Abstract
Rice rats (Oryzomys palustris) are a recognized animal model for studying periodontal disease and the photoperiodic regulation of reproduction. Here we share information regarding the breeding, husbandry, veterinary care, and hematologic findings about this animal species to facilitate its use in studies at other research institutions. Rice rats initially were quarantined and monitored for excluded pathogens by using microbiologic, parasitologic, and serologic methods with adult female Mus musculus and Rattus norvegicus sentinel animals. Breeders were paired in a monogamous, continuous-breeding system. Rats were housed in static filter-top cages, maintained on commercial chow under 14:10-h light:dark cycles at 68 to 79 °F (20.0 to 26.1 °C) and 30% to 70% humidity. Rice rats apparently adapt relatively well to standard laboratory conditions, despite their aggressive behavior toward conspecifics and humans. Our analysis of 97 litters revealed that dams gave birth to an average of 5.2 pups per dam and weaned 4.2 pups per dam. Several procedures and biologic reagents normally used in standard laboratory rodents (mice and rats) can be used with rice rats. In addition, we present hematologic and serum chemistry values that can be used as preliminary reference values for future studies involving rice rats.
Abbreviation: H-SC, high-sucrose and -casein
Rice rats (genus Oryzomys) were discovered by Bachman and described by Harlan in 1837.5 These animals were first described scientifically in 1858 as a pest of rice plantations in the southeastern United States during the Colonial period.48 Some disagreements exist regarding the taxonomic classification of this genus. According to Weksler and Percequillo,60 the genus Oryzomys, which includes several species, belongs to the order Rodentia, family Cricetidae, subfamily Sigmodontinae, and tribe Oryzomyini. The tribe can be diagnosed by 7 putative synapomorphies:59,61 1) the presence of a long palate with prominent posterolateral pits; 2) the absence of an alisphenoid strut; 3) the absence of a posterior suspensory process of the squamosal attached to tegment tympani; 4) the absence of a gall bladder; 5) 12 thoracic vertebrae; 6) the absence of hemal arches on the first caudal vertebra; and 7) fewer than 36 caudal vertebrae.
The marsh rice rat (Oryzomys palustris) is one species of this genus.60 These rats inhabit swamps, salt marshes, and forest clearings of North America to subtropical regions of South America.17,48 Marsh rice rats are medium-sized rodents that weigh about 40 to 80 g, with a total length of 226 to 305 mm.20,28,30,62 The dorsal coloration varies from grayish brown to brownish black, and the ventral areas, tail, and feet are gray-white to tan in color.20,62 Rice rats are nocturnal and good swimmers that are well adapted to life in water and that have water-repellent pelage.16,30,62 Breeding can occur throughout the year in rice rats.20,62 The gestation period is reported as 25 d in O. palustris texensis56 and 21 to 28 d in O. palustris natator.46,54 Each year, female rice rats produce several litters of 1 to 6 pups, with 4 to 6 pups per litter typical.9,30 Rice rats are preferentially carnivorous, eating insects and small crabs.52 However, they can be seasonally or opportunistically omnivorous, eating seeds, succulent plant parts, eggs of small birds, and baby turtles.20,30,31,38,44,52,56 Valuable data have been published about the biology, husbandry, and reproductive features of several different rodent species used in research.18,37,40,57,58 However, limited information is available about breeding, rearing, and husbandry of rice rats under laboratory conditions.
Periodontitis is a common disorder among dentate adults worldwide. According to The National Health and Nutrition Examination Survey (NHANES) III (data collected from 1999 to 2004), the prevalence of periodontitis in 20- to 64-y-olds is 8.5%.10 The marsh rice rat has been shown to be extraordinarily susceptible to the initiation and progression of a spontaneous form of periodontitis that requires no intraoral mechanical manipulation.2,24-26,51 These features have made this species an excellent model to study the pathophysiology of periodontal disease and to test treatments for its prevention, control, and resolution. Periodontal lesions in rice rats develop more rapidly when rats are fed a diet high in sucrose and casein (H-SC).6,21,24,25,32,51 Unlike the human disease, which takes years to develop, periodontal lesions in rice rats progress to a chronic destructive state within a short period of time (10 to 18 wk).21,24,41
In addition, the rice rat has also been used to investigate the effect of photoperiod on reproduction and reproductive development.11-15 These studies highlighted the importance of the pineal gland and melatonin on reproductive function. Briefly, rice rats in the laboratory are highly reproductively photoperiodic (they develop or maintain reproductive function under long photoperiods and fail to develop or have inhibited reproductive function under short photoperiods).11-15 In addition, the production and release of melatonin by the pineal gland are photoperiodically regulated, and melatonin plays an important role in reproductive function.11-15
More recently, we have also developed the rice rat model for anti-resorptive-related osteonecrosis of the jaw (ARONJ), a rare human condition characterized by exposed, necrotic bone in the maxillofacial region, now recognized in patients who have taken powerful antiresorptive drugs to prevent bone metastases in cancer patients, and skeletal fractures in postmenopausal osteoporosis patients.39,50 The purpose of the current report is to share information regarding the laboratory breeding and rearing of O. palustris. Herein, we describe various aspects of the biology, physiology, animal care, and husbandry conditions for O. palustris. We believe that this report will guide research institutions and investigators in the management of this valuable species as an animal model in compliance with appropriate animal care procedures and USDA regulations (http://www.aphis.usda.gov/animal_welfare/awa_info.shtml).
Materials and Methods
The Animal Care Services resource at the University of Florida is an AAALAC-accredited animal care and use program. All animal care and experimental procedures were approved by the IACUC before initiation and were completed in accordance with federal policies and guidelines.
Origin.
We originally received 17 pairs of marsh rice rats (Oryzomys palustris) from Dr Kent Edmonds (Department of Biology, Indiana University Southeast, New Albany, IN), whose colony was established in 1997 from animals trapped in the Canary Creek salt marsh (Lewes, DE). Immediately on their arrival at our facility, the colony-derived rodents were maintained in a BSL2 biocontainment facility for 1 y before being transferred to a conventional rodent housing room. During this period, rice rats as well as sentinel mice and rats (Rattus norvegicus) were monitored for the presence of excluded pathogens and clinical signs compatible with infectious diseases.
Monitoring for excluded pathogens.
During quarantine, sentinels (CD1 mice and CD rats), as well as randomly chosen rice rats, were euthanized and necropsied every 4 wk to monitor for the presence of clinical disease, pathogens, or antibodies to infectious agents. Sentinels were exposed to soiled bedding from rice rat cages for a minimum of 6 wk before being submitted for necropsy, serology, and parasitology. Sentinels were monitored for coronavirus, hantavirus, lymphocytic choriomeningitis virus, pneumonia virus of mice, rat parvoviruses (types 1 and 2, rat minute virus, Kilham rat virus, H1), reovirus type 3, Sendai virus, Theiler murine encephalomyelitis virus, ectromelia virus, epizootic diarrhea of infant mice virus, rotavirus, K virus, polyoma virus, minute virus of mice, mouse adenovirus (types 1 and 2), mouse cytomegalovirus, mouse hepatitis virus, and mouse parvoviruses. At least once every quarter, we surveyed for Mycoplasma pulmonis by serology and for fur mites and pinworms via tape tests and fecal flotation, respectively. The following bacteria and fungi were monitored at least annually: cilia-associated respiratory bacillus, Citrobacter rodentium, Clostridium piliforme, Corynebacterium kutcheri, Pasteurella pneumotropica, Salmonella spp., Streptobacillus moniliformis, Encephalitozoon cuniculi, and Helicobacter spp. In addition, we monitored for fur mites and pinworms annually. Superficial skin scrapes, perianal tape tests, and zinc sulfate fecal flotation preparations were examined microscopically for the presence of ecto- and endoparasites in breeders and experimental animals. In addition, direct pelt and cecal content examinations were performed in euthanized rice rats and sentinels.
Husbandry, breeding, rearing, and care.
Rice rats were housed in static filter-top cages (area, 143 in2) with pine shavings as bedding (Figure 1 A and B). Breeders were singly-pair housed (Figure 1 B and C), whereas experimental rice rats were allocated into compatible groups (2 to 5 rice rats per cage) of the same sex that were formed immediately after weaning. Small cardboard buckets (‘to-go cups’) were placed in breeder and experimental rat cages as shelters or nesting sites for litters (Figure 1 C). To avoid bites or other injuries from the rats, we used sanitized garden gloves and the scruff method when handling the animals (Figure 1 D). Breeders were fed a standard rat chow diet (8604 Teklad Rodent Diet, Harlan, Tampa, FL) and received water (in bottles) ad libitum. Experimental groups were fed a pelleted H-SC diet (TestDiet no. 5SXA AIN-93G, Purina Feeds, Richmond, IN) to accelerate the clinical occurrence of periodontitis or the standard rat chow.6,21,24,25,32,51 Eventually, water bottles were replaced by an automatic watering system without incident. The housing room was maintained at 68 to 79 °F (20.0 to 26.1 °C), with an average humidity between 30% to 70%, and under a 14:10-h light:dark cycle (which maintains reproductive function) for breeders or a 12:12-h light:dark cycle (which inhibits reproductive function) for experimental animals.13 Cages were changed once weekly. Experimental animals were weighed weekly by using a calibrated portable electronic scale (model SP-2001, Ohaus, Parsippany, NJ).
Figure 1.
Daily aspects of the housing and husbandry of rice rats. (A) Animal rack containing pair of breeders in static filter-top cages. (B) Male rice rat approaching a female rat minutes after they were paired. (C) A breeder cage containing a male rat in the entrance of a “to-go cup,” which is used as a rearing shelter; the female rat and pups are hidden inside the shelter. (D) The scruff method is used to restrain a rice rat when it needs to be checked or injected.
Hematology and serum chemistry.
Adult (age, 4 to 11 mo) male (n = 20) and female (n = 19) rice rats fed the standard rat chow were used for the analyses. Terminal sampling procedures were performed between 0900 and 1200. Each rat was placed in a polycarbonate container, which then was closed with a metal lid that is connected through a hose to a commercially supplied CO2 tank. Each rat was exposed gradually to CO2 at a flow rate of approximately 20%/min until death was verified by cessation of movement, absence of hind paw withdrawal reflex, and complete cessation of respiration.4 Rice rats were weighed and placed in lateral recumbency, and blood samples were collected by cardiocentesis by using a 25-gauge needle attached to a 1-mL syringe (Becton Dickenson, Franklin Lakes, NJ). The needle was inserted caudal to the xiphoid cartilage and directed cranially toward the heart. Once the heart was entered, percutaneous exsanguination was used to collect approximately 1 mL blood from each rat. After blood was drawn, thoracotomy was performed as a confirmatory method of euthanasia.4,36 Laboratory testing was performed as follows: immediately after collection, the blood for CBC analysis was placed in a 500-μL tube containing K-EDTA (Microtainer, Becton Dickinson). Samples were run inhouse on an automated hematology analyzer (Hemavet HV950 FS, Drew Scientific, Oxford, CT) according to the manufacturer's instructions. Blood smears were stained manually with modified Wright-Giemsa stain (Protocol Hema-3TM Stain Set, Fisher Scientific, Kalamazoo, MI), and differential analyses were completed.
The remainder of the blood sample (approximately 500 μL) was placed in a 1.5-mL collection vial without anticoagulant, allowed to clot at room temperature (20 to 25 °C) for 30 to 60 min, and then spun at a relative centrifugal force of 1800 × g for 5 min. Samples for serum biochemical analysis were tested inhouse in a serum chemistry analyzer (VetAce Clinical Chemistry System, Alfa Wasserman, West Caldwell, NJ) according to the manufacturer's instructions. The following 10 parameters were measured: glucose, BUN, creatinine, total protein, total bilirubin, cholesterol, AST, ALT, ALP, and creatine kinase. To characterize our findings, we followed previously published guidelines19 for sample sizes between 20 and 40. Accordingly, we reported the observed medians and ranges and robust 90% reference intervals for each variable, which were calculated according to described methods33 by using the R statistical software package (version 3.0.2, R Project, Vienna, Austria;). A 90% reference interval is defined as the set of values within which an estimated 90% of the values in the population would fall.33
Rice rats as a noninvasive model for periodontitis.
When rice rats eat a powdered H-SC diet from weaning,6,21,24,25,32,51 they develop periodontitis without intraoral mechanical manipulation.24-26,51 We reexamined the rice rat model for periodontitis and characterized the periodontal lesions.2 For this purpose, 4-wk-old rats were fed standard and H-SC diets for 0, 6, 12, and 18 wk. The composition of the H-SC diet was based on the formulas of the powdered Harvard high-sucrose 700 diet and the ration 100 diet.6,21,24-26,51
Results
Monitoring for excluded pathogens.
During quarantine, oxyurid nematodes of the genus Syphacia were identified in perianal tapes and in the cecal content of rice rats and sentinel mice and rats (Figure 2). Immediately after identification, rice rats were treated with fenbendazole-medicated rodent chow (150 ppm) for 6 wk.8,34 After this treatment, all rats tested negative by the perianal tape method. The medicated chow was continued for 6 more weeks, and all rats were retested afterward. Perianal tapes were negative in rice rats, and no nematodes were found in the cecal content or in perianal tapes of sentinels at this time. Importantly, except for Syphacia, none of the rats had a positive result for any of the pathogens on the exclusion list (see Materials and Methods) during quarantine or the experimental time period.
Figure 2.
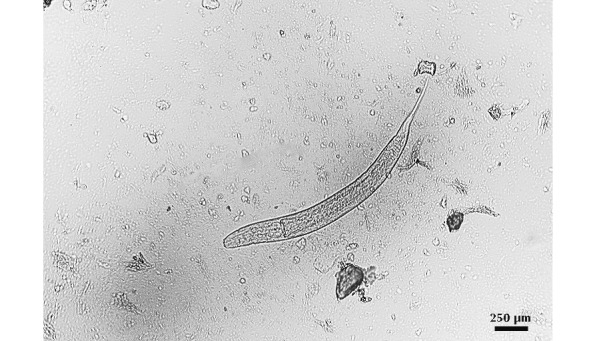
Oxyurid nematodes of the genus Syphacia were identified in rice rats during the quarantine period. Adult parasites were identified on perianal tapes and in cecal contents from sentinels and breeder rice rats. Wet mount sample from cecum; bar, 250 μm.
Animal husbandry, breeding, rearing, and care (Figure 1).
Rice rats can display very aggressive behavior. Both males and females engage in fights and physical interactions that can result in severe traumatic injuries and death. Females are particularly aggressive during the peripartum period. On several occasions, male rats were cannibalized by reproductive females. Litters were also vulnerable to the aggressive nature of reproductive females, and sometimes were also cannibalized after birth. This behavior is particularly an issue with primiparous female rats. To reduce this problem, we placed small cardboard buckets (to-go cups) in the breeder and experimental rat cages (Figure 1 C) as shelter for potential victims or as nesting sites for litters. These shelters were very effective, given that the number of injured and traumatized breeders and experimental rats decreased after their addition. Furthermore, pups in the pine shavings bedding might easily be overlooked during cage changes, possibly resulting in the disposal of a litter with the dirty bedding, particularly when the the bedding is deeper than 1 in. To prevent this problem, technicians were trained in performing weekly checks of all breeding cages to identify new litters.
Rice rats are belligerent and reluctant to be handled. To avoid bites or other injuries from the rats, we use sanitized garden gloves and practice the scruff method when we handle the animals (Figure 1 D). In addition, when rats are conditioned for the technique, the scoop method is very effective and practical and is particularly useful for simple husbandry duties, such as cage changes, pair breeding, weaning, and establishing experimental groups. Rats were conditioned to be moved into the container immediately after they were weaned.
Depending on the scale of the planned study, we used 5 to 54 breeder-pairs between 9 and 35 wk of age. When no study was ongoing, we used 5 to 10 breeder-pairs for colony maintenance purposes. To maintain breeders within the optimal age for breeding (2 to 8 mo), we bred 6 to 10 new pairs every 4 mo and replaced older breeders. Breeder rice rats older than 8 mo to 1 y were culled. Breeders assigned to generate experimental animals were paired by using a monogamous continuous-breeding system until the necessary number of rats was obtained for each experiment.
We analyzed a total of 97 litters from 56 pairs of breeders and found that dams gave birth to a total of 508 pups, representing an average of 5.24 pups/dam, and weaned 411 pups, corresponding to 4.24 pups/dam. Healthy weaned pups had an average weight of 28 to 36 g. The total number of breeders for an assigned experiment was calculated based on the production efficiency index that we observed in rice rats, which was 0.66 suitable pups each week per breeding female rat. 47
Female rice rats are generally attentive to their pups, and male rats appear to help in rearing the pups. After a lactation period of 25 to 28 d, litters were weaned, separated according to sex, and randomized to the various experimental groups.
Hematology and serum chemistry.
Hematologic and serum chemistry values for marsh rice rats (both sexes combined) are presented in Table 1. Data are reported as observed medians and ranges and robust 90% reference intervals for each variable. The 90% reference interval is defined as the set of values within which an estimated 90% of the values in the population would fall.
Table 1.
Hematologic and serum chemistry values for rice rats (Oryzomys palustris; n= 20 male, 19 female)
| Observed median | Observed range | Robust 90% referent interval | ||
| Hematology | ||||
| RBC (×106/μL) | 7.32 | 5.00–9.31 | 5.92–8.96 | |
| Hgb (g/dL) | 13.10 | 9.10–16.00 | 10.50 15.90 | |
| Hct (%) | 52.30 | 38.20–65.10 | 42.00–62.40 | |
| MicroHct (%) | 43.00 | 36.00–53.00 | 36.00–50.10 | |
| MCV (fL) | 70.30 | 62.30–77.60 | 63.80–76.70 | |
| MCH (pg) | 17.60 | 15.40–20.40 | 15.40–20.30 | |
| MCHC (g/dL) | 25.50 | 22.40–28.10 | 22.40–28.40 | |
| RBC distribution width (%) | 15.70 | 14.00–18.80 | 13.70–18.10 | |
| Mean platelet volume (fL) | 5.70 | 4.30–6.40 | 5.00–6.45 | |
| Platelet distribution width (%) | 19.70 | 16.10–31.60 | 14.40–24.30 | |
| WBC (×1000/μL) | 7.26 | 1.96–20.86 | 0.74–12.90 | |
| Neutrophils (×1000/μL) | 1.45 | 0.42–11.99 | 0.00–4.88 | |
| Lymphocytes (×1000/μL) | 4.81 | 1.46–10.34 | 0.81–8.49 | |
| Monocytes (×1000/μL) | 0.32 | 0.02–0.71 | 0.02–0.62 | |
| Eosinophils (×1000/μL) | 0.02 | 0.00–0.33 | 0.00–0.12 | |
| Basophils (×1000/μL) | 0.00 | 0.00–0.13 | 0.00–0.10 | |
| Platelets (×1000/μL) | 863 | 259–1178 | 399–1278 | |
| Reticulocytes (×1000/μL) | 0 | not applicable | not applicable | |
| WBC differential | ||||
| Neutrophils (%) | 23.98 | 10.27–57.50 | 6.72–48.90 | |
| Lymphocytes (%) | 71.10 | 41.35–85.76 | 51.20–86.70 | |
| Monocytes (%) | 4.87 | 0.78–9.20 | 1.22–8.12 | |
| Eosinophils (%) | 0.20 | 0.00–2.61 | 0.00–1.34 | |
| Basophils (%) | 0.05 | 0.00–1.60 | 0–1.04 | |
| Chemistry | ||||
| Glucose (mg/dL) | 124 | 71–205 | 78–172 | |
| BUN (mg/dL) | 24 | 17–34 | 17–32 | |
| Creatinine (mg/dL) | 0.40 | 0.30–0.50 | 0.30–0.40 | |
| Total protein (g/dL) | 5.10 | 2.10–6.10 | 3.97–6.38 | |
| Total bilirubin (mg/dL) | 0.30 | 0.20–0.70 | 0.20–0.70 | |
| Cholesterol (mg/dL) | 173 | 33–304 | 88–260 | |
| AST (IU/L) | 172 | 42–1209 | 0–539 | |
| ALT (IU/L) | 119 | 60–612 | 0–317 | |
| ALP (IU/L) | 100 | 34–407 | 0–240 | |
| Creatine kinase (U/L) | 1160 | 114–5353 | 0–3394 | |
Procedures routinely performed in rice rats.
As an identification method, we used an indelible dye as a temporary marker on the skin or an animal tattoo identification system as a permanent marker on the tail of the rice rats. When the tattoo system was used, rats were restrained for 1 to 2 min in a rat restrainer (Harvard Apparatus, Holliston, MA). A commercially available tattoo system (Kent Scientific, Torrington, CT) was used according to the manufacturer's instructions. No anesthesia was required to perform this procedure.
Rice rats routinely were injected intravenously in the tail vein. We found the procedure simple and easy to execute. Rats to be injected were transferred to a rat restrainer; 27-gauge needles with 1-mL syringes were used for the procedure. Warm water (approximately 40 °C) was used to dilate the tail vein. A lateral tail vein can be identified on either side of the tail. The area of the tail to be used for IV injection was swabbed with cotton soaked in isopropyl alcohol. The tail was held with one hand so that the vein faced upward. The needle was positioned as flat and parallel as possible to the tail. Penetration was near the tip of the tail. The needle then was moved slowly in a cranial direction. When the needle was removed from the vein, the site of injection was held firmly for 20 s with gauze, to stop all bleeding. The rat was removed from the restraint and returned to its home cage. This procedure can reliably be done once monthly for 5 consecutive months.
Rice rats as a noninvasive model for periodontitis.
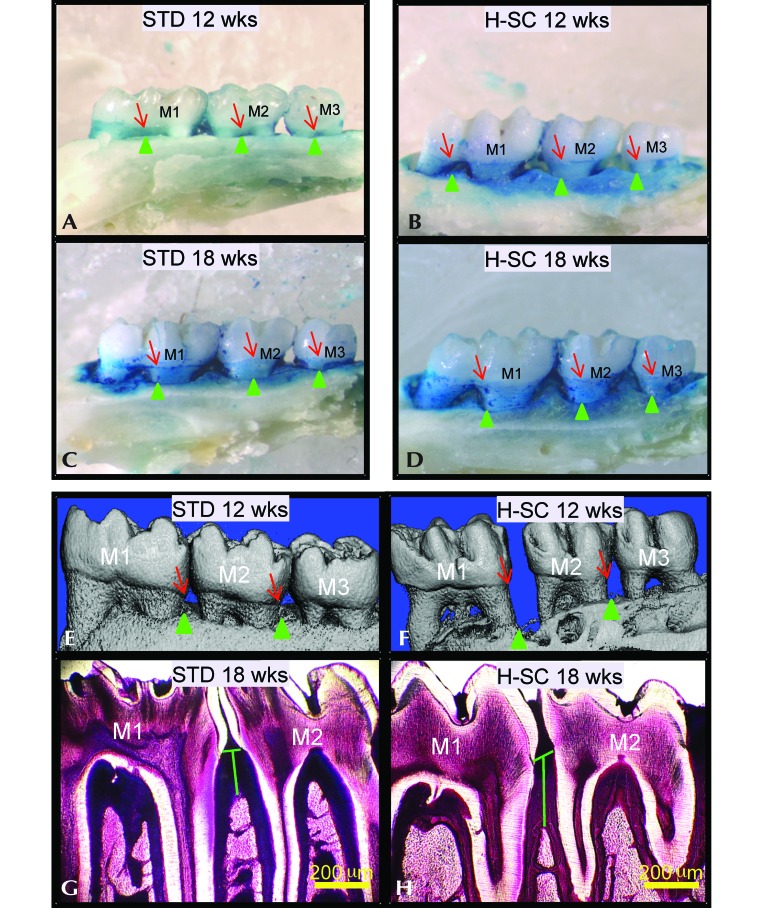
Rice rats that were fed the H-SC diet for 18 wk showed no significant differences in body weight or metabolic markers (including serum glucose, insulin, and cholesterol) compared with rats on the standard diet.3 However, both groups of rats showed a progressive increase in horizontal alveolar bone loss in the maxillae.2 The H-SC diet exacerbated horizontal alveolar bone loss at the palatal surface (Figure 3 A through D). Furthermore, increased vertical alveolar bone loss was detected in mandibles of rats fed the H-SC diet as evidenced by microCT (Figure 3 E and F) and histometry (Figure 3 G and H).2 These changes also were present in maxillae.2 In addition, H-SC rats had higher periodontitis scores and more plaque accumulation and gingival and alveolar bone inflammation than did standard-diet rats (Figure 4).2 Periodontitis was mild at 12 wk, and moderate to severe at 18 wk in H-SC rats but was nonexistent at 12 wk, mild at 18 wk, and moderate to severe at 26 wk in standard-diet rats.
Figure 3.
Characteristics of the periodontitis model in rice rats. Comparative images stained with methylene blue to delineate the cementoenamel (junction (red arrows) from the maxillary palatal surface of male rice rats fed the standard (standard) or high-sucrose and -casein (H-SC) diet for (A and B) 12 wk or (C and D) 18 wk. The green arrowheads depict the borders of the alveolar bone crest. The blue-stained area corresponds to the area of exposed tooth root which can serve as an index of horizontal alveolar bone height. The H-SC diet increases alveolar bone loss in rice rats. The methylene-blue–stained palatal surfaces of standard or H-SC diet rats for (A and B) 12 wk and (C and D) 18 wk show that rice rats present increased horizontal alveolar bone loss both over time and with H-SC diet feeding. In comparative reconstructed microCT images taken from the mandibles of female rice rats fed the (E) standard or (F) H-SC diets for 12 wk, note the increased vertical alveolar bone loss at the lingual surface in the interproximal spaces of mandibular molars (M) 1 and M2 and M2–M3 in a rat fed the (F) H-SC diet compared with (E) an age-matched control rat. Red arrows indicate the cementoenamel junction, and green arrows point to the alveolar bone crest. (H) Increased mandibular vertical alveolar bone loss (green vertical line) at the interproximal alveolar bone at M1–M2 in a male rat fed the H-SC diet for 18 wk compared with (G) an age-matched control rat fed the standard diet. Methacrylate-embedded section stained en bloc with basic fuchsin. Bar, 200 μm (G and H).
Figure 4.
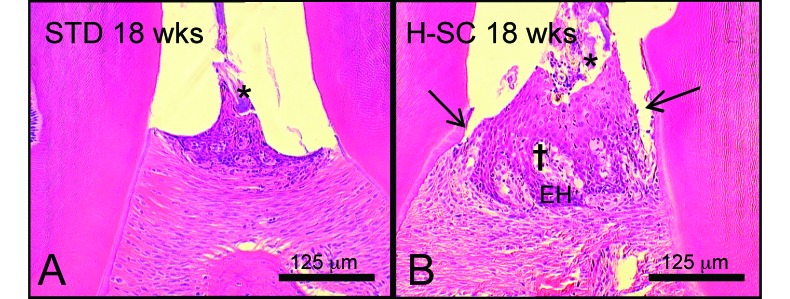
Histologic features of periodontitis in rice rats. Photographs taken at the interproximal space between the first and second mandibular molars of rice rats fed standard (standard) or high-sucrose and -casein (H-SC) diets for 18 wk. Bacterial plaques (*) were present in both (A) the rats fed the standard diet and (B) those fed the H-SC diet. (B) Periodontal lesions typically were moderate in rice rats fed the H-SC diet for 18 wk; mild migration of the junctional epithelium (black arrow), hyperplasia of the gingival epithelium (EH), and mild inflammatory cell infiltration in the lamina propria (†) also are present in this sample. Hematoxylin and eosin stain; bar, 125 μm.
Discussion
This report is the first published description of the husbandry, housing, breeding conditions, clinical pathology parameters, and veterinary care guidelines for marsh rice rats (O. palustris) under laboratory conditions. Although we followed the recommended minimal space for groups of rats (R. norvegicus) less than 200 g that are endorsed by the Guide for the Care and Use of Laboratory Animals,36 we would consider increasing the floor space per animal by using larger cages (17.8 in. × 9.5 in. × 8.0 in. instead of 14 in. × 10 in. × 7 in.) in the future to provide housing conditions that are perhaps more appropriate for a wild rodent species and possibly to reduce aggressive behavior in these animals. Importantly, we have found that several biologic reagents specifically developed for R. norvegicus can be used effectively with rice rats (data not shown). For instance, we found that several antibodies for immunocytochemistry specifically designed for rats (for example, CD31, Toll-like receptors 2 and 4, RANKL, and the epithelial cell proliferation marker Ki67 antigen) displayed strong immunoreactivity in tissues from rice rats. Furthermore, a rat serum insulin ELISA kit and labeled probes for use with a commercial microarray to determine gene expression levels have worked very well for rice rats. These results seem to suggest that other biologic reagents designed for use with the genus Rattus likely are suitable for use with Oryzomys as well. This situation might enhance the potential for using rice rats in biologic research.
Regarding periodontitis, a recent review succinctly summarizes a widely held opinion: “A simple and reproducible model that truly mimics human pathogenesis of periodontitis has yet to be discovered.”45 Rice rats (O. palustris) are indeed one of several relevant rat models for human periodontitis that is suboptimal.23-25,41,53,55 The rice rat model has the advantage that the onset of periodontitis is natural. No intraoral mechanical (for example, ligature placement and maintenance or injections 3 times weekly) or microbiologic intervention is required to initiate or maintain the disease state, making rice rat periodontitis an inflammatory condition that can be studied by investigators unfamiliar with correct execution of detailed, precise intraoral procedures. Although how well the intricate details of pathogenesis of rice rat periodontitis, including its microbiology, parallels events in humans is unknown, the changes in tissue levels bear reasonable similarity. Rice rats develop signs of gingivitis, followed by periodontitis and alveolar bone loss, while consuming their standard diet. The onset of periodontitis can be accelerated to occur between ages 4 to 22 wk by feeding a pelleted H-SC diet beginning at age 4 wk. Providing the standard diet until rats are 30 wk old achieves the same level of periodontitis as does feeding the H-SC diet until 22 wk.3 Previously, powdered H-SC diets were used in rice rats, inducing rapid severe periodontitis with frequent loss of teeth; disease progress is noticeably slower with pelleted diets.3 Although 30-wk-old rats on either diet may have mobile teeth, no tooth loss has occurred. Periodontal status can readily be evaluated during postnecropsy sampling of periodontal tissues by gross examination, histologic observation, and measurement of alveolar crest height at interdental regions.
To accelerate the onset of periodontitis, rice rats can be given a diet high in sucrose and casein.6,21,22,24,25,51 Long-term sucrose feeding in rats (R. norvegicus) is known to induce weight gain, hyperglycemia, and glucose intolerance, with variable insulin responses.29,42 In addition, rats fed diets high in sucrose can develop insulin resistance, hyperinsulinemia,27,35 or hypertriglyceridemia and hypertension without obesity or changes in fasting plasma glucose and leptin levels.1,7,35,43,49 We did not note these effects in rice rats (O. palustris) fed the H-SC diet3 suggesting that this rodent species is genetically adapted to tightly control glucose metabolism under extreme conditions.
In conclusion, we present data showing that rice rats adapt relatively well to standard laboratory conditions and that several procedures or biologic reagents usually used in standard laboratory rodents (mice and rats) are applicable to rice rats. Importantly, we have presented hematologic and serum chemistry values that can be used as reference values for future studies in this animal species. Because the rice rat is not currently commercially or centrally (National Center for Research Resources [http://www.nih.gov/about/almanac/organization/NCRR.htm]) available, this species requires investigator-directed collection, breeding, and rearing. We believe that the current report will help other investigators in this matter should they choose to use rice rats as an animal model in their studies. Another option would be to request healthy and clean rice rats for not-for-profit animal experimentation from the authors’ academic institutions.
Acknowledgments
This research was supported by NIH grant R03DE018924-01A1 from the National Institute of Dental and Craniofacial Research (NIDCR).
References
- 1.Agheli N, Kabir M, Berni-Canani S, Petitjean E, Boussairi A, Luo J, Bornet F, Slama G, Rizkalla SW. 1998. Plasma lipids and fatty acid synthase activity are regulated by short-chain fructo-oligosaccharides in sucrose-fed insulin-resistant rats. J Nutr 128:1283–1288. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre JI, Akhter M, Kimmel D, Pingel J, Xia X, Williams A, Jorgensen M, Edmonds K, Lee J, Reinhard M, Battles A, Kesavalu L, Wronski TJ. 2012. Enhanced alveolar bone loss in a model of noninvasive periodontitis in rice rats. Oral Dis 18:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguirre JI, Kimmel D, Akhter M, Leeper A, Trcalek C, Williams A, Rivera M, Kesavalu L, Ke HZ, Min L, Wronski T. 2013. Systemic sclerostin antibody treatment reduces alveolar bone loss in rice rats with active periodontitis. J Bone Miner Res 28:S75. [Google Scholar]
- 4.American Veterinary Medical Association 2013. AVMA Guidelines for euthanasia of animals: 2013 edition. Schaumburg (IL): American Veterinary Medical Association. [Google Scholar]
- 5.Audubon JJ, Bachman J. 1854. The quadrupeds of North America, p 348–350. New York (NY): John James Audubon. [Google Scholar]
- 6.Auskaps AM, Gupta O, Shaw J. 1957. Periodontal disease in the rice rat. III. Survey of dietary influences. J Nutr 63:325–343. [DOI] [PubMed] [Google Scholar]
- 7.Chevalier MM, Wiley JH, Leveille GA. 1972. Effect of dietary fructose on fatty acid synthesis in adipose tissue and liver of the rat. J Nutr 102:337–342. [DOI] [PubMed] [Google Scholar]
- 8.Coghlan LG, Lee DR, Psencik B, Weiss D. 1993. Practical and effective eradication of pinworms (Syphacia muris) in rats by use of fenbendazole. Lab Anim Sci 43:481–487. [PubMed] [Google Scholar]
- 9.Conaway CH. 1954. The reproductive cycle of rice rats (Oryzomys palustris palustris) in captivity. J Mammal 35:263–266. [Google Scholar]
- 10.Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, Eke PI, Beltran-Aguilar ED, Horowitz AM, Li CH. 2007. Trends in oral health status: United States, 1988–1994 and 1999–2004. Vital Health Stat 11 248:1–92. [PubMed] [Google Scholar]
- 11.Edmonds K, Riggs L, Masden T. 2005. Effects of photoperiod, melatonin, and the pineal gland on compensatory gonadal hypertrophy during postnatal development in the marsh rice rat (Oryzomys palustris). Zoolog Sci 22:763–774. [DOI] [PubMed] [Google Scholar]
- 12.Edmonds KE, Rollag MD, Stetson MH. 1995. Effects of photoperiod on pineal melatonin in the marsh rice rat (Oryzomys palustris). J Pineal Res 18:148–153. [DOI] [PubMed] [Google Scholar]
- 13.Edmonds KE, Stetson MH. 1993. Effect of photoperiod on gonadal maintenance and development in the marsh rice rat (Oryzomys palustris). Gen Comp Endocrinol 92:281–291. [DOI] [PubMed] [Google Scholar]
- 14.Edmonds KE, Stetson MH. 1994. Photoperiod and melatonin affect testicular growth in the marsh rice rat (Oryzomys palustris). J Pineal Res 17:86–93. [DOI] [PubMed] [Google Scholar]
- 15.Edmonds KE, Stetson MH. 2001. Effects of age and photoperiod on reproduction and the spleen in the marsh rice rat (Oryzomys palustris). Am J Physiol Regul Integr Comp Physiol 280:R1249–R1255. [DOI] [PubMed] [Google Scholar]
- 16.Esher RJ, Wolfe JL, Layne JN. 1978. Swimming behavior of rice rats (Oryzomys palustris) and cotton rats (Sigmodon hispidus). J Mammal 59:551–558. [Google Scholar]
- 17.Eubanks BW, Hellgren EC, Nawrot JR, Bluett RD. 2011. Habitat associations of the marsh rice rat (Oryzomys palustris) in freshwater wetlands of southern Illinois. J Mammal 92:552–560. [Google Scholar]
- 18.Felt SA, Hussein HI, Mohamed Helmy IH. 2008. Biology, breeding, husbandry, and diseases of the captive Egyptian fat-tailed jird (Pachyuromys duprasi natronensis). Lab Anim (NY) 37:256–261. [DOI] [PubMed] [Google Scholar]
- 19.Friedrichs KR, Harr KE, Freeman KP, Szladovits B, Walton RM, Barnhart KF, Blanco-Chavez J. 2012. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 41:441–453. [DOI] [PubMed] [Google Scholar]
- 20.Goldman EA. 1918. The rice rats of North America. U S Department of Agriculture. Washington (DC): Government Printing Office. [Google Scholar]
- 21.Gotcher JE, Jee WS. 1981. The progress of the periodontal syndrome in the rice rat. I. Morphometric and autoradiographic studies. J Periodontal Res 16:275–291. [DOI] [PubMed] [Google Scholar]
- 22.Gotcher JE, Jee WS. 1981. The progress of the periodontal syndrome in the rice rat. II. The effects of a diphosphonate on the periodontium. J Periodontal Res 16:441–455. [DOI] [PubMed] [Google Scholar]
- 23.Graves DT, Kang J, Andriankaja O, Wada K, Rossa C., Jr 2012. Animal models to study host–bacteria interactions involved in periodontitis. Front Oral Biol 15:117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta OP, Shaw J. 1956. Periodontal disease in the rice rat. I. Anatomic and histopathologic findings. Oral Surg Oral Med Oral Pathol 9:592–603. [DOI] [PubMed] [Google Scholar]
- 25.Gupta OP, Shaw J. 1956. Periodontal disease in the rice rat. II. Methods for the evaluation of the extent of periodontal disease. Oral Surg Oral Med Oral Pathol 9:727–735. [DOI] [PubMed] [Google Scholar]
- 26.Gupta OP, Shaw J. 1956. The relation of a chelating agent to smooth-surface lesions in the white rat. J Nutr 60:311–322. [DOI] [PubMed] [Google Scholar]
- 27.Gutman RA, Basilico MZ, Bernal CA, Chicco A, Lombardo YB. 1987. Long-term hypertriglyceridemia and glucose intolerance in rats fed chronically an isocaloric sucrose-rich diet. Metabolism 36:1013–1020. [DOI] [PubMed] [Google Scholar]
- 28.Hall ER, Kelson KR. 1959. The mammals of North America, vol. 2. New York (NY): Ronald Press. [Google Scholar]
- 29.Hallfrisch J, Lazar F, Jorgensen C, Reiser S. 1979. Insulin and glucose responses in rats fed sucrose or starch. Am J Clin Nutr 32:787–793. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton WJ. 1946. Habits of the swamp rice rat, Oryzomys palustris palustris (Harlan). Am Midl Nat 36:730–736. [Google Scholar]
- 31.Hamilton WJ, Whitaker JOJ. 1979. Mammals of the eastern United States, 2nd ed. Ithaca (NY): Cornell University Press. [Google Scholar]
- 32.Hattler AB, Snyder DE, Listgarten MA, Kemp W. 1977. The lack of pulpal pathosis in rice rats with the periodontal syndrome. Oral Surg Oral Med Oral Pathol 44:939–948. [DOI] [PubMed] [Google Scholar]
- 33.Horn PS, Pesce AJ, Copeland BE. 1998. A robust approach to reference interval estimation and evaluation. Clin Chem 44:622–631. [PubMed] [Google Scholar]
- 34.Huerkamp MJ, Benjamin KA, Zitzow LA, Pullium JK, Lloyd JA, Thompson WD, Webb SK, Lehner ND. 2000. Fenbendazole treatment without environmental decontamination eradicates Syphacia muris from all rats in a large, complex research institution. Contemp Top Lab Anim Sci 39:9–12. [PubMed] [Google Scholar]
- 35.Hulman S, Falkner B. 1994. The effect of excess dietary sucrose on growth, blood pressure, and metabolism in developing Sprague–Dawley rats. Pediatr Res 36:95–101. [DOI] [PubMed] [Google Scholar]
- 36.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): The National Academic Press. [Google Scholar]
- 37.Joyner CP, Myrick LC, Crossland JP, Dawson WD. 1998. Deer mice as laboratory animals. ILAR J 39:322–330. [DOI] [PubMed] [Google Scholar]
- 38.Kale HW. 1965. Ecology and bioenergetics of the long-billed marsh wren in Georgia salt marshes: (Telmatodytes balustris griseus [Brewster]). 1st ed, Cambridge (MA): Publications of the Nuttall Ornithological Club. [Google Scholar]
- 39.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S, McCauley LK, McGowan J, McKee MD, Mohla S, Pendrys DG, Raisz LG, Ruggiero SL, Shafer DM, Shum L, Silverman SL, Van Poznak CH, Watts N, Woo SB, Shane E. 2007. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 22:1479–1491. [DOI] [PubMed] [Google Scholar]
- 40.Kudo H, Oki Y. 1982. [Breeding and rearing of Japanese field voles (Microtus montebelli Milne-Edwards) and Hungarian voles (Microtus arvalis Pallas) as new herbivorous laboratory animal species] Jikken Dobutsu 31:175–183. [Article in Japanese]. [DOI] [PubMed] [Google Scholar]
- 41.Leonard EP. 1979. Periodontitis. Animal model: periodontitis in the rice rat (Oryzomys palustris). Am J Pathol 96:643–646. [PMC free article] [PubMed] [Google Scholar]
- 42.Lombardo YB, Drago S, Chicco A, Fainstein-Day P, Gutman R, Gagliardino JJ, Gomez Dumm CL. 1996. Long-term administration of a sucrose-rich diet to normal rats: relationship between metabolic and hormonal profiles and morphological changes in the endocrine pancreas. Metabolism 45:1527–1532. [DOI] [PubMed] [Google Scholar]
- 43.London E, Lala G, Berger R, Panzenbeck A, Kohli AA, Renner M, Jackson A, Raynor T, Loya K, Castonguay TW. 2007. Sucrose access differentially modifies 11β-hydroxysteroid dehydrogenase 1 and hexose-6-phosphate dehydrogenase message in liver and adipose tissue in rats. J Nutr 137:2616–2621. [DOI] [PubMed] [Google Scholar]
- 44.Negus NC, Gould E, Chipman RK. 1961. Ecology of the rice rats, Oryzomys palustris (Harlan), on Breton Island, Gulf of Mexico, with a critique of social stress theory. Tulane Studies Zool 8:93–123. [Google Scholar]
- 45.Oz HS, Puleo DA. 2011. Animal models for periodontal disease. J Biomed Biotechnol 2011:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park AW, Nowosielski-Slepowron BJ. 1972. Biology of the rice rat (Oryzomys palustris natator) in a laboratory environment. Z Saugetierkd 37:42–51. [PubMed] [Google Scholar]
- 47.Peters AG, Bywater PM, Festing MF. 2002. The effect of daily disturbance on the breeding performance of mice. Lab Anim 36:188–192. [DOI] [PubMed] [Google Scholar]
- 48.Rafferty JP. 2010. Rats, bats, and xenarthrans. Britannica guide to predators and prey, 1st ed, London (UK): Rosen Education Service. [Google Scholar]
- 49.Reaven GM, Ho H. 1991. Sugar-induced hypertension in Sprague–Dawley rats. Am J Hypertens 4:610–614. [DOI] [PubMed] [Google Scholar]
- 50.Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B. 2009. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J Oral Maxillofac Surg 67:2–12. [DOI] [PubMed] [Google Scholar]
- 51.Ryder MI. 1980. Histological and ultrastructural characteristics of the periodontal syndrome in the rice rat. I. General light microscopic observations and ultrastructural observations of initial inflammatory changes. J Periodontal Res 15:502–515. [DOI] [PubMed] [Google Scholar]
- 52.Sharp HF., Jr 1967. Food ecology of the rice rat, Oryzomys palustris (Harlan), in a Georgia salt marsh. J Mammal 48:557–563. [PubMed] [Google Scholar]
- 53.Shaw JH, Griffiths D. 1961. Relation of protein, carbohydrate, and fat intake to the periodontal syndrome. J Dent Res 40:614–621. [Google Scholar]
- 54.Steward JS. 1951. The swamp rice rat (Oryzomys palustris natator) as a possible laboratory animal for special purposes. J Hyg (Lond) 49:427–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Struillou X, Boutigny H, Soueidan A, Layrolle P. 2010. Experimental animal models in periodontology: a review. Open Dent J 4:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Svihla A. 1931. Life history of the Texas rice rat (Oryzomys palustris texensis). J Mammal 12:238–242. [Google Scholar]
- 57.Voss RS, Heideman periodontitis, Mayer VL, Donnelly TM. 1992. Husbandry, reproduction, and postnatal development of the neotropical muroid rodent Zygodontomys brevicauda. Lab Anim 26:38–46. [DOI] [PubMed] [Google Scholar]
- 58.Ward LE. 2001. Handling the cotton rat for research. Lab Anim (NY) 30:45–50. [PubMed] [Google Scholar]
- 59.Weksler M. 2006. Phylogenetic relationships of oryzomyine rodents (Muridae: Sigmodontinae): separate and combined analyses of morphological and molecular data. Bull Am Mus Nat Hist 296:1–149. [Google Scholar]
- 60.Weksler M, Percequillo R. 2011. Key to the genera of the tribe Oryzomyini (Rodentia: Cricetidae: Sigmodontinae). Mastozoologia Neotropical 18:281–292. [Google Scholar]
- 61.Weksler M, Percequillo R, Voss R. 2014. Ten new genera of oryzomyine rodents (Cricetidae: Sigmodontinae). Am Mus Novit 3537:1–29. [Google Scholar]
- 62.Wolfe JL. 1982. Oryzomys palustris. Mamm Species 176:1–5. [Google Scholar]