, or 89.90 ± 2.97, and the untreated control cells showed mean cell viability of 94.33 ± 1.53. There was a significant reduction in cell viability only to exposure of methylprednisolone 500 μg/mL when compared to untreated controls or the equivalent dose of preservative (P<.001).
After 6 hours, ARPE-19 cells exposed to methylprednisolone 500, 100, 50, or 25 μg/mL presented the following cell viabilities, respectively: 6.37 ± 0.47, 75.37± 2.96, 89.43 ± 1.45, and 94.07 ± 2.39. Cells exposed to the preservative at concentrations of 375, 75, 37.5, or 18.75 μg/mL had a mean cell viability ± SD of 95.47 ± 1.27, 94.47 ± 1.37, 95.57 ± 1.25, and 92.13 ± 1.03, and the untreated control cells had a mean cell viability of 92.67 ± 2.08. There was a significant reduction in cell viability of methylprednisolone 500 and 100 μg/mL when compared to untreated controls or the equivalent dose of preservative (P<.001). Cells exposed to methylprednisolone 50 μg/mL had a significant reduction in cell viability when compared to cells treated with the same amount of preservative (P<.01), but no difference was noted when compared to untreated controls.
After 24 hours of exposure, the ARPE-19 percentage mean cell viability was 5.57 ± 2.00, 59.40 ± 2.65, 84.13 ± 1.16, and 93.77 ± 1.98 to methylprednisolone 500, 100, 50, or 25 μg/mL, respectively. Cell exposed to the preservative at concentrations of 375, 75, 37.5, or 18.75 μg/mL had a mean cell viability ± SD of 94.57 ± 1.60, 94.47 ± 2.15, 95.37 ± 2.06, and 94.23 ± 2.31, and the untreated control cells had a mean cell viability of 94.73 ± 1.29. There was a significant reduction in cell viability of methylprednisolone 500, 100, or 50 μg/mL when compared to untreated controls or cell exposed to the equivalent dose of the preservative (P<.001).
For R28 cells, after 2 hours of commercial methylprednisolone exposure, the mean cell viability ± SD for methylprednisolone 500, 100, 50, and 25 μg/mL was, respectively: 9.27 ± 1.04, 55.30 ± 4.16, 85.73 ± 1.85, and 90.40 ± 0.53. Cells exposed to the preservative at concentrations of 375, 75, 37.5, or 18.75 μg/mL had a mean cell viability ± SD of 89.53 ± 0.75, 89.73 ± 2.27, 91.60 ± 1.28, and 91.67 ± 1.53, and the untreated control cells had a mean cell viability of 91.40 ± 0.83. There was a significant reduction in cell viability of methylprednisolone 500, 100, or 50 μg/mL when compared to untreated controls or cells treated with the equivalent dose of preservative (P<.001 for methylprednisolone 500 or 100; P<.05 for cells treated with methylprednisolone 50 μg/mL).
After 6 hours of exposure, mean percentage of R28 cell viability ± SD for methylprednisolone 500, 100, 50, and 25 μg/mL was, respectively: 10.80 ± 0.10, 62.50 ± 1.04, 86.57 ± 1.95, and 91.83 ± 0.95. Cells exposed to the preservative at concentrations of 375, 75, 37.5, or 18.75 μg/mL showed mean cell viability ± SD of 92.93 ± 2.54, 90.77 ± 1.33, 91.77 ± 2.25, and 92.90 ± 2.40, and the untreated control cells showed mean cell viability of 93.33 ± 0.83. There was a significant reduction in cell viability of methylprednisolone 500, 100, or 50 μg/mL when compared to untreated controls or cells treated with the equivalent dose of preservative (P<.001 for MPA 500 or 100; P<.01 for cells treated with methylprednisolone 50 μg/mL).
After 24 hours of exposure, mean percentage of R28 cell viability ± SD for methylprednisolone 500, 100, 50, and 25 μg/mL was, respectively 9.27 ± 1.04, 55.30 ± 4.16, 85.73 ± 1.85, and 90.40 ± 0.53. Cells exposed to the preservative at concentrations of 375, 75, 37.5, or 18.75 μg/mL showed mean cell viability ± SD of 89.53 ± 0.75, 89.73 ± 2.27, 91.60 ± 1.28, and 91.67 ± 1.53, and the untreated control cells showed mean cell viability of 93.00 ± 2.00. There was a significant reduction in cell viability of methylprednisolone 500, 100, or 50 μg/mL when compared to untreated controls or cells treated with the equivalent dose of preservative (P<.001 for methylprednisolone 500 or 100; P<.01 for cells treated with methylprednisolone 50 μg/mL when compared to untreated controls; P<.05 for cells treated with methylprednisolone 50 μg/mL when compared to cells treated with equivalent doses of the preservative).
At all time points and in both cell lines tested, cells exposed to any concentration of the preservative did not have statistically different mean cell viabilities when compared to untreated controls.
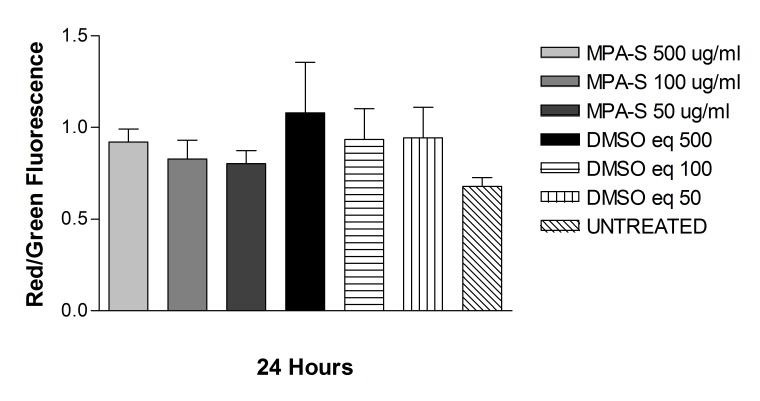
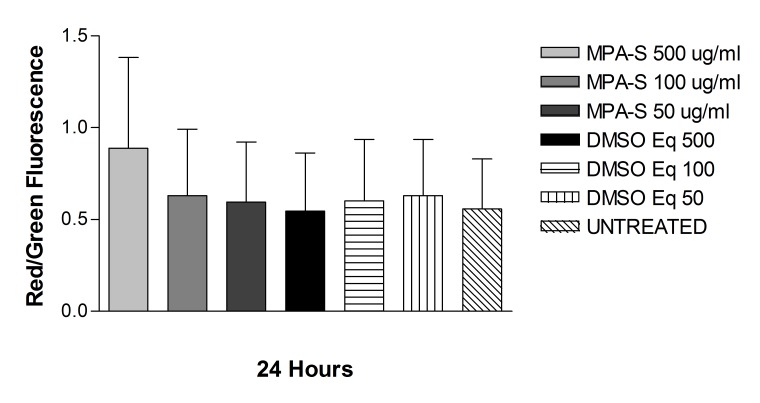
Solubilized Methylprednisolone (s-MPA)
The highest dose tested (s-MPA 1000 μg/mL, equivalent to the clinical dose) had a significant reduction in mean cell viability when compared to cells treated with the equivalent dose of DMSO or untreated controls (P<.001) (Figure 17). Exposure of ARPE-19 cells to solubilized methylprednisolone for 24 hours resulted in the mean percentage ± SD cell viabilities of 57.70 ± 13.85, 93.33 ± 2.27, 96.95 ± 0.50, and 97.90 ± 0.52, for solubilized methylprednisolone 1000, 500, 100, and 50 μg/mL, respectively. Cells treated with the highest concentration of DMSO used (30 μg/mL, which is the equivalent dose for solubilized methylprednisolone 1000 μg/mL) showed mean cell viability of 97.73 ± 0.21, and untreated controls had 96.85 ± 0.75.
FIGURE 17.
Cell viability of human retinal pigment epithelial cells exposed to methyl prednisolone solubilized in DMSO. Only the highest concentration tested (1000 μg/mL) showed a significant decrease in cell viability when compared to untreated cells or DMSO-treated controls (P<.001).
The solubilized methylprednisolone doses of 1000 and 500 μg/mL had a significant reduction in mean cell viability when compared to R28 cells treated with the equivalent dose of DMSO (P<.01) or untreated controls (P<.001). Exposure of R28 cells to solubilized methylprednisolone for 24 hours resulted in the following mean ± SD cell viabilities: 32.57 ± 19.95, 37.57 ± 14.78, 90.53 ± 2.89, and 90.50 ± 3.01, for solubilized methylprednisolone 1000, 500, 100, and 50 μg/mL, respectively. Cells treated with the highest concentration of DMSO presented mean cell viability of 75.17 ± 8.72, and untreated controls presented mean cell viability of 87.40 ± 5.19 (Figure 18).
FIGURE 18.
Cell viability of rat embryonal neurosensory precursor retinal cells exposed to methylprednisolone solubilized in DMSO. The concentration of 500 or 1000 μg/mL showed a significant decrease in cell viability when compared to untreated cells or DMSO-treated controls (P<.001).
Cells treated with DMSO in any concentration did not present any statistical reduction in cell viability when compared to untreated controls.
The mitochondrial membrane potential assay (JC-1) was tested for cells exposed for 24 hours to solubilized methylprednisolone 500, 100, and 50 μg/mL, its respective DMSO controls, and untreated cells. In both ARPE-19 and R28 cell lines, there was no difference between cells treated with solubilized methylprednisolone, cells treated with the equivalent doses of DMSO, or untreated controls (Figures 19 and 20). For ARPE-19 cells, the red/green ratio was 0.921 ± 0.10, 0.828 ± 0.15, 0.802 ± 0.101 for solubilized methylprednisolone 500, 100, or 50 μg/mL, respectively; 1.081 ± 0.388, 0.934 ± 0.238, 0.945 ± 0.234 for controls treated with equivalent doses of DMSO, respectively; and 0.680 ± 0.066 for untreated controls. For R28 cells, the red/green ratio was 0.889 ± 0.70, 0.630 ± 0.51, 0.594 ± 0.46 for solubilized methylprednisolone 500, 100, or 50 μg/mL, respectively; 0.546 ± 0.45, 0.602 ± 0.47, 0.630 ± 0.43 for controls treated with equivalent doses of DMSO, respectively, and 0.557 ± 0.39 for untreated controls.
FIGURE 19.
Mitochondrial membrane potential (ΔΨm) of human retinal pigment epithelial cells exposed to methylprednisolone solubilized in DMSO. No significant difference in mitochondrial potential was found between cells treated with solubilized methylprednisolone, DMSO-treated, or untreated controls.
FIGURE 20.
Mitochondrial membrane potential (ΔΨm) of rat embryonal neurosensory precursor retinal cells exposed to methylprednisolone solubilized in DMSO. No significant difference in mitochondrial potential was found between cells treated with solubilized methylprednisolone, DMSO-treated, or untreated controls.
LOTEPREDNOL ETABONATE (LE)
Crystalline Loteprednol Etabonate
ARPE-19 cells. Loteprednol etabonate significantly decreased the viability of ARPE-19 cells at all doses at 24 hours (P<.001 for all), except at the lowest dose tested (15.62 μg/mL, one-eighth the clinical dose equivalent of 125 μg/mL). The mean cell viabilities of ARPE-19 cells after 24 hours exposure to loteprednol concentrations of 125 μg/mL, 62.5 μg/mL, 31.25 μg/mL, and 15.62 μg/mL were 8.2 ± 3.8 %, 80.4 ± 0.9 %, 80.5 ± 1.5 %, and 94.4 ± 2.3%, respectively, when compared to control untreated ARPE-19 cells (98.15 ± 0.50; Figure 21). Significant toxic effects were additionally seen at 6 and 2 hours at the two higher concentrations (125 μg/mL and 62.5 μg/mL, P<.01). Loteprednol etabonate 31.25 or 15.62 μg/mL did not cause significant reductions in ARPE-19 cell viability after 2 or 6 hours of exposure (P>.05). The viability of these cells after 6 hours exposure to loteprednol etabonate concentrations of 125 μg/mL, 62.5 μg/mL, 31.25 μg/mL, and 15.62 μg/mL was 44.4 ± 2.%, 84.8 ± 3.3 %, 97.6 ± 2.0%, and 97.4 ± 1.5 %, respectively, and the mean cell viability of untreated controls was 96.05 ± 0.64 %. The mean cell viability of these cells after 2 hours exposure to the same concentrations of loteprednol etabonate was 74.1 ± 0.2 %, 88.6 ± 2.5 %, 97.4 ± 1.1 %, and 92.3 ± 0.5 %, respectively, and the mean cell viability of untreated controls was 98.50 ± 0.56 %.
FIGURE 21.
Cell viability of human retinal pigment epithelial cells exposed to commercially available loteprednol etabonate (LE). After 24 hours, there was a significant decrease in cell viability for cells exposed to 125, 62.5, or 31.25 μg/mL compared to untreated controls (P<.001). After 2 or 6 hours of exposure, cells treated with 125 or 62.5 μg/mL also showed a reduction in cell viability when compared to untreated controls (P<.01).
R28 Cells. After 24-hour exposure, loteprednol etabonate caused a significant decrease in cell viability at all concentrations tested when compared to untreated controls (P<.01; Figure 22). The mean cell viabilities after exposure to concentrations of 125 μg/mL, 62.5 μg/mL, 31.25 μg/mL, and 15.62 μg/mL were 22.3 ± 3.1%, 58.8 ± 4.6%, 69.7 ± 8.6%, 76.1 ± 6.5%, respectively, and the mean cell viability of untreated controls was 97.65 ± 0.84%. Toxic effects were seen at 6 and 2 hours (P<.01) only at the highest concentration tested (125 μg/mL, P<.01). After 6 hours of exposure to loteprednol concentrations of 125 μg/mL, 62.5 μg/mL, 31.25 μg/mL, and 15.62 μg/mL, the mean cell viabilities of R28 cells were 54.2 ± 15.4%, 91.7 ± 14.3 %, 107.0 ± 23.0%, and 104.7 ± 19.9 %, respectively, and the mean cell viability of untreated controls was 96.98 ± 1.24%. After 2 hours, mean cell viability was 63.8 ± 9.1%, 98.2 ± 1.2%, 93.3 ± 1.5%, and 96.3 ± 1.4 %, respectively, and the mean cell viability of untreated controls was 98.1 ± 0.43%.
FIGURE 22.
Cell viability of rat embryonal neurosensory precursor retinal cells exposed to commercially available loteprednol etabonate (LE). After 24 hours, there was a significant decrease in cell viability for cells exposed to 125, 62.5, or 31.25 μg/mL compared to untreated controls (P<.01). After 2 or 6 hours, only the highest concentration tested (125 μg/mL) showed a reduction in cell viability when compared to untreated controls (P<.01).
Solubilized Loteprednol Etabonate (s-LE)
ARPE-19 cells. Cells treated with the highest concentration tested of solubilized loteprednol etabonate (250 μg/mL, 2× the clinical dose equivalent) had a significant decrease in viability when compared to untreated controls or cells incubated with the same amount of DMSO (P<.001; Figure 23). The other concentrations tested did not show a statistical decrease in cell viability when compared to controls. The mean cell viabilities after exposure to concentrations of 250, 125, 62.5, or 31.25 μg/mL were 59.6 ± 15.6%, 85.9 ± 3.8%, 94.3 ± 0.5%, and 95.7 ± 1.3%, respectively. The mean cell viabilities after exposure to DMSO at doses equivalent to the ones used to solubilize loteprednol etabonate at concentrations of 250 or 125 μg/mL were, respectively, 88.1 ± 8.8% and 97.9 ± 0.4%, and were not statistically different than mean cell viability of untreated cells (96.8 ± 0.8%).
FIGURE 23.
Cell viability of human retinal pigment epithelial cells exposed for 24 hours to loteprednol etabonate solubilized in DMSO (LE-S). Cells treated with the highest concentration tested of LE-S (250 μg/mL) had a significant decrease in viability when compared to untreated controls or cells incubated with the same amount of DMSO (P<.001).
R28 cells. After 24 hours of exposure, no significant decrease in cell viability was noted for any concentration of solubilized loteprednol etabonate tested when compared to DMSO controls. However, cells treated with the highest dose of solubilized loteprednol etabonate and its corresponding DMSO control showed a significant reduction in cell viability when compared to untreated controls (P<.05; Figure 24). The mean cell viabilities after exposure to concentrations of 250, 125, 62.5, or 31.25 μg/mL were 52.4 ± 5.1%, 77.7 ± 13.9%, 80.9 ± 14.9%, and 85.0 ± 11.4%, respectively. The mean cell viabilities after exposure to DMSO at doses equivalent to the ones used to solubilize loteprednol etabonate at concentrations of 250 or 125 μg/mL were, respectively, 57.3 ± 4.7% and 75.2 ± 8.7%, and for untreated cells, 87.4 ± 5.2%.
FIGURE 24.
Cell viability of rat embryonal neurosensory precursor retinal cells exposed for 24 hours to loteprednol etabonate solubilized in DMSO (LE-S). No significant decrease in cell viability was noted when any concentration of LE-S was compared to its respective DMSO controls; cells treated with the highest dose tested of LE-S (250 μg/mL) and its respective DMSO controls had a significant reduction in cell viability when compared to untreated controls (P<.05).
Mitochondrial Membrane Potential and Solubilized Loteprednol Etabonate
The mitochondrial membrane potential JC-1 assay was tested for cells exposed for 24 hours to solubilized loteprednol etabonate 250, 125, 62.5, or 31.25 μg/mL; the DMSO controls for the three highest doses; and for untreated cells. In both the ARPE-19 and R28 cell lines, there was no difference between cells treated with solubilized loteprednol etabonate, cells treated with the equivalent doses of DMSO, or untreated controls (Figures 25 and 26). For ARPE-19 cells, the red/green ratio was 1.24 ± 0.25, 1.36 ± 0.16, 1.35 ± 0.14, 1.38 ± 0.07, 1.28 ± 0.11, 1.26 ± 0.08, 1.48 ± 0.05, and 1.39 ± 0.15 for s-LE 250, 125, 62.5, or 31.25 μg/mL, controls treated with equivalent doses of DMSO, or untreated controls, respectively. For R28 cells, the red/green ratio was 1.53 ± 0.08, 1.46 ± 0.06, 1.48 ± 0.07, 1.47 ± 0.13, 1.47 ± 0.190, 1.45 ± 0.05, and 1.52 ± 0.06 for solubilized loteprednol etabonate 125, 62.5 or 31.25 μg/mL, controls treated with equivalent doses of DMSO, or untreated controls, respectively.
FIGURE 25.
Mitochondrial membrane potential (ΔΨm) of human retinal pigment epithelial cells exposed to loteprednol etabonate solubilized in DMSO (LE-S). No significant difference in mitochondrial potential was found between cells treated with solubilized loteprednol, DMSO-treated, or untreated controls.
FIGURE 26.
Mitochondrial membrane potential (ΔΨm) of rat embryonal neurosensory precursor retinal cells exposed to loteprednol etabonate solubilized in DMSO (LE-S). No significant difference in mitochondrial potential was found between cells treated with solubilized loteprednol, DMSO-treated, or untreated controls.
FLUOCINOLONE ACETONIDE
Cell viability results from both cells obtained after 2, 6, or 24 hours of exposure to fluocinolone acetonide were statistically similar to each other. Among all concentrations of fluocinolone acetonide tested (1 μg/mL, 10 μg/mL, 150 μg/mL, 500 μg/mL, 1000 μg/mL) only 500 μg/mL and 1000 μg/mL were found to be toxic on both cell lines (P<.05). In the R28 cell line, a toxic effect was also observed after 24 hours for 150 μg/mL (P<.05) (Figures 27 and 28).
FIGURE 27.
Cell viability of human retinal pigment epithelial cells exposed to fluocinolone acetonide (FA). Cells treated with 500 or 1000 μg/mL FA showed a significant reduction in cell viability after 2, 6, or 24 hours of exposure (P<.05) when compared to ethyl alcohol (EtOH) or untreated controls.
FIGURE 28.
Cell viability of rat embryonal neurosensory precursor retinal cells exposed to fluocinolone acetonide (FA). Cells treated with 500 or 1000 μg/mL FA showed a significant reduction in cell viability after 2, 6, or 24 hours of exposure (P<.05). Cells exposed to 150 μg/mL FA showed a significant reduction in cell viability after 24 hours of exposure, when compared to ethyl alcohol (EtOH) or untreated controls (P<.05).
After 2 hours of exposure, the percentage of ARPE-19 viable cells was 99.6 ± 0.2%, 100.5 ± 0.8%, 98.3 ± 2.2%, 70.9 ± 22.5, and 22.3 ± 5.5%, for fluocinolone acetonide 1, 10, 150, or 1000 μg/mL. After 6 hours of exposure, the ARPE-19 cell viability was: 99.7 ± 0.4%, 99.4 ± 0.6%, 99.7 ± 1.8%, 68.4 ± 24.6%, and 12.2 ± 12.9%, and after 24 hours, it was 99.6 ± 0.7, 99.5 ± 1.5, 100.3 ± 1.8, 81.6 ± 9.0, and 23.4 ± 12.9, for fluocinolone acetonide 1, 10, 150, or 1000 μg/mL. Cell viabilities for ethanol controls after 24-hour exposure were 100.4 ± 2.4, 95.5 ± 5.1, and 28.5 ± 12.5, for equivalent doses of ethanol used to solubilize fluocinolone acetonide 150, 500, or 1000 μg/mL.
After 2 hours of exposure, the percentage of R28 viable cells was 103.4 ± 5.1%, 103.3 ± 4.5%, 98.7 ± 2.2%, 72.6 ± 14.1, and 16.5 ± 1.5%, for fluocinolone acetonide 1, 10, 150, or 1000 μg/mL. After 6 hours of exposure, the R28 cell viability was 102.0 ± 3.1%, 102.1 ± 3.2%, 101.7 ± 6.9%, 36.2 ± 23.2%, and 11.4 ± 4.3%, and after 24 hours, it was 91.8 ± 15.7, 97.1 ± 3.7, 81.0 ± 4.6, 24.8 ± 7.4, and 10.2 ± 0.1, for fluocinolone acetonide 1, 10, 150, or 1000 μg/mL. Cell viabilities for ethanol controls after 24-hour exposure were 87.3 ± 8.8, 50.19 ± 32.5, and 13.8 ± 9.6, for equivalent doses of ethanol used to solubilize fluocinolone acetonide 150, 500, or 1000 μg/mL.
DISCUSSION
Intravitreous injection of steroids remains one of the most commonly used interventions in ophthalmology with the bulk of intravitreal steroid injections utilizing one of several formulations of triamcinolone acetonide.130 The experiments presented here test, in a comparative manner under standard in vitro laboratory conditions, the main commercially available nontriamcinolone steroid compounds in an attempt to assess whether viable alternatives to triamcinolone acetonide could be considered for further testing and development for retinal conditions. Our in vitro steroid experiments aimed to create conditions similar to the ones found in clinical practice. Therefore, we had to define what we would consider the clinical dose for different steroids. If we take triamcinolone acetonide as an example, intravitreous injections range from 1 mg76,81 to 25 mg,11,13 but most reports in literature consider it to be 4 mg, which is the amount obtained when 0.1 mL is taken from a 40 mg/mL suspension. The human vitreous volume is approximately 4 mL, so that the clinical dose of triamcinolone acetonide, reached when 0.1 mL of the solution containing 4 mg of triamcinolone acetonide is injected intravitreally and assuming it disperses equally into the vitreous, would be 1 mg/mL. After the same considerations, the clinical dose for betamethasone would be 150 μg/mL, for loteprednol etabonate it is 125 μg/mL, and for methylprednisolone, 1 mg/mL.
Dexamethasone comes as either a 4 mg/mL or a 10 mg/mL solution. Therefore, 0.1 mL of the commercially available dexamethasone contains 0.4 to 1.0 mg of the drug, and the clinical dose for injections of dexamethasone solution would be 100 to 250 μg/mL. When considering drug levels associated with the dexamethasone drug delivery system, the maximum vitreous concentration generated by Ozurdex in an animal model is approximately 0.25 μg/mL, which is 1000 times less than the dose associated with intravitreal free dexamethasone injections.66
For fluocinolone acetonide, the determination of the clinical dose is not obvious, as there is no commercially available suspension, and our experiments were performed after solubilizing the powder drug in ethanol. The Retisert implant releases fluocinolone acetonide at an initial rate of 0.6 μg/day and reaches a steady state of 0.3 to 0.4 μg/day after around a month. Driot and associates58 have reported that the steady state fluocinolone acetonide vitreous concentration of the Retisert implant can reach 0.15 μg/mL.
Similar to what has been previously reported for triamcinolone acetonide,108 we have shown here that the other commercially available steroid formulations (betamethasone, loteprednol etabonate, methyprednisolone) in their crystalline commercially available form, are toxic to retinal cells in vitro at clinically relevant doses. This toxicity is less pronounced when the drug is solubilized in DMSO, though even after solubilization, methylprednisolone is still toxic to retinal cells.
Dexamethasone at concentrations of 0.125, 0.25, and 0.50 mg/mL (up to 5 times the clinical dose of dexamethasone solution) does not cause decreased cell viability in ARPE-19 and R28 cells in vitro. However, the more sensitive WST-1 assay shows that there may be some reduced mitochondrial function in response to dexamethasone solution and powder at the doses tested here.
Kwak and D’Amico’s in vivo study in rabbits59 demonstrated a lack of retinal toxicity following intravitreal injection of 440 μg of dexamethasone sodium phosphate, as a pure solution or in a commercially supplied preparation with paraben vehicle. However, doses from 800 to 4000 μg showed a dose-related retinal toxicity with increasing spectrum of disorganization in Müller and other retinal cells. Yeung and colleagues103 compared the relative in vitro toxicity of triamcinolone acetonide with dexamethasone and hydrocortisone. They reported the action of dexamethasone and hydrocortisone (but not triamcinolone acetonide) to be biphasic. There was an initial rise in cell proliferation in the presence of dexamethasone and hydrocortisone at concentrations of 0.01 to 0.1 mg/mL at 24 hours. However, they found that after 24 hours there was a significant reduction in cell numbers in concentrations from 0.01 to 1 mg/mL. Also, the relative degree of toxicity regardless of duration of exposure was triamcinolone acetonide > dexamethasone > hydrocortisone. This biphasic response of dexamethasone on human retinal pigment epithelial cells has been reported previously by He and coworkers.131 They found that a dexamethasone dose of 1 μg/mL causes cultured human retinal pigment epithelial cells to proliferate and suggest that it may have a growth factor–like action.
Previous studies from our laboratory have used the mitochondrial dehydrogenase WST-1 assay to evaluate the toxicity of triamcinolone acetonide, as well as the vital dyes indocyanine green and trypan blue, on ARPE-19 and R-28 cells.105,127,128 WST-1 is a tetrazolium dye containing an electron coupling reagent, which is cleaved by the mitochondrial dehydrogenase enzyme to a formazan dye, and the intensity of this reaction directly correlates to the number of metabolically active cells.
We found that dexamethasone solution at concentrations of 0.125 mg/mL, 0.25 mg/mL, and 0.50 mg/mL (at or above the dose normally used in clinical practice, which is 0.1 to 0.25 mg/mL) is not toxic to cells in vitro. However, the highest dose of dexamethasone (1 mg/mL, up to 10× the clinical dose of freely injected DEX) is toxic to ARPE-19 and R28 cells after 6 and 24 hours exposure. The toxicity observed can be at least partially related to the preservative itself, as a decrease in mitochondrial dehydrogenase activity in both cell lines was observed in our study after treatment with benzyl alcohol. Moreover, cells treated with dexamethasone powder showed less toxicity as compared to the commercially available solution.
Despite being a very potent steroid, few studies describe the effects of in vivo or in vitro exposure of retinal cells to betamethasone. Shimada and Matsui132 showed that doses less than 5 mg were safe to rabbit eyes, but injection of 10 mg caused localized inferior retinal degeneration. Hida and coworkers133 reported that injection of betamethasone caused significant retinal degeneration in its standard concentration. Citirik and coworkers16 injected different steroids in the vitreous cavity of rats and observed that low-dose betamethasone (0.075 mg) did not cause oxidative changes, whereas high doses (0.150 mg) significantly increased retinal malondialdehyde and glutathione levels, indicative of oxidative stress. However, a case report of inadvertent intravitreous injection of betamethasone has been described after cataract extraction, and in that reported case, the patient had good visual recovery.134 For crystalline commercially available betamethasone, our results show a decrease in cell viability for doses as low as 45 μg/mL (with the clinical dose being 150 μg/mL). Only the lowest concentration tested (30 μg/mL, or one-fifth of the clinical dose) was safe for both cell lines. However, no significant decrease in cell viability was observed in up to 2.5 times the clinical dose after the drug was solubilized in DMSO.
Most of the literature involving methylprednisolone and retinal cells comes from inadvertent intravitreous injections, leading to poor visual outcomes due to retinal detachment or optic nerve atrophy.135–137 Hida and coworkers133 observed significant retinal degeneration with preretinal membrane formation after exposure to methylprednisolone. Citirik and coworkers16 reported a significant change in malondialdehyde levels after intraocular injections of methylprednisolone in rat eyes in any concentration tested. Hence, the investigators recommend that intravitreal use of methylprednisolone should be avoided. Our findings point to a significant decrease in cell viability in both cell lines tested in doses as low as 1/20th of the calculated clinical dose. The solubilization of the drug shows less effect on cell viability compared to the commercial formulation, but even when solubilized, doses lower than the clinical dose caused a significant reduction in cell viability when compared to untreated controls.
The literature regarding the in vitro exposure of loteprednol etabonate or fluocinolone acetonide to retinal cells is scarce. We found here that loteprednol etabonate doses as low as one-eighth of the clinical dose were toxic to R28 cells in vitro, suggesting that this particular steroid may not be a good option for intraocular use. The observed toxicity decreased after the drug was solubilized in DMSO, but solubilized doses two times higher than the clinical dose still exhibited toxicity. For fluocinolone acetonide, doses less than 150 μg/mL did not cause a reduction in cell viability. Considering that the Retisert fluocinolone acetonide implant delivers a maximum vitreous concentration of 0.15 μg/mL,58 our experiments indicate that doses 1000 times higher than the ones observed were safe for retinal cells in vitro, similar to what was seen here with regard to the lack of dexamethasone toxicity relative to the intravitreal dexamethasone concentrations achieved with the Ozurdex implant.
The majority of the scientific literature assessing in vitro steroid toxicology on retinal cells has tested the effects of triamcinolone acetonide exposure due to its prevalent use in clinical settings. The toxicity of triamcinolone acetonide in vitro has been shown,103,105,107,114,115 but the mechanism of the toxicity has been controversial. Some investigators attribute the toxicity to components of the solution, such as the dispersion agent polysorbate 80 or its preservative, benzyl alcohol.123 Polysorbate 80 has been associated with hypersensitivity in intravenous formulations of multiple medications.138–140 Benzyl alcohol is an aromatic alcohol that has been incorporated into many parenteral preparations as a preservative.
However, it has not been clear as to whether benzyl alcohol is the cause of triamcinolone acetonide toxicity, as several investigators have shown that it is safe on retinal cells. In a study from our laboratory, Narayanan and coworkers105 treated ARPE-19 and R28 cells with the vehicle of triamcinolone acetonide solution and found no toxic effects. Yeung and colleagues103 found that benzyl alcohol is not toxic, in concentrations equal to or lower than clinical equivalents on ARPE and human glial cells; Dierks and coworkers117 and McGee and coworkers141 did not observe benzyl alcohol toxicity at concentrations of 0.0025% in vivo. Walter and colleagues142 showed that benzyl alcohol at concentrations of 0.3 mmol/L and 1 mol/L did not affect the b wave on the ERG in bovine retina. On the other hand, some evidence suggests toxicity of this vehicle.
Chang and coworkers123 found necrosis after in vitro exposure of retinal cells to the benzyl alcohol vehicle. Morrison and coworkers143 found that at a 3.3-fold higher concentration than that injected into a human eye, benzyl alcohol injected into rabbit eyes caused retinal whitening and intraretinal hemorrhages, which eventually led to atrophy as confirmed histologically. Moreover, at high concentrations of benzyl alcohol, the ERG responses of bovine retinas showed decreased b waves.142 This led some investigators to suggest that triamcinolone acetonide should be separated from most of its vehicle before injection.143
In the present study, different crystalline steroids (betamethasone, methylprednisolone, and loteprednol) were solubilized in DMSO to determine the role of the steroid crystals (found in the commercially available solution) on retinal cells. To obtain the solubilized steroids, we centrifuged the drug, removed the supernatant, and added the same amount of DMSO to dilute the drug. Previous studies have shown that the final concentration achieved by the centrifugation method is identical to the original commercial concentration.144 We chose the centrifugation technique because the sedimentation process reduced the expected dose by 25% and the filter technique showed a reduction between 45% and 75% of the original concentration, depending on the pore size.144 All steroids tested had better cell viability percentages after the drug was solubilized in DMSO. The results presented are similar to what was described when a triamcinolone acetonide formulation (Triesence) was solubilized108 and points to the direct contact of different steroid crystals on retinal cells as a causative factor for the decrease in cell viability.
Our findings suggest that the interaction of the steroid crystals with cultured cells may play a role in their observed cytotoxicity. Some studies have demonstrated that triamcinolone acetonide is not toxic if there is no direct contact of the triamcinolone acetonide crystals with the cell surface.114,115 The triamcinolone acetonide crystals seem to exert a strong, local, concentration-dependent chemical toxicity if directly adhering to the apical surface of retinal cells.116 If the triamcinolone acetonide concentration falls below the solubility equilibrium of 36 μg/mL, then no toxicity is found, but with the appearance of minute cell-adhering crystals, then rapid progressive toxicity has been observed. Utilizing a Boyden chamber with a filter that prevents direct contact of crystals with retinal cells, Spitzer and coworkers130 counted the number of retinal pigment epithelial cells with and without filter, and found 14 times less cell death after the filter was placed.
The decrease in cell viability caused by triamcinolone acetonide does not seem to be directly mechanical. When confluent cultured retinal ganglion cells were exposed to either triamcinolone acetonide crystals or glass pearls of the same size and weight, only triamcinolone acetonide crystals induced a rapid and strong toxic effect.114 These results suggest that the crystals exert a strong localized, dose-dependent toxicity when they enter into intimate contact with retinal cellular membranes, which can be either related to the shape of the crystals145 or related to a gradient concentration across the cell membrane. Slow-release lipophilic drugs like crystalline steroids show a diffusion-dependent local gradient around the particles that lead to accumulation of high amounts of the drug in the cell. In other words, steroid crystals in direct contact with the lipophilic cellular membranes may act as continuous releasing systems resulting in higher intracellular concentrations of the drug than the solubility equilibrium might suggest.130 This may cause a rapid disintegration of the cell membrane, in accordance with the very early phosphatidylserine externalization to the outer leaflet of the plasma membrane.115
Despite the cell culture experiments, which show that exposure to triamcinolone acetonide leads to decrease in cell viability by the induction of apoptosis,103,108 there is a dissociation between laboratory and clinical data, as there is a lack of overt clinical evidence for retinal toxicity after intravitreal administration of triamcinolone acetonide. This discrepancy between in vitro experiments and clinical practice may be explained by protective factors, such as the vitreous gel or the ILM, that would prevent the direct contact of steroid crystals and retinal cells.108,114,115,130
Szurman and coworkers114 used the anterior capsule removed from patients that had undergone phacoemulsification as a basement membrane sheet over a layer of ganglion cells in culture. They observed that areas covered by the overlying basement membrane during triamcinolone acetonide exposure were completely devoid of dead cells, and the interface between vital and dead cells was clearly delineated along the edge of the basement membrane. Similarly, in areas where the ganglion cells were covered by porcine vitreous, no dead cells were found. The ILM and the vitreous were considered to be protective factors. Those findings are in agreement with the present study, as the methodology we tested suggests that steroid (and not just triamcinolone acetonide) crystals play an important role in retinal cell toxicity in vitro.
In addition to a mechanical protective effect, it has been proposed that the ILM would act as a filter, so that the real concentration of the drug on the retinal pigment epithelium would be much lower than in the vitreous. Additionally, retinal and choriocapillaris circulation could potentially wash away high levels of drug concentration.115 Those protective effects mentioned above could explain the dissociation between tissue culture experiments showing toxicity of triamcinolone acetonide and most in vivo studies that show no to limited toxicity.
In summary, the current studies evaluating the comparative toxicities observed after exposure of retinal cells to different steroids in vitro seem to be related to an intimate contact of the steroids to cellular membranes. In most clinical scenarios, this effect can be reduced compared to in vitro settings, as there are natural protective factors, such as the vitreous gel or the ILM, that may prevent this contact. However, after vitrectomy with or without ILM removal, those factors may be lacking and the in vitro steroid toxic findings seen here may become clinically relevant. Based on the results from the data presented here, methylprednisolone and loteprednol etabonate do not seem to be good alternatives to triamcinolone acetonide due to the poor safety profile observed, with loteprednol showing cytotoxic effects even when solubilized. Betamethasone, a very potent steroid, was toxic to retinal cells in its crystalline, commercial formulation, but limited toxicity was observed after the drug was solubilized. The exposure of retinal cells to minute amounts of dexamethasone and fluocinolone acetonide, as observed with drug delivery systems such as the Iluvien and Retisert fluocinolone acetonide implants or the Ozurdex dexamethasone implant, seem to be good therapeutic alternatives to triamcinolone acetonide. The drug delivery systems prevent the direct contact of steroids to cellular membranes, and no signs of retinal toxicity are observed even at drug levels of a greater magnitude than achieved with these drug delivery systems.
The literature assessing the safety of various steroid formulations on retinal cells in culture, including the work presented here, suggests that dexamethasone may be the safest steroid to use because of its reduced amount of observed toxicity compared to the other formulations, even at the higher drug levels achieved with intravitreal injections of commercial dexamethasone solutions. However, the low dose of fluocinolone acetonide used in the two commercial drug delivery systems appears to be quite safe. The Iluvien and Ozurdex drug delivery systems appear to be nearing approval by the FDA for the treatment of diabetic macular edema as of this writing. Based on the laboratory experiments described here, both the Ozurdex and Iluvien implants are extremely unlikely to exhibit any direct retinal cytotoxic effects, in contrast to what was observed with the other crystalline steroid formulations tested here. Triamcinolone acetonide appears to exhibit retinal cellular toxicity at clinically relevant doses in cell culture experiments by our laboratory and elsewhere, though in most clinical settings this toxic effect is not observed, and is presumably much less than its net therapeutic benefit.
Finally, methylprednisolone, betamethasone, and loteprednol etabonate, in their commercially available formulations, do not seem to be good candidates for intravitreal clinical use because of their observed significant toxicity in the in vitro experiments presented here. In comparison, dexamethasone and fluocinolone acetonide, which exhibited fewer cytotoxic effects than other steroids, may be potentially viable alternatives to triamcinolone acetonide for clinical use.
Acknowledgments
Funding/Support: This work was supported by the Discovery Eye Foundation, Beckman Initiative for Macular Research, Polly and Michael Smith Foundation, Iris and B. Gerald Cantor Foundation, and a Challenge Grant from Research to Prevent Blindness.
Financial Disclosures: Dr Kuppermann has received consulting fees and/or grant support in the last 2 years from the following companies: AcuFocus, Ampio, Alcon Alimera, Allegro, Allergan, AquaTherapeutics, Genentech, Glaukos, GlaxoSmithKline, Jaeb (Diabetic Retinopathy Clinical Research Network), Novagali, Novartis, Neurotech, Ophthotech, Pfizer, Regeneron, Santen, SecondSight, Staar Surgical, Teva, and ThromboGenics. Dr Zacharias and Dr Kenney have received no corporate consulting fees or grant support in the past 2 years.
Author Contributions: Conception and design (B.D.K.); analysis and interpretation (B.D.K., L.C.Z., M.C.K.); writing the article (B.D.K., L.C.Z.); critical revision of the article (B.D.K., L.C.Z., M.C.K.); final approval of the article (B.D.K., L.C.Z., M.C.K.); data collection (B.D.K., L.C.Z., M.C.K.); provision of materials, patients or resources (B.D.K., M.C.K.); statistical expertise (B.D.K., L.C.Z., M.C.K.); obtaining funding (B.D.K., M.C.K.); literature search (B.D.K., L.C.Z., M.C.K.); administrative, technical or logistical support (B.D.K., L.C.Z., M.C.K.).
Other Acknowledgments: The following individuals who were fellows at the University of California, Irvine, during the time of the study are to be recognized for their participation in data collection: Ana Laura Gramajo, MD; Ashish Sharma, MD; Jayaprakash Patil, MD; Aneesh Neekhra, MD; and Saurabh Luthra, MD.
REFERENCES
- 1.Leopold IH. Nonsteroidal and steroidal anti-inflammatory agents. In: Sears ML, Tarkkanen A, editors. Surgical Pharmacology of the Eye. New York: Raven Press; 1985. [Google Scholar]
- 2.Pagano G, Bruno A, Cavallo-Perin P, Cesco L, Imbimbo B. Glucose intolerance after short-term administration of corticosteroids in healthy subjects. Prednisone, deflazacort, and betamethasone. Arch Intern Med. 1989;149(5):1098–1101. [PubMed] [Google Scholar]
- 3.Saag KG. Glucocorticosteroid-induced osteoporosis. Endocrinol Metab Clin North Am. 2003;32(1):135–157. doi: 10.1016/s0889-8529(02)00064-6. [DOI] [PubMed] [Google Scholar]
- 4.Stanbury RM, Graham EM. Systemic corticosteroid therapy—side effects and their management. Br J Ophthalmol. 1998;82(6):704–708. doi: 10.1136/bjo.82.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weijtens O, van der Sluijs FA, Schoemaker RC, et al. Peribulbar corticosteroid injection: vitreal and serum concentration after dexamethasone disodium phosphate injection. Am J Ophthalmol. 1997;123(3):358–363. doi: 10.1016/s0002-9394(14)70131-x. [DOI] [PubMed] [Google Scholar]
- 6.Weijtens O, Schoemaker RC, Cohen AF, et al. Dexamethasone concentration in vitreous and serum after oral administration. Am J Ophthalmol. 1998;125(5):673–679. doi: 10.1016/s0002-9394(98)00003-8. [DOI] [PubMed] [Google Scholar]
- 7.Weijtens O, Feron EJ, Schoemaker RC, et al. High concentration of dexamethasone in aqueous and vitreous after subconjunctival injection. Am J Ophthalmol. 1999;128(2):192–197. doi: 10.1016/s0002-9394(99)00129-4. [DOI] [PubMed] [Google Scholar]
- 8.Weijtens O, Schoemaker RC, Romjin FP, Cohen AF, Lentjes EG, van Meurs JC. Intraocular penetration and systemic absorption after topical application of dexamethasone disodium phosphate. Ophthalmology. 2002;109(10):1887–1891. doi: 10.1016/s0161-6420(02)01176-4. [DOI] [PubMed] [Google Scholar]
- 9.Herrero-Vanrell R, Cardillo JA, Kuppermann BD. Clinical applications of the sustained-release dexmethasone implant for treatment of macular edema. Clin Ophthalmol. 2011;5:139–146. doi: 10.2147/OPTH.S15783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HB, Pulido JS, McCannel CA, Buettner H. Role of inflammation in retinal vein occlusion. Can J Ophthalmo. 2007;42:131–133. doi: 10.1139/i06-101. [DOI] [PubMed] [Google Scholar]
- 11.Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide as treatment of macular edema in central retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2002;240:782–783. doi: 10.1007/s00417-002-0529-0. [DOI] [PubMed] [Google Scholar]
- 12.Sivaprasad S, McCluskey P, Lightman S. Intravitreal steroids in the management of macular oedema. Acta Ophthalmol Scand. 2006;84:722–733. doi: 10.1111/j.1600-0420.2006.00698.x. [DOI] [PubMed] [Google Scholar]
- 13.Jonas JB, Kreissig I, Kamppeter B, Degenring RF. Intravitreal triancinolone acetonide for the treatment of intraocular edematous and neovascular diseases. Ophthalmologe. 2004;101:113–120. doi: 10.1007/s00347-003-0982-0. [DOI] [PubMed] [Google Scholar]
- 14.Spaide RF. Rationale for combination therapies for choroidal neovascularization. Am J Ophthalmol. 2006;141:149–156. doi: 10.1016/j.ajo.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 15.Spaide RF. Combination therapies for choroidal neovascularization. In: Holz FG, Spaide RF, editors. Medical Retina (Essentials in Ophthalmology series) Berlin: Springer; 2007. pp. 90–104. [Google Scholar]
- 16.Citirik M, Dilsiz N, Batman C, Zilelioglu O. Comparative toxicity of 4 commonly used intravitreal corticosteroids on rat retina. Can J Ophthalmol. 2009;44:e3–8. doi: 10.3129/i09-059. [DOI] [PubMed] [Google Scholar]
- 17.Glybina IV, Kennedy A, Ashton P, Abrams GW, Iezzi R. Photoreceptor neuroprotection in RCS rats via low-dose intravitreal sustained-delivery of fluocinolone acetonide. Invest Ophthalmol Vis Sci. 2009;50:4847–4857. doi: 10.1167/iovs.08-2831. [DOI] [PubMed] [Google Scholar]
- 18.Glybina IV, Kennedy A, Ashton P, Abrams GW, Iezzi R. Intravitreous delivery of the corticosteroid fluocinolone acetonide attenuates retinal degeneration in S334ter-4 rats. Invest Ophthalmol Vis Sci. 2010;51:4243–4252. doi: 10.1167/iovs.09-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naveh N, Weissman C. Prolonged corticosteroid treatment exerts transient inhibitory effect on prostaglandin E2 release from rabbits’ eyes. Prostaglandins Leukot Essent Fatty Acids. 1991;42:101–105. doi: 10.1016/0952-3278(91)90075-g. [DOI] [PubMed] [Google Scholar]
- 20.Lewis GD, Campbell WB, Johnson AR. Inhibition of prostaglandin synthesis by glucocorticoids in human endothelial cells. Endocrinology. 1986;119:62–69. doi: 10.1210/endo-119-1-62. [DOI] [PubMed] [Google Scholar]
- 21.Heffernan JT, Futterman S, Kalina RE. Dexamethasone inhibition of experimental endothelial cell proliferation in retinal venules. Invest Ophthalmol Vis Sci. 1978;17:565–568. [PubMed] [Google Scholar]
- 22.Bhattacherjee P, Williams RN, Eakins KE. A comparison of the ocular anti-inflammatory activity of steroidal and nonsteroidal compounds in the rat. Invest Ophthalmol Vis Sci. 1983;24:1143–1146. [PubMed] [Google Scholar]
- 23.Antonetti DA, Wolpert EB, DeMaio L, Harhaj NS, Scaduto RC., Jr Hydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludin. J Neurochem. 2002;80:667–677. doi: 10.1046/j.0022-3042.2001.00740.x. [DOI] [PubMed] [Google Scholar]
- 24.Ando N, Sen HA, Berkowitz BA, Wilson CA, de Juan E., Jr Localization and quantification of blood-retinal barrier breakdown in experimental proliferative vitreoretinopathy. Arch Opthalmol. 1994;112:117–122. doi: 10.1001/archopht.1994.01090130127029. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda S, Gomi F, Oshima Y, Tohyama M, Tano Y. Vascular endothelial growth factor reduced and connective tissue growth factor induced by triamcinolone in ARPE19 cells under oxidative stress. Invest Ophthalmol Vis Sci. 2005;46:1062–1068. doi: 10.1167/iovs.04-0761. [DOI] [PubMed] [Google Scholar]
- 26.Nauck M, Karakiulakis G, Perruchoud AP, Papakonstantinou E, Roth M. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998;341:309–315. doi: 10.1016/s0014-2999(97)01464-7. [DOI] [PubMed] [Google Scholar]
- 27.Sohn HJ, Han DH, Kim IT, et al. Changes in aqueous concentrations of various cytokines after intravitreal triamcinolone versus bevacizumab for diabetic macular edema. Am J Ophthalmol. 2011;152:686–694. doi: 10.1016/j.ajo.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 28.Penfold PL, Wen L, Madigan MC, Gillies MC, King NJ, Provis JM. Triamcinolone acetonide modulates permeability and intercellular adhesion molecule-1 (ICAM-1) expression of the ECV304 cell line: implications for macular degeneration. Clin Exp Immunol. 2000;121:458–465. doi: 10.1046/j.1365-2249.2000.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamura H, Miyamoto K, Kiryu J, et al. Intravitreal injection of corticosteroid attenuates leukostasis and vascular leakage in experimental diabetic retina. Invest Ophthalmol Vis Sci. 2005;46:1440–1444. doi: 10.1167/iovs.04-0905. [DOI] [PubMed] [Google Scholar]
- 30.Miyamoto N, Iossifov D, Metge F, Behar-Cohen F. Early effects of intravitreal triamcinolone acetonide on macular edema. Mechanistic implication. Ophthalmology. 2006;113:2048–2053. doi: 10.1016/j.ophtha.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 31.Graham RO, Peyman GA. Intravitreal injection of dexamethasone. Treatment of experimentally induced endophthalmitis. Arch Ophthalmol. 1974;92(2):149–154. doi: 10.1001/archopht.1974.01010010155016. [DOI] [PubMed] [Google Scholar]
- 32.Peyman GA, Rose M, Sanders D. Intravitreal antibiotic injection and vitrectomy in acute bacterial endophthalmitis. Can J Ophthalmol. 1976;11:188–190. [PubMed] [Google Scholar]
- 33.Peyman GA, Vastine DW, Meisels HI. The experimental and clinical use of intravitreal antibiotics to treat bacterial and fungal endophthalmitis. Doc Ophthalmol. 1975;39:183–201. doi: 10.1007/BF00578762. [DOI] [PubMed] [Google Scholar]
- 34.Peyman GA, Vastine DW, Diamond JG. Vitrectomy and intraocular gentamicin management of Herellea endophthalmitis after incomplete phacoemulsification. Am J Ophthalmol. 1975;80:764–765. doi: 10.1016/0002-9394(75)90409-2. [DOI] [PubMed] [Google Scholar]
- 35.Machemer R, Sugita G, Tano Y. Treatment of intraocular proliferations with intravitreal steroids. Trans Am Ophthalmol Soc. 1979;77:171–180. [PMC free article] [PubMed] [Google Scholar]
- 36.Tano Y, Sugita G, Abrams G, Machemer R. Inhibition of intraocular proliferations with intravitreal corticosteroids. Am J Ophthalmol. 1980;89:131–136. doi: 10.1016/0002-9394(80)90239-1. [DOI] [PubMed] [Google Scholar]
- 37.Tano Y, Chandler D, Machemer R. Treatment of intraocular proliferation with intravitreal injection of triamcinolone acetonide. Am J Ophthalmol. 1980;90:810–816. doi: 10.1016/s0002-9394(14)75196-7. [DOI] [PubMed] [Google Scholar]
- 38.Ishibashi T, Miki K, Sorgente N, Patterson R, Ryan SJ. Effects of intravitreal administration of steroids on experimental subretinal neovascularization in the subhuman primate. Arch Ophthalmol. 1985;103:708–711. doi: 10.1001/archopht.1985.01050050100026. [DOI] [PubMed] [Google Scholar]
- 39.Penfold PL, Gyory JF, Hunyor AB, Billson FA. Exudative macular degeneration and intravitreal triamcinolone. A pilot study. Aust N Z J Ophthalmol. 1995;23:29–38. doi: 10.1111/j.1442-9071.1995.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 40.Danis RP, Ciulla TA, Pratt LM, Anliker W. Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina. 2000;20:244–250. [PubMed] [Google Scholar]
- 41.Shah A, Branley M. Use of intravitreal triamcinolone in the management of birdshot retinochoroidopathy associated with cystoid macular oedema: a case study over a three-year period. Clin Experiment Ophthalmol. 2005;33:442–444. doi: 10.1111/j.1442-9071.2005.01048.x. [DOI] [PubMed] [Google Scholar]
- 42.Karacorlu M, Arf Karacorlu S, Ozdemir H. Intravitreal triamcinolone acetonide in Vogt-Koyanagi-Harada syndrome. Eur J Ophthalmol. 2006;16:481–483. doi: 10.1177/112067210601600322. [DOI] [PubMed] [Google Scholar]
- 43.Pathengay A. Intravitreal triamcinolone acetonide in serpiginous choroidopathy. Indian J Ophthalmol. 2005;53:77–79. doi: 10.4103/0301-4738.15295. [DOI] [PubMed] [Google Scholar]
- 44.Adigüzel U, Sari A, Ozmen C, Oz O. Intravitreal triamcinolone acetonide treatment for serpiginous choroiditis. Ocul Immunol Inflamm. 2006;14:375–378. doi: 10.1080/09273940601025974. [DOI] [PubMed] [Google Scholar]
- 45.Ozdemir H, Karacorlu M, Karacorlu S. Intravitreal triamcinolone acetonide in sympathetic ophthalmia. Graefes Arch Clin Exp Ophthalmol. 2005;243:734–736. doi: 10.1007/s00417-004-1064-y. [DOI] [PubMed] [Google Scholar]
- 46.Karacorlu M, Ozdemir H, Karacorlu S. Intravitreal triamcinolone acetonide for the treatment of chronic pseudophakic cystoid macular oedema. Acta Ophthalmol Scand. 2003;81:648–652. doi: 10.1046/j.1395-3907.2003.0146.x. [DOI] [PubMed] [Google Scholar]
- 47.Ip M, Kahana A, Altaweel M. Treatment of central retinal vein occlusion with triamcinolone acetonide: an optical coherence tomography study. Semin Ophthalmol. 2003;18:67–73. doi: 10.1076/soph.18.2.67.15860. [DOI] [PubMed] [Google Scholar]
- 48.Ciardella AP, Klancnik J, Schiff W, Barile G, Langton K, Chang S. Intravitreal triamcinolone for the treatment of refractory diabetic macular oedema with hard exudates: an optical coherence tomography study. Graefes Arch Clin Exp Ophthalmol. 2004;242:1024–1027. doi: 10.1136/bjo.2004.041707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Massin P, Audren F, Haouchine B, et al. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004;111:218–224. doi: 10.1016/j.ophtha.2003.05.037. [DOI] [PubMed] [Google Scholar]
- 50.Shields CL, Demirci H, Dai V, et al. Intravitreal triamcinolone acetonide for radiation maculopathy after plaque radiotherapy for choroidal melanoma. Retina. 2005;25:868–874. doi: 10.1097/00006982-200510000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Shields CL, Demirci H, Marr BP, et al. Intravitreal triamcinolone acetonide for acute radiation papillopathy. Retina. 2006;26:537–544. doi: 10.1097/00006982-200605000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Chaudhary V, Mao A, Hooper PL, Sheidow TG. Triamcinolone acetonide as adjunctive treatment to verteporfin in neovascular age-related macular degeneration: a prospective randomized trial. Ophthalmology. 2007;114:2183–2189. doi: 10.1016/j.ophtha.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 53.Gilman AG, Rall TW, Nies AS, Taylor P. The pharmacological basis of therapeutics. Ed 8, rev. New York: Pergamon Press; 1990. [Google Scholar]
- 54.Nehmé A, Lobenhofer EK, Stamer WD, Edelman JL. Glucocorticoids with different chemical structures but similar glucocorticoid receptor potency regulate subsets of common and unique genes in human trabecular meshwork cells. BMC Med Genomics. 2009;2:58–68. doi: 10.1186/1755-8794-2-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edelman JL. Differentiating intraocular glucocorticoids. Ophthalmologica. 2010;224(suppl 1):25–30. doi: 10.1159/000315158. [DOI] [PubMed] [Google Scholar]
- 56.Hardman J, Limbird L, Goodman A. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. Chicago, IL: McGraw-Hill; 2001. [Google Scholar]
- 57.Inoue M, Takeda K, Morita K, Yamada M, Tanigawara Y, Oguchi Y. Vitreous concentrations of triamcinolone acetonide in human eyes after intravitreal or subtenon injection. Am J Ophthalmol. 2004;138:1046–1048. doi: 10.1016/j.ajo.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 58.Driot JY, Novack GD, Rittenhouse KD, Milazzo C, Pearson PA. Ocular pharmacokinetics of fluocinolone acetonide after Retisert intravitreal implantation in rabbits over a 1-year period. J Ocul Pharmacol Ther. 2004;20:269–275. doi: 10.1089/1080768041223611. [DOI] [PubMed] [Google Scholar]
- 59.Kwak HW, D’Amico DJ. Evaluation of the retinal toxicity and pharmacokinetics of dexamethasone after intravitreal injection. Arch Ophthalmol. 1992;110(2):259–266. doi: 10.1001/archopht.1992.01080140115038. [DOI] [PubMed] [Google Scholar]
- 60.Gan IM, Ugahary LC, van Dissel JT, van Meurs JC. Effect of intravitreal dexamethasone on vitreous vancomycin concentrations in patients with suspected postoperative bacterial endophthalmitis. Graefes Arch Clin Exp Ophthalmol. 2005;243(11):1186–1189. doi: 10.1007/s00417-005-1182-1. [DOI] [PubMed] [Google Scholar]
- 61.Ozurdex [package insert] Irvine, CA: Allergan Inc; 2009. [Google Scholar]
- 62.Haller JA, Kuppermann BD, Blumenkranz MS, et al. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128(3):289–296. doi: 10.1001/archophthalmol.2010.21. [DOI] [PubMed] [Google Scholar]
- 63.Kuppermann BD, Blumenkranz MS, Haller JA, et al. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol. 2007;125(3):309–317. doi: 10.1001/archopht.125.3.309. [DOI] [PubMed] [Google Scholar]
- 64.Williams GA, Haller JA, Kuppermann BD, et al. Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or Irvine-Gass syndrome. Am J Ophthalmol. 2009;147(6):1048–1054. doi: 10.1016/j.ajo.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 65.Haller JA, Dugel P, Weinberg DV, Chou C, Whitcup SM. Evaluation of the safety and performance of an applicator for a novel intravitreal dexamethasone drug delivery system for the treatment of macular edema. Retina. 2009;29(1):46–51. doi: 10.1097/IAE.0b013e318188c814. [DOI] [PubMed] [Google Scholar]
- 66.Chang-Lin JE, Attar M, Acheampong A, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52(1):80–86. doi: 10.1167/iovs.10-5285. [DOI] [PubMed] [Google Scholar]
- 67.Brumm M, Nguyen QD. Fluocinolone acetonide intravitreal sustained release device—a new addition to the armamentarium of uveitic management. Int J Nanomedicine. 2007;2(1):55–64. doi: 10.2147/nano.2007.2.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jaffe GJ, McCallum RM, Branchaud B, et al. Long-term follow-up results of a pilot trial of fluocinolone acetonide implant to treat posterior uveitis. Ophthalmology. 2005;112:1192–1198. doi: 10.1016/j.ophtha.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 69.Goldstein DA, Godfrey DG, Hall A, et al. Intraocular pressure in patients with uveitis treated with fluocinolone acetonide implants. Arch Ophthalmol. 2007;125:1478–1485. doi: 10.1001/archopht.125.11.ecs70063. [DOI] [PubMed] [Google Scholar]
- 70.Campochiaro PA, Brown DM, Pearson A, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118:626–635. doi: 10.1016/j.ophtha.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 71.Zacharias LC, Lin T, Migon R, et al. Assessment of the differences in pharmacokinetics and pharmacodynamics between four distinct formulations of triamcinolone acetonide. Retina. 2013;33:522–531. doi: 10.1097/IAE.0b013e3182647f69. [DOI] [PubMed] [Google Scholar]
- 72.Edelman JL, Lutz D, Castro MR. Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res. 2005;80:249–258. doi: 10.1016/j.exer.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 73.Dyer D, Callanan D, Bochow T, et al. Clinical evaluation of the safety and efficacy of preservative-free triamcinolone (triesence [triamcinolone acetonide injectable suspension] 40 mg/ml) for visualization during pars plana vitrectomy. Retina. 2009;29:38–45. doi: 10.1097/IAE.0b013e318188c6e2. [DOI] [PubMed] [Google Scholar]
- 74.Triesence prescribing information. Available at: http://ecatalog.alcon.com/pi/Triesence_us_en.pdf. Accessed September 8, 2012.
- 75.TRIVARIS. Prescribing Information. Available at: http://www.allergan.com/assets/pdf/trivaris_pi.pdf. Accessed September 8, 2012.
- 76.The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study Report 6. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard of care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion. Arch Ophthalmol. 2009;127:1115–1129. doi: 10.1001/archophthalmol.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447–1459. doi: 10.1016/j.ophtha.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wingate RJ, Beaumont PE. Intravitreal triamcinolone and elevated intraocular pressure. Aust N Z J Ophthalmol. 1999;27:431–432. doi: 10.1046/j.1440-1606.1999.00238.x. [DOI] [PubMed] [Google Scholar]
- 79.Jonas JB, Kreissig I, Degenring R. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol. 2003;87:24–27. doi: 10.1136/bjo.87.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bakri SJ, Beer PM. The effect of intravitreal triamcinolone acetonide on intraocular pressure. Ophthalmic Surg Lasers Imaging. 2003;34:386–390. [PubMed] [Google Scholar]
- 81.The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study Report 5. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion. Arch Ophthalmol. 2009;127:1101–1114. doi: 10.1001/archophthalmol.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaushik S, Gupta V, Gupta A, Dogra MR, Singh R. Intractable glaucoma following intravitreal triamcinolone in central retinal vein occlusion. Am J Ophthalmol. 2004;137:758–760. doi: 10.1016/j.ajo.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 83.Haller JA, Bandello F, Belfort R, Jr, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–1146. doi: 10.1016/j.ophtha.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 84.Haller JA, Bandello F, Belfort R, Jr, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Twelve-month study results. Ophthalmology. 2011;118:2453–2460. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 85.Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003;136:791–796. doi: 10.1016/s0002-9394(03)00483-5. [DOI] [PubMed] [Google Scholar]
- 86.Jonas JB, Kreissig I, Degenring RF. Endophthalmitis after intravitreal injection of triamcinolone acetonide. Arch Ophthalmol. 2003;121:1663–1669. doi: 10.1001/archopht.121.11.1663-b. [DOI] [PubMed] [Google Scholar]
- 87.Gilles MC, Simpson JM, Billson FA, et al. Safety of intravitreal injection of triamcinolone: results from a clinical trial. Arch Ophthalmol. 2004;122:336–342. doi: 10.1001/archopht.122.3.336. [DOI] [PubMed] [Google Scholar]
- 88.Jonas JB, Kreissig I, Degenring RF. Retinal complications of intravitreal injections of triamcinolone acetonide. Graefes Arch Clin Exp Ophthalmol. 2004;242:184–188. doi: 10.1007/s00417-003-0841-3. [DOI] [PubMed] [Google Scholar]
- 89.Jonas JB, Kreissig I, Spandau UH, et al. Infectious and noninfectious endophthalmitis after intravitreal high-dosage triamcinolone acetonide. Am J Ophthalmol. 2006;141:579–580. doi: 10.1016/j.ajo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 90.Nelson ML, Tennant MT, Sivaligan A, et al. Infectious and presumed noninfectious endophthalmitis after intravitreal triamcinolone injection. Retina. 2003;23:686–691. doi: 10.1097/00006982-200310000-00014. [DOI] [PubMed] [Google Scholar]
- 91.Wang LC, Yang CM. Sterile endophthalmitis following intravitreal injection of triamcinolone acetonide. Ocul Immunol Inflamm. 2005;13:295–300. doi: 10.1080/09273940590951007. [DOI] [PubMed] [Google Scholar]
- 92.Stepien KE, Eaton AM, Jaffe GJ, et al. Increased incidence of sterile endophthalmitis after intravitreal triamcinolone acetonide in spring 2006. Retina. 2009;29:207–213. doi: 10.1097/IAE.0b013e31818eccb3. [DOI] [PubMed] [Google Scholar]
- 93.Maia M, Farah ME, Belfort RN, Penha FM, et al. Effects of intravitreal triamcinolone acetonide injection with and without preservative. Br J Ophthalmol. 2007;91:1122–1124. doi: 10.1136/bjo.2007.115386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song JH, Hong YT, Kwon OW. Acute syphilitic posterior placoid chorioretinitis following intravitreal triamcinolone acetonide injection. Graefes Arch Clin Exp Ophthalmol. 2008;246:1775–1778. doi: 10.1007/s00417-008-0928-y. [DOI] [PubMed] [Google Scholar]
- 95.Kocabora MS, Durmaz S, Kandemir N. Exacerbation of central serous chorioretinopathy following intravitreal triamcinolone injection. Graefes Arch Clin Exp Ophthalmol. 2008;246:1783–1786. doi: 10.1007/s00417-008-0932-2. [DOI] [PubMed] [Google Scholar]
- 96.Kahook MY, Noecker RJ, Abdelghani WM, Schuman JS. Filtering bleb rupture after intravitreal triamcinolone acetonide injection. Ophthalmic Surg Lasers Imaging. 2008;39:232–233. doi: 10.3928/15428877-20080501-08. [DOI] [PubMed] [Google Scholar]
- 97.Sarraf D, Vyas N, Jain A, et al. Triamcinolone-associated crystalline maculopathy. Arch Ophthalmol. 2010;128:685–690. doi: 10.1001/archophthalmol.2010.109. [DOI] [PubMed] [Google Scholar]
- 98.Pearson A, Levy B, the Fluocinolone Acetonide Implant Study Group Fluocinolone acetonide intravitreal implant to treat diabeic macular edema: 2 year results of a multicenter clinical trial. Invest Ophthalmol Vis Sci. 2005;46 E-Abstract 1795. [Google Scholar]
- 99.Yeh WS, Haller JA, Lanzetta P, et al. Effect of the duration of macular edema on clinical outcomes in retinal vein occlusion treated with dexamethasone intravitreal implant. Ophthalmology. 2012;119:1190–1198. doi: 10.1016/j.ophtha.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 100.Kuppermann BD, Chou C, Weinberg D, Whitcup SM, Haller JA, Blumenkranz MS. Intravitreous dexamethasone effects on different patterns of diabetic macular edema. Arch Ophthalmol. 2010;128(5):642–643. doi: 10.1001/archophthalmol.2010.44. [DOI] [PubMed] [Google Scholar]
- 101.Boyer DS, Faber D, Gupta S, et al. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31(5):915–923. doi: 10.1097/IAE.0b013e318206d18c. [DOI] [PubMed] [Google Scholar]
- 102.Schlaegel TF, Jr, Wilson FM. Accidental intraocular injection of depot corticosteroids. Trans Am Acad Ophthalmol Otolaryngol. 1978;78:847. [Google Scholar]
- 103.Yeung CK, Chan KP, Chiang SW, Pang CP, Lam DS. The toxic and stress responses of cultured human retinal pigment epithelium (ARPE19) and human glial cells (SVG) in the presence of triamcinolone. Invest Ophthalmol Vis Sci. 2003;44:5293–5300. doi: 10.1167/iovs.03-0490. [DOI] [PubMed] [Google Scholar]
- 104.Shaikh S, Ho S, Engelmann LA, Klemann SW. Cell viability effects of triamcinolone acetonide and preservative vehicle formulations. Br J Ophthalmol. 2006;90:233–236. doi: 10.1136/bjo.2005.076190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Narayanan R, Mungkal JK, Kenney MC, Seigel GM, Kuppermann BD. Toxicity of triamcinolone acetonide on retinal neurosensory and pigment epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:722–728. doi: 10.1167/iovs.05-0772. [DOI] [PubMed] [Google Scholar]
- 106.Oh J, Jung YS, Kim GS, Oh IK, Rho B, Huh K. The effect of short-term exposure of triamcinolone acetonide on fibroblasts and retinal pigment epithelial cells. Acta Ophthalmol Scand. 2007;85:786–790. doi: 10.1111/j.1600-0420.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- 107.Chung H, Hwang JJ, Koh JY, Kim J, Yoon YH. Triamcinolone acetonide-mediated oxidative injury in retinal cell culture: comparison with dexamethasone. Invest Ophthalmol Vis Sci. 2007;48:5742–5749. doi: 10.1167/iovs.07-0566. [DOI] [PubMed] [Google Scholar]
- 108.Zacharias LC, Estrago-Franco MF, et al. The effects of commercially available preservative-free FDA-approved triamcinolone (Triesence) on retinal cells in culture. J Ocul Pharmacol Ther. 2011;27(2):143–150. doi: 10.1089/jop.2010.0143. [DOI] [PubMed] [Google Scholar]
- 109.Sharma A, Pirouzmanesh A, Patil J, et al. Evaluation of the toxicity of triamcinolone acetonide and dexamethasone sodium phosphate on human lens epithelial cells (HLE B-3) J Ocul Pharmacol Ther. 2011;27(3):265–271. doi: 10.1089/jop.2010.0120. [DOI] [PubMed] [Google Scholar]
- 110.Kuppermann BD, Patil AJ, Sharma A, et al. Effects of triamcinolone acetonide on human trabecular meshwork cells in vitro. Invest Ophthalmol Vis Sci. 2008;49 doi: 10.4103/0301-4738.121143. E-Abstract 1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lang Y, Zemel E, Miller B, Perlman I. Retinal toxicity of intravitreal Kenalog in albino rabbits. Retina. 2007;27:778–788. doi: 10.1097/IAE.0b013e318030c517. [DOI] [PubMed] [Google Scholar]
- 112.Kai W, Yanrong J, Xiaoxin L. Vehicle of triamcinolone acetonide is associated with retinal toxicity and transient increase of lens density. Graefes Arch Clin Exp Ophthalmol. 2006;244:1152–1159. doi: 10.1007/s00417-005-0251-9. [DOI] [PubMed] [Google Scholar]
- 113.Macky T, Helmy D, Shazly NE. Retinal toxicity of triamcinolone’s vehicle (benzyl alcohol): an electrophysiological and electron microscopic study. Graefes Clin Exp Ophthalmol. 2007;245:817–824. doi: 10.1007/s00417-006-0459-3. [DOI] [PubMed] [Google Scholar]
- 114.Szurman P, Sierra A, Kaczmarek R, et al. Different biocompatibility of crystalline triamcinolone on retinal cells in vitro and in vivo. Exp Eye Res. 2007;85:44–53. doi: 10.1016/j.exer.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 115.Szurman P, Kaczmarek R, Spitzer MS, et al. Differential toxic effect of dissolved TA and its crystalline crystals on cultured human retinal pigment epithelium (ARPE19) cells. Exp Eye Res. 2006;83:584–592. doi: 10.1016/j.exer.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 116.Lüke M, Januschowski K, Beutel J, et al. The effects of triamcinolone crystals on retinal function in a model of isolated perfused vertebrate retina. Exp Eye Res. 2008;87:22–29. doi: 10.1016/j.exer.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 117.Dierks D, Lei B, Zhang K, Hainsworth DP. Electroretinographic effects of an intravitreal injection of triamcinolone in rabbit retina. Arch Ophthalmol. 2005;123:1563–1569. doi: 10.1001/archopht.123.11.1563. [DOI] [PubMed] [Google Scholar]
- 118.Kim H, Csaky C, Gravlin L, et al. Safety and pharmacokinetics of a preservative-free triamcinolone acetonide formulation for intravitreal administration. Retina. 2006;26:523–530. doi: 10.1097/00006982-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 119.Albini TA, Abd-El-Barr MM, Carvounis PE, et al. Long-term retinal toxicity of intravitreal commercially available preserved triamcinolone acetonide (Kenalog) in rabbit eyes. Invest Ophthalmol Vis Sci. 2007;48:390–395. doi: 10.1167/iovs.06-0145. [DOI] [PubMed] [Google Scholar]
- 120.Zenghyu S, Fang W, Ying F. Vehicle used for triamcinolone is toxic to ocular tissues of the pigmented rabbit. Cur Eye Res. 2009;34:769–776. doi: 10.1080/02713680903063082. [DOI] [PubMed] [Google Scholar]
- 121.Maia M, Penha FM, Farah ME, et al. Subretinal injection of preservative-free triamcinolone acetonide and supernatant vehicle in rabbits: an electron microscopy study. Graefes Arch Clin Exp Ophthalmol. 2008;246:379–388. doi: 10.1007/s00417-007-0718-y. [DOI] [PubMed] [Google Scholar]
- 122.Charcoal stripped fetal bovine serum. Manufacturer information. Available at: http://www.lifetechnologies.com/br/en/home/life-science/cell-culture/mammalian-cell-culture/fbs/specialty-serum/charcoal-stripped-fbs.html on December 26, 2013.
- 123.Chang YS, Wu CL, Tseng SH, Kuo PY, Tseng SY. Cytotoxicity of triamcinolone acetonide on human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:2792–2798. doi: 10.1167/iovs.06-1146. [DOI] [PubMed] [Google Scholar]
- 124.Seigel GM. Establishment of an E1A-immortalized retinal cell culture. In Vitro Cell Dev Biol Anim. 1996;32(2):66–68. doi: 10.1007/BF02723034. [DOI] [PubMed] [Google Scholar]
- 125.Seigel GM, Sun W, Wang J, et al. Neuronal gene expression and function in the growth-stimulated R28 retinal precursor cell line. Curr Eye Res. 2004;28(4):257–269. doi: 10.1076/ceyr.28.4.257.27831. [DOI] [PubMed] [Google Scholar]
- 126.Sun W, Seigel GM, Salvi RJ. Retinal precursor cells express functional ionotropic glutamate and GABA receptors. Neuroreport. 2002;13:2421–2424. doi: 10.1097/00001756-200212200-00009. [DOI] [PubMed] [Google Scholar]
- 127.Narayanan R, Kenney MC, Kamjoo S, et al. Toxicity of indocyanine green (ICG) in combination with light on retinal pigment epithelial cells and neurosensory retinal cells. Curr Eye Res. 2005;30(6):471–478. doi: 10.1080/02713680590959312. [DOI] [PubMed] [Google Scholar]
- 128.Narayanan R, Kenney MC, Kamjoo S, et al. Trypan blue: effect on retinal pigment epithelial and neurosensory retinal cells. Invest Ophthalmol Vis Sci. 2005;46(1):304–309. doi: 10.1167/iovs.04-0703. [DOI] [PubMed] [Google Scholar]
- 129.Cellerino A, Bahr M, Isenmann S. Apoptosis in the developing visual system. Cell Tissue Res. 2000;301:53–69. doi: 10.1007/s004410000178. [DOI] [PubMed] [Google Scholar]
- 130.Spitzer M, Mlynczak T, Schultheiss M, et al. Preservative-free triamcinolone injectable suspension versus “traditional” triamcinolone preparations: Impact of aggregate size on retinal biocompatibility. Retina. 2011;31(10):2050–2057. doi: 10.1097/IAE.0b013e318214d076. [DOI] [PubMed] [Google Scholar]
- 131.He S, Wang HM, Ye J, Ogden TE, Ryan SJ, Hinton DR. Dexamethasone induced proliferation of cultured retinal pigment epithelial cells. Curr Eye Res. 1994;13(4):257–261. doi: 10.3109/02713689408995786. [DOI] [PubMed] [Google Scholar]
- 132.Shimada H, Matsui M. Effects of intravitreal injection on rabbit eye. Nippon Ganka Gakkai Zasshi. 1989;93:501–510. [PubMed] [Google Scholar]
- 133.Hida T, Chandler D, Arena JE, Machemer R. Experimental and clinical observations of the intraocular toxicity of commercial corticosteroid preparations. Am J Ophthalmol. 1986;101:190–195. doi: 10.1016/0002-9394(86)90593-3. [DOI] [PubMed] [Google Scholar]
- 134.Chen V, Bartov E, Blumenthal M. Inadvertent intravitreal injection of betamethasone. Metab Pediatr Syst Ophthalmol. 1988;11:61–62. [PubMed] [Google Scholar]
- 135.Piccolino FC, Pandolfo A, Polizzi A, Eandi C, Ventre L. Retinal toxicity from accidental intraocular injection of depomedrol. Retina. 2002;22(1):117–119. doi: 10.1097/00006982-200202000-00025. [DOI] [PubMed] [Google Scholar]
- 136.Wilson DR, Kimble JA, Witherspoon CD, Morris R. Retinal toxicity from accidental intraocular injection of a depot corticosteroid: a case report. Ann Ophthalmol Glaucoma. 1994;26:126–130. [Google Scholar]
- 137.Zinn KM. Iatrogenic intraocular injection of depot corticosteroid and its surgical removal using the pars plana approach. Ophthalmology. 1981;88:13–17. doi: 10.1016/s0161-6420(81)35081-7. [DOI] [PubMed] [Google Scholar]
- 138.Pesce AJ, McKean DL. Toxic susceptibilities in the newborn with special consideration of polysorbate toxicity. Ann Clin Lab Sci. 1989;19:70–73. [PubMed] [Google Scholar]
- 139.Levy M, Dupuis LL. Parenteral nutrition hypersensitivity. J Parenter Enteral Nutr. 1990;14:213–215. doi: 10.1177/0148607190014002213. [DOI] [PubMed] [Google Scholar]
- 140.Shelley WB, Talanin N, Shelley ED. Polysorbate 80 hypersensitivity. Lancet. 1995;345:1312–1313. doi: 10.1016/s0140-6736(95)90963-x. [DOI] [PubMed] [Google Scholar]
- 141.McGee DH, Dembinska O, Gruebbel MM. Evaluation of triamciolone acetonide following intravitreal injection in New Zealand white rabbits. Int J Toxicol. 2005;24:419–425. doi: 10.1080/10915810500366864. [DOI] [PubMed] [Google Scholar]
- 142.Walter P, Luck C, Sickel W. Antibiotics and light responses in superfused bovine retina. Cell Mol Neurobiol. 1999;19:87–92. doi: 10.1023/a:1006968608825. [DOI] [PubMed] [Google Scholar]
- 143.Morrison VL, Koh HJ, Cheng L, Bessho K, Davidson MC, Freeman W. Intravitreal toxicity of the kenalog vehicle (Benzyl Alcohol) in rabbits. Retina. 2006;26:339–344. doi: 10.1097/00006982-200603000-00014. [DOI] [PubMed] [Google Scholar]
- 144.Garcia-Arumi J, Boixadera A, Giralt J, et al. Comparison of different techniques for purification of triamcinolone acetonide suspension for intravitreal use. Br J Ophthalmol. 2005;89:1112–1114. doi: 10.1136/bjo.2005.067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Petersen S, Steiniger F, Fischer D, Fahr A, Bunjes H. The physical state of lipid nanoparticles influences their effect on in vitro cell viability. Eur J Biopharm. 2011;79:150–161. doi: 10.1016/j.ejpb.2011.03.022. [DOI] [PubMed] [Google Scholar]