Abstract
The hypothalamic–pituitary–adrenal (HPA) axis is a key factor in the trajectory of the addiction-like cycle (a pattern of behavior characterized by escalating drug use, withdrawal, and relapse) in preclinical and clinical studies. Concentrations of HPA hormones change in laboratory animals in response to standard experimental procedures, including handling and vehicle injections. We compared HPA activity in adult male Lewis (inbred) and Sprague–Dawley (outbred) rats, 2 common strains in rodent models of addiction, after different schedules of handling and saline injections, to explore the extent to which HPA responses differ by strain and whether interindividual differences underlie addiction vulnerability. The 4 treatment conditions were no, short, or long handling and saline injections. In handled groups, rats were handled for 1 to 2 min for 3 times daily and were euthanized after 7 d (short handling) or 14 d (long handling). The injection schedule in the saline injection group mimicked that in a model of binge-like cocaine exposure. Across all treatment groups, concentrations of adrenocorticotropic hormone were higher in Sprague–Dawley than in Lewis rats. In Sprague–Dawley rats, corticosterone concentrations decreased after continued handling but remained constant in Lewis rats. Interindividual variability in hormone levels was greater in Sprague–Dawley than Lewis rats, although corticosterone variability decreased after continued handling. Prolactin did not differ between groups of either Sprague–Dawley and Lewis rats before or after handling. This study underscores the importance of prolonged handling before experimenter-provided drug-administration paradigms and of strain-associated differences that may affect study outcomes.
Abbreviation: ACTH, adrenocorticotropic hormone; HPA, hypothalamic–pituitary–adrenal
Studies in medical and life science often require repeated handling or injection of rodent subjects over the course of the experiment. Of primary concern is the ability to minimize stress associated with these procedures, which are necessary and unavoidable aspects of most behavioral, pharmacologic, and endocrine studies. A growing body of research suggests that experimental conditions, such as the presence and type of handling3,7,30 and providing saline injections,11,33 affect basal physiologic measures and, potentially, performance on behavioral assays.
Inconsistencies and high variability in behavioral data have been attributed, in part, to differences in the laboratory environment, including animal housing and husbandry. In addition, several environmental factors have been shown to influence experimental outcomes. These include cage changes,1,12 placement on cage racks,19 and environmental noise,20,27,36 both in studies using mice8,37 and rats.13,19 These environmental differences may act as physical stressors, which are known to alter basal neuroendocrine stress responses and contribute to psychologic stress.11 Although the specific process of handling or injection has been shown to affect endocrine stress responses in mice,11 less is known about how these responses manifest in commonly used rat strains.
Several groups have investigated the effects of various types of stress on rat hormone concentrations as well as the different hormonal response patterns of various rat strains. For example, corticosterone concentrations increased in response to amphetamine more dramatically in Sprague–Dawley rats than in Lewis rats.21 A similar pattern occurred after the rats experienced restraint stress.20 Another study found that the initial rise in corticosterone levels due to restraint stress is attenuated in both Sprague–Dawley and Lewis rats after prolonged exposure to the stressor.10 In addition, a study comparing Lewis and Fischer rats found that during an extended-access self-administration protocol, neither ACTH nor corticosterone was elevated at 24 h after the rats’ final cocaine self-administration session in Lewis rats but remained increased in Fischer rats.29
The present study expands on these data by examining how standard laboratory techniques affect neuroendocrine stress responses. The techniques in this study were designed to mimic standard animal handling and injection protocols frequently used in neurobiologic endocrine studies. It is important to quantify the effect of these practices to understand how laboratory techniques contribute to changes in stress hormone concentrations and thereby influence study outcomes.
Routine laboratory procedures such as handling and injections present stimuli that can be associated with mild to moderate physical or psychologic stress.16 The hypothalamic–pituitary–adrenal (HPA) axis regulates physiologic processes that enable adaptive responses to external stressors, including distress caused by handling or injections, and helps to maintain homeostasis. The intensity and duration of a stressful stimulus influences the magnitude of physiologic stress response produced by the subject,35 and repeated exposure to the same stressor results in a reduction in response over time, that is, desensitization or habituation.17,35 Minimizing disruptions to basal HPA activity is particularly important in pharmacologic and endocrine studies,31 because HPA responses prior to, during, and after exposure to drugs of abuse contribute importantly to the trajectory of drug dependence and addiction.23,25,34
The purpose of the current study was to compare changes in HPA response between 2 commonly used rat strains by using different handling and injection protocols, 2 typical laboratory procedures that can elicit stress. We compared these responses in outbred Sprague–Dawley rats with those from the inbred Lewis strain. Lewis rats have an HPA axis that is relatively hyporesponsive to stress, and this strain responds strongly to multiple drugs in several behavioral tests.24,29
We also measured prolactin, which, although not part of the HPA axis, is under hypothalamic control. An increased prolactin level has been associated with acute stress.2 Therefore, handling may affect prolactin concentrations. In addition, measuring the prolactin concentration allows us to identify the extent to which handling influences nonHPA activity. Furthermore, prolactin affects the activity of tuberoinfundibular dopamine neurons26 and can be used in translational research as an indirect biomarker for dopamine concentration.6 Given the established role of dopaminergic systems in several neurobiologic diseases and disorders, examining the role of prolactin response to handling stress may offer novel insight into the dysregulated circuits underlying these diseases.
Materials and Methods
All rats were purchased from a single vendor to maintain consistency and avoid potential differences between vendors in rats’ basal and stress-induced HPA responsivity.28
Young adult male Lewis and Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA) were housed in a stress-minimized suite in the animal colony at The Rockefeller University, which is AAALAC-accredited. Each rat was 73 to 74 d old at the start of the study (Day 0) and was singly housed in a standard clear cage with nest material and ad libitum access to food and water. Lewis rats weighed 273.8 ± 10.8 g (mean ± 1 SD), and Sprague–Dawley rats were 311.8 ± 12.6 g. Eight rats of each strain were randomly assigned to 1 of 4 groups: no handling, short (7 d) handling, long (14 d) handling, and saline injections. Rats were housed in temperature- and humidity-controlled rooms and maintained on a 12:12-h reversed light:dark cycle (lights on, 0900). Cages were changed during the second handling bout (or injection) on days 2, 6, 9, and 12. Because exposure to a novel (for example, clean) cage can increase corticosterone concentrations,12 the last regularly scheduled cage change occurred more than 26 h before euthanasia in all groups. All animal care and experimental protocols were in compliance with the Guide for the Care and Use of Laboratory Animals18 and approved by the IACUC of The Rockefeller University.
Experimental interventions began on day 1, the day after rats arrived at the colony (day 0). Rats in the no-handling group remained in their homecages without being handled or receiving injections. Rats were weighed during regularly scheduled cage changes on day 2, 6, 9, and 12; rats were picked up and quickly lowered into a metal bowl for weighing so that handling was minimized. For short- and long-handling groups, each rat was handled gently 3 times daily at 1-h intervals, mimicking the schedule of saline injections (see following section). All handling and injections were done by the same investigator. During handling, rats were gently held in the experimenter's hand or on her arm next to her torso, without being restrained except for a light hold at the base of the tail. Each handling bout lasted 1 to 2 min, with the exception of days 1 and 2, when handling bouts lasted 3 to 4 min. During the first 2 d of the experiment, handling bouts were slightly longer in an effort to expose rats to the experimenter and help them to adapt to the start of the experiment. Rats were euthanized after either 7 d (short-handling group) or 14 d (short-handling group). Weights were recorded at the start of the first handling session each day. Rats in the saline-injections group each received an intraperitoneal injection of physiologic (0.9%) saline (0.2 mL) 3 times each day at 1-h intervals, on a schedule mimicking that of a rodent model of binge-like cocaine use.4,5,31,38 Injections took place at 0930, 1030, and 1130 and were administered daily for 14 d. Injections were given by gently placing each rat on the experimenter's torso, lifting its rear leg to expose its ventrum, and inserting the syringe. Rats were only handled during the process of administering their injections, with the exception of days 1 and 2, when rats were handled for 3 to 4 min prior to injection. Weights were recorded each day immediately before rats’ first injection.
At 30 min after their last handling bout or injection, rats were transferred in their homecage to the necropsy room (transport time, approximately 15 s), where the lid on their homecage was promptly replaced with a clean metal lid connected to a CO2 regulator and flowmeter. Rats were exposed to CO29 until anesthetized, recumbent, and breathing deeply (100 to 120 s) and were then decapitated by using a guillotine.
After decapitation, trunk blood was collected in EDTA-coated tubes and placed in ice before being centrifuged at 1839 × g at 4 °C for 15 min. Plasma was removed promptly and stored at −80 °C. Brains were removed, frozen in powdered dry ice, and stored at −80 °C for future mRNA analysis, according to standard laboratory protocols. Concentrations of circulating ACTH were measured by using an ACTH 125I radioimmunoassay kit (DiaSorin, Stillwater, MN). Corticosterone concentrations were measured by using a corticosterone 125I radioimmunoassay kit (MP Biomedicals, Solon OH). Prolactin concentrations were measured by using a prolactin ELISA (Abcam, Cambridge MA). All neuroendocrine values were measured in duplicate samples from each rat in a single assay. To minimize circadian variability, all euthanasias occurred approximately 3 h after the start of the light cycle, when basal corticosterone concentrations reach their circadian nadir.15
Analyses were performed using Statistica (version 5.5, StatSoft, Tulsa, OK). We performed Bartlett tests to determine equal variance before running ANOVA. All ANOVA were followed by a Newman–Keuls posthoc test between treatment groups or across days. A P value of 0.05 was used to define statistical significance.
Results
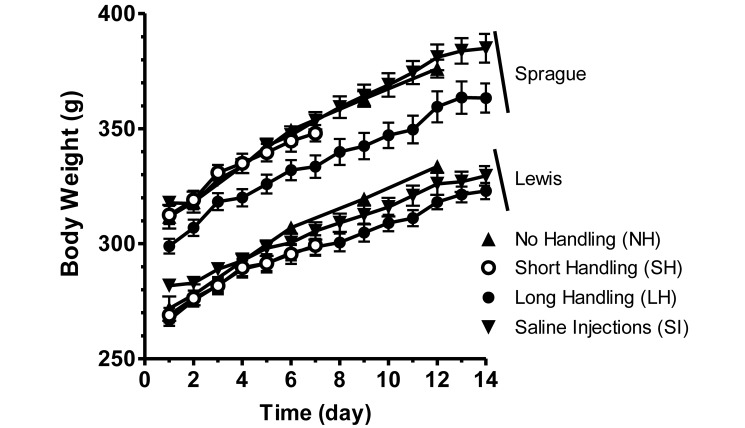
The average weight of each group of Lewis or Sprague–Dawley rats increased over the duration of the study (7 to 14 d; Figure 1). Weights of rats in the short- and long-handling groups were compared over the first 7 d by using 3-way ANOVA (group × strain × day). This analysis revealed a significant effect of strain (F1,28 = 124.5, P < 0.01) as well as a significant interaction between strain and day (F6,168 = 5.27, P < 0.01). Weights of rats in the long-handling and saline injection groups also increased over the entire 14-d period (main effect of day, F13,364 = 549.4, P < 0.01), and there was a significant interaction between strain and day (F13,364 = 5.05, P < 0.01). There were no significant interactions between handling group with either of the other 2 variables.
Figure 1.
Daily body weight. Rats in the NH group were weighed on days 2, 6, 9, and 12; all other groups were measured daily. All groups showed increases in weight. Sprague–Dawley rats were heavier at baseline and gained significantly (P < 0.01) more weight across days than did Lewis rats. Error bars represent 1 SD.
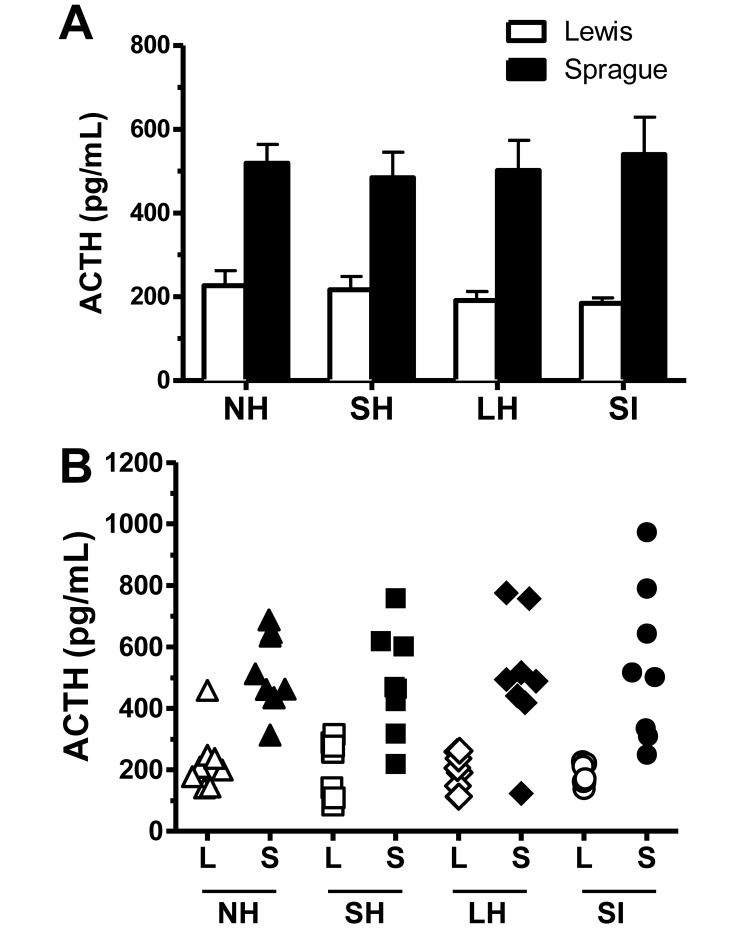
Hormone concentrations were analyzed by using 2-way ANOVA with group and strain as independent measures. Values that were beyond a 2-SD range were omitted, as were samples that fell outside of the 20% confidence range in 2 separate RIA assays. Analyses showed that, compared with Lewis rats, Sprague–Dawley rats had significantly higher ACTH concentrations across handling groups (main effect of strain, F1,54 = 71.29, P < 0.01; Figure 2 A). ACTH concentrations did not differ as a result of handling in either strain, suggesting that handling manipulations did not influence ACTH concentrations circulating at the time of euthanasia. As expected, interindividual variability of ACTH concentrations was more pronounced in Sprague–Dawley rats as compared with Lewis rats (Figure 2 B).
Figure 2.
(A) ACTH concentrations in blood collected 30 min after the last handling bout or injection. ACTH was significantly (P < 0.01) higher in Sprague–Dawley rats than in Lewis rats, but ACTH concentration did not differ between handling groups. Error bars represent 1 SD. (B) Scatter plot showing individual ACTH concentrations. Sprague–Dawley rats showed greater interindividual ACTH variability than did Lewis rats, whose concentrations remained consistent across handling groups. In addition, Lewis rats showed a decrease in interindividual variability after extended handling. L, Lewis; S, Sprague–Dawley. NH, no handling; SH, short handling; LH, long handling; SI, saline injection.
Corticosterone concentrations differed significantly between strains: Sprague–Dawley rats had significantly higher corticosterone than did Lewis rats (F1,53 = 10.08, P < 0.01). However, corticosterone concentrations in Sprague–Dawley rats decreased significantly according to handling practices, in that continued handling led to reduced corticosterone concentrations (F3, 25 = 4.58, P < 0.05; Figure 3 A). Corticosterone concentrations in Lewis rats did not differ as a result of handling.
Figure 3.
(A) Corticosterone concentrations in blood collected 30 min after the last handling bout or injection. Basal corticosterone concentrations were significantly (P < 0.01) higher in Sprague–Dawley rats and decreased with continued handling and saline injections. Concentrations for Lewis rats remained constant across groups. Error bars represent 1 SD. (B) Scatter plot showing individual corticosterone concentrations. Sprague–Dawley rats again showed greater interindividual variability as compared with Lewis rats, although this variability decreased with continued handling. There was no change in Lewis interindividual variability with continued handling. L, Lewis; S, Sprague. NH, no handling; SH, short handling; LH, long handling; SI, saline injection.
Variability between strains was calculated by using a Bartlett test for the equality of variance (strain × group). This test revealed an unequal variance between strains for both ACTH (P < 0.01) and corticosterone (P < 0.01). Interindividual variability was more pronounced in Sprague–Dawley rats as compared with Lewis rats (Figures 2 B and 3 B).
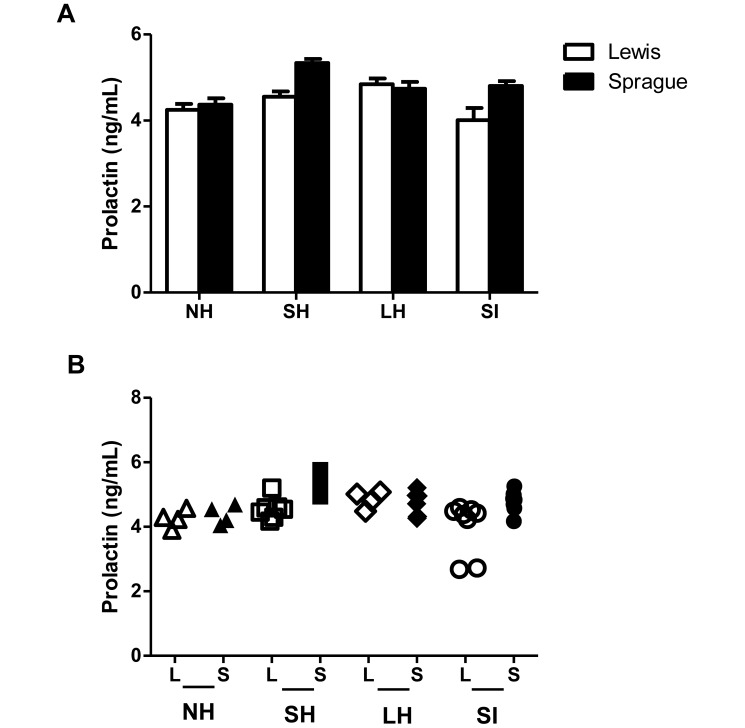
Prolactin concentrations did not show any significant difference between strains or across groups (Figure 4 A). In addition, unlike ACTH and corticosterone concentrations, prolactin levels showed similar interindividual variability within groups of Lewis and Sprague–Dawley rats. This variability did not change as a result of extended handling or providing saline injections in either strain (Figure 4 B).
Figure 4.
(A) Prolactin concentrations in blood collected 30 min after the last handling bout or injection. Concentrations were similar for both strains and did not change as a result of handling. Error bars represent 1 SD. (B) Scatter plot showing individual prolactin concentrations. There was no difference in variability between strains, and variability remained consistent for both Lewis and Sprague–Dawley rats across handling groups. L, Lewis; S, Sprague–Dawley. NH, no handling; SH, short handling; LH, long handling; SI, saline injection.
Discussion
The goal of the present work was to study the effects of 2 standard laboratory stressors, regular handling and intraperitoneal injections, on 2 commonly used strains of laboratory rats. Differences in stress response, mediated primarily by HPA activity, may be a key factor contributing to inconsistencies in data collected by different laboratories or different investigators, even when experimental variables and subjects are carefully described and controlled. In addition, HPA responsiveness contributes to rats’ response to pharmacologic interventions and trajectory of drug addiction,22,23 emphasizing the importance of characterizing different strains’ HPA activity in response to routine aspects of experimental protocols. The present data demonstrate clear HPA-associated differences between an inbred strain of rats (Lewis) and an outbred strain (Sprague–Dawley) in their responses to various handling and injection procedures.
In the present study, ACTH concentrations were higher in Sprague–Dawley than Lewis rats, regardless of handling group, and remained consistent across groups. Corticosterone concentrations were initially higher among Sprague–Dawley rats, as observed in the no-handling and short-handling groups. In contrast, corticosterone concentrations in Sprague–Dawley rats after continued handling dropped to levels equal those of the Lewis rats. The fact that this attenuation affected corticosterone only and not ACTH suggests an effect at the adrenal level. Given that prolactin concentrations were similar for both strains, the hormonal differences between Sprague–Dawley and Lewis rats appears to be HPA-axis–specific.
Interindividual differences in ACTH and corticosterone concentrations were more pronounced in Sprague–Dawley than Lewis rats, as expected in light of the wider genetic variability characterizing outbred strains.14 For Sprague–Dawley rats, the high degree of interindividual variability in ACTH and corticosterone again seem to be HPA-specific, because little variability in plasma prolactin emerged in this group. Our results are consistent with the broad genetic variation within outbred strains, such as Sprague–Dawley, and limited genetic variation within inbred strains, like Lewis.
The present study extends existing literature examining the effects of several stressors on various rat strains. One group of researchers identified a rise in corticosterone concentrations after rats were placed in restrain cages; however, this effect was attenuated when rats remained in the cages for extended periods of time.10 These previous results were consistent in both Sprague–Dawley and Lewis rats, in contrast to the present findings of corticosterone elevation followed by habituation exclusively in Sprague–Dawley rats. This difference may be due to the fact that the stress in our study is much milder than is a restrain cage and therefore does not cause any change in Lewis rats’ corticosterone concentrations.
Prior work that examined the effect of restraint stress on corticosterone concentration after amphetamine administration identified a larger increase in corticosterone concentration in Sprague–Dawley rats than Lewis.21 Our results are consistent with this finding. In addition, the previous study20 noted an almost immediate (15 min after being placed in a restrain cage) rise in prolactin in both strains. At the 30-min time point, Sprague–Dawley rats showed a decreased prolactin concentration whereas Lewis rats did not.21 The discrepancy between these data and the findings from the present study may be explained by the type and severity of stressors used in the 2 studies. Furthermore, samples previously were drawn from the rats only 15 and 30 min after the induction of stress,21 whereas in the present study, rats were handled for a minimum of 7 d. This difference suggests that stress may cause a temporary spike in prolactin concentration that dissipates over time.
In conclusion, neuroendocrine markers of stress differ between Sprague–Dawley and Lewis rats after a series of standard and universally used laboratory techniques. The present data highlight the specific effects of investigator handling and the importance of sufficient handling and sham injections to habituate rats to handlers and to decrease neuroendocrine stress responses prior to initiating an experiment. These practices may minimize preexisting interindividual variability in the experimental data, particularly in outbred strains, and facilitate the interpretation of the primary behavioral and molecular results.
Acknowledgments
This work was supported by grants from NIH-NIDA P50DA05130 (MJK), NIH-NIDA F32DA030831 (KSC), and The Adelson Medical Research Foundation (MJK). We thank Brian Reed for his help with the statistical analyses and Michele Buonora and Adam Brownstein for their help in performing neuroendocrine assays.
References
- 1.Armario A, Lopez-Calderon A, Jolin T, Castellanos JM. 1986. Sensitivity of anterior pituitary hormones to graded concentrations of psychological stress. Life Sci 39:471–475. [DOI] [PubMed] [Google Scholar]
- 2.Armario A, Marti O, Molina T, de Pablo J, Valdes M. 1996. Acute stress markers in humans: response of plasma glucose, cortisol, and prolactin to 2 examinations differing in the anxiety they provoke. Psychoneuroendocrinology 21:17–24. [DOI] [PubMed] [Google Scholar]
- 3.Asanuma M, Ogawa N, Hirata H, Chou H, Tanaka K, Mori A. 1992. Opposite effects of rough and gentle handling with repeated saline administration on c-fos mRNA expression in the rat brain. J Neural Transm Gen Sect 90:163–169. [DOI] [PubMed] [Google Scholar]
- 4.Bailey A, Gianotti R, Ho A, Kreek MJ. 2007. Downregulation of κ opioid receptors in basolateral amygdala and septum of rats withdrawn for 14 days from an escalating dose “binge” cocaine administration paradigm. Synapse 61:820–826. [DOI] [PubMed] [Google Scholar]
- 5.Bailey A, Yuferov V, Bendor J, Schlussman SD, Zhou Y, Ho A, Kreek MJ. 2005. Immediate withdrawal from chronic “binge” cocaine administration increases µ opioid receptor mRNA concentrations in rat frontal cortex. Brain Res Mol Brain Res 137:258–262. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Jonathan N. 1985. Dopamine: a prolactin-inhibiting horemone. Endocr Rev 6:564–589. [DOI] [PubMed] [Google Scholar]
- 7.Costa R, Tamascia ML, Nogueira MD, Casarini DE, Marcondes FK. 2012. Handling of adolescent rats improves learning and memory and decreases anxiety. J Am Assoc Lab Anim Sci 51:548–553. [PMC free article] [PubMed] [Google Scholar]
- 8.Crabbe JC, Wahlsten D, Dudek BC. 1999. Genetics of mouse behavior: interactions with laboratory environment. Science 284:1670–1672. [DOI] [PubMed] [Google Scholar]
- 9.Danneman PJ, Stein S, Walshaw SO. 1997. Humane and practical implications of using carbon dioxide mixed with oxygen for anesthesia or euthanasia of rats. Lab Anim Sci 47:376–385. [PubMed] [Google Scholar]
- 10.Dhabhar FS, McEwen B, Spencer R. 1997. Adaptation to prolonged or repeated stress—comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology 65:360–368. [DOI] [PubMed] [Google Scholar]
- 11.Drude S, Geissler A, Olfe J, Starke A, Domanska G, Schuett C, Kiank-Nussbaum C. 2011. Side effects of control treatment can conceal experimental data when studying stress responses to injection and psychological stress in mice. Lab Anim (NY) 40:119–128. [DOI] [PubMed] [Google Scholar]
- 12.Fluttert M, Dalm S, Oitzl MS. 2000. A refined method for sequential blood sampling by tail incision in rats. Lab Anim 34:372–378. [DOI] [PubMed] [Google Scholar]
- 13.Gadek-Michalska A, Bugajski J. 2003. Repeated handling, restraint, or chronic crowding impair the hypothalamic–pituitary–adrenocortical response to acute restraint stress. J Physiol Pharmacol 54:449 –459. [PubMed] [Google Scholar]
- 14.George FR, Goldberg SR. 1989. Genetic approaches to the analysis of addiction processes. Trends Pharmacol Sci 10:78–83. [DOI] [PubMed] [Google Scholar]
- 15.Griffin AC, Whitacre CC. 1991. Sex and strain differences in the circadian rhythm fluctuation of endocrine and immune function in the rat: implications for rodent models of autoimmune disease. J Neuroimmunol 35:53–64. [DOI] [PubMed] [Google Scholar]
- 16.Grandin T. 1997. Assessment of stress during handling and transport. J Anim Sci 75:249–257. [DOI] [PubMed] [Google Scholar]
- 17.Grissom N, Bhatnagar S. 2009. Habituation to repeated stress: get used to it. Neurobiol Learn Mem 92:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 19.Izidio GS, Lopes DM, Spricigo L, Jr, Ramos A. 2005. Common variations in the pretest environment influence genotypic comparisons in models of anxiety. Genes Brain Behav 4:412–419. [DOI] [PubMed] [Google Scholar]
- 20.Jauregui-Huerta F, Garcia-Estrada J, Ruvalcaba-Delgadillo Y, Trujillo X, Huerta M, Feria-Velasco A, Gonzalez-Perez O, Luquin S. 2011. Chronic exposure of juvenile rats to environmental noise impairs hippocampal cell proliferation in adulthood. Noise Health 13:286–291. [DOI] [PubMed] [Google Scholar]
- 21.Klenerova V, Sida P, Hynie S, Jurcovivoca J. 2001. Rat strain differences in responses of plasma prolactin and PRL mRNA expression after acute amphetamine treatment or restrain stress. Cell Mol Neurobiol 21:91–100. [DOI] [PubMed] [Google Scholar]
- 22.Koob GF. 2008. A role for brain stress systems in addiction. Neuron 59:11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koob GF, Kreek MJ. 2007. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry 164:1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosten TA, Ambrosio E. 2002. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology 27:35–69. [DOI] [PubMed] [Google Scholar]
- 25.Kreek MJ, Koob GF. 1998. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend 51:23–47. [DOI] [PubMed] [Google Scholar]
- 26.Lyons DJ, Hellysaz A, Broberger C. 2012. Prolactin regulates tuberoinfundibular dopamine neuron discharge pattern: novel feedback control mechanisms in the lactotrophic axis. J Neurosci 32:8074–8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naqvi F, Haider S, Batool Z, Perveen T, Haleem DJ. 2012. Subchronic exposure to noise affects locomotor activity and produces anxiogenic and depressive-like behavior in rats. Pharmacol Rep 64:64 –69. [DOI] [PubMed] [Google Scholar]
- 28.Pecoraro N, Ginsberg AB, Warne JP, Gomez F, la Fleur SE, Dallman MF. 2006. Diverse basal and stress-related phenotypes of Sprague Dawley rats from 3 vendors. Physiol Behav 89:598–610. [DOI] [PubMed] [Google Scholar]
- 29.Picetti R, Ho A, Butelman ER, Kreek MJ. 2010. Dose preference and dose escalation in extended-access cocaine self-administration in Fischer and Lewis rats. Psychopharmacology (Berl) 211:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pritchard LM, Van Kempen TA, Zimmerberg B. 2013. Behavioral effects of repeated handling differ in rats reared in social isolation and environmental enrichment. Neurosci Lett 536:47–51. [DOI] [PubMed] [Google Scholar]
- 31.Reed B, Varon J, Chait BT, Kreek MJ. 2009. Carbon dioxide-induced anesthesia results in a rapid increase in plasma concentrations of vasopressin. Endocrinology 150:2934–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlussman SD, Ho A, Zhou Y, Curtis AE, Kreek MJ. 1998. Effects of “binge” pattern cocaine on stereotypy and locomotor activity in C57BL/6J and 129/J mice. Pharmacol Biochem Behav 60:593–599. [DOI] [PubMed] [Google Scholar]
- 33.Sharp FR, Sagar SM, Hicks K, Lowenstein D, Hisanaga K. 1991. c-fos mRNA, Fos, and Fos-related antigen induction by hypertonic saline and stress. J Neurosci 11:2321–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinha R. 2008. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 1141:105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulrich-Lai YM, Herman JP. 2009. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uran SL, Aon-Bertolino ML, Caceres LG, Capani F, Guelman LR. 2012. Rat hippocampal alterations could underlie behavioral abnormalities induced by exposure to moderate noise concentrations. Brain Res 1471:1–12. [DOI] [PubMed] [Google Scholar]
- 37.Wahlsten D, Metten P, Phillips TJ, Boehm SL, 2nd, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC. 2003. Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol 54:283–311. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ. 1996. Corticotropin-releasing factor and type 1 corticotropin-releasing factor receptor messenger RNAs in rat brain and pituitary during “binge”-pattern cocaine administration and chronic withdrawal. J Pharmacol Exp Ther 279:351–358. [PubMed] [Google Scholar]