Abstract
Purpose
The mechanisms by which depressive symptoms negatively impacts clinical outcomes in patients with CAD remains poorly understood. Previous interventions that have attempted to treat depressive symptoms in CAD patients in order to improve clinical outcomes have been disappointing. Our objectives were to evaluate the impact of depressive symptoms over time, controlling for comorbidity, in determining both successful long-term lifestyle change (i.e., increased physical activity), and cardiovascular morbidity and mortality outcomes. In addition, we examined the impact of physical activity changes over time on two known mediators of cardiovascular morbidity: parasympathetic tone and inflammation.
Methods
Clinical data were previously collected (2004-2006) from 242 elective/urgent coronary angioplasty patients who participated in a prospective randomized controlled trial evaluating the efficacy of a behavioral intervention vs. an educational control to motivate physical activity over 12 months. Exclusion criteria included: 1) inability to walk; 2) enrollment in other risk-reduction trials; 3) non-English speaking; and 4) lack of cardiologist's permission to increase physical activity. Participants were assessed every 2 months for interval clinical events and physical activity. In addition, biomarkers were collected at baseline and 12 months in a subset of 54 participants, including low and high frequency heart rate variability (lfHRV and hfHRV), serum C-reactive protein (CRP) and interleukin-6 (IL-6), and salivary cortisol.
Findings
The mean age of participants was 63 years and 30% were female. Overall, 37% had high depressive symptoms at baseline. Patients with high depressive symptoms who achieved an increase in physical activity of ≥336 kilocalories(kcal)/week by 12 months had significantly lower rates of cardiovascular morbidity/mortality (5.1% vs. 21.3%; odds ratio [OR], 0.20, [95% CI, 0.04–0.98]; P = 0.03). In a multivariate model examining cardiovascular morbidity/mortality in patients with high depressive symptoms, an increase in physical activity of ≥336 kcal/week reduced the risk of new cardiovascular morbidity/mortality (OR, 0.11 [95% CI, 0.02–0.81]; P < 0.03), and comorbidity increased the risk (OR, 1.58 [95% CI, 1.18–2.13]; P = 0.002).
Implications
This study demonstrates a threshold in physical activity in depressed CAD patients that is associated with a decrease in cardiovascular morbidity and mortality. Exercise maintenance at this level may improve clinical outcomes via enhanced parasympathetic tone and decreased inflammation.
Keywords: Coronary artery disease, Depression, Exercise, Interleukin-6, Heart rate variability
INTRODUCTION
Depression is common among patients with coronary artery disease (CAD), including both major depressive disorder and depressive symptoms.1-3 CAD patients who have major depression or high levels of depressive symptoms are at increased risk for morbidity and mortality.2,3 In older adults, it is well established that patients with depressive symptoms frequently present with increased medical comorbidity.4 Evidence also suggests that, particularly in older individuals, depression may have an underlying vascular etiology.4,5 Given that depression, depressive symptoms, and medical comorbidity6 are all important predictors of subsequent adverse outcomes, including increased morbidity and mortality, decreased functional status and greater resource utilization, the potential for confounding is considerable.4
The mechanisms by which depressive symptoms negatively impact clinical outcomes (e.g. cardiovascular morbidity and mortality) in people with CAD remains poorly understood. In a five-year longitudinal study of more than 1,000 participants with CAD, the association between depressive symptoms and adverse cardiovascular events was largely explained by physical inactivity, which was associated with a 44% higher rate of cardiovascular events.7 CAD patients who increase physical activity experience significant reductions in morbidity and mortality.8,9 However, randomized trial data are sparse. Unfortunately, and for many psychosocial, medical and motivational reasons, only a small percentage of people with CAD are successful at maintaining long-term lifestyle changes such as increased physical activity.10
Physical activity may play a particularly important role in reducing the risk of cardiovascular events among CAD patients with depressive symptoms. Of note, increased physical activity may ameliorate depressive symptoms; by itself, an intensive 16-week aerobic exercise regimen has been shown to be equally effective to pharmacologic therapy in treating older adults for major depressive disorder.11
However, CAD patients with depressive symptoms are less successful in increasing physical activity over 12 months when compared to those without depressive symptoms.12
Physical activity may also reduce the risk of cardiovascular events by suppressing inflammation. Inflammation is known to be an important pathophysiologic factor in cardiovascular disease progression,13,14 and a large body of work has shown that depressive symptoms are associated with increased inflammation.15,16 Exercise interventions have been shown to reduce inflammation in people with CAD.17 Moreover, low parasympathetic tone has been linked to poor prognosis, more severe symptoms and mortality from cardiovascular disease.18,19 Physical activity can increase parasympathetic tone20,21 which is low in patients with major depression,22 and functions to both increase emotional regulation23 and suppress inflammation.24,25
Our objectives were to evaluate the impact of depressive symptoms over time, controlling for comorbidity, in determining: 1) successful long-term lifestyle change (i.e., increased physical activity); and 2) cardiovascular morbidity and mortality outcomes. In addition, we examined the impact of physical activity changes over time on two known mediators of cardiovascular morbidity: parasympathetic tone and inflammation.
PATIENTS AND METHODS
Study Design
Data were collected between October 2004 and October 2006 at a tertiary academic medical center in New York City. Participants were enrolled in an NHLBI-funded prospective randomized, controlled trial (NCT 00248846) evaluating the efficacy of a behavioral medicine intervention vs. an educational control to motivate physical activity over 12 months. The methods have been previously described.26 A subgroup of patients were recruited from the randomized trial for a biological substudy. The randomized trial and biological measures substudy were both approved by the Weill Medical College Institutional Review Board and participants gave written informed consent for both studies.
Participants
In brief, 242 eligible participants were identified following elective or urgent percutaneous coronary intervention during hospitalization following the index procedure. Exclusion criteria included: 1) inability to walk; 2) enrollment in other risk-reduction trials; 3) non-English speaking; and 4) lack of cardiologist’ permission to increase physical activity. At baseline, all participants received an educational workbook.27 The Consort diagram of the participants has been previously reported.26 Fifty-four participants agreed to enroll in the biological measures substudy which had no additional exclusion criteria.
Follow-up
At two weeks following discharge, participants selected a physical activity goal, and agreed to a behavioral contract.28 Participants were then contacted by telephone at 2, 4, 6, 8, 10 and 12 months, and a standardized follow-up was conducted that included surveillance for new clinical events and physical activity level and also reinforced the workbook content.
Participants in the positive affect/self-affirmation intervention group received bimonthly induction of positive affect/self-affirmation at the end of each follow-up call, and also received small unexpected gifts in the mail several weeks prior to each follow-up. The positive affect/self-affirmation intervention has been previously described.26,29
Primary Outcome
Physical activity was evaluated at baseline and every 2 months thereafter with the Paffenbarger Exercise and Activity Index.30 The main trial outcome was a within-patient increase in expenditure of ≥ 336 kilocalories (kcal)/week at 12 months, assessed by the Paffenbarger. The Paffenbarger is a widely used valid and reliable31 self-report physical activity measure.
Demographic and Psychosocial Measures
We documented demographic and clinical characteristics at enrollment, including age, sex, race/ethnicity, marital status, body mass index, the Charlson Comorbidity Index,6 and the Seattle Angina Questionnaire.32 We administered the Short Form Center for Epidemiologic Studies Depression Scale (CES-D 10)33,34 at baseline and 12 months, and considered a score >10 as indicative of a high level of depressive symptoms.
Biological Measures
Biological measures were collected one month following the coronary angioplasty/stent procedure and again one year later.
• Inflammation
At both assessments, patients had blood drawn for measurement of Interleukin-6 (IL-6) and C-reactive protein (CRP) (Human IL-6 HS and CRP Quantikine ELISA, R & D Systems, Minneapolis, MN). IL-6 is a pro-inflammatory cytokine produced by macrophages and adipocytes in response to a wide range of inflammatory conditions including infections, autoimmune disease, and tissue injury. CRP is a protein synthesized by the liver which activates the complement system and is released in response to IL-6 and other inflammatory mediators.35 While Il-6 has direct effects on cardiovascular disease,36 CRP is likely to be a biomarker of inflammation in cardiovascular disease patients rather than a direct contributor to heart disease.37
• Parasympathetic Activity
Patients also had ambulatory heart monitoring (Lifeshirt, Vivometrics Corp, Ventura, CA) while awake for a minimum of 4 hours at baseline and one year later. High frequency heart rate variability (hfHRV) and low frequency variability (lfHRV) were calculated using Cardio Batch software (Mind Body Institute, University of Illinois, Chicago).38 hfHRV is determined primarily by parasympathetic cardiac afferent input from the vagus nerve.39 lfHRV reflects contributions of both parasympathetic and sympathetic activity.39 Heart rate recordings were edited to remove movement artifacts, ectopy, and paced beats using Cardio Edit software (Mind Body Institute, University of Illinois, Chicago). Next, using Cardio Batch software, the heart period time series were resampled at successive 500-ms intervals, smoothed using a 21-point moving cubic polynomial filter, subtracted from the original series to produce a residual time series, and then processed by a digital bandpass filter with 25 coefficients to extract the variance in the frequency band of 0.12– 0.40 Hz (the frequency of spontaneous breathing for adults) for hfHRV and 0.06-0.10 Hz for lfHRV. Both hfHRV and lfHRV were log transformed for quantification.
• Hypothalamic-pituitary adrenal (HPA) axis
Salivary cortisol was used to measure HPA axis activity because it can be collected outside the hospital setting, and can reflect circadian rhythm as well as relative hyper- or hypocortisolism. Participants collected saliva samples for 3 days on awakening, 45 minutes later, and at 5PM and at 11PM using cotton swabs (Salivette, Sarstedt Corp). Saliva samples were frozen –80°C then assayed using a commercial ELISA kit developed specifically for saliva (Cortisol Salivary Immunoassy, Salimetrics, State College, PA). Three cortisol measures were derived: mean cortisol over the 3 days, morning rise and circadian amplitude. Morning rise in cortisol was calculated by subtracting wake up cortisol from wake up +45 minutes value of each of the three collection days and averaged across the three days. Circadian amplitude was calculated by subtracting the 11PM value from the peak morning value on each of the three collection days and averaged across the three days.
Clinical Outcomes
Clinical outcomes were ascertained every 2 months with standardized follow-up calls. These included surveillance for cardiovascular and non-cardiovascular events that would impede the participant's ability to engage in physical activity (i.e., myocardial infarction, congestive heart failure, percutaneous coronary intervention, cardiac surgical procedures, ischemic colitis, stroke, and other major medical complications including shock and metastatic disease). All information was corroborated by treating physicians and clinical records whenever possible. Two blinded clinicians reviewed all clinical events (JCP and MEC).
Statistical Analyses
We calculated means and standard deviations for continuous variables, and counts and percentages were determined for categorical variables. For the comparison between the low vs. high depressive symptom groups, and within-patient changes in biomarkers over time, we employed x2 tests to investigate the categorical data and conducted t-tests for continuous variables (SAS version 9.3 and Stata version 13.1). The multivariate model for cardiac complications in patients with high depressive symptoms was performed using logistic regression. In the analysis of the biological data that examined predictors of change in IL-6 and change in CRP as dependent variables, we employed a multivariate regression modeling with robust standard errors,40 clustered by comorbidity score, to correct the variance estimates due to the presence of conditional dependence of comorbidity status. The path model (Figure 3) that summarizes the overall clinical and biological findings uses generalized structural equation modeling with a logit link for the binary cardiac complications outcome and identity links for the normal distributed change in IL-6 and hfHRV outcomes. Separate models were constructed using IL-6 and CRP as markers of inflammation. The modest number of cases in the biological database meant that the number of predictor variables that we were able to simultaneously consider in the path model was limited. For this reason, cortisol measures, which have relatively little evidence supporting a pathophysiological role in cardiovascular complications and lfHRV, which is a less specific reflection of autonomic influences, were excluded from the model.
Figure 3.
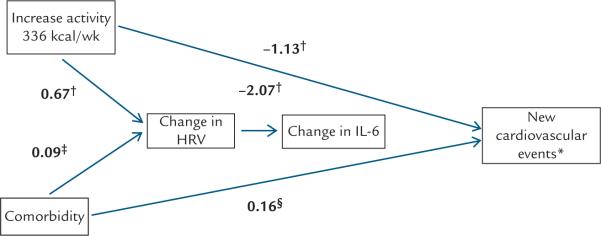
Generalized structural equation model integrating physical activity, comorbidity, and biological findings. *New cardiovascular events include all-cause mortality, percutaneous coronary intervention, coronary bypass surgery, congestive heart failure, new ischemia, and myocardial infarction. †P < 0.01, ‡P < 0.10, §P < 0.05. An increase in energy expenditure of ≥ 336 kcal/week at 12 months was associated with a significant reduction in new cardiovascular events (Coeff = 1.13, P = 0.006), and greater medical comorbidity significantly increased the risk of cardiovascular events (Coeff = 0.16, P = 0.02). In addition, both increased physical activity (Coeff = 0.67, P = 0.006), but not comorbidity (Coeff = 0.09, P = 0.09), significantly predicted an increase in high-frequency heart rate variability (HRV), which, in turn predicted a reduction in interleukin-6 (IL-6) (b = - 2.08, P = 0.001). C-reactive protein behaved similarly to IL-6 in a separate path model (data not shown).
RESULTS
Overall, 2,605 CAD patients were screened between October 2004 and October 2006.26 Of these, 242 patients were randomized and enrolled in the study. Attrition was 4.5% and 2.1% of the participants patients died.
Baseline characteristics
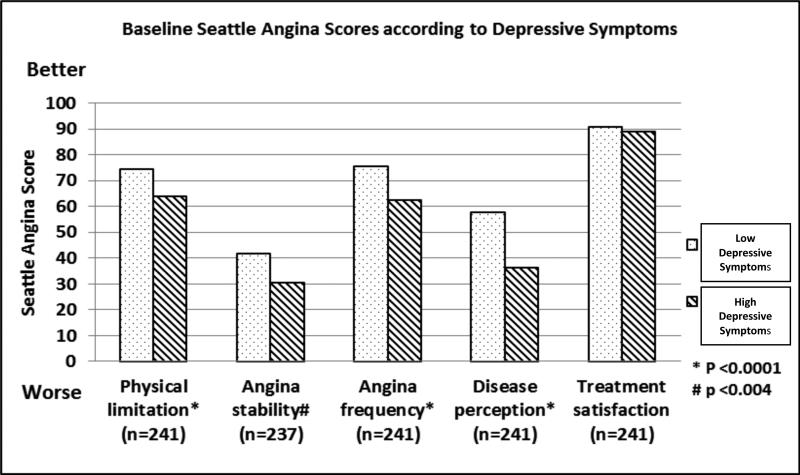
With a mean age of 63 years, the participants were predominantly male, Caucasian, married, and college graduates (Table 1). Overall, 37% of participants had high depressive symptoms. Those with high depressive symptoms were significantly more likely to be Black or multiracial (p<.02) and have lower education (p<.05) (Table 1). Participants with high depressive symptoms were also more likely to have concomitant pulmonary disease (p=0.03) and peripheral vascular disease (p=.001). As shown in Table 1 and Figure 1, participants with high depressive symptoms reported significantly worse scores on the Seattle angina questionnaire32 for physical limitation (p<.0001), angina stability (p<.003), angina frequency (p<.0001) and disease perception (p<.0001) compared to those with low depressive symptoms.
Table 1.
Sociodemographic and clinical characteristics of the cohort (N=242) stratified according to depressive symptoms (Baseline CES-D > 10). All are n (%) unless otherwise notated.
| Demographic characteristics | Total N=242 | CESD <10 N=153 | CES-D ≥ 11 N=89 | p |
|---|---|---|---|---|
| Age, mean (SD) | 63.2 (±11.2) | 64.2 (±10.9) | 61.5 (±11.5) | 0.07 |
| Women | 73 (30.2%) | 40 (26.1) | 33 (37.1%) | 0.07 |
| Race | ||||
| Caucasian | 196 (81.0%) | 130 (85.0) | 66 (74.2%) | <0.02 |
| Black | 26 (10.7%) | 12 (7.8) | 14 (15.7%) | |
| Asian | 10 (4.1%) | 8 (5.2) | 2 (2.3%) | |
| Multiracial | 10 (4.1%) | 3 (2.0) | 7 (7.9) | |
| Hispanic ethnicity | 31 (12.8%) | 16 (10.5) | 15 (16.9%) | 0.15 |
| Married/Partnered | 170 (70.2%) | 112(73.7) | 55 (63.2%) | 0.09 |
| Widowed | 15 (6.2%) | 9 (5.9) | 6 (6.9%) | |
| Separated/Divorced | 31 (12.8%) | 17 (11.2) | 14 (16.1%) | |
| Never married | 26 (10.7%) | 14 (9.2) | 12 (13.8%) | |
| Education | ||||
| < High School | 18 (7.4%) | 10 (6.5) | 8 (9.0%) | 0.05 |
| Completed High School | 91 (37.6%) | 51 (33.3) | 40 (44.9%) | |
| College or > | 133 (54.9%) | 92 (60.1) | 41 (46.1%) | |
| Clinical characteristics | ||||
| Body Mass Index* | ||||
| Normal, < 25 | 59 (24.5%) | 39 (25.5) | 20 (22.7%) | 0.45 |
| Overweight, ≥ 25 to < 30 | 95 (39.4%%) | 62 (40.5) | 33 (37.5%) | |
| Obese, ≥ 30 | 87 (36.1%) | 52 (34.0) | 35 (39.8%) | |
| Seattle Angina Questionnaire,32 mean score (SD) | ||||
| Physical limitation (n=241) | 70.7 (±18.0) | 74.6 (±14.3) | 64.1 (±21.6) | <0.0001 |
| Angina stability (n=237) | 37.9 (±29.1) | 42.0 (±27.4) | 30.5 (±30.8) | 0.003 |
| Angina frequency (n=241) | 70.8 (±23.1) | 75.6 (±21.6) | 62.1 (±24.2) | <0.0001 |
| Treatment satisfaction (n=241) | 90.3 (±8.9) | 89.0 (±10.2) | 89.0 (±8.6) | 0.13 |
| Disease perception (n=241) | 50.1 (±25.3) | 57.9 (±24.7) | 36.3 (±20.3) | <0.0001 |
| MI | 70 (28.9%) | 44 (28.8) | 26 (29.2%) | 0.94 |
| CHF | 12 (5.0%) | 6 (3.9) | 6 (6.7%) | 0.37 |
| Asthma/bronchitis | 41 (16.9%) | 20 (13.1) | 21 (23.6%) | 0.03 |
| Previous Angioplasty | 91 (37.6%) | 57 (37.3) | 34 (38.2%) | 0.88 |
| Diabetes | 61 (25.2%) | 34 (22.2) | 27 (30.3%) | 0.16 |
| Smoking history | 166 (68.6%) | 101(66.0) | 65 (73.0%) | 0.26 |
| Rheumatic disease | 16 (6.6%) | 8 (5.2) | 8 (9.0%) | 0.26 |
| Peripheral vascular disease | 32 (13.2%) | 12 (7.8) | 20 (22.5%) | 0.001 |
| Stroke | 16 (6.6%) | 8 (5.2) | 8 (9.0%) | 0.26 |
| Cancer | 34 (14.0%) | 22(14.4) | 12(13.5%) | 0.85 |
| Liver disease | 4 (1.7%) | 3 (2.0) | 1 (1.1%) | 1.0 |
| Charlson Index6 | ||||
| 0-1 | 137 (56.6%) | 92 (60.1) | 45 (50.6%) | 0.16 |
| 2-3 | 49 (20.3%) | 29(18.9) | 20 (22.5%) | |
| ≥ 4 | 56 (23.1%) | 32 (20.9) | 24 (26.9%) |
calculated as weight in kilograms divided by height in meters squared
Figure 1.
Baseline Seattle Angina Scores32 according to baseline low vs. high depressive symptoms.
Physical activity expenditure
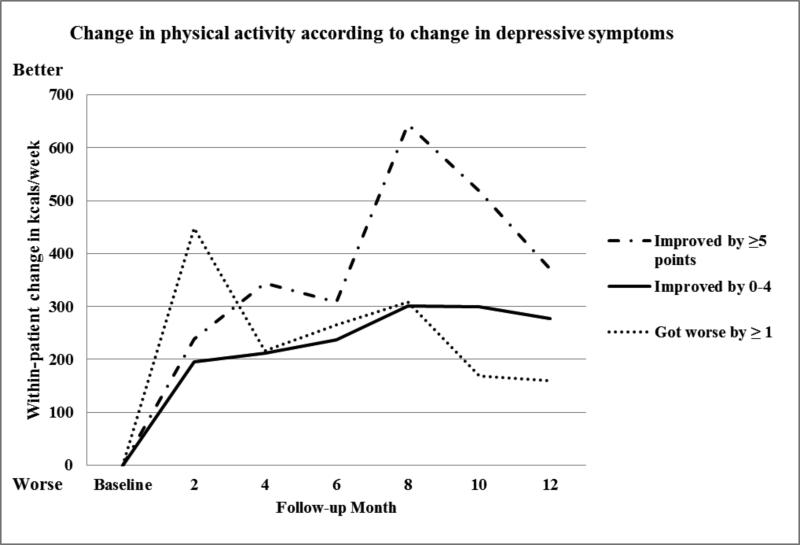
At baseline, participants with high depressive symptoms reported significantly lower energy expenditure (log(kcal/week), (p=0.043). The mean within-patient change in kcal/week at 12 months was not different for patients with high vs. low depressive symptoms. We examined trends in physical activity according to whether depressive symptoms got worse, stayed the same, or improved, and found no differences between the groups (Figure 2). However, when we compared kcal/week expenditure among those whose depressive symptoms improved by 5 or more points on the CES-D vs. those whose depressive symptoms stayed the same/got worse, we found that those who improved had a significant within-patient improvement in expenditure between baseline and 8 months (798 vs. 233 kcal/week, p=0.01). Between 8 and 12 months, physical activity declined in all three groups, but the differences between the groups were not significant.
Figure 2.
Change in kcal/week according to whether depressive symptoms improved, stayed the same or got worse according to the CES-D 10.33,34 N=237.
Cardiovascular morbidity and mortality
Patients with high depressive symptoms who achieved the main trial outcome of increasing physical activity by ≥336 kcal/week had a significantly lower rate of cardiovascular morbidity and mortality at 12 months (all-cause mortality, repeat percutaneous coronary intervention, coronary bypass surgery, congestive heart failure, new ischemia, or myocardial infarction) (5.1% vs 21.3%; odds ratio [OR], 0.20 [95% CI, 0.04–0.98]; P = 0.03). A multivariate model evaluated predictors of 12-month morbidity and mortality among patients with high depressive symptoms; these predictors included sex, education, and randomization group. We found that an increase in physical activity (≥336 kcal/week) reduced the risk of new cardiovascular morbidity/mortality (OR, 0.11 [95% CI, 0.02–0.81]; P < 0.03), and comorbidity increased the risk (OR, 1.58 [95% CI, 1.18–2.13]; P = 0.002).
Biological results
Fifty-four patients enrolled in the biological sub-study. Baseline and within-patient change (baseline - 12 months) results for the biological variables are presented in Table 2. Mean daily cortisol level significantly increased at 12 months (t=2.1, p<0.05), but there was no change in the magnitude of the AM rise in cortisol or the circadian cortisol amplitude over the course of the study. There was also no consistent change in CRP and IL-6 levels over the course of the study. There was a significant reduction in lfHRV (t= −2.9, p< 0.01) over the year, but no change in hfHRV. There were no differences in the baseline scores or the within-patient change scores (baseline - 12 months) in patients with high depressive symptoms (N=40) vs. low depressive symptoms (N=14), (data not displayed).
Table 2.
Baseline and within-patient change (baseline-12 months) (± SEM) for the physiological measures, N=54.
| Measure | N | Baseline | Within-patient change (baseline - 12 months) |
|---|---|---|---|
| IL-6 pg/ml | 46 | 3.8 ± 0.4 | 0.9 ± 1.0 |
| CRP nmol/L | 45 | 3.7 ± 0.7 | −0.2 ± 0.7 |
| hfHRV (log unit) | 40 | 4.1 ± 0.2 | −0.2 ± 0.1 |
| lfHRV (log unit) | 36 | 4.7 ± 0.2 | −0.6 ± 0.2 * |
| Mean cortisol (nmol/L) | 50 | 5.8 ± 0.5 | 1.09 ± 0.5 * |
| Cortisol AM rise (nmol/L) | 50 | 2.3 ± 0.8 | −0.2 ± 0.9 |
| Circadian cortisol amp (nmol/L) | 50 | 9.9 ± 0.3 | 2.8 ± 2.9 |
significant at p < 0.05
In a regression model with change in IL-6 as the dependent variable (incorporating age, baseline depressive symptoms, and change from baseline to 12 months in: physical activity, hfHRV, lfHRV, mean cortisol level, morning increase in cortisol, and circadian amplitude of cortisol) (Table 3), we found that a decrease in IL-6 over 12 months was significantly predicted by low depressive symptoms at baseline (t= 4.43, p= 0.004), increase in mean cortisol over 12 months (t= −2.56, p= 0.043), increase in the awakening cortisol rise (t= −2.96, p= 0.025), and an increase in hfHRV (t= −2.72, p= 0.035). In addition, older patients were less likely to have a decrease in IL-6 over 12 months (t= 2.67, p= 0.037).
Table 3.
Linear regression of factors predicting change in IL-6 over 12 months, N=30
| Coeff | Robust Std Err | t | p>/t/ | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| hfHRV Δ | −2.15 | .789 | −2.72 | 0.035 | −4.08 | −0.22 |
| lfHRV Δ | −.60 | .332 | −1.80 | 0.122 | −1.41 | 0.22 |
| Mean cortisol Δ | −9.93 | 7.79 | −2.56 | 0.043 | −38.99 | −0.89 |
| Cortisol amplitude Δ | 8.19 | 2.22 | 3.68 | 0.010 | 2.75 | 13.63 |
| Cortisol AM rise Δ | −7.05 | 2.39 | −2.96 | 0.025 | −12.89 | −1.22 |
| Activity (Kcal) Δ | .0001 | .0001 | 1.05 | 0.33 | −.0001 | .0005 |
| CES-Depression > 10 | 1.81 | 0.41 | 4.43 | .004 | 0.81 | 2.82 |
| Age | 0.10 | .037 | 2.67 | 0.037 | 0.01 | 0.19 |
R2=0.67
Generalized structural equation model combining the clinical and biological data
We constructed a generalized structural equation model (Figure 3) that combines both the clinical and biological data. As displayed in the model, an increase in energy expenditure of ≥336 kcal/week at 12 months was associated with a significant reduction in cardiovascular morbidity and mortality (Coeff = -1.13, P = 0.006), and greater medical comorbidity significantly increased the risk of complications (Coeff = 0.16, P = 0.02). In addition, increased physical activity (Coeff = 0.67, P = 0.006), but not medical comorbidity (Coeff = 0.09, P = 0.09), predicted an increase in hfHRV, which, in turn, predicted a reduction in IL-6 (b = –2.08, P = 0.001). CRP behaved similarly to IL-6 in a separate path model (data not shown).
DISCUSSION
To our knowledge, this is the first report of a threshold in physical activity in CAD patients with depressive symptoms that is associated with a reduction in cardiovascular morbidity and mortality. The notion that exercise augmentation at this level may improve clinical outcomes via enhanced parasympathetic tone and decreased inflammation is supported by our data. In this secondary data analysis of a randomized controlled trial that focused on patients with high depressive symptoms, we found that an increase in energy expenditure of ≥336 kcal/week at 12 months was associated with a significant reduction in cardiovascular morbidity and mortality (OR, 0.11 [95%CI, 0.02– 0.81]; P < 0.03). We also found that greater medical comorbidity significantly increased the risk of complications (OR, 1.58 [95% CI, 1.18–2.13]; P = 0.002).
Patients with depressive symptoms have more difficulty initiating and sustaining increased physical activity.41,42 In older adults in particular, depressive symptoms can be accompanied by executive dysfunction, functional disability, psychomotor retardation and apathy.43 These impairments, in turn, may lead to a further increase in depressive symptoms, and additional decreases in physical activity. We found that CAD patients with high vs. low depressive symptoms had significantly worse scores on the Seattle angina questionnaire,32 indicating greater CAD-related physical limitation (p<.0001), greater angina instability (p<.003), more frequent symptoms (p<.0001) and greater perceived burden of disease (p<.0001) (Figure 1). Those patients with high depressive symptoms were also significantly more likely to have pulmonary disease (p<0.03) and peripheral vascular disease (p<0.001) (Table 1). Given these physical limitations, it is perhaps not surprising that patients with high vs. low depressive symptoms are not able to increase their exercise.
It is well recognized that cardiac rehabilitation that results in even minor improvements in fitness can reduce depressive symptoms in older adults with cardiovascular disease and also decreases morbidity and mortality, yet remains widely underutilized (see Lavie44-46 for further perspective on benefits of cardiac rehabilitation and activity expenditure thresholds in CAD); however, more recent work has shown a possibly toxic effect of very high levels of activity in CAD patients, which is associated with increased morbidity and mortality.47 Contextually, these potentially toxic levels of activity are at least 10-fold higher than the threshold of 336 kcal/week suggested in the current study, which is the equivalent of walking approximately 4.2 miles/week).
Accumulating evidence indicates that CAD patients with depressive symptoms would benefit from physical activity, cardiac rehabilitation, and other behavioral activity interventions that provide structured support for physical activity. If CAD patients with high depressive symptoms can be motivated to increase their physical activity, they may not only improve cardiovascular fitness, but exercise may relieve their depressive symptoms. For example, in the UPBEAT trial, participants with CAD and major depressive disorder or subclinical depressive symptoms who engaged in aerobic exercise three times/week achieved a reduction in depressive symptoms that was comparable to that observed in participants randomized to antidepressant therapy.48 However, this study followed participants for only 16 weeks and did not report cardiovascular endpoints. We found those participants with the greatest improvement in depressive symptoms over 12 months expended the most kcal/week at every follow-up (Figure 2). Also, compared to participants whose depressive symptoms stayed the same/got worse, those whose depressive symptoms improved at 12 months had significant within-patient improvements in energy expenditure detected at 8 months (798 vs. 233 kcal/week, p=0.01), (Figure 2). Energy expenditure declined in all three groups between eight and 12 months, indicating difficulty sustaining the increased physical activity.
Many CAD patients have progressive disease, leading to worsening function and adverse events. Within one year of coronary angioplasty, 20% of patients experience major morbidity or mortality49-51 and by two years, over 30% have complications.10,52 Our results are consistent with multiple studies demonstrating an association between inflammation and progression of CAD. In the laboratory, coronary angioplasty patients have significantly elevated levels of C-reactive protein in response to mental challenges, indicating an exaggerated inflammatory response.53 Coronary angioplasty patients with elevated C-reactive protein levels have significantly increased rates of adverse cardiovascular events over 12 months (p< 0.001).54 Among patients with unstable angina, an elevated Il-6 level conferred nearly a 3.5-fold increased risk of death over a year.55 In healthy men, those with elevated IL-6 levels had a 2.3-fold increased risk of myocardial infarction over 6 years.13
Our longitudinal study of cortisol, autonomic function, and inflammation demonstrated an inverse relationship between changes in hfHRV and IL-6, a marker of inflammation, supporting multiple cross-sectional and preclinical studies.24,25 Longitudinal studies such as this one strengthen the evidence for a functional relationship between hfHRV and inflammation in patients with CAD by better controlling for confounding variables. We also found that changes in mean cortisol and the morning rise in cortisol had an inverse relationship with inflammation in the same regression model (Table 3), consistent with evidence that inflammation is exacerbated by hypocortisolism.56 Of note, baseline hypocortisolism has been associated with low activity and chronic illness56,57 and blunting of the AM rise in cortisol has been associated with chronic illness,57 fatigue,58 and pain,59 all of which are common in patients with progressive CAD. The increase in cortisol on awakening has also been shown to reflect the reactive capacity of the HPA axis required to respond adequately to acute and chronic inflammatory challenges.60 Although increased cortisol levels are usually interpreted as a sign of stress, depression, and increased allostatic load, our findings suggest that increased cortisol levels within the physiological range may, in fact, be beneficial in terms of reducing inflammation in this patient population.
We found in our generalized structural equation model that increased physical activity (≥ 336 kcal/week) was predictive of change in hfHRV (Figure 3). An increase in hfHRV in response to increased activity is consistent with studies of exercise training in patients with cardiovascular disease21 and in healthy subjects.20 The UPBEAT study enrolled 101 CAD patients with major depression or depressive symptoms and randomized them to aerobic exercise, antidepressant therapy or placebo, and found that participants randomized to exercise or antidepressant therapy had improved heart rate variability compared to the placebo group, and those randomized to exercise vs. antidepressant therapy had improvements in IL-6.48 Our results are similar and add to these findings.
Previous interventions that have attempted to treat depressive symptoms in order to improve adverse clinical outcomes in CAD patients have been disappointing. For example, the ENRICHD trial randomized over 2,400 patients with major or minor depression who had sustained a myocardial infarction (MI) to cognitive behavioral therapy vs. an educational control, along with pharmacologic treatment for depression as medically necessary.61 After an average of 29 months, depressive symptoms improved but there were no differences in event-free survival between the randomization arms. A secondary analysis of the ENRICHD trial showed that participants who exercised had significantly lower rates of non-fatal MI and mortality.62 Those who exercised had fewer depressive symptoms at baseline and a greater reduction in depressive symptoms over six months; however, exercise and a decrease in depressive symptoms were both independently related to mortality.62
Study Limitations
The optimal dose of physical activity for CAD patients with high depressive symptoms remains unknown. Our results suggest that an increase in physical activity of ≥ 336 kcal/week may be a clinically important threshold for this population in order to decrease cardiovascular morbidity and mortality at one year.
Another limitation of this study is the small number of patients who had complete sets of biological data and were able to be included in the regression analyses. Assessment of anxiety symptoms may also have strengthened the analysis. Major depressive disorder and comorbid anxiety are associated with greater reductions in heart rate variability than major depressive disorder alone,63 and anxiety comorbid with major depressive disorder increases the risk of cardiovascular disease two-to three-fold.64 Strengths of the biological marker substudy were the longitudinal within-patient design, a patient sample with significant cardiovascular disease, and simultaneous assessment of autonomic, hypothalamic-pituitary adrenal axis and inflammatory measures.
In conclusion, our results reinforce the benefits of physical activity in high-risk CAD patients with high depressive symptoms and extend the literature in several important ways. First, we have documented a clinically important improvement in kcal/week (i.e., ≥ 336 kcal/week) that is associated with a significant decrease in cardiovascular morbidity and mortality in CAD patients with high depressive symptoms. Second, we have the benefit of 12 months of follow-up, which underscores the challenges of physical activity maintenance; patients with high depressive symptoms would benefit from customized interventions that focus on maintaining long-term physical activity beyond 8 months. Finally, we have reported biological data that helps to inform the biological mechanisms by which physical activity may improve clinical outcomes in CAD patients.
Acknowledgements
The study was supported by grant 1N01-HC-25196 from the National Heart, Lung and Blood Institute.
Dr. Peterson is the recipient of a Paul B. Beeson Award from the National Institute on Aging, the American Federation for Aging Research, The John A. Hartford Foundation and The Atlantic Philanthropies under award K23AG042869.
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT00248846
References
- 1.Pirraglia PA, Peterson JC, Williams-Russo P, Gorkin L, Charlson ME. Depressive symptomatology in coronary artery bypass graft surgery patients. Int J Geriatr Psychiatry. 1999 Aug;14(8):668–680. doi: 10.1002/(sici)1099-1166(199908)14:8<668::aid-gps988>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Peterson JC, Charlson ME, Williams-Russo P, et al. New postoperative depressive symptoms and long-term cardiac outcomes after coronary artery bypass surgery. Am J Geriatr Psychiatry. 2002 Mar-Apr;10(2):192–198. [PubMed] [Google Scholar]
- 3.Frasure-Smith N, Lesperance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995 Feb 15;91(4):999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- 4.Charlson M, Peterson JC. Medical comorbidity and late life depression: what is known and what are the unmet needs? Biol Psychiatry. 2002 Aug 1;52(3):226–235. doi: 10.1016/s0006-3223(02)01422-1. [DOI] [PubMed] [Google Scholar]
- 5.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. Vascular depression' hypothesis. Arch Gen Psychiatry. 1997 Oct;54(10):915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 6.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 7.Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA : the journal of the American Medical Association. 2008 Nov 26;300(20):2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agency for Healthcare Research and Quality (AHRQ) Technology Assessment Program . Randomized trials of secondary prevention programs in coronary artery disease: a systematic review. Rockville, MD.: 2005. [PubMed] [Google Scholar]
- 9.Taylor RS, Unal B, Critchley JA, Capewell S. Mortality reductions in patients receiving exercise-based cardiac rehabilitation: how much can be attributed to cardiovascular risk factor improvements? Eur J Cardiovasc Prev Rehabil. 2006 Jun;13(3):369–374. doi: 10.1097/01.hjr.0000199492.00967.11. [DOI] [PubMed] [Google Scholar]
- 10.Charlson ME, Peterson JC, Boutin-Foster C, et al. Changing health behaviors to improve health outcomes after angioplasty: a randomized trial of net present value versus future value risk communication. Health Educ Res. 2008 Oct;23(5):826–839. doi: 10.1093/her/cym068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999 Oct 25;159(19):2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 12.Peterson JC. The adaptive neuroplasticity hypothesis of behavioral maintenance. Neural Plast. 2012;2012:516364. doi: 10.1155/2012/516364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000 Apr 18;101(15):1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 14.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005 Apr 21;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 15.Panagiotakos DB, Pitsavos C, Chrysohoou C, et al. Inflammation, coagulation, and depressive symptomatology in cardiovascular disease-free people; the ATTICA study. Eur Heart J. 2004 Mar;25(6):492–499. doi: 10.1016/j.ehj.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009 Feb;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 17.Swardfager W, Herrmann N, Cornish S, et al. Exercise intervention and inflammatory markers in coronary artery disease: a meta-analysis. Am Heart J. 2012 Apr;163(4):666–676. doi: 10.1016/j.ahj.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010 May 28;141(2):122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 19.Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation. 1993 Sep;88(3):927–934. doi: 10.1161/01.cir.88.3.927. [DOI] [PubMed] [Google Scholar]
- 20.Levy WC, Cerqueira MD, Harp GD, et al. Effect of endurance exercise training on heart rate variability at rest in healthy young and older men. Am J Cardiol. 1998 Nov 15;82(10):1236–1241. doi: 10.1016/s0002-9149(98)00611-0. [DOI] [PubMed] [Google Scholar]
- 21.Iellamo F, Legramante JM, Massaro M, Raimondi G, Galante A. Effects of a residential exercise training on baroreflex sensitivity and heart rate variability in patients with coronary artery disease: A randomized, controlled study. Circulation. 2000 Nov 21;102(21):2588–2592. doi: 10.1161/01.cir.102.21.2588. [DOI] [PubMed] [Google Scholar]
- 22.Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol Psychol. 2007 Feb;74(2):200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Porges SW. The polyvagal perspective. Biol Psychol. 2007 Feb;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev. 2012 Jul;248(1):188–204. doi: 10.1111/j.1600-065X.2012.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCaffery JM, Marsland AL, Strohacker K, Muldoon MF, Manuck SB. Factor structure underlying components of allostatic load. PLoS One. 2012;7(10):e47246. doi: 10.1371/journal.pone.0047246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson JC, Charlson ME, Hoffman Z, et al. A randomized controlled trial of positive-affect induction to promote physical activity after percutaneous coronary intervention. Arch Intern Med. 2012 Feb 27;172(4):329–336. doi: 10.1001/archinternmed.2011.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson JC, Link AR, Jobe JB, Winston GJ, Marina Klimasiewfski E, Allegrante JP. Developing self-management education in coronary artery disease. Heart Lung. 2014 Mar-Apr;43(2):133–139. doi: 10.1016/j.hrtlng.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorig K, Fries JF. The arthritis helpbook: A tested self-management program for coping with your arthritis. Addison-Wesley; Reading, MA: 1990. [Google Scholar]
- 29.Peterson JC, Czajkowski S, Charlson ME, et al. Translating basic behavioral and social science research to clinical application: the EVOLVE mixed methods approach. Journal of consulting and clinical psychology. 2013 Apr;81(2):217–230. doi: 10.1037/a0029909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paffenbarger RS, Jr., Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993 Jan;25(1):60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994 Oct;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 32.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995 Feb;25(2):333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 33.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993 May;5(2):179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 34.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement. 1977;1:385–401. [Google Scholar]
- 35.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003 Jun;111(12):1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hedayat M, Mahmoudi MJ, Rose NR, Rezaei N. Proinflammatory cytokines in heart failure: double-edged swords. Heart Fail Rev. 2010 Nov;15(6):543–562. doi: 10.1007/s10741-010-9168-4. [DOI] [PubMed] [Google Scholar]
- 37.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008 Oct 30;359(18):1897–1908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 38.Porges SW, Bohrer RE. The analysis of periodic processes in psychophysiological research. In: Cacioppo JT, Tassinary IG, editors. Principles of Psychophysiology: Physical, Social, and Inferential Elements. Cambridge University Press; New York: 1990. pp. 708–753. [Google Scholar]
- 39.Berntson GG, Bigger JT, Jr., Eckberg DL, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997 Nov;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 40.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–838. [Google Scholar]
- 41.Farmer ME, Locke BZ, Moscicki EK, Dannenberg AL, Larson DB, Radloff LS. Physical activity and depressive symptoms: the NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1988 Dec;128(6):1340–1351. doi: 10.1093/oxfordjournals.aje.a115087. [DOI] [PubMed] [Google Scholar]
- 42.Camacho TC, Roberts RE, Lazarus NB, Kaplan GA, Cohen RD. Physical activity and depression: evidence from the Alameda County Study. Am J Epidemiol. 1991 Jul 15;134(2):220–231. doi: 10.1093/oxfordjournals.aje.a116074. [DOI] [PubMed] [Google Scholar]
- 43.Alexopoulos GS. Role of executive function in late-life depression. J Clin Psychiatry. 2003;64(Suppl 14):18–23. [PubMed] [Google Scholar]
- 44.Menezes AR, Lavie CJ, Forman DE, Arena R, Milani RV, Franklin BA. Cardiac rehabilitation in the elderly. Progress in cardiovascular diseases. 2014 Sep-Oct;57(2):152–159. doi: 10.1016/j.pcad.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 45.O'Keefe JH, Franklin B, Lavie CJ. Exercising for Health and Longevity vs Peak Performance: Different Regimens for Different Goals. Mayo Clinic proceedings. 2014 Sep;89(9):1171–1175. doi: 10.1016/j.mayocp.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Milani RV, Lavie CJ. Impact of cardiac rehabilitation on depression and its associated mortality. The American journal of medicine. 2007 Sep;120(9):799–806. doi: 10.1016/j.amjmed.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 47.Williams PT, Thompson PD. Increased cardiovascular disease mortality associated with excessive exercise in heart attack survivors. Mayo Clinic proceedings. 2014 Sep;89(9):1187–1194. doi: 10.1016/j.mayocp.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Blumenthal JA, Sherwood A, Babyak MA, et al. Exercise and pharmacological treatment of depressive symptoms in patients with coronary heart disease: results from the UPBEAT (Understanding the Prognostic Benefits of Exercise and Antidepressant Therapy) study. J Am Coll Cardiol. 2012 Sep 18;60(12):1053–1063. doi: 10.1016/j.jacc.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Javaid A, Steinberg DH, Buch AN, et al. Outcomes of coronary artery bypass grafting versus percutaneous coronary intervention with drug-eluting stents for patients with multivessel coronary artery disease. Circulation. 2007 Sep 11;116(11 Suppl):I200–206. doi: 10.1161/CIRCULATIONAHA.106.681148. [DOI] [PubMed] [Google Scholar]
- 50.Palmerini T, Marzocchi A, Marrozzini C, et al. Comparison between coronary angioplasty and coronary artery bypass surgery for the treatment of unprotected left main coronary artery stenosis (the Bologna Registry). Am J Cardiol. 2006 Jul 1;98(1):54–59. doi: 10.1016/j.amjcard.2006.01.070. [DOI] [PubMed] [Google Scholar]
- 51.van Domburg RT, Lemos PA, Takkenberg JJ, et al. The impact of the introduction of drug-eluting stents on the clinical practice of surgical and percutaneous treatment of coronary artery disease. Eur Heart J. 2005 Apr;26(7):675–681. doi: 10.1093/eurheartj/ehi088. [DOI] [PubMed] [Google Scholar]
- 52.Walther C, Mobius-Winkler S, Linke A, et al. Regular exercise training compared with percutaneous intervention leads to a reduction of inflammatory markers and cardiovascular events in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2008 Feb;15(1):107–112. doi: 10.1097/HJR.0b013e3282f29aa6. [DOI] [PubMed] [Google Scholar]
- 53.Kop WJ, Weissman NJ, Zhu J, et al. Effects of acute mental stress and exercise on inflammatory markers in patients with coronary artery disease and healthy controls. Am J Cardiol. 2008 Mar 15;101(6):767–773. doi: 10.1016/j.amjcard.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Buffon A, Liuzzo G, Biasucci LM, et al. Preprocedural serum levels of C-reactive protein predict early complications and late restenosis after coronary angioplasty. J Am Coll Cardiol. 1999 Nov 1;34(5):1512–1521. doi: 10.1016/s0735-1097(99)00348-4. [DOI] [PubMed] [Google Scholar]
- 55.Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or noninvasive strategy. JAMA : the journal of the American Medical Association. 2001 Nov 7;286(17):2107–2113. doi: 10.1001/jama.286.17.2107. [DOI] [PubMed] [Google Scholar]
- 56.Raison C, Miller A. When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. American Journal of Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 57.Vreeburg SA, Kruijtzer BP, van Pelt J, et al. Associations between sociodemographic, sampling and health factors and various salivary cortisol indicators in a large sample without psychopathology. Psychoneuroendocrinology. 2009 Sep;34(8):1109–1120. doi: 10.1016/j.psyneuen.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 58.Kumari M, Badrick E, Chandola T, et al. Cortisol secretion and fatigue: Associations in a community based cohort. Psychoneuroendocrinology. 2009;13:121–125. doi: 10.1016/j.psyneuen.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Fabian L, McGuire L, Page G, Goodin B, Edwards R, Haythornthwaite J. The association of the cortisol awakening response with experimental pain ratings. Psychoneuroendocrinology. 2009;34:1247–1251. doi: 10.1016/j.psyneuen.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmidt-Reinwald A, Pruessner J, Hellhammer D, et al. he cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci. 1999;64:1653–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- 61.Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA : the journal of the American Medical Association. 2003 Jun 18;289(23):3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 62.Blumenthal JA, Babyak MA, Carney RM, et al. Exercise, depression, and mortality after myocardial infarction in the ENRICHD trial. Med Sci Sports Exerc. 2004 May;36(5):746–755. doi: 10.1249/01.mss.0000125997.63493.13. [DOI] [PubMed] [Google Scholar]
- 63.Kemp A, Quintana D, KL Felmingham SM, Jelinek H. Depression, cormorbid anxiety disorders, and heart rate variability in physically healthy, unmedicated patients: implications for cardiovascular risk. PLoS One. 2012;7:e30777. doi: 10.1371/journal.pone.0030777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phillips A, Batty G, Gale C, et al. Generalized anxiety disorder, major depressive disorder, and their comorbidity as predictors of all-cause and cariovascular mortality: the Vietnam experience study. Psychosom Med. 2009;71:395–403. doi: 10.1097/PSY.0b013e31819e6706. [DOI] [PubMed] [Google Scholar]