Abstract
Background
Endoscopic therapy for the treatment of high-grade dysplasia (HGD) and intramucosal cancer (IMC) in Barrett’s esophagus (BE) may not always result in complete remission of dysplasia (CRD).
Objective
To determine if genetic alterations in the Barrett’s mucosa can predict response to endoscopic therapy.
Design
Retrospective cohort study.
Setting
Tertiary-care institution.
Patients
Selected patients who underwent endoscopic therapy for BE containing HGD/IMC between 2003 and 2010.
Interventions
Endoscopic therapy combining mucosal resection and different ablation modalities were performed based on patient characteristics, endoscopic findings, and technique evolution. Fluorescence in situ hybridization (FISH) was used to evaluate genetic alterations on baseline endoscopic cytology brushings using probes directed to loci 8q24 (MYC), 9p21 (CDKN2A; alias P16), 17q12 (ERBB2; alias Her-2/neu), and 20q13.2 (ZNF217).
Main outcome measurements
Genetic biomarkers predicting achievement of CRD after endoscopic therapy.
Results
A total of 181 patients were included (145 males; age 66 ±10 years). There were 130 patients (72%) who responded to endoscopic therapy with CRD. Multiple gains detected by FISH was found to be a negative predictor (HR 0.57; 95% CI, 0.40-0.82) after adjusting for potential clinical confounders. Similar results were found when analyses were restricted to patients (n = 66) undergoing radiofrequency ablation (HR 0.58; 95% CI, 0.31-1.09).
Limitations
Retrospective study, heterogeneity of treatment modalities.
Conclusion
Patients with multiple gains detected by brush cytology specimens may have a lower response rate to endoscopic therapy. The presence of multiple gains can be an adjunct to standard histology in prognosticating BE patients with HGD/IMC undergoing endoscopic therapy.
Keywords: Barrett’s esophagus, endoscopic therapy, biomarkers, fluorescence in situ hybridization, radiofrequency ablation
BACKGROUND
High grade dysplasia (HGD) and intramucosal cancer (IMC) associated with Barrett’s esophagus (BE) carry a high risk of progression to esophageal adenocarcinoma (EAC).1,2 Endoscopic therapies including endoscopic mucosal resection (EMR) and radiofrequency ablation (RFA) have shown to be effective for the treatment of HGD and IMC and are less invasive alternatives to conventional esophagectomy.3-5 Despite initial response to endoscopic therapy with downstaging of dysplasia, a significant number of patients will not achieve complete remission of dysplasia (CRD).6-8 This has intensified the interest whether biomarkers can help predict outcome after endoscopic therapy.
The majority of EACs associated with BE exhibit chromosomal abnormalities.9,10 In addition, a number of chromosomal alterations have been associated with increased risk of progression from non-dysplastic BE and low-grade dysplasia to HGD or EAC. Among them are several markers of chromosomal instability including DNA content abnormalities such as aneuploidy (i.e. a cell containing either extra or missing copies of particular chromosomes), loss or inactivation of tumor suppressor genes (e.g. P53, P16), and amplification of oncogenes (e.g. Her-2/neu, MYC).11-17 It has been shown that these genetic alterations can be found in large areas of the Barrett’s mucosa, even in areas without morphological features of dysplasia.18,19 Hage et al. investigated the effect of ablation therapy on molecular abnormalities (cellular proliferation, aneuploidy and p53 overexpression) in the Barrett’s mucosa and found that they were still present in a subset of patients with persistent Barrett’s epithelium, despite intensive ablation with argon plasma coagulation and photodynamic therapy (PDT).20 In a longitudinal case series of 19 patients undergoing RFA, somatic mutations (i.e. P16, P53) were evaluated pre- and postablation therapy. Five patients demonstrated persistence of HGD/IMC as well as persistent mutations post-ablation, suggesting that persistence of genetic abnormalities may be associated with persistent pathology.21 However, few studies have investigated the ability of genetic markers to predict outcome after endoscopic therapy.22,23 If biomarkers can be identified that can predict outcome after endoscopic treatment, this may help to optimize both treatment and surveillance strategies.
Fluorescence in situ hybridization (FISH) is a technique that can be applied to cytology specimens obtained by easily collected endoscopic brushings. It uses fluorescently labeled DNA probes that hybridize to multiple chromosomal locations. Abnormal gains or losses of chromosomes, chromosomal regions or specific chromosomal loci can be visualized with a fluorescence microscope. We have found that the use of cytological specimens are more likely representative of the entire Barrett’s mucosa and less subject to sampling bias as biopsies. Our multi-target FISH assay consisting of locus-specific probes to 8q24 (MYC), 9p21 (CDKN2A; alias P16), 17q12 (ERBB2; alias Her-2/neu), and 20q13.2 (ZNF217) can be used to detect gains, amplification, or deletions of four genes that are frequently altered during neoplastic progression in Barrett’s esophagus.10,24 Gains of multiple probes is an indicator of increasing chromosomal instability and has been associated with advanced stages of dysplasia as well as with an increased risk of neoplastic progression. 10
Previously, we demonstrated that allelic loss of P16 detected by FISH is a predictor of a decreased response in patients undergoing PDT for Barrett’s dysplasia.23 At present, a variety of endoscopic techniques including endoscopic resection and ablation techniques are used for the treatment of Barrett’s dysplasia. The aim of this study was to determine the predictive value of genetic biomarkers in BE patients undergoing multiple-mode endoscopic therapy. Recently, RFA has become the standard of care for patients with HGD due to the increasing evidence for its efficacy and its favorable side effect profile. Therefore, in addition, we performed a sub-analysis in patients who underwent RFA.
METHODS
Study design
This was a retrospective cohort study. Medical records of all BE patients who had undergone endoscopic therapy at our tertiary referral center between April 2003 and December 2010 as well as had cytology obtained for FISH analysis were reviewed. This analysis was performed with approval from the Institutional Review Board of the Mayo Clinic.
Patients
We included BE patients with histologically confirmed HGD or IMC who underwent endoscopic therapy with or without preceding EMR in case of any visible lesions. BE was defined as the presence of a columnar-lined distal esophagus, visible as pink mucosa extending above the top of the gastric folds during endoscopy confirmed by the presence of specialized intestinal metaplasia on biopsies. Inclusion was limited to (1) patients in which endoscopic brushing specimens used for FISH analysis were obtained up to 3 months before endoscopic therapy, and (2) availability of biopsy results after endoscopic treatment. Patients with signs of lymph node metastasis or distant metastasis on EUS or CT scan were excluded from the study as well as patients who were diagnosed with invasive EAC during the initial treatment endoscopy. Relevant clinical and endoscopic data were collected from a prospectively maintained database of electronic medical records and FISH results were obtained by review of our FISH database. Extracted data included age, sex, smoking, alcohol use, body mass index (BMI), details of endoscopic treatment, Barrett’s segment length, and results of post-treatment biopsies.
Fluorescence in situ hybridization
Endoscopic brushings were obtained using a standard cytology brush (Hobbs Medical Inc, Stafford Springs, CT) by brushing the whole Barrett’s segment during upper endoscopy. Brushings were collected during the referral endoscopy or at the first treatment endoscopy before any treatment was performed. Cytology specimens were prepared for FISH analysis as previously described.24 Slides were evaluated by FISH with a multicolor probe set consisting of probes to 8q24 (MYC), 9p21 (CDKN2A; alias P16), 17q12 (ERBB2; alias Her-2/neu), and 20q13.2 (ZNF217) (Abbott Molecular Inc, Des Plaines, IL) to detect allelic loss of P16, single locus gain (SLG), and multiple gains (gain of ≥ 2 probes). FISH analysis was performed using a fluorescence microscope with unique band filters designed for imaging each of the probes by an experienced cytotechnologist trained in FISH analysis who was blinded to the histopathological diagnosis and the clinical history. Cut-off values for the different probes were adapted from a previous study in which they were determined by the use of receiver operator characteristic curves.24 A specimen was considered positive for P16 loss if at least 6% of the cells showed homozygous 9p21 loss (i.e. zero copies of the 9p21 probe) or if at least 11% of the cells showed hemizygous 9p21 loss (i.e. one copy of the 9p21 probe) or if at least 11% of the cells showed a mixture of hemizygous and homozygous 9p21 loss. A case was scored as showing “single locus gain” (SLG) if ≥5% of the cells demonstrated gains of 8q24, 17q12, or 20q13.2. Multiple gains was defined as gains of 2 or more of the 4 probes in ≥4 cells.
Surveillance
During follow-up, all patients received proton-pump inhibitors (dose 40-80 mg/day) and were enrolled in an active surveillance protocol with endoscopies performed every 3 months during the first year after treatment. The surveillance interval was increased to 6 months in the second year, if biopsies showed an absence of IM. All histological diagnoses were based on biopsies obtained using the Seattle biopsy protocol (4-quadrant biopsies for every 1-2 centimeters of the entire length of the BE segment and neosquamous epithelium). From the third year onwards, patients with absence of IM underwent annual follow-up. An expert gastrointestinal pathologist assessed all biopsies and resection specimens. Every diagnosis of dysplasia or carcinoma had to be confirmed by a second experienced gastrointestinal pathologist.
Outcomes
The main outcome was based on analysis of achievement of CRD during follow-up. CRD was defined as the endoscopic and histologic absence of dysplasia and IMC, after 2 serial endoscopies at least 3 months apart with biopsies from every 1-2 centimeters of the (former) BE segment. Follow-up time was measured from the date of the first treatment endoscopy to the date of CRD or to the date of the last follow-up endoscopy for those who did not achieve CRD.
Statistical Analysis
Data were analyzed using JMP 9.0.3. (SAS Institute, Cary, NC). Continuous variables were expressed as mean with standard deviation (SD) or median with interquartile range (IQR, 25%- 75%), based on normality of distribution. The value of all clinical parameters and FISH abnormalities for predicting response to therapy were evaluated using univariable Cox regression analysis. All variables associated with CRD on univariable analysis with P < 0.10 were entered in a multivariable Cox regression model. Individual hazard ratios (HR) for each of the biomarkers were adjusted for the clinically relevant variables. For all analyses, a two-sided P value less than 0.05 was considered statistically significant. Time-to event data were analyzed with the use of Kaplan-Meier estimation and the log-rank test.
RESULTS
A total of 181 patients (145 male patients) with a mean age of 66.0 (± 9.5) years and with a median BE length of 4 cm were included. There were 147 patients (81%) with a baseline diagnosis of HGD and 34 patients (19%) with IMC. Baseline characteristics are summarized in Table 1. In 174 patients (96%), endoscopic resection was performed. In addition, a variety of ablative techniques were used including RFA, n=66 (37%), PDT, n=27 (15%), cryotherapy, n=7 (4%), bipolar coagulation, n=38 (21%), multipolar coagulation, n=48 (27%), and argon plasma coagulation, n=15 (8.3%). A combination of treatment modalities was used in 177 patients (98%). All endoscopic procedures were performed by two expert endoscopists. Median follow-up after the initial treatment endoscopy was 36 months (IQR, 22-59). There were 130 patients (72%) who achieved CRD with 2 consecutive negative endoscopies for dysplasia and carcinoma after a median follow-up time of 13 months (IQR 7-25). From the 51 patients (28%) who did not achieve CRD, there were 28 patients (15%) who showed absence of dysplasia at only one of the follow-up endoscopies. Recurrence of dysplasia occurred in 21/130 patients (16%) who had achieved CRD. Recurrence was observed in only 12% of patients (11/92) who had achieved complete remission of intestinal metaplasia at two consecutive endoscopies (5 cases of HGD, 6 cases of LGD), but in 26% of patients (10/38) with residual non-dysplastic BE in which 3 cases of HGD and 7 cases of LGD were observed (P=0.07).
Table 1.
Baseline patient characteristics and FISH results
| Variable | Whole cohort | Patients receiving RFA |
|---|---|---|
|
| ||
| No. of subjects | 181 | 66 |
|
| ||
| Age, yrs | 66.0 ± 9.5 | 65.6 ± 8.7 |
|
| ||
| Sex, N (%) | ||
| Men | 145 (80) | 60 (91) |
| Women | 36 (20) | 6 (9) |
|
| ||
| Barrett length, cm ¶ | 4 (2-7) | 6 (4-8) |
|
| ||
| Baseline histology | ||
| HGD, N (%) | 147 (81) | 61 (92) |
| IMC, N (%) | 34 (19) | 5 (8) |
|
| ||
| Body mass index, mean (SD), kg/m2 | 30.0 ± 4.5 | 30.7 ± 4.5 |
|
| ||
| Smoking, N (%) | 13 (7) | 6 (9) |
|
| ||
| Alcohol use, N (%) † | 31 (17) | 10 (15) |
|
| ||
| Normal FISH result, N (%) ‡ | 69 (38) | 17 (26) |
|
| ||
| P16 loss, N (%) | 52 (29) | 22 (33) |
|
| ||
| Single locus gain, N (%) | 35 (19) | 13 (20) |
|
| ||
| Multiple gains, N (%) | 80 (44) | 37 (56) |
FISH, fluorescence in situ hybridization; SD, standard deviation; HGD, high-grade dysplasia; IMC, intramucosal carcinoma.
Plus-minus values are means ± SD.
Data are shown as median (IQR).
Alcohol use was defined as a self-reported intake of more than two units a day.
The expected normal signal pattern for all probes is two signals per nucleus. If the percentage of nuclei showing abnormal signal patterns is less than the cut-off value for all possible abnormalities (i.e. P16 loss, single locus gain, multiple gains), the result is designated as normal.
Observed FISH abnormalities included P16 loss; single locus gain of 8q24 (MYC), 20q13.2 (ZNF217), or 17q12 (Her-2/neu or ERBB2); and multiple gains (see Figure 1). P16 loss was observed in 52 patients (29%) and single locus gain in 35 patients (19%). Multiple gains was the most common abnormality, being observed in 88 patients (44%). Some patients showed 2 or more genetic abnormalities (e.g. single locus gain and P16 loss). There were 69 patients (38%) with a normal FISH result (i.e. no P16 loss, SLG or multiple gains was observed).
Figure 1. FISH signal patterns.
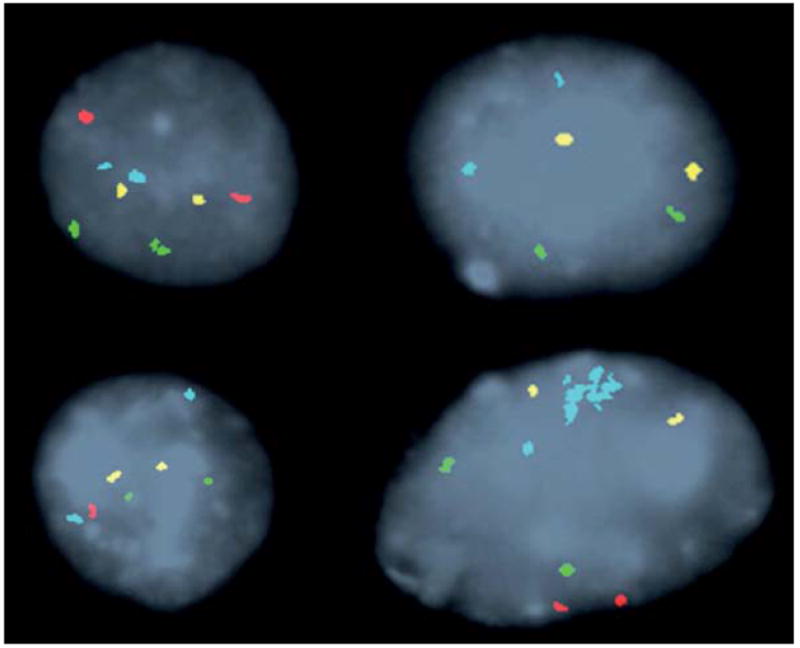
Representative FISH signal patterns in Barrett’s esophagus with a probe set for 8q24 (MYC) [aqua], 9p21 (P16) [red], 17q12 (ERBB2) [green], and 20q13.2 (ZNF217) [yellow]. Clockwise from top left: a normal cell (2 signals of each probe), homozygous P16 loss (no red signals), hemizygous P16 loss (one red signal), single locus gain (amplification of MYC probe).
Univariable analysis identified increasing BE length (HR 0.83; 95% CI, 0.78-0.88), BMI (HR 0.94; 95% CI, 0.91-0.98), and multiple gains (HR 0.57; 95% CI, 0.40-0.82) as significant negative predictors of response to therapy and a normal FISH result (HR 1.77; 95% CI, 1.24-2.52) as a positive predictor. Sex, age, smoking, and a baseline diagnosis of HGD were not significantly associated with achievement of CRD (Table 2).
Table 2.
Predictors of complete remission of dysplasia or adenocarcinoma after endoscopic therapy on univariable analysis
| Whole cohort (N=181) |
Patients receiving RFA (N=66) |
|||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P value | HR | 95% CI | P value |
| Age (yrs) | 0.99 | 0.97-1.01 | 0.16 | 0.99 | 0.95-1.02 | 0.42 |
| Male sex | 1.25 | 0.78-1.99 | 0.36 | 2.41 | 0.74-7.83 | 0.14 |
| Barrett’s segment length (cm) | 0.83 | 0.78-0.88 | <0.001 | 0.90 | 0.81-1.01 | 0.06 |
| Histology (HGD) | 0.95 | 0.61-1.48 | 0.82 | 0.70 | 0.27-1.78 | 0.45 |
| BMI (kg/m2) | 0.94 | 0.91-0.98 | 0.003 | 0.96 | 0.90-1.02 | 0.15 |
| Smoking | 1.07 | 0.80-1.45 | 0.64 | 0.75 | 0.46-1.23 | 0.25 |
| Alcohol use | 1.15 | 0.92-1.44 | 0.21 | 1.07 | 0.73-1.58 | 0.72 |
| Normal FISH result | 1.77 | 1.24-2.52 | 0.001 | 1.44 | 0.74-2.79 | 0.28 |
| P16 loss | 0.74 | 0.49-1.09 | 0.13 | 0.73 | 0.38-1.39 | 0.34 |
| Single locus gain | 0.71 | 0.45-1.11 | 0.13 | 1.14 | 0.58-2.27 | 0.70 |
| Multiple gains | 0.57 | 0.40-0.82 | 0.002 | 0.52 | 0.28-0.96 | 0.038 |
HR, hazard ratio; FISH, fluorescence in situ hybridization; HGD, high-grade dysplasia.
Values were calculated with the use of univariable Cox regression analysis.
Multivariable Cox regression analysis demonstrated that multiple gains detected by FISH was an independent negative predictor of CRD (HR 0.60; 95% CI, 0.41-0.86) when adjusted for age, BMI, and BE segment length (Table 3). In total, 60% of patients with multiple gains achieved CRD, whereas 81% of patients in which no multiple gains were observed achieved CRD. Within all patients who did not achieve CRD, multiple gains was observed in 32 of 51 patients (63%) whereas it was observed in 48 of 130 patients (37%) who did achieve CRD (P =0.003).
Table 3.
Genetic biomarkers as potential predictors of a complete response to endoscopic therapy.
| a. Whole cohort (N=181) | |||
|---|---|---|---|
|
| |||
| Variable | HR | 95% CI | P value |
| Normal FISH result | 1.48 | 1.03-2.14 | 0.035 |
| Barrett’s segment length, cm | 0.84 | 0.78-0.90 | <0.001 |
| BMI | 0.94 | 0.90-0.98 | 0.002 |
|
| |||
| P16 loss | 0.97 | 0.64-1.48 | 0.90 |
| Barrett’s segment length, cm | 0.83 | 0.77-0.88 | <0.001 |
| BMI | 0.94 | 0.90-0.98 | 0.005 |
|
| |||
| Single locus gain | 0.67 | 0.43-1.06 | 0.09 |
| Barrett’s segment length, cm | 0.83 | 0.77-0.88 | <0.001 |
| BMI | 0.94 | 0.90-0.98 | 0.003 |
|
| |||
| Multiple gains | 0.60 | 0.41-0.86 | 0.006 |
| Barrett’s segment length, cm | 0.83 | 0.78-0.89 | <0.001 |
| BMI | 0.94 | 0.90-0.98 | 0.003 |
|
| |||
| b. Patients undergoing RFA (N=66) | |||
|
| |||
| Variable | HR | 95% CI | P value |
|
| |||
| Normal FISH result | 1.23 | 0.62-2.44 | 0.55 |
| Barrett’s segment length, cm | 0.91 | 0.82-1.02 | 0.10 |
|
| |||
| P16 loss | 0.87 | 0.45-1.69 | 0.68 |
| Barrett’s segment length, cm | 0.91 | 0.81-1.02 | 0.09 |
|
| |||
| Single locus gain | 1.25 | 0.63-2.48 | 0.53 |
| Barrett’s segment length, cm | 0.90 | 0.81-1.00 | 0.06 |
|
| |||
| Multiple gains | 0.58 | 0.31-1.09 | 0.09 |
| Barrett’s segment length, cm | 0.92 | 0.83-1.03 | 0.14 |
HR, hazard ratio; FISH, fluorescence in situ hybridization
A complete response was defined as the absence of dysplasia and/or carcinoma after 2 serial endoscopies with Seattle protocol biopsies.
Values were calculated with the use of multivariable Cox regression analysis.
Patients with a normal FISH results were significantly more likely to achieve CRD (HR 1.53; 95% CI, 1.06-2.21). Kaplan-Meier analysis revealed that there was a significant difference in time to achieve CRD between BE patients with multiple gains and those without multiple gains (P = 0.001, Log-rank; Figure 2).
Figure 2.
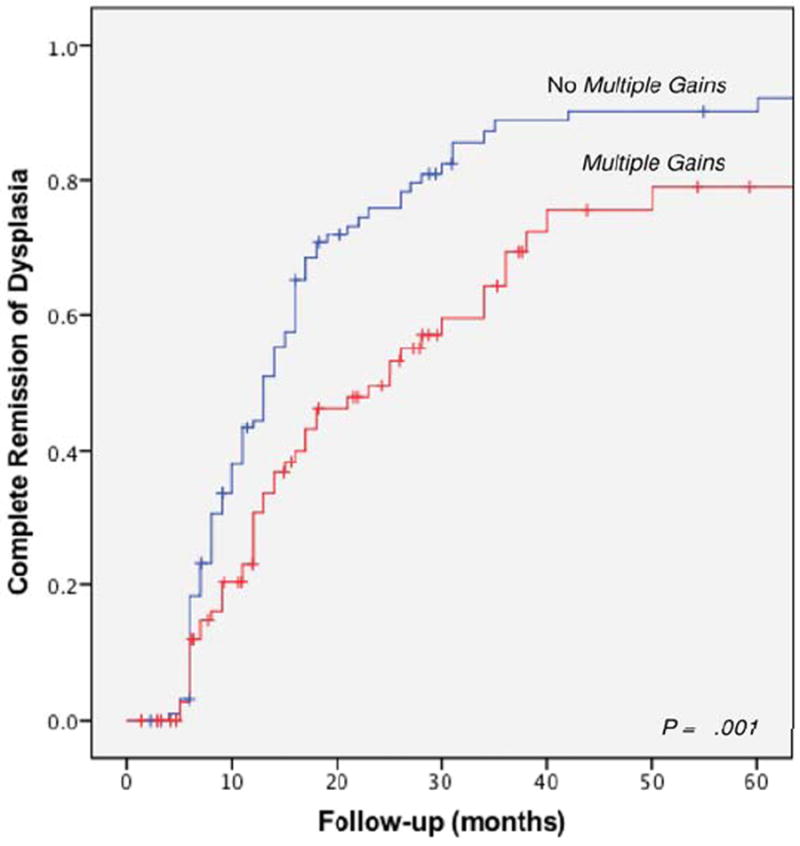
Time to achievement of complete remission of dysplasia based on the presence of multiple gains.
A subgroup of 66 patients underwent RFA, of which 62 patients (94%) also underwent endoscopic resection. RFA was performed using the HALO system consisting of the HALO360 Balloon catheter for circumferential ablation and the smaller HALO90 electrode for focal ablation. RFA patients underwent a median of 2 ablation sessions (IQR 2-3) per patient. CRD was achieved in 71% (47/66) of these patients. BE segment length and multiple gains were predictors of a decreased response, but not age and BMI (Table 2). However, on multivariable analysis, the predictive value of multiple gains was not a significant finding (HR 0.58; 95% CI, 0.31-1.09, Table 3). Kaplan Meier analysis restricted to patients who underwent RFA resulted in largely similar results when patients with multiple gains were compared to patients without multiple gains (Figure 3).
Figure 3.
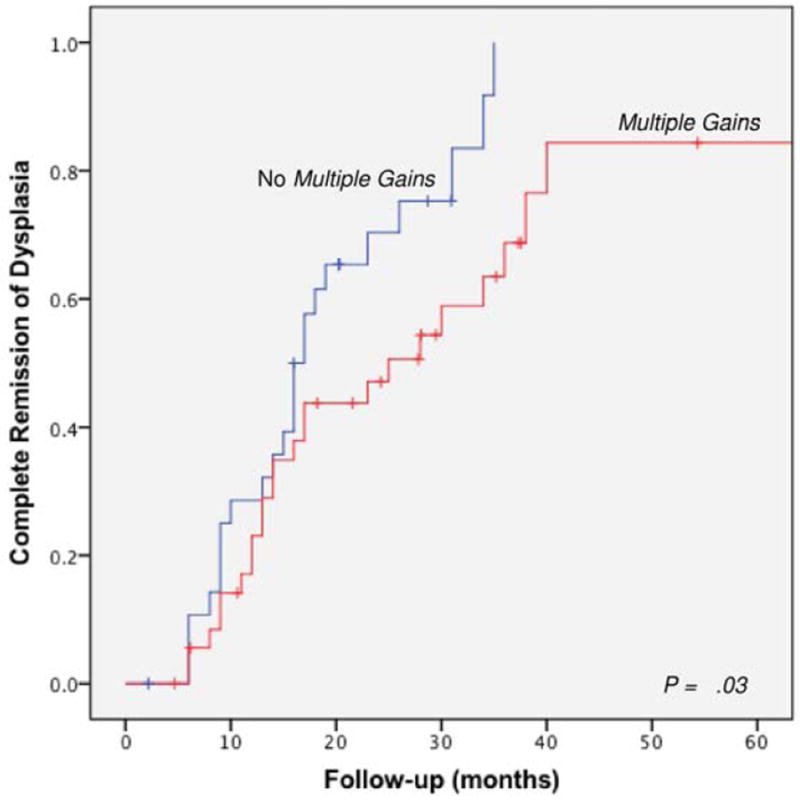
Time to achievement of complete remission of dysplasia based on the presence of multiple gains in a subgroup of 66 patients who underwent RFA.
DISCUSSION
In this large cohort study, we evaluated the predictive value of FISH analysis for determining response to endoscopic therapy in BE patients having HGD or IMC. The finding of multiple gains by FISH was associated with a lower probability of achieving CRD, which was defined as the absence of dysplasia/IMC after 2 serial surveillance endoscopies. Other clinical factors that were independent predictors of a decreased response included BE length and higher BMI.
Endoscopic therapy has become a well-established treatment for HGD and IMC in BE. Various endoscopic techniques have been introduced in the last decades with different success rates.3,5,25 However, no technique is perfect and residual dysplasia may be present, sometimes despite multiple treatment sessions. The use of biomarkers such as the alterations detected with the FISH probe set used in this study may help predict treatment response and can eventually provide knowledge to individualize treatment strategies for each patient.
During follow-up, there were 130 patients (72%) patients who achieved CRD. The CRD rate in our study was lower than has been described in previous studies although results vary considerably among the different endoscopic techniques.4,5,26-28 Due to the heterogeneity of our study population and the different treatment modalities used it is difficult to make a direct comparison of our results with other studies. One of the reasons for this discrepancy may be our more stringent definition of CRD as being an absence of dysplasia/IMC at 2 serial surveillance endoscopies instead of negative biopsies at a single surveillance endoscopy. We chose for a more stringent definition of CRD to decrease the chance that the absence of dysplasia on biopsies was due to sampling error. Moreover, patients in our cohort had a relatively long BE segment which has been associated with lower response rates.23,29
Few studies have investigated whether genetic markers such as FISH can improve prediction of response to endoscopic therapy beyond that provided by clinical risk factors. We previously reported that P16 allelic loss is a predictor of decreased response to PDT where “response” was defined as the absence of carcinoma and dysplasia at 3-months follow-up.23 In our current study, patients received a variety of different endoscopic treatment modalities including RFA, cryotherapy, and multipolar coagulation. P16 loss was also associated with a decreased response to endoscopic therapy on univariable analysis, but not in a multivariable model.
Chromosomal instability refers to an abnormal cellular DNA content due to chromosomal gains or losses and is a hallmark of cancer cells. The gains and losses of chromosomes are often a result of chromosome segregation errors during mitosis and are found commonly when chromosomal instability occurs. Defects in mitotic checkpoints may lead to aneuploidy and contribute to carcinogenesis. In BE, aneuploidy has been associated with increased risk of malignant progression using different techniques including flow cytometry, image cytometry, and FISH.10,15,30 We have previously shown that multiple gains, involving the assessment of gains of particular oncogenes, detected by the FISH assay used in this study has a higher sensitivity and specificity than DNA ploidy analysis by digital image analysis for the detection of HGD and EAC in BE. In addition, patients with multiple gains progressed to HGD or EAC significantly earlier than patients with a normal FISH results or with other FISH abnormalities such as SLG or P16 loss.10 In our study, we detected a new predictor of a decreased response to endoscopic therapy; multiple gains detected by FISH. However, It should be noted that the sensitivity and specificity of multiple gains as a biomarker is moderate. This test would identify the majority of nonresponders to endoscopic therapy. However, about a third of patients without multiple gains detected by FISH in their brush specimen, would still have persistence of dysplasia.
It could be argued that BE cell clones that have acquired multiple genetic abnormalities (e.g. multiple gains) may be more resistant to endoscopic therapy or may be typically spared from ablation resulting in persistent dysplasia despite these interventions. This has very important clinical ramifications as it suggests that the population of patients at highest risk of progression may well be those that are resistant to endoscopic therapy. This would add evidence to the recommendation to completely eliminate Barrett’s esophagus in these patients.
The use of RFA has expanded rapidly over the last decade and has become the standard of care for patients with HGD.31 In a sub-analysis restricted to patients who underwent RFA, similar results were found with regard to the predictive value of multiple gains. (HR 0.58; 95% CI, 0.31- 1.09). Although this was not a significant finding, it still may be of clinically importance, as this study was probably underpowered to assess the genetic markers in the subgroup of RFA patients.
To our knowledge, this is the largest study to date examining the use of biomarkers in predicting response to endoscopic therapy. The study provides support for the hypothesis that genetic abnormalities may be associated with persistence of dysplasia. However, a few limitations should be considered. In this retrospective cohort, we identified multiple gains as a marker of decreased response to endoscopic therapy. However, clinical implementation of such a marker would require independent validation in a prospective setting. In addition, a wide variety of endoscopic treatment modalities were used in this study. Therefore, the evidence generated by this study is insufficient to draw firm conclusions about the association of genetic abnormalities and response to a specific endoscopic treatment.
In conclusion, multiple gains detected by FISH on cytology brushings may predict a decreased response to endoscopic therapy. The presence of multiple gains can be an adjunct to standard histology in prognosticating patients at high risk undergoing endoscopic therapy.
These findings need to be confirmed in large prospective studies in the context of currently used endoscopic treatment modalities like RFA. Eventually, this may help to stratify patients and select the optimal management strategies.
Acknowledgments
Funding: Supported by NCI and NIH U54 CA163004, P30 CA015083, and UL1 TR000135.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sharma P, Falk GW, Weston AP, Reker D, Johnston M, Sampliner RE. Dysplasia and cancer in a large multicenter cohort of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2006 May;4(5):566–72. doi: 10.1016/j.cgh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Brown LM, Devesa SS, Chow W-H. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008 Aug 20;100(16):1184–7. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaheen NJ, Overholt BF, Sampliner RE, Wolfsen HC, Wang KK, Fleischer DE, et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology. 2011 Aug;141(2):460–8. doi: 10.1053/j.gastro.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pouw RE, Wirths K, Eisendrath P, Sondermeijer CM, Ten Kate FJ, Fockens P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett’s esophagus with early neoplasia. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2010 Jan;8(1):23–9. doi: 10.1016/j.cgh.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Overholt BF, Wang KK, Burdick JS, Lightdale CJ, Kimmey M, Nava HR, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett’s high-grade dysplasia. Gastrointest Endosc. 2007 Sep;66(3):460–8. doi: 10.1016/j.gie.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 6.Haidry RJ, Dunn JM, Butt MA, Burnell MG, Gupta A, Green S, et al. Radiofrequency ablation and endoscopic mucosal resection for dysplastic barrett’s esophagus and early esophageal adenocarcinoma: outcomes of the UK National Halo RFA Registry. Gastroenterology. 2013 Jul;145(1):87–95. doi: 10.1053/j.gastro.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 7.Anders M, Bär C, El-Masry MA, Marx AH, Koch M, Seewald S, et al. Long-term recurrence of neoplasia and Barrett’s epithelium after complete endoscopic resection. Gut. 2014 Jan 3; doi: 10.1136/gutjnl-2013-305538. [DOI] [PubMed] [Google Scholar]
- 8.Bulsiewicz WJ, Kim HP, Dellon ES, Cotton CC, Pasricha S, Madanick RD, et al. Safety and efficacy of endoscopic mucosal therapy with radiofrequency ablation for patients with neoplastic Barrett’s esophagus. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2013 Jun;11(6):636–42. doi: 10.1016/j.cgh.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rygiel AM, Milano F, Ten Kate FJ, Schaap A, Wang KK, Peppelenbosch MP, et al. Gains and amplifications of c-myc, EGFR, and 20.q13 loci in the no dysplasia-dysplasiaadenocarcinoma sequence of Barrett’s esophagus. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2008 Jun;17(6):1380–5. doi: 10.1158/1055-9965.EPI-07-2734. [DOI] [PubMed] [Google Scholar]
- 10.Fritcher EGB, Brankley SM, Kipp BR, Voss JS, Campion MB, Morrison LE, et al. A comparison of conventional cytology, DNA ploidy analysis, and fluorescence in situ hybridization for the detection of dysplasia and adenocarcinoma in patients with Barrett’s esophagus. Hum Pathol. 2008 Aug;39(8):1128–35. doi: 10.1016/j.humpath.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabinovitch PS, Longton G, Blount PL, Levine DS, Reid BJ. Predictors of progression in Barrett’s esophagus III: baseline flow cytometric variables. Am J Gastroenterol. 2001 Nov;96(11):3071–83. doi: 10.1111/j.1572-0241.2001.05261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulmann K, Sterian A, Berki A, Yin J, Sato F, Xu Y, et al. Inactivation of p16, RUNX3, and HPP1 occurs early in Barrett’s-associated neoplastic progression and predicts progression risk. Oncogene. 2005 Jun 9;24(25):4138–48. doi: 10.1038/sj.onc.1208598. [DOI] [PubMed] [Google Scholar]
- 13.Reid BJ, Prevo LJ, Galipeau PC, Sanchez CA, Longton G, Levine DS, et al. Predictors of progression in Barrett’s esophagus II: baseline 17p (p53) loss of heterozygosity identifies a patient subset at increased risk for neoplastic progression. Am J Gastroenterol. 2001 Oct;96(10):2839–48. doi: 10.1111/j.1572-0241.2001.04236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galipeau PC, Li X, Blount PL, Maley CC, Sanchez CA, Odze RD, et al. NSAIDs modulate CDKN2A, TP53, and DNA content risk for progression to esophageal adenocarcinoma. PLoS Med. 2007 Feb;4(2):e67. doi: 10.1371/journal.pmed.0040067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bird-Lieberman EL, Dunn JM, Coleman HG, Lao-Sirieix P, Oukrif D, Moore CE, et al. Population-based study reveals new risk-stratification biomarker panel for Barrett’s esophagus. Gastroenterology. 2012 Oct;143(4):927–935.e3. doi: 10.1053/j.gastro.2012.06.041. [DOI] [PubMed] [Google Scholar]
- 16.Jin Z, Cheng Y, Gu W, Zheng Y, Sato F, Mori Y, et al. A multicenter, double-blinded validation study of methylation biomarkers for progression prediction in Barrett’s esophagus. Cancer Res. 2009 May 15;69(10):4112–5. doi: 10.1158/0008-5472.CAN-09-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merlo LMF, Shah NA, Li X, Blount PL, Vaughan TL, Reid BJ, et al. A comprehensive survey of clonal diversity measures in Barrett’s esophagus as biomarkers of progression to esophageal adenocarcinoma. Cancer Prev Res Phila Pa. 2010 Nov;3(11):1388–97. doi: 10.1158/1940-6207.CAPR-10-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brankley SM, Fritcher EGB, Smyrk TC, Keeney ME, Campion MB, Voss JS, et al. Fluorescence in situ hybridization mapping of esophagectomy specimens from patients with Barrett’s esophagus with high-grade dysplasia or adenocarcinoma. Hum Pathol. 2012 Feb;43(2):172–9. doi: 10.1016/j.humpath.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Barrett MT, Sanchez CA, Prevo LJ, Wong DJ, Galipeau PC, Paulson TG, et al. Evolution of neoplastic cell lineages in Barrett oesophagus. Nat Genet. 1999 May;22(1):106–9. doi: 10.1038/8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hage M, Siersema PD, Vissers KJ, Steyerberg EW, Haringsma J, Kuipers EJ, et al. Molecular evaluation of ablative therapy of Barrett’s oesophagus. J Pathol. 2005 Jan;205(1):57–64. doi: 10.1002/path.1685. [DOI] [PubMed] [Google Scholar]
- 21.Zeki SS, Haidry R, Graham TA, Rodriguez-Justo M, Novelli M, Hoare J, et al. Clonal Selection and Persistence in Dysplastic Barrett’s Esophagus and Intramucosal Cancers After Failed Radiofrequency Ablation. Am J Gastroenterol. 2013 Oct;108(10):1584–92. doi: 10.1038/ajg.2013.238. [DOI] [PubMed] [Google Scholar]
- 22.Dunn JM, Mackenzie GD, Oukrif D, Mosse CA, Banks MR, Thorpe S, et al. Image cytometry accurately detects DNA ploidy abnormalities and predicts late relapse to high-grade dysplasia and adenocarcinoma in Barrett’s oesophagus following photodynamic therapy. Br J Cancer. 2010 May 25;102(11):1608–17. doi: 10.1038/sj.bjc.6605688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasad GA, Wang KK, Halling KC, Buttar NS, Wongkeesong L-M, Zinsmeister AR, et al. Utility of biomarkers in prediction of response to ablative therapy in Barrett’s esophagus. Gastroenterology. 2008 Aug;135(2):370–9. doi: 10.1053/j.gastro.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brankley SM, Wang KK, Harwood AR, Miller DV, Legator MS, Lutzke LS, et al. The development of a fluorescence in situ hybridization assay for the detection of dysplasia and adenocarcinoma in Barrett’s esophagus. J Mol Diagn JMD. 2006 May;8(2):260–7. doi: 10.2353/jmoldx.2006.050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Attwood SEA, Lewis CJ, Caplin S, Hemming K, Armstrong G. Argon beam plasma coagulation as therapy for high-grade dysplasia in Barrett’s esophagus. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2003 Jul;1(4):258–63. [PubMed] [Google Scholar]
- 26.Guarner-Argente C, Buoncristiano T, Furth EE, Falk GW, Ginsberg GG. Long-term outcomes of patients with Barrett’s esophagus and high-grade dysplasia or early cancer treated with endoluminal therapies with intention to complete eradication. Gastrointest Endosc. 2013 Feb;77(2):190–9. doi: 10.1016/j.gie.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Lyday WD, Corbett FS, Kuperman DA, Kalvaria I, Mavrelis PG, Shughoury AB, et al. Radiofrequency ablation of Barrett’s esophagus: outcomes of 429 patients from a multicenter community practice registry. Endoscopy. 2010 Apr;42(4):272–8. doi: 10.1055/s-0029-1243883. [DOI] [PubMed] [Google Scholar]
- 28.Ganz RA, Overholt BF, Sharma VK, Fleischer DE, Shaheen NJ, Lightdale CJ, et al. Circumferential ablation of Barrett’s esophagus that contains high-grade dysplasia: a U.S. Multicenter Registry. Gastrointest Endosc. 2008 Jul;68(1):35–40. doi: 10.1016/j.gie.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Pech O, Behrens A, May A, Nachbar L, Gossner L, Rabenstein T, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008 Sep;57(9):1200–6. doi: 10.1136/gut.2007.142539. [DOI] [PubMed] [Google Scholar]
- 30.Reid BJ, Levine DS, Longton G, Blount PL, Rabinovitch PS. Predictors of progression to cancer in Barrett’s esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol. 2000 Jul;95(7):1669–76. doi: 10.1111/j.1572-0241.2000.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American Gastroenterological Association Medical Position Statement on the Management of Barrett’s Esophagus. Gastroenterology. 2011 Mar;140(3):1084–91. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]


