Abstract
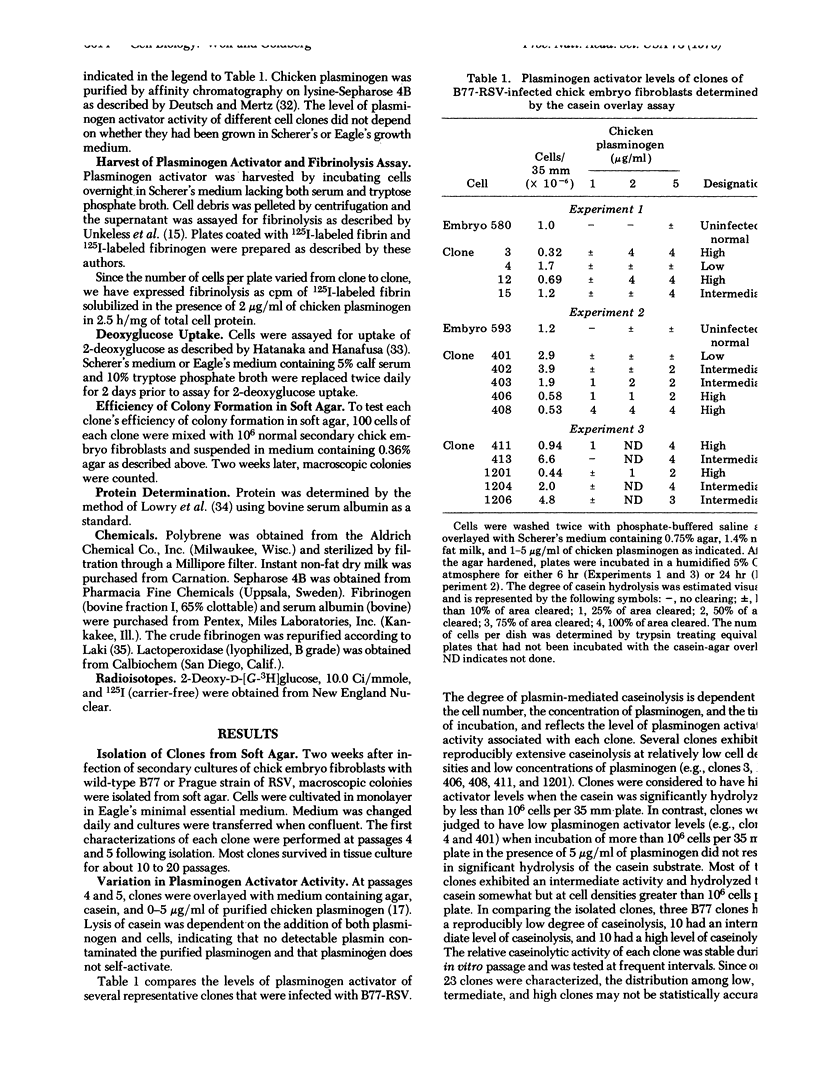
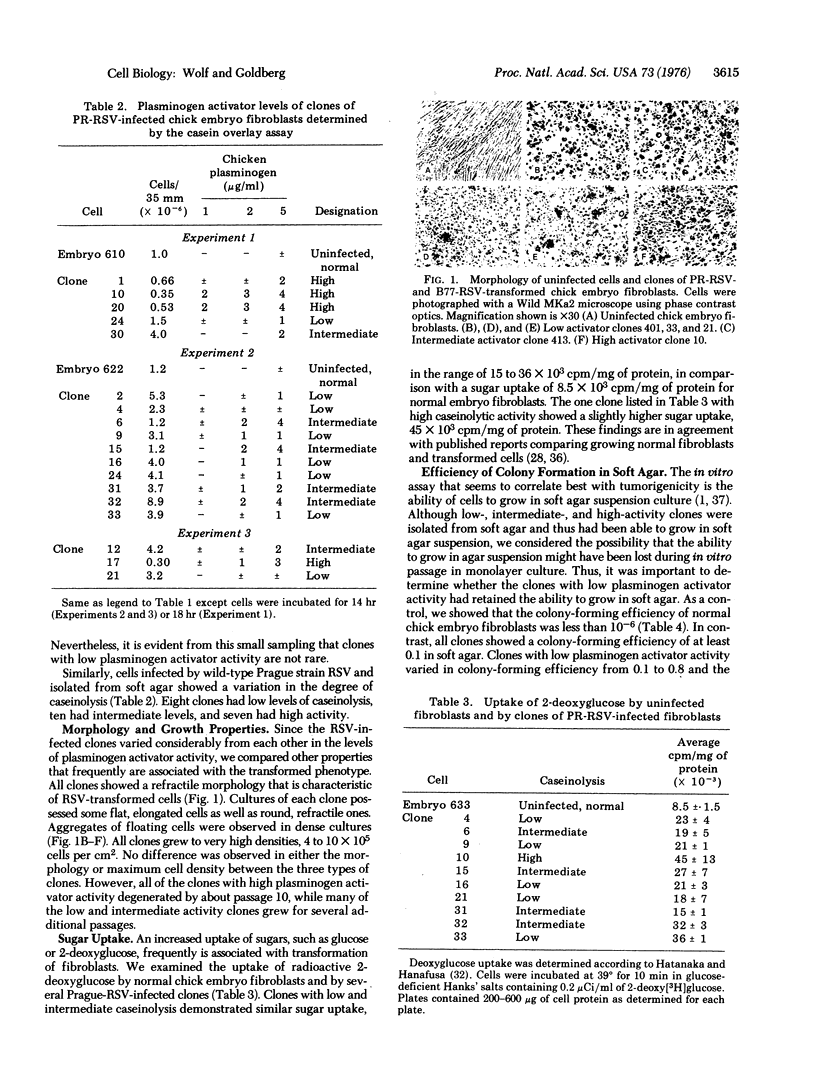
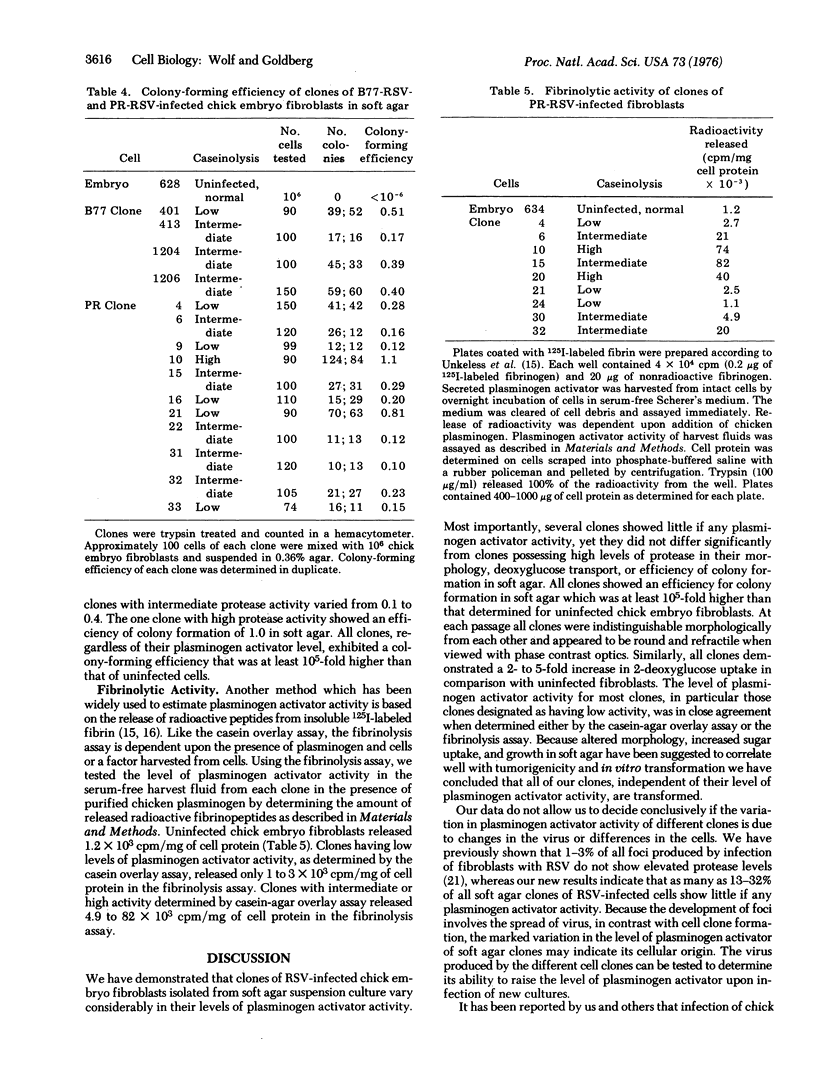
Several clones of transformed chick embryo fibroblasts infected with wild-type B77-C or Prague-C strain of Rous sarcoma virus have been isolated from soft agar suspension. These clones were screened for plasminogen activator activity by overlaying monolayer cultures with medium containing agar, casein, and chicken plasminogen. Twenty-three percent of all of the isolated clones showed little caseinolytic activity, 42% had intermediate activity, and 35% had high activity. Although the clones with low plasminogen activator activity had no more than twice the activity shown by uninfected fibroblasts, they did not differ significantly from clones possessing high levels of plasminogen activator in their morphology, 2-deoxyglucose transport, or efficiency of colony formation in soft-agar.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P., Brown N. R. Induction of mutations in an RNA tumour virus by an analogue of a DNA precursor. Nat New Biol. 1971 Nov 3;234(44):11–12. doi: 10.1038/newbio234011a0. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Tumor viruses: 1974. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1187–1200. doi: 10.1101/sqb.1974.039.01.137. [DOI] [PubMed] [Google Scholar]
- Biquard J. M., Vigier P. Isolement et étude d'un mutant conditionnel du virus de Rous à capacité transformante thermosensible. C R Acad Sci Hebd Seances Acad Sci D. 1970 Dec 21;271(25):2430–2433. [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M. Proteolytic enzymes initiating cell division and escape from contact inhibition of growth. Nature. 1970 Jul 11;227(5254):170–171. doi: 10.1038/227170a0. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Buchanan J. M. Plasminogen-independent fibrinolysis by proteases produced by transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1132–1136. doi: 10.1073/pnas.72.3.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou I. N., Black P. H., Roblin R. O. Non-selective inhibition of transformed cell growth by a protease inhibitor. Proc Natl Acad Sci U S A. 1974 May;71(5):1748–1752. doi: 10.1073/pnas.71.5.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1673–1680. doi: 10.1073/pnas.67.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Friis R. R., Toyoshima K., Vogt P. K. Conditional lethal mutants of avian sarcoma viruses. I. Physiology of ts 75 and ts 149. Virology. 1971 Feb;43(2):375–389. doi: 10.1016/0042-6822(71)90310-2. [DOI] [PubMed] [Google Scholar]
- Goetz I. E., Weinstein C., Roberts E. Effects of protease inhibitors on growth of hamster tumor cells in culture. Cancer Res. 1972 Nov;32(11):2469–2474. [PubMed] [Google Scholar]
- Goldberg A. R. Increased protease levels in transformed cells: a casein overlay assay for the detection of plasminogen activator production. Cell. 1974 Jun;2(2):95–102. doi: 10.1016/0092-8674(74)90097-x. [DOI] [PubMed] [Google Scholar]
- Goldberg A. R. Involvement of cell and serum proteases in exposing tumor-specific agglutinin sites. Ann N Y Acad Sci. 1974;234(0):348–354. doi: 10.1111/j.1749-6632.1974.tb53047.x. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Hanafusa H. Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970 Aug;41(4):647–652. doi: 10.1016/0042-6822(70)90429-0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A., Laug W. E., Benedict W. F. Fibrinolytic activity in a human fibrosarcoma cell line and evidence for the induction of plasminogen activator secretion during tumor formation. Cell. 1975 Oct;6(2):245–252. doi: 10.1016/0092-8674(75)90015-x. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- LAKI K. The polymerization of proteins; the action of thrombin on fibrinogen. Arch Biochem Biophys. 1951 Jul;32(2):317–324. doi: 10.1016/0003-9861(51)90277-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin G. S. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970 Sep 5;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Venuta S., Weber M., Rubin H. Temperature-dependent alterations in sugar transport in cells infected by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2739–2741. doi: 10.1073/pnas.68.11.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski L., Unkeless J. C., Tobia A., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. II. Mammalian fibroblast cultures transformed by DNA and RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):112–126. doi: 10.1084/jem.137.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Risser R., Conlon S., Rifkin D. Plasminogen activator production accompanies loss of anchorage regulation in transformation of primary rat embryo cells by simian virus 40. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4792–4796. doi: 10.1073/pnas.71.12.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley J. P., Ossowski L., Reich E. Plasminogen, the serum proenzyme activated by factors from cells transformed by oncogenic viruses. J Biol Chem. 1974 Jul 10;249(13):4306–4311. [PubMed] [Google Scholar]
- Schnebli H. P., Burger M. M. Selective inhibition of growth of transformed cells by protease inhibitors. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3825–3827. doi: 10.1073/pnas.69.12.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Rubin H. Release from density dependent growth inhibition by proteolytic enzymes. Nature. 1970 Aug 22;227(5260):843–845. doi: 10.1038/227843a0. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Guntaka R. V., Varmus H. E., Bishop J. M. Purification of DNA complementary to nucleotide sequences required for neoplastic transformation of fibroblasts by avian sarcoma viruses. J Mol Biol. 1976 Mar 5;101(3):349–365. doi: 10.1016/0022-2836(76)90152-2. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Tobia A., Ossowski L., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. I. Chick embryo fibroblast cultures transformed by avian RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):85–111. doi: 10.1084/jem.137.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J., Dano K., Kellerman G. M., Reich E. Fibrinolysis associated with oncogenic transformation. Partial purification and characterization of the cell factor, a plasminogen activator. J Biol Chem. 1974 Jul 10;249(13):4295–4305. [PubMed] [Google Scholar]
- Weber M. J. Inhibition of protease activity in cultures of rous sarcoma virus-transformed cells: effect on the transformed phenotype. Cell. 1975 Jul;5(3):253–261. doi: 10.1016/0092-8674(75)90100-2. [DOI] [PubMed] [Google Scholar]



