Abstract
In research and development studies for human and veterinary medicine, relevant comparators for interpreting clinical pathology results are matched with concurrent control animals. However, reference intervals (RI) provide a comparator database and important aids for interpreting clinical pathology data, especially in laboratory beagle dogs. Furthermore, RI incorporate biologic variation, which includes analytical, intraindividual, and interindividual variation. No studies to date have established RI and studied the effect of biologic variation on hematologic variables in a large group of laboratory dogs. The purpose of this retrospective study was to establish hematologic RI for laboratory beagles according to international recommendations and estimate the effect of biologic variation in routinely measured hematologic analytes by using the databank at a pharmaceutical center. Blood specimens from 340 healthy beagles (age, 9 to 36 mo) were evaluated by using a flow-cytometry-based hematology analyzer. RI and their 90% confidence intervals were established by using a nonparametric method. Effects of sex, age, and weight were investigated. Weight had no effect on any analyte. RBC, Hgb, Hct, MCV, MCH, RBC distribution width, and platelet count increased with age, whereas WBC count decreased. The only clinically relevant effect of sex was observed for platelets, which were lower in male beagles than in female and warranted 2 different RI. The calculated index of individuality showed that population-based RI were appropriate for almost all hematologic analytes, as might be expected for a homogeneous group of laboratory beagles.
Abbreviation: CLSI, Clinical and Laboratory Standards Institute; RCV, reference change value; RI, reference intervals
Routine hematology is a basic examination that is performed in laboratory beagles to monitor health and evaluate the toxicologic potential of various drugs, pollutants, or treatments. A recent statistical survey showed that 3000 laboratory dogs were used for scientific purposes in 2010 in France,27 half of them for toxicological studies and the other half for safety evaluations. The interpretation of clinical pathology results primarily is based on the statistical comparison of results from controls with those of treated animals. This constitutes only a crude use of the data, because it does not take into account basic knowledge of reference intervals (RI) and inter- and intraindividual factors of variation of the analytes.
Recently, population-based RI for hematologic variables have been determined according to international recommendations in various breeds of companion dogs by using modern cell counters, which are partially or completely based on flow cytometry.4,6,28,34 These efforts demonstrated an occurrence of breed effects, which had not been previously established. Paradoxically, similar studies involving modern hematology analyzers and techniques for determining RI in laboratory beagles have not been published. Today, the most frequently cited hematologic canine RI30 are taken from textbooks published in the 1960s and 1970s,31-33 and the reference values were obtained from both mongrels and beagles, both kept under laboratory animal conditions. Similarly, previous studies done on large populations of beagles used for biomedical research were focused on blood reference values obtained from animals of different age classes.1-3,13,26,35,43 To our knowledge, the latest report of comprehensive hematology and chemistry analyte RI in laboratory beagles was published in 198643 and involved a now-outdated analyzer. In consequence, none of these studies meets the currently available international recommendations of the International Federation of Clinical Chemistry and Clinical and Laboratory Standards Institute,8 because the preanalytical and analytical conditions, animal population characteristics, and statistical procedures are not fully documented. Because laboratory beagles typically are young animals, with low genetic variability and bred under fully controlled conditions, we surmised that the RI of other breeds would not be transferrable and that interindividual variability would be lower than that in companion dogs.
Moreover, information regarding the biologic variation of an analyte is important to consider before reference values are determined. If the intraindividual variability is considerably less than the interindividual variability, a population-based RI is not sufficiently sensitive to detect changes in a subject over time.24 Although biologic variation has been acknowledged and accommodated in human medicine over the last 15 y,10,17,29 corresponding data in veterinary medicine remain scant.40 A Danish team interested in animal biologic variation, particularly in dogs,21,23,42 published a study 16 y ago on the variability of some hematologic analytes, especially in beagles.22 The conclusions were that population-based RI were relevant for RBC count, Hct, Hgb, and WBC count and that subject-based RI consequently were unnecessary.22
We therefore performed the current study to investigate the biologic variation of hematologic analytes from laboratory beagles by using the database of control dogs of a pharmaceutical company. The objectives were: to establish an a posteriori procedure for the determination of population-based RI according to the American Society of Veterinary Clinical Pathology12 and the International Federation of Clinical Chemistry and Clinical and Laboratory Standards Institute;8 to estimate indexes of individuality; to consider the use of population-based compared with subject-based reference values; and to estimate the reference change value (RCV) to identify the significance of any change between consecutive results.
Materials and Methods
Experimental design and animals.
This retrospective study was designed to obtain population-based and subject-based reference values, and was performed using the database of routine hematology measurements in control beagle dogs during acclimation or wash-out periods. The data were compiled by the Sanofi research center (Alfortville, France) between 2007 and 2012. Population-based RI were established by using the a posteriori procedure recommended by the American Society of Veterinary Clinical Pathology12 and the International Federation of Clinical Chemistry and Clinical and Laboratory Standards Institute;8 subject-based RI were generated from repeated analyses in some of the beagles. These data also were used to determine inter- and intraindividual variability and the RCV (critical difference) for each analyte.
The Sanofi center complies with the European Directive 2010/63/EU43 on the protection of animals used for scientific purposes, is accredited by AAALAC, and is in compliance with good laboratory practices (Organization for Economic Cooperation and Development principles on good laboratory practices). This site is licensed by the French authorities as an institution for experimental animal housing (agreement no. E94-002-4). All procedures were approved by the ethics committee for the protection of laboratory animals (Comité no. 21) registered by the French Ministère de l'Enseignement Supérieur et de la Recherche.
The male and female beagle dogs included were all younger than 3 y and provided by Marshall Farms (North Rose, NY). They were housed either individually or in groups of 2 or 3, were provided daily and individually with 250 g of a certified pelleted feed for 2 h (Extruded Dog Maintenance Diet 326, Aston Pharma, London, United Kingdom), and supplied with water ad libitum.
The animals’ health status was checked regularly by the support staff and veterinary management. Dogs were excluded when they had any sign of illness or had been treated 1 mo before or after a time of sampling. In addition, animals in which an in-dwelling catheter had been inserted before sampling were excluded from the study population.
Blood sampling and analysis.
Between 0800 and 1100, overnight-fasted dogs were gently taken individually from their cages into a neighboring room, to minimize possible stress. They were kept under minimal physical restraint while experienced animal technicians obtained blood from the jugular vein by using a 21-gauge needle and syringe and placed it into a 1.2-mL EDTA-K2 tube (Monovette EDTA-K2; Sarstedt, Haute-Saone, France). The tubes were gently homogenized, identified, and stored at room temperature until analyzed within 1 h.
Analyses were performed by using an automated hematology analyzer with a veterinary module (ADVIA 2120, Siemens Healthcare Diagnostics, Saint-Denis, France) and by using the settings for canine blood (software version 5.9, Siemens). Because canine control solutions and specimens were not commercially available, analyses of human low-, medium-, and high-levels controls were performed daily before analysis of the canine samples (ADVIA 3-in-1 [Siemens Healthcare Diagnostics] for normal and low control levels and Eurocell Control H [Eurocell Diagnostics, Noyal chatillon-sur-Seiches France] for the high control level). Routine measurements included the following variables: RBC count, Hgb, Hct, MCV, MCH, MCHC, RBC distribution width, and WBC, neutrophil, eosinophil, lymphocyte, monocyte, and platelet counts. Basophils and large unstained cell counts were not reported, given that they have been shown to be unreliable in canine specimens.25,38
The CV for analyzer imprecision was determined in accordance with CLSI guidelines, by performing duplicate measurements of human low- and high-level controls in the morning and afternoon for 5 consecutive days.7
Statistical analysis.
When multiple specimens had been collected from the same animal, only the first was used for the population-based RI determination. Histograms were visually inspected to detect possible outliers, which were confirmed by using the Dixon test. Normality of the distributions was tested by using the Anderson–Darling test. Reference limits and their 90% CI were determined according to recommendations from the American Society of Veterinary Clinical Pathology and the International Federation of Clinical Chemistry and Clinical and Laboratory Standards Institute8,12 by using the nonparametric method provided through the Reference Value Advisor freeware.14
Effects of sex, age, and weight on results were tested by using a general linear model. When significant effects of sex were observed, the relevance of partitioning was tested by using the Harris–Boyd test. Significant effects of age or weight were studied by regression-based analysis.39 Effects were considered as significant when the P value was less than 0.005.
The following mixed-effects model was used to estimate inter- and intraindividual variabilities:
 |
where:
Yij is the analyte concentration measured on the ith dog at age Ageij; b is the population effect of age on the analyte; Dogi is the random effect of the ith subject (which is assumed to be distributed according to a N(0;SDg2) distribution; and ϵij is a residual term that is assumed to be N(0; SDi2)-distributed. The variances SDi2 and SDg2 are, respectively, the intraindividual and interindividual variances after removing a possible effect of age on the variables.
Intraindividual variability was estimated from repeated measurements in the same animal, whereas the entire reference sample group was used to estimate interindividual variability. These variabilities were expressed as CV (%) = 1 SD / mean × 100% by using the overall mean for each variable.
The index of individuality was calculated as (CVi2 + CVa2)0.5 / CVg, where CVi is the CV of intraindividual variability, CVa is the CV for analyzer imprecision, and CVg is the CV of interindividual variability.
The RCV (also known as the critical difference) was estimated as:
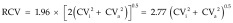 |
Calculations were performed by using Systat 13 (IBM, Chicago, IL), Analyse-It (Leeds, United Kingdom), and the Reference Value Advisor freeware.14
Results
Demographic characteristics of the reference sample group.
Of the 527 laboratory beagles represented in the database, 147 female and 193 male dogs were included in the study population according to the previously defined criteria. Of these 340 animals, 106 underwent 2 to 7 blood samplings over 23 to 378 d.
The age of the beagles (mean, 13.1 mo; range, 9.4 to 34.7 mo) did not differ according to sex, and most animals were younger than 18 mo (299 of 340, 88%). Male beagles weighed significantly (P < 0.001) more than did female beagles (male: median, 8.9 kg; range, 5.6 to 15.2 kg; female: median, 7.6 kg; range, 4.1 to 12.8 kg). Weight increased moderately but significantly (P < 0.018) with age.
Population-based RI.
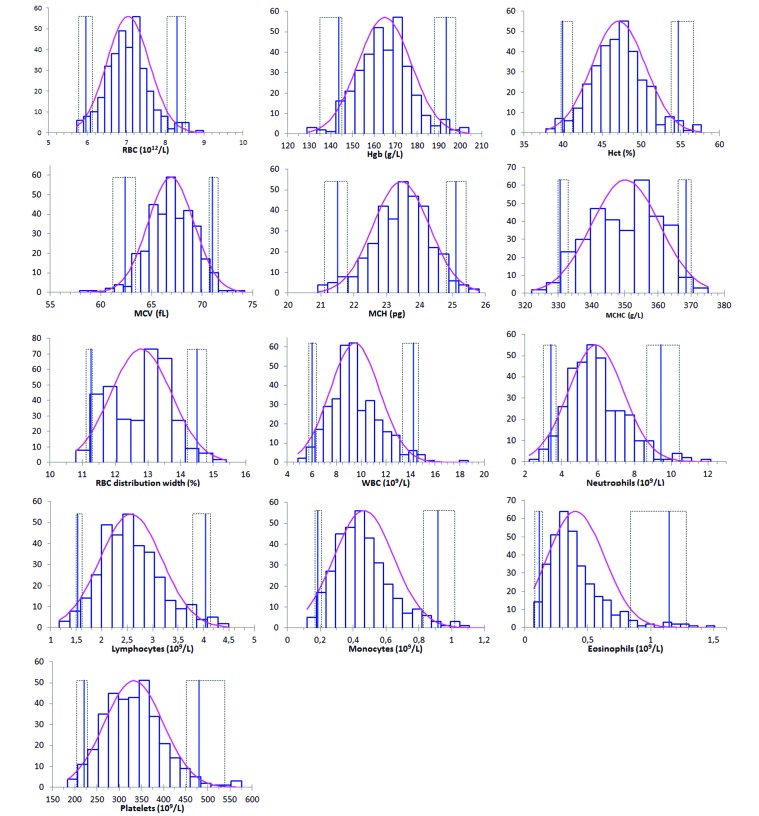
RI for hematologic variables from our laboratory beagle population are provided in Table 1, and the corresponding histograms in Figure 1. Most distributions were significantly different from Gaussian. The medians and means were very close to each other, but most distributions were skewed toward higher values (Figure 1).
Table 1.
Data regarding hematologic variables obtained for laboratory beagles (current study) or various breeds of dogs (previous studies)
| Current study |
Previous studies |
||||||||||
| n | Mean | Median (range) | 2.5th centile (90% CI) | 97.5th centile (90% CI) | Normality P | Significant effects (P) | Reference 28 (n = 46) | Reference 20 (n = 130) | Reference 4 (n = 132) | Equipment not reporteda (n unknown) | |
| RBC, ×1012/L | 340 | 7.1 | 7.0 (5.7–9.0) | 6.0 (5.8–6.1) | 8.3 (8.1–8.5) | 0.041 | Age: <0.001 | 5.68–9.08 | 5.7–8.3 | 5.1–7.6 | 5.5–8.5 |
| Hgb, g/L | 340 | 164.9 | 164.9 (129–204) | 143.5 (135.0–145.0) | 193.5 (188.0–198.0) | 0.176 | Age: <0.001 | 137.7–203.8 | 140–200 | 124.0–191.5 | 120–180 |
| Hct, L/L | 340 | 0.471 | 0.471 (0.378–0.577) | 0.399 (0.397–0.412) | 0.547 (0.539–0.567) | 0.044 | Age: <0.001 | 0.42–0.62 | 0.40–0.56 | 0.35–0.52 | 0.37–0.55 |
| MCV, fL | 340 | 66.9 | 66.9 (57.9–74.2) | 62.4 (61.2–63.4) | 71.0 (70.7–71.6) | 0.216 | Sex: 0.032 | 62.7–74.56 | 64–74 | 60–71 | 60.0–77.0 |
| MCH, pg | 340 | 23.4 | 23.5 (20.9–25.8) | 21.5 (21.1–21.8) | 25.1 (24.8–25.4) | 0.001 | Age: 0.004 | 20.46–24.81 | 22–26 | 22–26 | 19.5–24.5 |
| MCHC, g/L | 340 | 350.3 | 351.0 (322.0–375.0) | 330.5 (330.0–333.0) | 368.5 (366.0–370.0) | 0.0002 | Age: <0.001 | 316.1–343.5 | 330–380 | 344–381 | 320–360 |
| RBC distribution width, % | 340 | 12.8 | 13.0 (10.8–15.4) | 11.3 (11.1–11.3) | 14.5 (14.2–14.8) | <0.0001 | Age: 0.002 | 12.00–13.15 | 11–14 | 13.2–19.1 | none |
| WBCb, × 109/L | 339 | 9.5 | 9.2 (4.9–18.7) | 6.0 (5.7–6.4) | 14.3 (13.3–14.7) | <0.0001 | Age: 0.018 | 5.84–20.26 | 5.0–13.0 | 5.6– 20.4 | 6.0–17.0 |
| Neutrophils,b ×109/L | 339 | 5.9 | 5.7 (2.2–12.2) | 3.4 (3.0–3.7) | 9.5 (8.7–10.4) | <0.0001 | none | 4.27–9.06 | 2.7–8.9 | 2.9–13.6 | 3.0–11.5 |
| Lymphocytes, ×109/L | 340 | 2.6 | 2.5 (1.2–4.5) | 1.5 (1.5–1.6) | 4.0 (3.8–4.1) | 0.0002 | Age: <0.001 Sex: <0.001 | 2.04–4.66 | 0.9–3.4 | 1.14–5.28 | 1.0–4.8 |
| Monocytes,b ×109/L | 339 | 0.5 | 0.4 (0.1–1.5) | 0.2 (0.2–0.2) | 0.9 (0.8–1.0) | <0.0001 | Age: <0.001 Sex: <0.001 | 0.24–2.04 | 0.1–0.8 | 0.35–1.56 | 0.15–1.35 |
| Eosinophils,c ×109/L | 339 | 0.4 | 0.4 (0.1–1.5) | 0.1 (0.1–0.1) | 1.1 (0.8–1.3) | <0.0001 | Age: 0.008 | 0.10–1.20 | 0.1–1.3 | 0.13–3.05 | 0.10–1.25 |
| Platelets,d ×109/L | 339 | 332.8 | 331.0 (183.0–576.0) | 220.5 (204.0–228.0) | 480.5 (451.0–538.0) | 0.018 | Age: 0.001 Sex: <0.001 | 173.1–486.5 | 134–396 | 108–562 | 200–500 |
| Platelets, ×109/L | 147 ♀ | 346 | 340.0 (204.0–576.0) | 241.5 (204.0–254.0) | 542.5 (460.0–576.0) | ||||||
| 192d ♂ | 323 | 321.0 (183.0–509.0) | 214.5 (190.0–225.0) | 470.0 (432.0–506.0) | |||||||
References 30 through 33
Outliers for WBC (27.04 x 109/L), neutrophils (22.74 x 109/L), and monocytes (1.96 x109/L)
Outlier for eosinophils (2.72 x109/L)
Data missing
Figure 1.
Histograms of reference values for routine hematologic variables in laboratory beagles. The curved pink line represents the fitted distribution; vertical bars are reference limits, with their 90% confidence intervals as dotted lines.
Weight did not significantly affect any variable. Significant effects of sex did not warrant the use of 2 different RI, according to the Harris–Boyd test (z > z*) for any parameter except the platelet count, which was higher in female than in male beagles (Table 1).
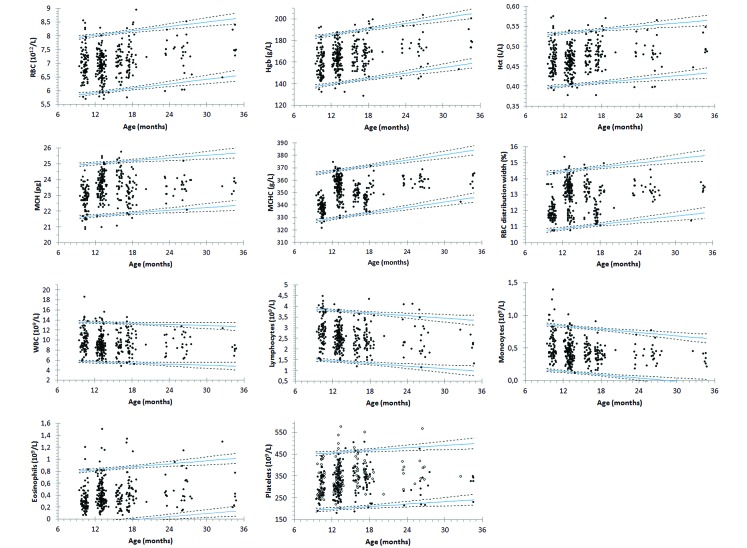
RBC count, Hgb, Hct, MCH, MCHC, and RBC distribution width increased with age (Figure 2). Platelet counts increased with age and increased similarly in male and female beagles. WBC counts decreased with age due to decreases in lymphocyte and monocyte counts, whereas eosinophils increased moderately. The average age-associated changes in the hematologic variables of 9- and 36-mo-old laboratory beagles are summarized in Table 2.
Figure 2.
Regression-based reference limits (pink line, mean) and their 90% confidence intervals for hematologic variables that are significantly affected by age in laboratory beagles.
Table 2.
Average changes (range) in routine hematologic variables of laboratory beagles between 9 and 36 mo of age
| Unit | a × n | b | |
| RBC | ×1012/L | 0.016 (0.008–0.023) | + 6.81 (6.68–6.95) |
| Hgb | g/L | 0.504 (0.337–0.670) | + 156.8 (153.7–159.8) |
| Hct | L/L | 0.093 (0.045–1.14) | + 45.2 (45.0–46.7) |
| MCH | pg | 0.008 (-0.012–0.029) | + 23.2 (22.9–23.5) |
| MCHC | g/L | 0.364 (0.233–0.295) | + 432.7 (340.3–345.1) |
| RBC distribution width | % | 0.033 (0.021–0.045) | + 12.2 (12.0–12.4) |
| WBC | ×109/L | −0.037 (-0.065–-0.010) | + 10.1 (9.6–10.6) |
| Lymphocytes | ×109/L | −0.021 (-0.029–-0.014) | + 2.90 (2.76–3.05) |
| Monocytes | ×109/L | −0.007 (-0.009–-0.005) | + 0.57 (0.53–0.61) |
| Eosinophils | ×109/L | 0.011 (0.007–0.015) | + 0.28 (0.21–0.35) |
| Platelets | ×109/L | 1.227 (0.306–2.148) | + 309.6 (292.5–326.6) |
Concentration at age n (in months) = a × n + b; 95% confidence intervals in parentheses
Variability, index of individuality, and RCV.
Analytical variability was very low, that is, ≤ 2.6%, except for those associated with the lymphocyte, monocyte, and eosinophil counts (Table 3). Intraindividual variability was low (CV < 6.2%) for RBC variables and indexes; moderate (7% to 14%) for the monocyte, lymphocyte, and platelet counts; intermediate (20% to 30%) for the WBC and neutrophil counts, and very high (almost 100%) for the eosinophil count. Interindividual variability was slightly lower than intraindividual variability for RBC, HGB, Hct, MCHC, and WBC and neutrophil counts, whereas interindividual variability was slightly higher than intraindividual variability for the other variables. The eosinophil, monocyte, and lymphocyte counts demonstrated high interindividual variability. Most indexes of individuality were 0.5 to 1.5, except for MCHC and the WBC and neutrophil counts, for which they were higher than 1.5, and the monocyte and eosinophil counts, for which indexes of individuality were lower than 0.5. RCV were low (less than 20% of mean value) for the RBC variables and indexes but higher than 20% for the platelet, WBC, and WBC differential counts.
Table 3.
CV (%), RCV (%), and index of individuality (IoI) for routine hematologic variables in 9- to 36-mo-old laboratory beagles
| Unit | CVa | CVg | CVi | RCV | IoI | |
| RBC | ×1012/L | 1.8 | 4.8 | 6.0 | 17.2 | 1.3 |
| Hgb | g/L | 1.5 | 4.4 | 6.1 | 17.5 | 1.4 |
| Hct | L/L | 2.1 | 4.3 | 6.2 | 18.1 | 1.5 |
| MCV | fL | 1.4 | 2.6 | 2.1 | 7.0 | 1.0 |
| MCH | pg | 1.2 | 3.3 | 1.6 | 5.5 | 0.6 |
| MCHC | g/L | 2.1 | 1.1 | 2.6 | 9.2 | 2.9 |
| RBC distribution width | % | 1.0 | 6.0 | 4.0 | 11.4 | 0.7 |
| WBC | ×109/L | 2.2 | 11.8 | 19.6 | 54.7 | 1.7 |
| Neutrophils | ×109/L | 1.1 | 14.0 | 25.9 | 71.8 | 1.8 |
| Lymphocytes | ×109/L | 4.3 | 40.2 | 11.1 | 32.9 | 0.3 |
| Monocytes | ×109/L | 7.0 | 47.5 | 7.0 | 27.5 | 0.2 |
| Eosinophils | ×109/L | 7.6 | 191.1 | 96.4 | 267.8 | 0.5 |
| Platelets | ×109/L | 2.6 | 15.2 | 14.0 | 39.6 | 0.9 |
a, analytical; g, interindividual; i, intraindividual
Discussion
This study is the first evaluation of the biologic variation of hematology variables in clinically healthy laboratory beagles by using the latest generation of analyzers. The study revealed that the population-based reference values are close to those previously reported for canine species; that almost all of the hematologic analytes exhibit low intraindividual and interindividual variability, such that subject-based reference values are of limited interest.
RI in laboratory beagles, in accordance with international recommendations, have not been established previously. These recommendations advocate that prospective studies be performed whenever possible, to limit possible factors of variations.12,15 In the case of laboratory animals bred in the standard environment of a controlled experimental center, a retrospective study can be based on a large amount of reliable data, with the possibility of eliminating outliers (limited to 3 specimens in the current study).
The beagles included in our study ranged in age from 9 to 36 mo, which is the most common age range of dogs at pharmaceutical experimental centers. Despite this range, relatively few dogs were older than 2 y, a factor that limited the relevance of age-related observations, at least for dogs older than 24 mo.
For most of the variables, analytical variability was very low, as can be expected with most modern hematology analyzers. However, the WBC differential count, even with these instruments, was less precise when leukocyte concentrations were low. Between-day imprecision was within the range specified by the manufacturer but was tested with human controls, because stabilized control specimens of canine blood are unavailable commercially.
Many distributions, especially of RBC count and the derived variables, were quite symmetrical and close to Gaussian, as noted visually and confirmed by comparing the means and medians. Normality testing was somewhat biased and not fully relevant, because most variables showed significant effects (mostly increases) of age. These effects may have led to the skewing toward high values noted for some variables, including neutrophil, monocyte, eosinophil, and platelet counts and broadening of the confidence interval for the upper limit of the RI, which exceeded 20% of the RI, the limit suggested by CLSI.8 Given that this retrospective study was based on routine hematologic analyses to monitor the health of control or untreated beagles, analyses were limited to routine hematologic analytes according to the internal procedure of the pharmaceutical center. Moreover, the differential count of the analyzer we used included a cell type designated as ‘large unstained cells,’ which we did not report because it did not represent a known cell type in the official hematologic nomenclature. Finally, the basophil count was not evaluated, because this variable has not been validated in dogs.25,38
As shown in Table 1, we obtained only moderate differences between the RI for the laboratory beagles in this study and those previously reported for various breeds of companion dogs and obtained by using similar analyzers.4,20,28 The width of the RI for the RBC count and derived analytes was slightly narrower than those in previous studies. For example, the width of the RI for Hgb was 50 g/L in the current study and ranged from 60 to 67.5 g/L in previous studies.4,20,28,30
This difference may partly be explained by the homogeneous genetics and living conditions of laboratory beagles, although this trend was not as marked for WBC and platelet counts. Older literature data for beagle blood RI had not been obtained in accordance with the new recommendations, thus precluding truly meaningful comparisons. However, high similarity between current and previous means for Hct, Hgb, and RBC count13,26,43 and ranges for RBC count, Hgb, Hct and WBC count16 were observed.
Effects of age on canine hematologic variables have already been reported. Increases in RBC count, Hgb, and Hct during the first to second year of life and decreases in WBC counts, particularly lymphocyte counts, were reported in beagles.5,9,16,26,36 Most age-related variations associated with the narrow age range of the beagles in the current study (9 to 36 mo) were moderate, with changes for most variables (except for monocytes and RBC distribution width) accounting for less than 10% of the RCV over 6 mo. Accordingly, the short-term age-related changes in hematologic variables can likely be considered as negligible in laboratory beagles.
Sex-associated effects on canine hematology have been investigated only infrequently. Various authors have reported slightly higher RBC counts and derived variables1,2,26 and slightly lower leukocyte values in male than female dogs.2 In the current study, sufficient numbers of animals were available to permit the use of parametric procedures to determine any effects of sex. This partitioning was relevant only for platelets, warranting 2 separate RI for platelet counts from male and female beagles. The increased platelet counts in female dogs had already been reported for a population of beagles with the same age range.43 In the current study, the intraindividual variability was slightly lower than or close to the interindividual variability for most of the hematologic variables. The intraindividual variability that we obtained for RBC count, Hct, and Hgb was similar to that determined over 10 mo in the previously mentioned study, which involved 23 beagles (age, 1 to 7 y), where coefficients of variation of intraindividual variability were 5.4%, 6.4%, and 5.9% respectively.22 Paradoxically, however, intraindividual variability for WBC count was higher in the current study, even though the population of beagles in the present study was larger and more homogeneous than that previously.22
“A […] test will contribute to individuality only if the combination of personal and analytic variance components is substantially less than inter-individual variability.”18 This concept is expressed by the index of individuality as defined earlier, for which arbitrary limits of less than or equal to 0.6 and greater than or equal to 1.4 were proposed to be used preferably with subject-based and reference-based RI, respectively.40 In the present study, most indexes of individuality were close to or higher than 1.4, thus confirming the relevance of population-based RI for these variables.40 Exceptions were sometimes observed for lymphocyte and monocyte counts and, to a lesser extent, eosinophil count and MCH, suggesting that subject-based RI would be more sensitive for detecting changes in subjects.24,40 Moreover, interindividual variability was high for eosinophil and monocyte counts due to their low concentrations. Surprisingly, we obtained high interindividual variability for lymphocyte counts in the current study, which could not be compared with previous studies. Nevertheless, in human clinical pathology, a coefficient of interindividual variability of 35.3% is reported for lymphocyte count.41
Arbitrary limits were accepted for the index of individuality. However, “the algebra indicates that the normal range will tend to be slightly less sensitive than expected to departures from the individual's own normal mean.”17 This is one reason why individual follow-ups are used as often as possible in both experimental and clinical settings. In such cases, the main criterion of interest is the “reference change, defined as that difference between two consecutive test results in an individual that is statistically significant in a given proportion of all similar persons”19—that is, the value accounting for analytical and intraindividual variability. In practical terms, the RCV “may help to judge whether the difference between two serial test results may be safely ascribed to natural variation or not.”22 However, in the present study, most of the variables were affected significantly, even if only mildly, by age. Therefore, it was necessary to calculate RCV without the possible influence of age, which is an intraindividual factor of variation for which the average effect can be evaluated. However, the RCV thus calculated were almost identical to those previously reported in laboratory beagles for RBC count, Hct, and Hgb but slightly higher for WBC count.22 Accordingly, for example, an increase or decrease of more than 17% in 2 consecutive RBC results can be ascribed to treatment or disease. For RBC indexes, the RCV were less than 10% of the average values. In contrast, the RCV for WBC subpopulations, especially for neutrophil and eosinophil counts, tended to be greater than 10% of the means, likely because of the low cellular counts, which led to high intraindividual variability. These RCV estimated from healthy animals were, in general, also relevant to diseased animals, as was shown in humans.11
Laboratory beagles are, as expected, a homogenous group of animals exhibiting low intra- and interindividual variability for most hematologic analytes. Therefore, population-based RI are relevant for monitoring the possible effects of a test compound or procedure on the dogs’ health status. The coefficients of variation of intraindividual variability and RCV that we established likely will facilitate more efficient monitoring when repeated samplings in the same dog are possible.
References
- 1.Andersen AC, Gee W. 1958. Normal blood values in the beagle. Vet Med 53:135–156. [Google Scholar]
- 2.Andersen AC, Schalm OW. 1970. Hematology, p 261–284 In: Andersen AC, editor. The beagle as an experimental dog. Ames (IA): Iowa State University Press [Google Scholar]
- 3.Berger J. 1981. Hematology reference values for dogs of beagle stock. Z Versuchstierkd 23:278–283. [PubMed] [Google Scholar]
- 4.Bourges-Abella N, Geffre A, Concordet D, Braun JP, Trumel C. 2011. Canine reference intervals for the Sysmex XT-2000iV hematology analyzer. Vet Clin Pathol 40:303–315. [DOI] [PubMed] [Google Scholar]
- 5.Bulgin MS, Munn SL, Gee W. 1970. Hematologic changes to 4 and 1/2 years of age in clinically normal beagles. J Am Vet Med Assoc 157:1064–1070. [PubMed] [Google Scholar]
- 6.Campora C, Freeman KP, Lewis FI, Gibson G, Sacchini F, Sanchez-Vazquez MJ. 2011. Determination of haematological reference intervals in healthy adult greyhounds. J Small Anim Pract 52:301–309. [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2006. User verification of performance for precision and trueness: approved guideline. Clinical and Laboratory Standards Institute (CLSI) document EP15-A2. Wayne (PA): Clinical and Laboratory Standards Institute. [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2008. Defining, establishing, and verifying reference intervals in the clinical laboratory: approved guideline, 3rd ed. Clinical and Laboratory Standards Institute (CLSI) document C28–A3. Wayne (PA): Clinical and Laboratory Standards Institute. [Google Scholar]
- 9.Dougherty JH, Rosenblatt LS. 1965. Changes in the hemogram of the beagle with age. J Gerontol 20:131–138. [DOI] [PubMed] [Google Scholar]
- 10.Fraser CG. 2004. Inherent biological variation and reference values. Clin Chem Lab Med 42:758–764. [DOI] [PubMed] [Google Scholar]
- 11.Fraser CG. 2012. Reference change values. Clin Chem Lab Med 50:807–812. [DOI] [PubMed] [Google Scholar]
- 12.Friedrichs KR, Harr KE, Freeman KP, Szladovits B, Walton RM, Barnhart KF, Blanco-Chavez J. 2012. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 41:441–453. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda S, Kawashima N, Iida H, Aoki J, Tokita K. 1989. Age dependency of hematological values and concentrations of serum biochemical constituents in normal beagles from 1 to 14 years of age. Nihon Juigaku Zasshi 51:636–641. [DOI] [PubMed] [Google Scholar]
- 14.Geffre A, Concordet D, Braun JP, Trumel C. 2011. Reference Value Advisor: a new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. Vet Clin Pathol 40:107–112. [DOI] [PubMed] [Google Scholar]
- 15.Geffre A, Harr K, Concordet D, Trumel C, Braun JP. 2009. Reference values: a review. Vet Clin Pathol 38:288–298. [DOI] [PubMed] [Google Scholar]
- 16.Harper EJ, Hackett RM, Wilkinson J, Heaton PR. 2003. Age-related variations in hematologic and plasma biochemical test results in beagles and Labrador retrievers. J Am Vet Med Assoc 223:1436–1442. [DOI] [PubMed] [Google Scholar]
- 17.Harris EK. 1974. Effects of intra- and interindividual variation on the appropriate use of normal ranges. Clin Chem 20:1535–1542. [PubMed] [Google Scholar]
- 18.Harris EK, Kanofsky P, Shakarji G, Cotlove E. 1970. Biological and analytic components of variation in long-term studies of serum constituents in normal subjects. II. Estimating biological components of variation. Clin Chem 16:1022–1027. [PubMed] [Google Scholar]
- 19.Harris EK, Yasaka T. 1983. On the calculation of a ‘reference change’ for comparing 2 consecutive measurements. Clin Chem 29:25–30. [PubMed] [Google Scholar]
- 20.Harvey JW. 2012. Veterinary hematology: a diagnosis guide and color atlas. New York (NY): Elsevier Saunders. [Google Scholar]
- 21.Iversen L, Jensen AL, Hoier R, Aaes H. 1999. Biological variation of canine serum thyrotropin (TSH) concentration. Vet Clin Pathol 28: 16–19. [DOI] [PubMed] [Google Scholar]
- 22.Jensen AL, Iversen L, Petersen TK. 1998. Study on biological variability of haematological components in dogs. Comp Haematol Int 8:202–204. [Google Scholar]
- 23.Jensen AL, Aaes H, Iversen L, Petersen TK. 1999. The long-term biological variability of fasting plasma glucose and serum fructosamine in healthy beagle dogs. Vet Res Commun 23:73–80. [DOI] [PubMed] [Google Scholar]
- 24.Kjelgaard-Hansen M, Jensen AL. 2010. Reference intervals. In: Weiss DJ, Wardrop KJ, editors. Schalm's veterinary hematology, 6th ed. Ames (IA): Wiley–Blackwell. [Google Scholar]
- 25.Lilliehook I, Tvedten HW. 2011. Errors in basophil enumeration with 3 veterinary hematology systems and observations on occurrence of basophils in dogs. Vet Clin Pathol 40:450–458. [DOI] [PubMed] [Google Scholar]
- 26.Michaelson SM, Scheer K, Gilt S. 1966. The blood of the normal beagle. J Am Vet Med Assoc 148:532–534. [PubMed] [Google Scholar]
- 27.Ministère de l'Enseignement Supérieur et de la Recherche. [Internet]. Utilisation des animaux à des fins scientifiques. [Cited May 2014]. Available at: www.enseignementsup-recherche.gouv.fr/cid70613/enquete-statistique-sur-l-utilisation-des-animaux-a-des-fins-scientifiques.html.
- 28.Moritz A, Fickenscher Y, Meyer K, Failing K, Weiss DJ. 2004. Canine and feline hematology reference values for the ADVIA 120 hematology system. Vet Clin Pathol 33: 32–38. [DOI] [PubMed] [Google Scholar]
- 29.Ricos C, Iglesias N, Garcia-Lario JV, Simon M, Cava F, Hernandez A, Perich C, Minchinela J, Alvarez V, Domenech MV, Jimenez CV, Biosca C, Tena R. 2007. Within-subject biological variation in disease: collated data and clinical consequences. Ann Clin Biochem 44:343–352. [DOI] [PubMed] [Google Scholar]
- 30.Rizzi TE, Meinkoth JH, Clinkenbeard KD. 2010. Normal hematology of the dog, p 799–810 In: Weiss DJ, Wardrop KJ, editors. Schalm's veterinary hematology, 6th ed. Ames (IA): Wiley-Blackwell. [Google Scholar]
- 31.Schalm OW. 1961. Normal values in blood morphology, p 132–204 In: Schalm OW, editor. Veterinary Hematology. Philadelphia (PA): Lea and Febiger. [Google Scholar]
- 32.Schalm OW. 1965. Normal Values in blood morphology, p 190–292 In: Schalm OW, editor. Veterinary Hematology. Second edition Philadelphia (PA): Lea and Febiger. [Google Scholar]
- 33.Schalm OW, Jain NC, Carroll EJ. 1975. Normal values in blood morphology with comments on species characteristics in response to disease, p 82–218 In: Schalm OW, Jain NC, Carroll EJ, editors. Veterinary hematology. Third edition Philadelphia (PA): Lea and Febiger. [Google Scholar]
- 34.Serra M, Freeman kp, Campora C, Sacchini F. 2012. Establishment of canine hematology reference intervals for the Sysmex XT-2000iV hematology analyzer using a blood-donor database. Vet Clin Pathol 41:207–215. [DOI] [PubMed] [Google Scholar]
- 35.Shifrine M, Munn SL, Rosenblatt LS, Bulgin MS, Wilson FD. 1973. Hematologic changes to 60 days of age in clinically normal beagles. Lab Anim Sci 23:894–898. [PubMed] [Google Scholar]
- 36.Strasser A, Niedermuller H, Hofecker G, Laber G. 1993. The effect of aging on laboratory values in dogs. Zentralbl Veterinarmed A 40:720–730. [DOI] [PubMed] [Google Scholar]
- 37.The European Parliament and the Council of the European Union. 2010. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off J Eur Communities L276:33–79. [Google Scholar]
- 38.Tvedten HW, Lilliehook IE. 2011. Canine differential leukocyte counting with the CellaVision DM96Vision, Sysmex XT-2000iV, and Advia 2120 hematology analyzers and a manual method. Vet Clin Pathol 40:324–339. [DOI] [PubMed] [Google Scholar]
- 39.Virtanen A, Kairisto V, Irjala K, Rajamaki A, Uusipaikka E. 1998. Regression-based reference limits and their reliability: example on hemoglobin during the first year of life. Clin Chem 44:327–335. [PubMed] [Google Scholar]
- 40.Walton RM. 2012. Subject-based reference values: biological variation, individuality, and reference change values. Vet Clin Pathol 41:175–181. [DOI] [PubMed] [Google Scholar]
- 41.Westgard J, Westgard QC. [Internet]. Desirable biological variation database specifications. [Cite May 2014]. Available at: www.westgard.com/biodatabase1.htm.
- 42.Wiinberg B, Jensen AL, Kjelgaard-Hansen M, Rojkjaer R, Johansson PI, Gade LP, Gram DX, Kristensen AT. 2007. Study on biological variation of haemostatic parameters in clinically healthy dogs. Vet J 174:62–68. [DOI] [PubMed] [Google Scholar]
- 43.Wolford ST, Schroer RA, Gohs FX, Gallo PP, Brodeck M, Falk HB, Ruhren R. 1986. Reference range data base for serum chemistry and hematology values in laboratory animals. J Toxicol Environ Health 18:161–188. [DOI] [PubMed] [Google Scholar]