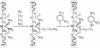Abstract
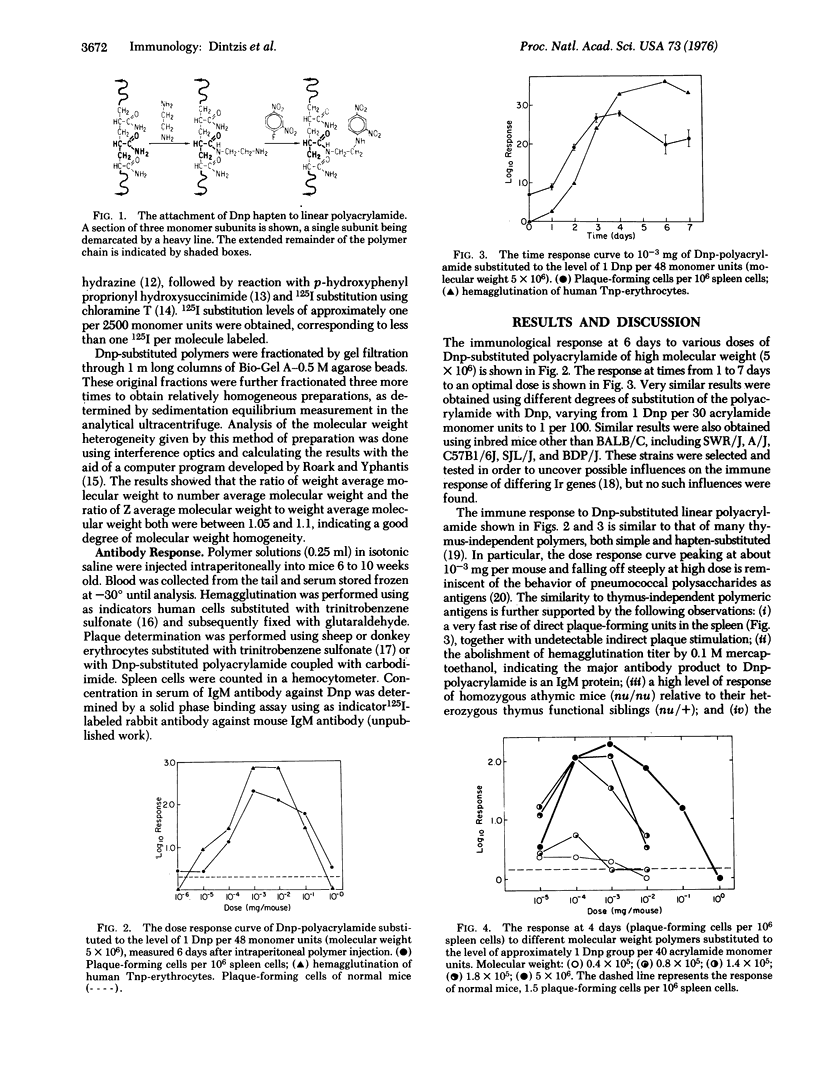
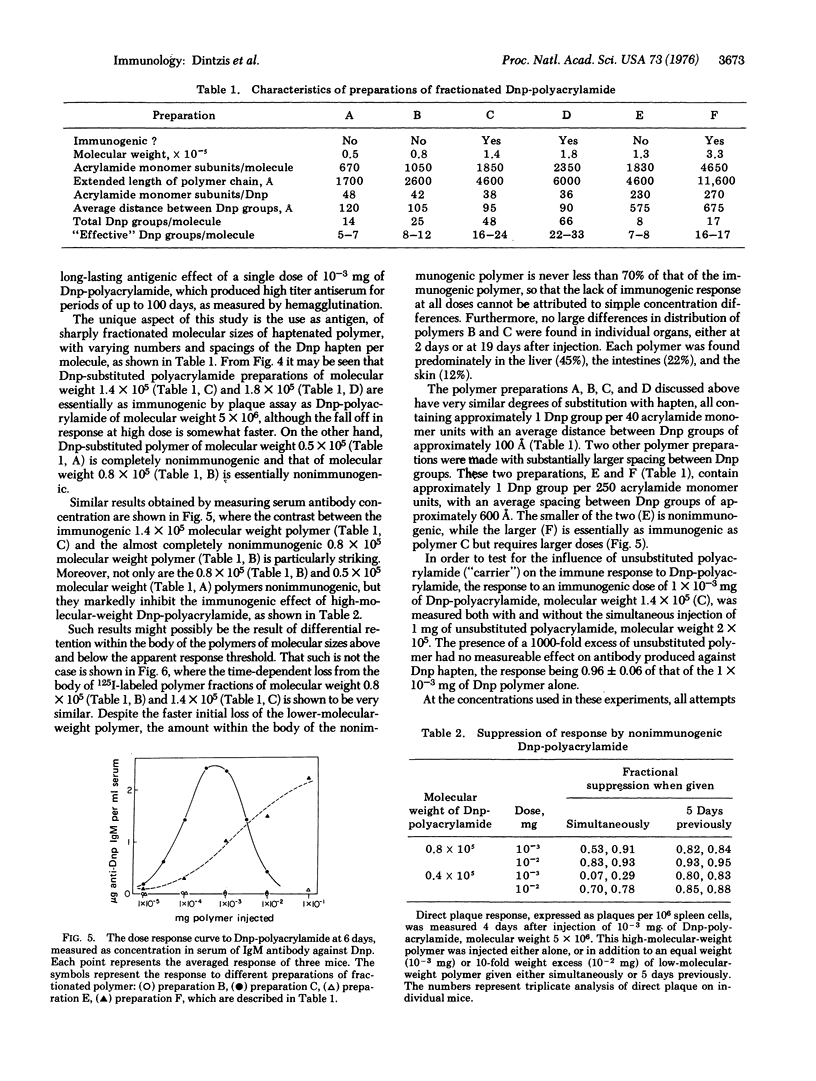
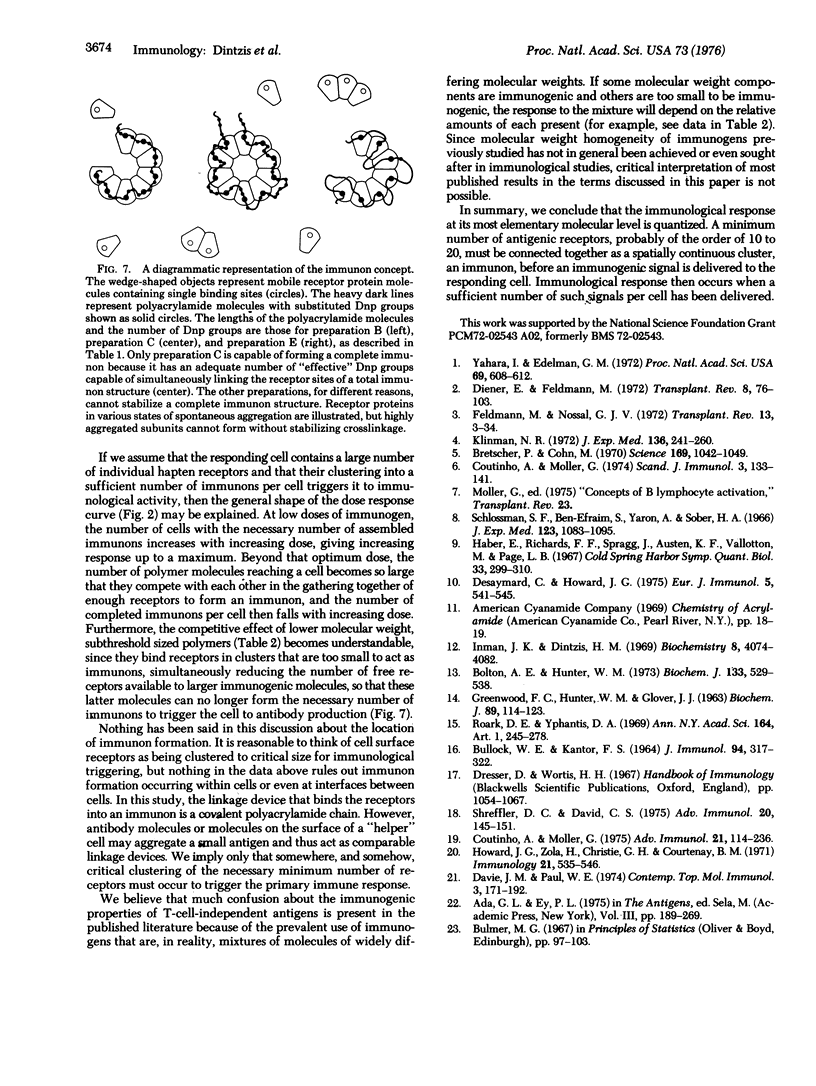
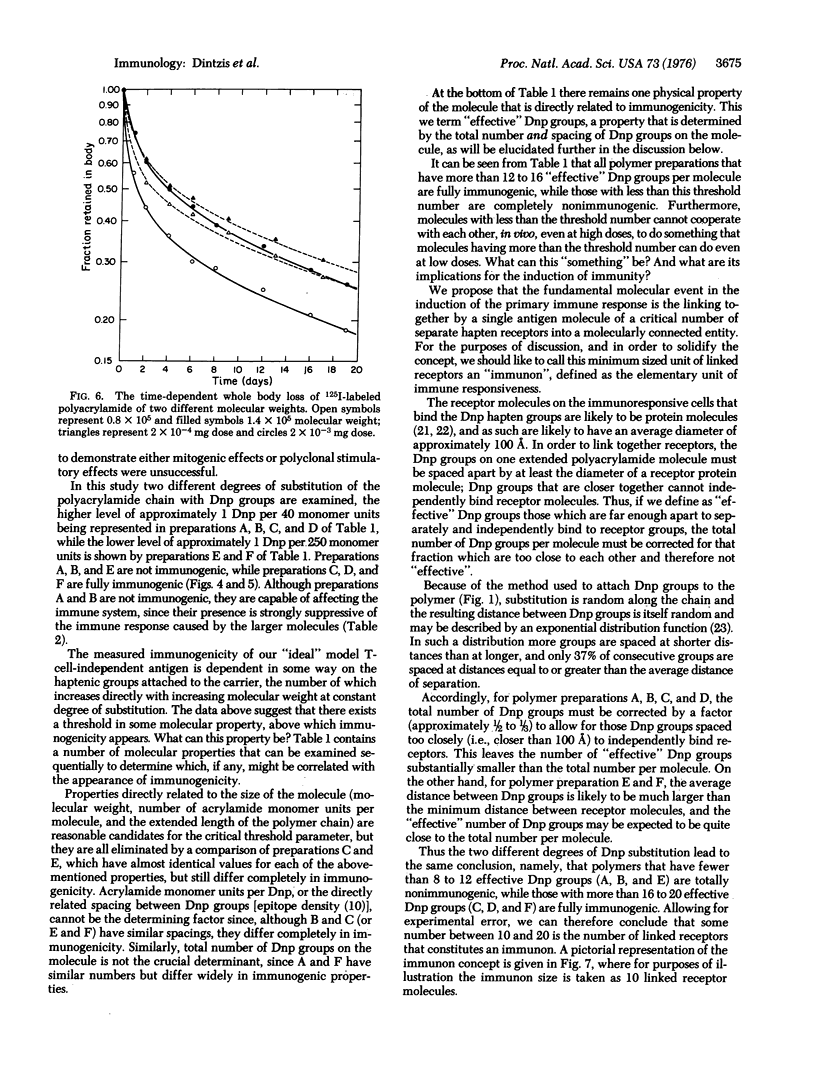
The immunological response in vivo to a series of size-fractionated linear polymers of acrylamide substituted with hapten has been measured in mice. A sharp threshold was observed in immunogenic response elicited by various polymer preparations. All polymers with less than 12 to 16 appropriately spaced hapten groups per molecule were nonimmunogenic, while those polymers with greater than this number were fully immunogenic. The results lead to the conclusion that the immunological response at its most elementary level is quantized, i.e., a minimum specific number of antigen receptors (approximately 12 to 16) must be connected together as a spatially continuous cluster, an immunon, before an immunogenic signal is delivered to the responding cell.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULLOCK W. E., KANTOR F. S. HEMAGGLUTINATION REACTIONS OF HUMAN ERYTHROCYTES CONJUGATED COVALENTLY WITH DINITROPHENYL GROUPS. J Immunol. 1965 Mar;94:317–322. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher P., Cohn M. A theory of self-nonself discrimination. Science. 1970 Sep 11;169(3950):1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Möller G. Editorial: Immune activation of B cells: evidence for 'one nonspecific triggering signal' not delivered by the Ig receptors. Scand J Immunol. 1974;3(2):133–146. [PubMed] [Google Scholar]
- Coutinho A., Möller G. Thymus-independent B-cell induction and paralysis. Adv Immunol. 1975;21:113–236. doi: 10.1016/s0065-2776(08)60220-5. [DOI] [PubMed] [Google Scholar]
- Davie J. M., Paul W. E. Antigen-binding receptors on lymphocytes. Contemp Top Immunobiol. 1974;3:171–192. doi: 10.1007/978-1-4684-3045-5_7. [DOI] [PubMed] [Google Scholar]
- Desaymard C., Howard J. G. Role of epitope density in the induction of immunity and tolerance with thymus-independent antigens. II. Studies with 2,4-dinitrophenyl conjugates in vivo. Eur J Immunol. 1975 Aug;5(8):541–545. doi: 10.1002/eji.1830050807. [DOI] [PubMed] [Google Scholar]
- Diener E., Feldmann M. Relationship between antigen and antibody-induced suppression of immunity. Transplant Rev. 1972;8:76–103. doi: 10.1111/j.1600-065x.1972.tb01565.x. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Nossal G. J. Tolerance, enhancement and the regulation of interactions between T cells, B cells and macrophages. Transplant Rev. 1972;13:3–34. doi: 10.1111/j.1600-065x.1972.tb00058.x. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Zola H., Christie G. H., Courtenay B. M. Studies on immunological paralysis. V. The influence of molecular weight on the immunogenicity, tolerogenicity and antibody-neutralizing activity of the 3 pneumococcal polysaccharide. Immunology. 1971 Sep;21(3):535–546. [PMC free article] [PubMed] [Google Scholar]
- Inman J. K., Dintzis H. M. The derivatization of cross-linked polyacrylamide beads. Controlled introduction of functional groups for the preparation of special-purpose, biochemical adsorbents. Biochemistry. 1969 Oct;8(10):4074–4082. doi: 10.1021/bi00838a026. [DOI] [PubMed] [Google Scholar]
- Klinman N. R. The mechanism of antigenic stimulation of primary and secondary clonal precursor cells. J Exp Med. 1972 Aug 1;136(2):241–260. doi: 10.1084/jem.136.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roark D. E., Yphantis D. A. Studies of self-associating systems by equilibrium ultracentrifugation. Ann N Y Acad Sci. 1969 Nov 7;164(1):245–278. doi: 10.1111/j.1749-6632.1969.tb14043.x. [DOI] [PubMed] [Google Scholar]
- Schlossman S. F., Ben-Efraim S., Yaron A., Sober H. A. Immunochemical studies on the antigenic determinants required to elicit delayed and immediate hypersensitivity reactions. J Exp Med. 1966 Jun 1;123(6):1083–1095. doi: 10.1084/jem.123.6.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Restriction of the mobility of lymphocyte immunoglobulin receptors by concanavalin A. Proc Natl Acad Sci U S A. 1972 Mar;69(3):608–612. doi: 10.1073/pnas.69.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]