Abstract
The isomeric configuration of the 3,4-didehydroretinal chromophore of goldfish porphyropsin was determined by high performance liquid chromatography (HPLC) and by the regeneration of this visual pigment with authentic isomers of 3,4-didehydroretinal. A nonisomerizing, quantitative method using hydroxylamine and methylene chloride was employed to extract the 3,4-didehyroretinal chromophore from the rod outer segment membrane (containing the porphyropsin). When this extracted chromophore was injected into the HPLC, only a single major peak was observed and this peak coeluted with the authentic 11-cis 3,4-didehydroretinyl oxime. This suggests that the chromophore of goldfish porphyropsin is 11-cis 3,4-didehydroretinal. When the bleached rod outer segments (containing the opsin) were incubated with different 3,4-didehydroretinal isomers (13-cis, 11-cis, 9-cis, and all-trans), only the 11-cis isomer resulted in the degeneration of porphyropsin. This also suggests that the porphyropsin chromophore exists in the 11-cis configuration.
Rhodopsin and porphyropsin are rod visual pigments found in the vertebrate retina (for reviews, see Crescitelli, ’72; Bridges, ’72). These visual pigments are derived respectively from the conjugation of retinal and 3,4-didehydroretinal to a species-specific opsin (Wald, ’38, ’39). Although the isomeric configuration of the retinal chromophore of rhodopsin is well established (Hubbard and Wald, ’52; Pilkiewicz et al., ’77; Groenendijk et al, ’80; Tsuda, ’82; Mathies, ’82; Baumann, ’72; Bridges, ’77; Cusanovich, ’82), similar information on the 3,4-didehydroretinal chromophore of porphyropsin is lacking.
The chemical nature of the visual pigment chromophore can be determined by analyzing the prosthetic group extracted directly from the visual pigment. A nonisomerizing and sensitive method of extraction and analysis (with a recovery of more than 93%) was reported by Groenendijk et al, ’80). This method entails reacting the retinal chromophore with hydroxylamine, followed by the extraction of retinyl oxime with methylene chloride. The retinyl oxime is then analyzed by high performance liquid chromatography (HPLC). This method was later used successfully by other investigators to study visual pigment chromophores (Tsuda, ’82; Suzuki and Makino-Tasaka, ’83). In the present work, we have employed this method to study the chemical nature of the visual pigment chromophore of the goldfish porphyropsin. Goldfish were chosen in this study because they possess pure porphyropsin retinas under natural conditions (Tsin and Beatty, ’78, ’79).
The isomeric configuration of the retinal chromophore can also be identified by the regeneration of the visual pigment with known retinal isomers. This visual pigment regeneration is performed by providing the appropriate retinal isomer to the bleached retina, rod outer segments, or visual pigment extract (for reviews, see Baumann, ’72; Cusanovich, ’82; Pepperberg, ’82) and then analyzing the regenerated visual pigment. In this study, we have also investigated the chemical nature of the goldfish porphyropsin by visual pigment regeneration.
Using these two different approaches, we found that the visual pigment chromophore of the goldfish porphyropsin is 11-cis 3,4-didehydroretinal.
MATERIALS AND METHODS
Materials
All-trans retinol was obtained from the Sigma Chemical Co. (St. Louis, MO). All-trans 3,4-didehydroretinol was obtained as a gift from Hoffman-La Roche (Nutley, NJ). They were purified by HPLC before use. All-trans retinal was prepared by the oxidation of all-trans retinol with manganese dioxide (Bridges and Alvarez, ’82). Similarly, all-trans 3,4-didehydroretinal was prepared from the all-trans 3,4-didehydroretinol. Isomers of these aldehydes were prepared by the photoisomerization of the all-trans retinal and all-trans 3,4-didehydroretinal (Bridges and Alvarez, ’82). These isomers were then separated by normal phase HPLC (for the separation of retinals, see Bridges and Alvarez, ’82; for 3,4-didehydroretinals, see Tsukida et al., ’80) and individually collected from the eluant of the HPLC. HPLC grade solvents came from Burdick and Jackson (Delmon Craft Co., Houston, TX) and Fisher Scientific Co. (Houston, TX). N-octylglucoside was obtained from the Sigma Chemical Co.
Goldfish were obtained from the Grassy-fork Fisheries (Martinsville, IN). They were either used immediately upon arrival or kept in 120-1 aquaria at room temperature until use.
HPLC
The HPLC equipment consisted of a Beckman model 112 solvent delivery pump, a Rheodyne injector with a 250-μl loop, a Beckman model 160 UV monitor with a 340-nm filter, a Linear model 1210 chart recorder, and a Hewlett-Packard model 3390A recording integrator. The HPLC column (25 cm × 0.46 cm) was packed with 5 μm Ultrasphere silica. The mobile phase and the flow rate are indicated in the figure legends. Quantitation was performed by relating the integrated peak areas to the amounts of injected standards.
Preparation of rod outer segments
Goldfish were dark adapted overnight before decapitation. After enucleation, the retinas were dissected out and homogenized in 4 ml of sucrose solution (1.17 gm/ml). This homogenate was successively overlaid with 4-ml volumes of 1.15, 1.13, and 1.11 gm/ml sucrose and centrifuged at 27,000 rpm (for 1 h) in a Beckman model L5-50 ultracentrifuge (SW 27 rotor). The rod outer segments were recovered from the 1.11/1.13 interface (Papermaster, ’82; Fong et al., ’82), diluted with an equal volume of distilled water and then pelleted by centrifugation (10,000 rpm for 30 min in a Sorvall model RC-5B high speed centrifuge, using an SS 34 rotor). These procedures were carried out under dim red light (Wratten series 2 filter) or in total darkness.
Analysis of visual pigments and retinoids
Visual pigments were extracted from the rod outer segments by 1% octyglucoside (pH = 7.0) according to the method previously described (Fong et al., ’82). Visual pigment extracts were analyzed by a Bausch and Lomb model 2000 scanning spectrophotometer connected to an Apple II Plus computer and an Epson FX 80 dot matrix printer. Absorbance values were recorded between 700–300 nm before and after bleaching (room light, for 10 min) to derive the difference spectrum. The extinction coefficient of porphyropsin was taken as 30,000 (Brown et al., ’63). The porphyropsin nomogram values were derived from Ebrey and Honig (’77).
Retinoids were analyzed spectrophotometrically in n-hèxane or in ethanol. Their UV spectra were compared to the published spectra of authentic standards (Schwieter et al., ’62). Their extinction coefficients were summarized by Hubbard et al. (’71). The HPLC analyses of retinoids followed methods described previously (Bridges and Alvarez, ’82; Tsin et al., ’84).
Extraction of the porphyropsin chromophore
In this study, we have adopted the extraction method of Makino et al. (’83), which is based on that reported by Groenendijk et al. (’80). This method is outlined as followed. The washed rod outer segments were resuspended in 0.2 ml water, mixed with 40 μl 1 M NH2OH (pH = 7.0) and then with 0.6 ml methanol at 0°C. Methylene chloride (0.6 ml) was then added to the mixture, followed by 0.4 ml water and 1.2 ml n-hexane. The mixture was then agitated by a vortex mixer and centrifuged to ensure complete phase separation. The n-hexane containing the retinyl oxime was subsequently removed and evaporated to dryness under nitrogen, redissolved in a small volume of 12% diethyl ether in n-hexane and injected into the HPLC. Authentic retinal isomers were similarly treated with methanol, NH2OH, and methylene chloride after redissolving in 0.2 ml ethanol.
Regeneration of porphyropsin
Visual pigment regeneration was carried out in vitro according to the method of Bridges (’76). Four isomers of 3,4-didehydroretinal (13-cis, 11-cis, 9-cis, and all-trans) were individually collected from the HPLC eluant. The purity of these isomers was confirmed by comparing their absorbance spectra to those of authentic compounds and by the reinjection of collected isomers into the HPLC. From 2–5 molar excess of the 3,4-didehydroretinal was added (in 10 μl ethanol) to bleached rod outer segments (containing 5 nmol porphyropsin before bleaching) in 2 ml Ringer solution and the mixture incubated at room temperature. After 2 h in darkness, 5 ml NH2OH (pH = 7.0) was added to stop the reaction. The mixture was then centrifuged and the pellet extracted by 1% octylglucoside and analyzed as described before.
RESULTS
In order to obtain pure isomers of the 3,4-didehydroretinal for the HPLC analysis and for visual pigment regeneration, we first produced a mixture of these isomers by the illumination of all-trans 3,4-didehydroretinal according to the method described by Bridges and Alvarez (’82; for details, see legend to Fig. 1). When this photoisomerate was injected into the HPLC, individual isomers were eluted in a sequence as described by Tsukida et al. (’80). They were then collected from the HPLC eluant and their identity confirmed by the UV spectrum. Figure 1 shows the absorbance spectrum of 11-cis, 3,4-didehydroretinal collected from HPLC. This spectrum together with the spectra of the other collected isomers (13-cis, 9-cis, and all-trans) were consistent with the published spectra of 3,4-didehydroretinals (Schwieter et al., ’62). When each of these isomers was reinjected into the HPLC, only a single peak was observed, confirming their purity.
Fig. 1.
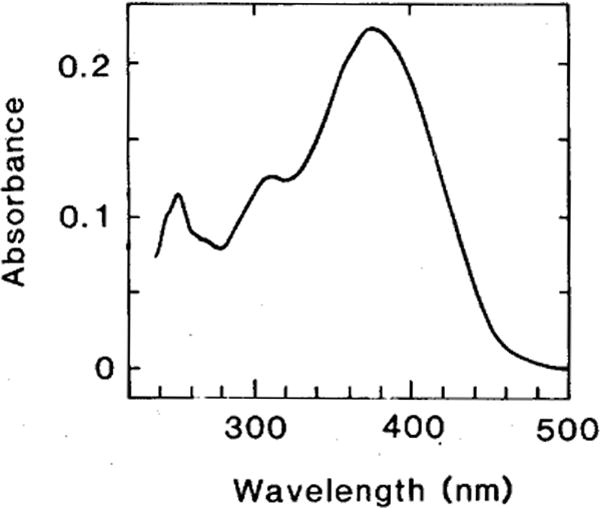
Absorbance spectrum of 11-cis 3,4-didehydroretinal in n-hexane. A purified sample of all-trans 3,4-didehydroretinal in ethanol (127 nmol/ml) was illuminated for 2 h (at 4°C) with a 150 W tungsten bulb. This photoisomerate of the all-trans isomer was injected into the HPLC (column, Ultrasphere; eluant, 12% diethyl ether in n-hexane; flow, 1 ml/min.; see Tsukida et al., ’80 for the sequence of isomer elution) and the 11-cis and other isomers were collected from the HPLC eluant. The spectrum shown in the figure is consistent with the published spectrum of the authentic 11-cis 3,4-didehydroretinal (Schwieter et al., ’62; the spectra of the other isomers were also consistent with published data). When this isomer was reinjected into the HPLC, only a single peak was eluted.
In our first approach to study the porphyropsin chromophore, the 3,4-didehydroretinal was extracted from rod outer segments of ten goldfish. In order to prevent the isomerization of 3,4-didehydroretinal during extraction, this 3,4-didehydroretinal was converted to 3,4-didehydroretinyl oxime by reacting with hydroxylamine before extracting with methylene chloride. The methylene chloride extract was then dried by nitrogen and injected into the HPLC. Figure 2A shows that a single major peak was eluted from the HPLC and this peak coeluted (Fig. 2C) with the authentic 11-cis 3,4-didehydroretinyl oxime (Fig. 2B). The methylene chloride extract of the rod outer segments also contained another component, which eluted as a smaller peak with a longer retention time than the 11-cis 3,4-didehydroretinyl oxime (see Fig. 2A). This component was identified as the all-trans 3,4-didehydroretinyl oxime. Its presence was the result of incomplete dark adaptation or isomerization during extraction. In the latter case, this isomerization was not sufficiently extensive to affect our conclusion. This experiment was repeated once with similar results.
Fig. 2.
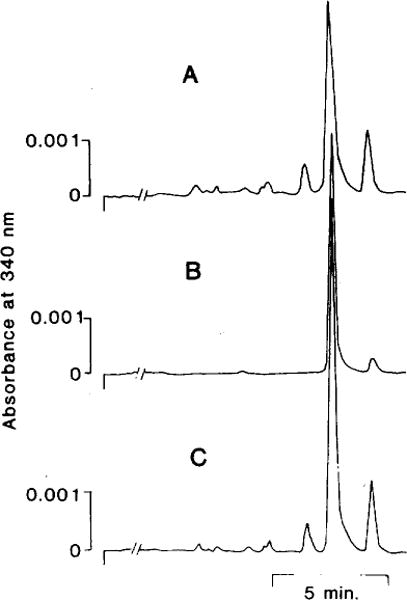
Identification of porphyropsin chromophore by co-chromatography. Purified goldfish rod outer segments were treated with methanol to release the chromophore, which was then reacted with hydroxylamine to form the oxime derivative. This 3,4-didehydroretinyl oxime was then extracted by methylene chloride before injection into the HPLC (tracing A). Pure 11-cis 3,4-didehydroretinal (see Fig. 1) was similarly treated to form the 11-cis 3,4-didehydroretinyl oxime (syn and anti isomers; tracing B, only the syn isomer is shown), which had an identical retention time as the 3,4-didehydroretinyl oxime extracted from the rod outer segments (tracing A). When the rod outer segment extract and the 11-cis 3,4-didehydroretinyl oxime were mixed and injected into the HPLC, only a single peak was observed (tracing C). This suggested that the compound extracted from goldfish rod outer segments is 11-cis 3,4-didehydroretinal. Column, Ultrasphere silica; eluant, 12% diethyl ether in n-hexane; flow, 1 ml/min; detection was made at 340nm. This HPLC system eluted all isomers of 3,4-didehydroretinyl oxime in a similar sequence as that reported by Tsuda (’82).
When the rod outer segments of the goldfish retina were regenerated with different isomers of 3,4-didehydroretinal (11-cis, 13-cis, 9-cis, and all-trans), only the 11-cis and the 9-cis isomers resulted in the regeneration of visual pigments. Figure 3 shows the difference spectra of the porphyropsin (absorbance maximum: 520 nm) resulting from the 11-cis isomer and the isoporphyropsin (absorbance maximum: 505 nm) from the 9-cis isomer of 3,4-didehydroretinal. The relative amount of pigment regenerated is indicated in Table 1. In comparison to the amount of porphyropsin regenerated by incubating 11-cis 3,4-didehydroretinal with bleached rod outer segments, the 9-cis isomer similarly regenerated about one-third to one-half of that amount (values from two experiments). In both our experiments, the 13-cis and the all-trans isomers did not regenerate any detectable amount of visual pigments (Table 1).
Fig. 3.
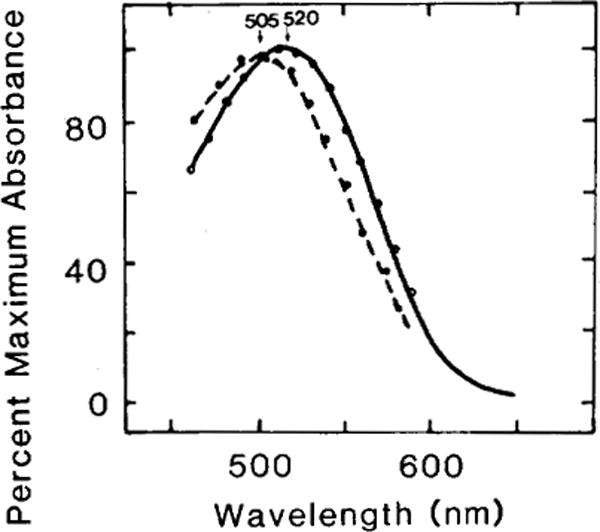
Difference spectra of porphyropsin and isoporphyropsin regenerated from the incubation of 11-cis and 9-cis isomers of 3,4-didehydroretinal with bleached rod outer segments of goldfish. Purified rod outer segments were completely bleached (room light, 10 min) before isomers of 3,4-didehydroretinal (13-cis, 11-cis, 9-cis, and all-frans) were added to them (for details, see Methods). Only the 11-cis and the 9-cis isomers resulted in the regeneration of visual pigments. The former regenerated the native goldfish porphyropsin (solid line, absorbance maximum: 520 nm) whereas the latter regenerated the isoporphyropsin (broken line, absorbance maximum: 505nm). The open and solid circles are nomogram values for these porphyropsins (Ebrey and Honig, ’77).
TABLE 1.
Percent regeneration of visual pigment from the incubation of different isomers of 3,4-didehydroretinal with rod outer segmentsa
| Type of isomer | Percent maximum regeneration |
|---|---|
| 11-Cis 3,4-didehydroretinal | 100 |
| 9-Cis 3,4-didehydroretinal | 32–48 |
| 13-Cis 3,4-didehyrdoretinal | 0 |
| All-trans 3,4-didehydroretinal | 0 |
Values were obtained from two experiments. For details, see Methods.
DISCUSSION
Since Wald published this report on porphyropsin some 45 years ago (Wald, ’39), the nature of the chromophore of this visual pigment has not been completely elucidated. Specifically, it is not known whether the chromophore of porphyropsin is 11-cis 3,4-didehydroretinal, although such information is assumed in many publications (Bridges, ’84; Hubbard et al., ’71; Suzuki and Makino-Tasaka, ’83). This is partly because the chromophore of rhodopsin, another rod visual pigment, is 11-cis retinal (Hubbard and Wald, ’52) and also because some unpublished observations have suggested that the 11-cis 3,4-didehydroretinal is the porphyropsin chromophore (Hubbard et al., ’71). However, no experimental evidence has been provided.
In the present study, we used two different approaches to investigate the chromophore of porphyropsin. In the first approach, we used a quantitative, nonisomerizing method (Groenendijk et al., ’80) to extract the porphyropsin chromophore from the goldfish rod outer segments. This resulted in a single isomer of 3,4-didehydroretinal, which was identified by co-chromatography in HPLC as the 11-cis isomer. This clearly suggests that the native chromophore of porphyropsin is 11-cis 3,4-didehydroretinal. In our second approach, the bleached visual pigment was incubated with different isomers of 3,4-didehydroretinal (13-cis, 11-cis, 9-cis, and all-trans) and only the 11-cis isomer regenerated the native porphyropsin. This further confirmed our conclusion that 11-cis 3,4-didehydroretinal is the chromophore of goldfish porphyropsin.
Based on results from other studies (Suzuki and Makino-Tasaka, ’83), it is also likely that the 3,4-didehydroretinal chromophores of other porphyropsins (such as that of the bullfrog) also exist in the 11-cis configuration.
Acknowledgments
We would like to thank Hoffman-La Roche for the generous gift of the all-trans 3,4-didehydroretinol. This research was supported by research grants from the National Science Foundation (BNS-82-03064) and the National Institutes of Health (Division of Research Resources, RR08194-04; and the Division of General Medical Science, 5T34-GM07717).
LITERATURE CITED
- Baumann C. The regeneration and renewal of visual pigment in vertebrates. In: Dartnall HJA, editor. Handbook of Sensory Physiology. Vol. 7. Springer-Verlag; New York: 1972. pp. 395–416. Part 1. [Google Scholar]
- Bridges CDB. The rhodopsin-porphyropsin visual system. In: Dartnall HJA, editor. Handbook of Sensory Physiology. Vol. 7. Springer-Verlag; New York: 1972. pp. 417–480. Part 1. [Google Scholar]
- Bridges CDB. Vitamin A and the role of the pigment epithelium during bleaching and regeneration of rhodopsin in the frog eye. Exp Eye Res. 1976;22:435–455. doi: 10.1016/0014-4835(76)90182-2. [DOI] [PubMed] [Google Scholar]
- Bridges CDB. Rhodopsin regeneration in the rod outer segments: Utilization of 11-cis retinal and retinol. Exp Eye Res. 1977;24:571–580. doi: 10.1016/0014-4835(77)90114-2. [DOI] [PubMed] [Google Scholar]
- Bridges CDB. Retinoids in photosensitive systems. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids. Vol. 2. Academic Press; New York: 1984. pp. 125–176. [Google Scholar]
- Bridges CDB, Alvarez RA. Measurement of the vitamin A cycle. In: Packer L, editor. Methods in Enzymology. Vol. 81. Academic Press; New York: 1982. pp. 463–485. [DOI] [PubMed] [Google Scholar]
- Brown PK, Gibbons IR, Wald G. The visual cells and visual pigment of the muddpuppy. Necturus J Cell Biol. 1963;19:79–106. doi: 10.1083/jcb.19.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescitelli F. The visual cells and visual pigments of the vertebrates. In: Dartnall HJA, editor. Handbook of Sensory Physiology. Vol. 7. Springer Verlag; New York: 1972. pp. 245–363. Part 1. [Google Scholar]
- Cusanovich MA. Kinetics and mechanism of rhodopsin regeneration with 11-cis retinal. In: Packer L, editor. Methods in Enzymology. Vol. 81. Academic Press; New York: 1982. pp. 443–447. [DOI] [PubMed] [Google Scholar]
- Ebrey TG, Honig B. New wavelength dependent visual pigment nomograms. Vision Res. 1977;17:147–151. doi: 10.1016/0042-6989(77)90213-9. [DOI] [PubMed] [Google Scholar]
- Fong S-L, Tsin ATC, Bridges CDB, Liou GI. Detergents for extraction of visual pigments: Types, solubilization and stability. In: Packer L, editor. Methods in Enzymology. Vol. 81. Academic Press; New York: 1982. pp. 133–140. [DOI] [PubMed] [Google Scholar]
- Groenendijk GWK, DeGrip WJ, Daemen FJM. Quantitative determination of retinals with complete retention of their geometric configuration. Biochim Biophys Acta. 1980;617:430–438. doi: 10.1016/0005-2760(80)90009-0. [DOI] [PubMed] [Google Scholar]
- Hubbard R, Brown PK, Bownds D. Methodology of vitamin A and visual pigments. In: McCormick DB, Wright LD, editors. Methods in Enzymology. Vol. 18. Academic Press; New York: 1971. pp. 615–683. [Google Scholar]
- Hubbard R, Wald G. Cis-trans isomers of vitamin A and retinene in the rhodopsin system. J Gen Physiol. 1952;36:269–315. doi: 10.1085/jgp.36.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino M, Nagai K, Suzuki T. Seasonal variation of the vitamin A2-based visual pigment in the retina of the adult bullfrog. Rana catesbeiana Vision Res. 1983;23:199–204. doi: 10.1016/0042-6989(83)90143-8. [DOI] [PubMed] [Google Scholar]
- Mathies R. Resonance Raman spectroscopy of rhodopsin and bacteriorhodopsin isotopic analogs. In: Packer L, editor. Methods in Enzymology. Vol. 88. Academic Press; New York: 1982. pp. 633–643. [Google Scholar]
- Papermaster DS. Preparation of retinal rod outer segments. In: Packer L, editor. Methods in Enzymology. Vol. 81. Academic Press; New York: 1982. pp. 48–51. [DOI] [PubMed] [Google Scholar]
- Pepperberg D. Generation of rhodopsin and “artificial” visual pigments in electrophysiologically active photoreceptors. In: Packer L, editor. Methods in Enzymology. Vol. 81. Academic Press; New York: 1982. pp. 452–459. [DOI] [PubMed] [Google Scholar]
- Pilkiewicz FG, Pettei MJ, Yudd AP, Nakanishi K. A simple and non-isomerizing procedure for the identification of protein-linked retinals. Exp Eye Res. 1977;24:421–423. doi: 10.1016/0014-4835(77)90157-9. [DOI] [PubMed] [Google Scholar]
- Schwieter U, Saucy G, Montavon M, Planta CV, Ruegg R, Isler O. Synthesis of vitamin A2. Hel Chim Acta. 1962;45:517–561. [Google Scholar]
- Suzuki T, Makino-Tasaka M. Analysis of retinal and 3-dehydroretinal in the retina by high-pressure liquid chromatography. Anal Biochera. 1983;129:111–119. doi: 10.1016/0003-2697(83)90059-3. [DOI] [PubMed] [Google Scholar]
- Tsin ATC, Beatty DD. Goldfish rhodopsin: P4991. Vision Res. 1978;18:1453–1455. doi: 10.1016/0042-6989(78)90243-2. [DOI] [PubMed] [Google Scholar]
- Tsin ATC, Beatty DD. Scotopic visual pigment composition in the retina and vitamin A in the pigment epithelium of the goldfish. Exp Eye Res. 1979;29:15–26. doi: 10.1016/0014-4835(79)90163-5. [DOI] [PubMed] [Google Scholar]
- Tsin ATC, Alvarez RA, Fong S-L, Bridges CDB. Use of high-performance liquid chromatography in the analysis of retinyl and 3,4-didehydroretinyl compounds in tissue extracts of bullfrog tadpoles and goldfish. Vision Res. 1984;24:1835–1840. doi: 10.1016/0042-6989(84)90015-4. [DOI] [PubMed] [Google Scholar]
- Tsuda M. Methods for extraction of pigment chromophore. In: Packer L, editor. Methods in Enzymology. Vol. 88. Academic Press; New York: 1982. pp. 552–561. [Google Scholar]
- Tsukida K, Masahara R, Ita M. High-performance liquid chromatographic analysis of cis-trans stereoisomeric 3-dehydroretinals in the presence of retinal isomers. J Chromatogr. 1980;192:395–401. [Google Scholar]
- Wald G. On rhodopsin in solution. J Gen Physiol. 1938;21:795–832. doi: 10.1085/jgp.21.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G. The porphyropsin visual system. J Gen Physiol. 1939;22:775–794. doi: 10.1085/jgp.22.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]


