Abstract
When sampling blood from mice, several different techniques can be used, with retroorbital sinus sampling traditionally being the most common. Given the severe tissue trauma caused by retroorbital sampling, alternative methods such as the facial vein route have been developed. The aim of this study was to evaluate 2 techniques for facial vein bleeding in conscious mice to ascertain whether differences in clinical outcomes, practicability of sample collection, and hematologic parameters were apparent. Blood samples were obtained from the facial vein of 40 BALB/c mice by using either a 21-gauge needle or a lancet. Subsequently, the protocol was repeated with isoflurane-anesthetized mice sampled by using the lancet method (n = 20). Behavior immediately after sampling was observed, and sample quantity, sampling time, and time until bleeding ceased were measured. Clinical pathology data and hematoma diameter at necropsy were analyzed also. The mean sample quantity collected (approximately 0.2 mL) was comparable among methods, but sampling was much more rapid when mice were anesthetized by using isoflurane. The only other noteworthy finding was a significantly reduced number of platelets in samples from anesthetized mice. Adverse, ongoing clinical signs were rare regardless of the method used. The results revealed no significant differences in welfare implications or blood sample quality among the methods or between conscious and anesthetized mice. Therefore, any of the methods we evaluated for obtaining blood samples from the facial vein are appropriate for use in research studies.
Stress has been defined as a change in biologic equilibrium caused by external or internal factors (stressors).19 Signs of stress have been noted in rats and mice during routine laboratory procedures, including those that involve high or low temperature, restraint, excess noise, and movement of cages.5,10,15,17,19 These stressors can cause an adaptive response, resulting in changes in physiology, psychologic state, and behavior, which can be assessed effectively by evaluating behavioral patterns, physiologic changes, or biochemical alterations.19 Such markers include grooming, vocalization, posture, response to handling, and blood cell counts.7,19 Blood collection is a common procedure in mice during scientific investigations and is a potential source of stress. As such, methods of blood collection should be assessed for potential implications on animals’ wellbeing.
For collecting blood samples from mice, several techniques can be considered, each with its own advantages and disadvantages. Several factors should be evaluated when choosing the optimal method, including collection volume, ease and rapidity of collection, requirement for anesthesia, and, most importantly, welfare implications for the animals.
Empirically, the most common route for blood collection in mice has been the retroorbital sinus route.2,4,16 This technique is renowned for yielding high volumes of blood (0.2 to 0.5 mL) when performed by an experienced technician.9 However, the use of this method has evoked considerable controversy due to the possibility of resultant tissue damage, in addition to its nonaesthetic qualities.4,11 Therefore the method has fallen out of vogue, and some regulatory bodies or institutions have banned it altogether.9 Incision of the tail vein is another method that has been used frequently in the past and is popular due to the minimal trauma caused and the lack of anesthesia required to perform the procedure.2 Tail vein incision involves lacerating the ventral blood vessels in the tail by using a razor or scalpel blade.6 However, this method typically yields only small volumes (0.1 to 0.15 mL), is difficult to perform in pigmented mice, and often requires preheating of the tail to ensure sufficient vasodilation to obtain adequate blood flow.2,4 Alternatively, blood can be collected by venipuncture of the saphenous vein.13 This method has been described as being suitable for multiple samplings, does not require anesthesia, and has few complications.1,4 However, tail vein incision has also been described as requiring increased restraint and as a somewhat time-consuming technique.1,9
The practical difficulties that arise when using the previously described sampling methods present a potential issue, because a viable alternative to the retroorbital sinus route must yield high volumes. Sublingual vein bleeding could meet this need. This method accommodates the collection of high volumes at frequent intervals, without severely decreasing animal welfare.4,11,12,22 Sublingual sampling is associated with much less tissue damage than is the retroorbital technique10 but requires greater restraint of animals and more practice. In addition, sublingual sampling often requires 2 people, and hematomas have been reported.4,11,16
Recently, a technique involving puncture of the facial vein has been developed as a rapid, humane method for blood collection in mice.8,9 This site is commonly, and perhaps erroneously, referred to as the submandibular vein.21 The decreased handling needed with facial vein sampling in comparison to other techniques makes it an optimal choice for ease of procedure.14 However, disadvantages associated with this method include the possibility of strangulation, contamination of samples by tissue fluid, and the high potential for missed punctures due to the lack of visibility of the vasculature. Facial vein sampling has currently been described to involve using needles of various gauges or an animal-bleeding lancet to pierce the vascular bundle in the caudal part of the jaw.9 Some researchers have suggested that using a needle for this method causes cheek laceration and produces poor sample yields due to coagulation within the needle.9 However little literature specifically addresses this assertion in particular, and the facial vein technique in general. As such, the current study aimed to investigate the effects of these 2 techniques on animal wellbeing and blood sample quantity and quality in mice. These were determined by completing behavioral observations and evaluating hematology parameters. In addition, we sought to determine whether isoflurane anesthesia altered these measured outcomes, in light of anecdotal reports of reduced volumes of blood samples obtained from anesthetized mice.
Materials and Methods
Animals.
Inbred BALB/c female mice were obtained from a barrier-maintained SPF commercial mouse production facility (University of Adelaide Laboratory Animal Services, South Australia, Australia). These animals were free of ecto- and endoparasites and serologically negative for a panel of viral and bacterial mouse pathogens including mouse hepatitis virus, minute virus of mice, and mouse parvovirus and rotavirus. The animals were approximately 5 wk old. The lancet group had a mean weight of 21.0 g (range, 18.6 to 24.8 g), whereas the needle group had a mean weight of 21.5 g (range, 18.0 to 26.7 g). According to the principle of reduction, the mice used were all surplus stock as a result of commercial breeding operations.
Experimental design.
The study received ethical approval from the University of Adelaide and was conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.18 A total of 60 animals were used, with mice being randomly allocated to 3 groups of 20 mice each in such a way that there were no significant weight differences between groups. The groups were allocated as lancet, needle, and isoflurane-anesthetized with lancet. Each animal was weighed prior to the procedure. Facial vein venipuncture was then performed according to a previously described method as a guide for both lancet and needle use.9 All procedures were completed by a single researcher who was trained and experienced in the techniques. The anesthetized group was exposed to approximately 3% isoflurane (Isoflurane, Laser Animal Health, Sydney, Australia) in oxygen, via induction chamber, until loss of righting reflex. Sampling of these animals was performed immediately on removal from the chamber. The mouse was restrained by the tail and the scruff of the neck to prevent movement, before puncturing of the vascular point at the back of the jaw, slightly behind the hinge of the jawbones and toward the ear. Venipuncture was performed by using either the point of a 25-mm, 21-gauge needle (Henry Schein, Melville, NY) grasped firmly in a pencil-like grip or a 5-mm disposable animal lancet (Goldenrod Animal Lancer, Medipoint, Mineola, NY) according to group allocation. For both instruments, depth of insertion was estimated to be approximately 4 mm. The dripping blood was collected into a 1-mL EDTA hematology tube, mixed by inversion of the tube, and placed on ice for transport. Because these were survival bleeds, blood collection ceased and pressure was applied when approximately 0.3 mL blood was collected, to prevent a hypovolemic episode. For volumes less than 0.3 mL, the end point for blood collection occurred when natural flow of blood ceased. The time needed for sample collection was defined as the time from venipuncture until bleeding had ceased or until the 0.3-mL maximal blood volume had been obtained and was recorded, as was the time until bleeding ceased. A maximum of 2 attempts to achieve blood flow was permitted per mouse.
Measurement of blood parameters.
Blood was collected into 1-mL microtubes containing EDTA for use in hematology evaluation. Blood samples were weighed (in grams) for increased accuracy. Total WBC count, differential WBC count, RBC count, Hgb, Hct, MCV, MCHC, red cell distribution width, platelet count, and mean platelet volume were evaluated (Cell Dyne, Abbott Diagnostics, New South Wales, Australia).
Clinical observations.
The mice were monitored continuously for 1 h after bleeding to evaluate recovery and to observe clinical signs that might be indicative of adverse effects. Individual mice were observed for signs including: circling, profuse bleeding, reduced activity, excessive vocalization, bleeding from the ear canal, struggling, difficulties of balance, and collapse. These signs had been reported in other studies.12,19 Signs were scored as either present or absent. The recorded number of mice in each group that exhibited signs was used to compare techniques.
Euthanasia and necropsy.
Once postbleeding observation was complete, mice were euthanized by using CO2 asphyxiation delivered by increasing fill, at a flow rate of 20% of the chamber volume per minute. After confirmation of death, the face and neck were dissected to determine the extent of trauma and to measure any hematoma in the mandibular region across its widest dimension (adapted from reference 14).
Statistical analysis.
For numerical data, which were parametric, a 2-tailed Student t test was performed; these parameters included sample quantity, time needed for sampling, hematoma size, and hematology parameters. No statistical evaluation was performed for qualitative data such as clinical observations. Values are reported as mean ± SEM unless otherwise detailed. Values obtained for the anesthetized group were compared (t test) only against those obtained from the conscious lancet group. Correlations were performed by using a Pearson correlation test. For statistical analysis, a P value less than 0.05 was considered significant. Statistical analyses were performed by using Megastat Excel Add-In (version 10.2, McGraw–Hill Higher Education, NY).
Results
The average duration for blood collection was approximately 5 s greater for the group in which needles (32.0 ± 3.7 s) were used than for the lancet-bled group (26.4 ± 3.1 s; Table 1). Blood collection time was significantly shorter (P = 9.26 × 10−8) for the anesthetized group compared with conscious lancet bleeding (6.3 ± 0.5 s). The average blood quantity collected was greater for mice bled by using a lancet (0.19 ± 0.04 g) than for those bled by using a needle (0.16 ± 0.02 g) or for anesthetized animals (0.12 ± 0.04 g); in addition, the time taken for the bleeding to cease was 5.57 s for lancet-bled mice as compared with 4.56 s for animals bled with a needle. These differences did not achieve statistical significance. By using a blood density value of 1060 kg/m3 and defining density as mass per unit volume, the values obtained equated to blood volumes of 0.26 mL, 0.2 mL, and 0.15 mL for the lancet, needle, and anesthetized groups, respectively.
Table 1.
Sample collection time, duration of bleeding, and volume collected from the facial vein of mice by various techniques
| Needle | Lancet | Anesthetized + Lancet | |
| Duration of blood collection (s) | 31.2 ± 3.71 | 26.4 ± 3.11a | 6.3 ± 0.5a |
| Time until bleeding ceased (s) | 4.6 ± 1.35 | 5.6 ± 2.67 | 0 |
| Sample quantity (g) | 0.16 ± 0.02 | 0.19 ± 0.04 | 0.12 ± 0.04 |
Data are given as mean ± SE.
Values differ significantly (P ≤ 0.05).
Hematology parameters were relatively consistent between groups (Table 2), with only slight variations. Values for all parameters were lowest in the anesthetized group, with platelet counts being significantly decreased (P = 1 × 10−4). Total WBC, platelet, and neutrophil counts were increased nonsignificantly for the lancet method in unanesthetized mice compared with the needle method.
Table 2.
Hematologic parameters of blood samples obtained from the facial vein of mice by using various techniques
| Needle | Lancet | Anesthetized + Lancet | |
| WBC (× 109/L) | 5.66 ± 0.48 | 6.46 ± 0.91 | 5.04 ± 0.33 |
| Neutrophils (%) | 0.93 ± 0.11 | 1.07 ± 0.08 | 0.83 ± 0.14 |
| Lymphocytes (%) | 4.38 ± 0.40 | 4.80 ± 1.00 | 3.90 ± 0.55 |
| Monocytes (%) | 0.30 ± 0.16 | 0.17 ± 0.03 | 0.09 ± 0.02b |
| Eosinophils (%) | 0.13 ± 0.01 | 0.15 ± 0.03 | 0.14 ± 0.01 |
| Basophils (%) | 0.09 ± 0.04 | 0.19 ± 0.08 | 0.08 ± 0.04 |
| RBC (× 1012/L) | 10.13 ± 0.14 | 10.08 ± 0.22 | 9.65 ± 0.15 |
| Hgb (g/L) | 165.4 ± 2.5 | 162.0 ± 2.0 | 153.8 ± 2.9 |
| Hct (L/L) | 0.48 ± 0.01 | 0.47 ± 0.02 | 0.45 ± 0.01 |
| MCV (fL) | 47.48 ± 0.23 | 47.06 ± 0.39 | 46.90 ± 0.32 |
| MCH (pg) | 16.34 ± 0.09 | 16.08 ± 0.22 | 15.94 ± 0.09 |
| MCHC (g/L) | 344.4 ± 1.3 | 341.8 ± 2.8 | 339.6 ± 3.12 |
| RBC distribution width (%CV) | 17.22 ± 0.16 | 17.54 ± 0.35 | 15.54 ± 0.26b |
| Platelets (× 109/L) | 1023 ± 113 | 1043 ± 89a | 692 ± 93a |
Data are given as mean ± SE.
Indicated values within the same row differ significantly (P ≤ 0.05).
Indicated value is significantly (P ≤ 0.05) different from the others in the same row.
As shown in Table 3, 4 mice from the group bled by using a needle showed adverse signs on return to the cage. One mouse appeared unsteady when walking after being released but soon recovered. A second mouse from this group appeared inactive briefly on release. The remaining 2 mice showed signs of bleeding from the ear canal, presumably due to puncture of the tympanic membrane during the procedure, and proceeded to remain hunched and inactive after release. One mouse from the anesthetized group seemed inactive and displayed a hunched posture. Mice that exhibited these clinical signs only did so for approximately 10 min before appearing to recover fully. On release, all mice proceeded to groom the puncture site to remove any residual blood.
Table 3.
Adverse clinical signs noted throughout the study
| Clinical signs during blood collection | |
| Needle | Unsteady on release (n = 1) |
| Inactive after collection (n = 1) | |
| Hunched with ear bleeding (n = 2) | |
| Lancet | No adverse clinical signs noted |
| Anesthetized + Lancet | Hunched with ear bleeding (n = 1) |
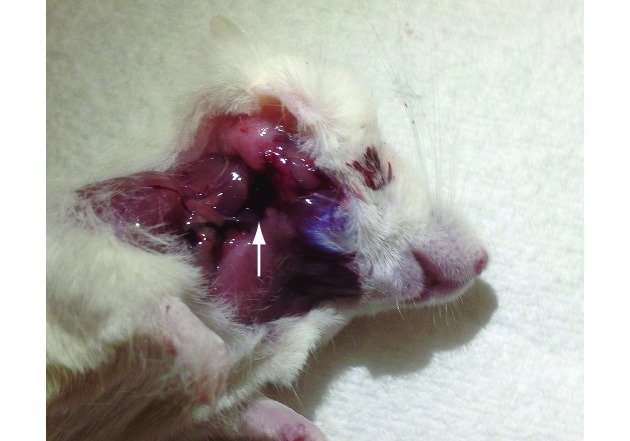
During necropsy, any hematomas (Figure 1) found were measured and recorded; mice bled with a needle had hematomas that were almost 1 mm larger than those in other groups, but there was marked variability in the measures. Hematomas were 5.75 ± 3.2 mm in diameter in the lancet group, 6.7 ± 2 mm in the needle group, and 5.4 ± 3.8 mm in the anesthetized group; these measures did not differ significantly. There was no correlation (R2 = 0.007; P = 0.96) between the quantity of blood collected and the size of the hematoma under the skin.
Figure 1.
An example of a typical hematoma (arrow) present in the mandibular region and extending caudally.
During sampling, the quantity of blood collected seemed to vary dramatically regardless of method. We hypothesized that the volume of blood collected might be directly related to the weight of the mice. Because there were no significant differences between groups regarding weight and blood quantity obtained in conscious mice, their group data were pooled, and a correlation analysis was performed. Mouse weight ranged from 18.2 to 26.7 g, and the correlation coefficient obtained was R2 = 0.004 (P = 0.98). Therefore, the hypothesis is unsupported, in that mouse weight did not influence the quantity of blood collected.
In some animals across all groups, venipuncture had to be repeated, because the correct site was not always located on the initial attempt. As such, the targeted structures were not accessed correctly, leading to inadequate blood flow.
Discussion
Blood collection by using either method of facial vein puncture caused no significant adverse effects on the wellbeing of the mice or on sample quality in this study, suggesting that either needles or lancets can be used successfully. Mice bled by using the lancet method yielded equivalent amounts of blood to those sampled by using the needle, indicating that both methods were equally reliable. However, the quantities we collected, approximately 0.2 mL, are less than those obtained by other investigators, who reported consistent volumes of 0.3 mL.12 We hypothesized that the lower collection volumes were due to low body weights of the mice, given that our mice were younger and smaller than those used in the previous study.12 Although we found no correlation between body weight and blood volume collected in our study, the mice in the current study occupied both a tighter and lower weight range (18.2 to 26.7 g) than did those of the earlier study (29.6 to 45.1 g).12 This smaller range may have affected the ability to determine the correlation expected. In addition, the variation in the quantity of blood collected may have arisen due to interstrain differences or to the use of inbred as compared with outbred mice. Additional studies need to be conducted to test these possibilities. Age- and sex-associated differences may be present as well. Furthermore, differences in experimental design between the studies may explain the inconsistency in blood quantities obtained, in that we limited the number of collection attempts to 2, whereas no such limits were imposed in the previous study.12
Some investigators surmise that the volume of blood collected is lower from anesthetized as compared with conscious mice. The results of the current study do not support this assertion. The rationale of this assumption is the anticipated reduction in blood pressure due to anesthesia. According to this proposed mechanism, the use of inhalational agents would have less of an effect on collected quantities than would some of the common rodent injectable anesthetic formulations using α2 adrenoreceptor agonist agents, given the less dramatic decrease in blood pressure associated with the use of gaseous agents.20 In addition, we used a relatively low induction flow rate of 3%, given that our aim was to briefly immobilize the mice only. If higher flow rates and consequently deeper anesthesia were attained, then blood pressure likely would be reduced further, and the amount of blood collected would be still lower.
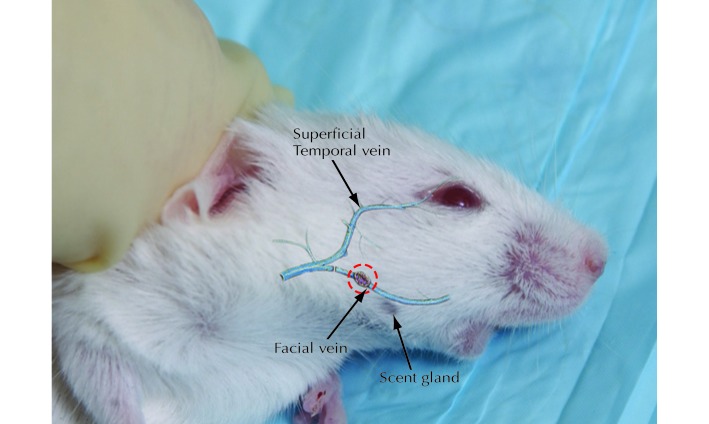
Two mice that were bled by using 21-gauge needles exhibited noteworthy clinical signs, appearing to be hunched with bleeding from the ear canal. These signs occur when the vein is pierced too high above the jaw, thus entering the ear canal and causing trauma, or when the needle is inserted too deeply, which is prevented when the lancet is used, given that its design allows for improved control of depth.9 Another mouse bled by using the needle method exhibited an unsteady gait on release. This behavior might have resulted from an excessively tight restraint that impaired the mouse's breathing. The need to cause the eye to bulge slightly to get sufficient blood flow with the needle method necessitates tighter restraint than that for the lancet method; therefore an unsteady gait may be more prevalent when using needles for blood collection. Another complication of the procedure was incorrectly accessing the puncture site during the first attempt, resulting in the need for a second attempt. Although the scent gland on the side of the face acts as a marker for the puncture site (Figure 2), it can still be difficult to estimate the exact location of the vascular bundle. Visualizing this marker is more difficult in dark and haired animals, perhaps contributing to variability in this technique across strains. Incorrect access also was reported as a complication in previous studies comparing facial vein venipuncture with sublingual venipuncture.12 The incidence of repuncture may decrease as experience with the procedure is gained.
Figure 2.
The vascular bundle targeted during blood collection via the facial vein. The dashed circle indicates the puncture site. Reprinted from reference 9 by permission from Macmillan Publishers.
The size of the hematoma did not differ significantly according to the method of blood sampling, and the previously reported hematomas (1 to 7 mm) in C57BL/6NTac mice14 were similar in size to those we observed. Given the diffuse nature of some of the hematomas (Figure 1), perhaps consequent effects on mouse wellbeing were not evaluated in our study. A similar study assessing the facial vein method of venipuncture evaluated long-term implications of the procedure, such as effects on body weight and food consumption.11 Furthermore, the tissue at the puncture site was evaluated histologically to more accurately determine the degree of damage in the previous study; this analysis found moderate hemorrhage, edema, and inflammation.11 Although similar evaluations might have added value to the current study, they were not feasible due to the scope and time frame and may provide the basis for future investigation.
The sample quality was consistently high between the 2 bleeding procedures we used and was sufficient to allow standard hematologic evaluation. In conscious mice, there was slight albeit nonsignificant variation in various blood cell indices between the methods. Researchers should be aware of the decreased values (especially for platelets and monocytes) from anesthetized mice and should consider this effect when comparing studies in which these parameters are of scientific importance. A previous study reported the problem of platelet clumping, leading to reduced counts.11 This was more prevalent in mice that were bled while conscious and restrained than while anesthetized. The authors postulated that the effect on platelets was due to struggling and defense movements, which significantly prolonged the blood collection time.11 However, we did not encounter a similar problem in the current study, and we therefore assume that the values reported here are accurate platelet values.
Perhaps the population size we used in the current study was inadequate to provide an accurate representation of the methods, given that there was insufficient previous research on which reliable power calculations could be based. However in the spirit of the principle of reduction, we included 20 mice per group, similar to the group sizes reported in comparable studies.1,11,13 However, a retrospective power calculation based on the values obtained for monocytes showed a statistical power of 87%. Not unsurprisingly due to the inherent variability in blood collection data, the statistical power for that part of the study was rather low (mean of 34% across measured parameters). Future studies might use larger populations and evaluate interstrain differences in the outcome measures evaluated.
Despite the lack of any significant difference between the 2 methods of facial vein access, we preferred using a lancet throughout the remainder of the study, on the basis of qualitative observations. Whereas 2 mice in the needle group exhibited bleeding from the ear canal and another was inactive after the procedure, no unanesthetized mice that underwent the lancet method showed any adverse clinical signs. In addition, the lancet method was faster than the needle method, and subsequently reduced the restraint time and the level of handling stress. In addition, the purpose-designed lancet ensures a controlled depth of access, preventing the occurrence of accidental trauma due to excess penetration. As such, the use of a lancet reduces the risk of puncturing the ear canal. Although the blood collection time was significantly shorter in the anesthetized group, the time required to perform anesthesia results in a longer procedure overall, which may be a consideration when deciding which method to use. Anesthesia has previously been recommended for use when facial vein sampling is performed.11 Our results do not provide any evidence to support this recommendation, and in fact, sample quantities seemed lower and platelet values were lower after isoflurane use. However, to provide detailed recommendations on the use of anesthesia with lancets to access the facial vein for blood collection, long-term studies evaluating recovery need to be performed. Regardless, we recommend anesthesia as a practical help when novice handlers are involved or when training users in the method, because the efficacy of restraint is likely to be critical to the success of blood collection from the facial vein of mice.
In conclusion, the absence of statistically significant intergroup differences in the parameters evaluated throughout this study indicate that blood collection via the facial vein can be completed successfully by using either a 21-gauge needle or a 5-mm animal lancet in conscious or anesthetized mice. Although both access methods are acceptable, we prefer the lancet method due to the rapid processing of animals and the lack of any clinically significant signs throughout the procedure.
References
- 1.Abatan OI, Welch KB, Nemzek JA. 2008. Evaluation of saphenous venipuncture and modified tail-clip blood collection in mice. J Am Assoc Lab Anim Sci 47:8–15. [PMC free article] [PubMed] [Google Scholar]
- 2.Conybeare G, Leslie GB, Angles K, Barrett RJ, Luke JSH, Gask DR. 1988. An improved simple technique for the collection of blood samples from rats and mice. Lab Anim 22:177–182. [DOI] [PubMed] [Google Scholar]
- 3.Cutnell J, Johnson K. 1998. Physics. New Jersey (NJ): Wiley. [Google Scholar]
- 4.Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, van de Vorstenbosch C. 2001. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol 21:15–23. [DOI] [PubMed] [Google Scholar]
- 5.Drozdowicz CK, Bowman TA, Webb ML, Lang M. 1990. Effect of inhouse transport on murine plasma corticosterone concentration and blood lymphocyte populations. Am J Vet Res 51:1841–1846. [PubMed] [Google Scholar]
- 6.Duerschlag M, Wuerbel H, Stauffacher M, Von Holst D. 1996. Repeated blood collection in the laboratory mouse by tail incision: modification of an old technique. Physiol Behav 60:1565–1568. [DOI] [PubMed] [Google Scholar]
- 7.Esterling B, Rabin BS. 1987. Stress-induced alterations of lymphocyte-T subsets and humoral immunity in mice. Behav Neurosci 101:115–119. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez I, Pena A, Del Teso N, Perez V, Rodriguez-Cuesta J. 2010. Clinical biochemistry parameters in C57BL/6J mice after blood collection from the submandibular vein and retroorbital plexus. J Am Assoc Lab Anim Sci 49:202–206. [PMC free article] [PubMed] [Google Scholar]
- 9.Golde WT, Gollobin P, Rodriguez LL. 2005. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 34:39–43. [DOI] [PubMed] [Google Scholar]
- 10.Harris RBS, Mitchell TD, Simpson J, Redmann SM, Youngblood BD, Ryan DH. 2002. Weight loss in rats exposed to repeated acute restraint stress is independent of energy or leptin status. Am J Physiol Regul Integr Comp Physiol 282:R77–R88. [DOI] [PubMed] [Google Scholar]
- 11.Heimann M, Kaesermann HP, Pfister R, Roth DR, Buerki K. 2009. Blood collection from the sublingual vein in mice and hamsters: a suitable alternative to retrobulbar technique that provides large volumes and minimizes tissue damage. Lab Anim 43:255–260. [DOI] [PubMed] [Google Scholar]
- 12.Heimann M, Roth DR, Ledieu D, Pfister R, Classen W. 2010. Sublingual and submandibular blood collection in mice: a comparison of effects on body weight, food consumption and tissue damage. Lab Anim 44:352–358. [DOI] [PubMed] [Google Scholar]
- 13.Hem A, Smith AJ, Solberg P. 1998. Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guinea pig, ferret, and mink. Lab Anim 32:364–368. [DOI] [PubMed] [Google Scholar]
- 14.Holmberg H, Kiersgaard MK, Mikkelsen LF, Tranholm M. 2011. Impact of blood sampling technique on blood quality and animal welfare in haemophilic mice. Lab Anim 45:114–120. [DOI] [PubMed] [Google Scholar]
- 15.Kvetnansky R, Mikulaj L. 1970. Adrenal and urinary catecholamines in rats during adaptation to repeated immobilization stress. Endocrinology 87:738-743. [DOI] [PubMed] [Google Scholar]
- 16.Mahl A, Heining P, Ulrich P, Jakubowski J, Bobadilla M, Zeller W, Bergmann R, Singer T, Meister L. 2000. Comparison of clinical pathology parameters with 2 different blood sampling techniques in rats: retrobulbar plexus versus sublingual vein. Lab Anim 34:351–361. [DOI] [PubMed] [Google Scholar]
- 17.Naff KA, Riva CM, Craig SL, Gray KN. 2007. Noise produced by vacuuming exceeds the hearing thresholds of C57Bl/6 and CD1 mice. J Am Assoc Lab Anim Sci 46:52–57. [PubMed] [Google Scholar]
- 18.National Health and Medical Research Council. 2004. Australian code for the care and use of animals for scientific purposes. Canberra (Australia): Australian Government. [Google Scholar]
- 19.Pekow C. 2005. Defining, measuring, and interpreting stress in laboratory animals. Contemp Top Lab Anim Sci 44:41–45. [PubMed] [Google Scholar]
- 20.Szczesny G, Veihelmann A, Massberg S, Nolte D, Messmer K. 2004. Long-term anaesthesia using inhalatory isoflurane in different strains of mice—the haemodynamic effects. Lab Anim 38:64–69. [DOI] [PubMed] [Google Scholar]
- 21.Silverman J. 2010. Clinical biochemistry parameters in C57BL/6J mice after blood collection from the submandibular vein and retroorbital plexus. J Am Assoc Lab Anim Sci 49:400. [PMC free article] [PubMed] [Google Scholar]
- 22.Zeller W, Weber H, Panoussis B, Burge T, Bergmann R. 1998. Refinement of blood sampling from the sublingual vein of rats. Lab Anim 32:369–376. [DOI] [PubMed] [Google Scholar]