Significance
The gecko adhesive system has garnered considerable interest from scientists over the past several decades. Most research has focused on the function and microanatomy of the adhesive system. However, it is currently unclear what impact the secondary loss or simplification of the adhesive system might have on the evolutionary biomechanics of gecko locomotion, which is critical for pinpointing the constraints that accompany such an innovation, and ultimately the release from these constraints. We found that geckos exhibit elevated rates of morphological and kinematic evolution when their adhesive system is lost/simplified, providing evidence that the loss of an innovation can have profound evolutionary impacts on organismal structural and functional divergence
Keywords: biomechanics, toepads, Pachydactylus, adaptation, Namibia
Abstract
Innovations permit the diversification of lineages, but they may also impose functional constraints on behaviors such as locomotion. Thus, it is not surprising that secondary simplification of novel locomotory traits has occurred several times among vertebrates and could potentially lead to exceptional divergence when constraints are relaxed. For example, the gecko adhesive system is a remarkable innovation that permits locomotion on surfaces unavailable to other animals, but has been lost or simplified in species that have reverted to a terrestrial lifestyle. We examined the functional and morphological consequences of this adaptive simplification in the Pachydactylus radiation of geckos, which exhibits multiple unambiguous losses or bouts of simplification of the adhesive system. We found that the rates of morphological and 3D locomotor kinematic evolution are elevated in those species that have simplified or lost adhesive capabilities. This finding suggests that the constraints associated with adhesion have been circumvented, permitting these species to either run faster or burrow. The association between a terrestrial lifestyle and the loss/reduction of adhesion suggests a direct link between morphology, biomechanics, and ecology.
Structural innovations involving coordinated changes in multiple anatomical systems [Frazzetta’s “complex adaptations” (1)] are associated with the diversification of many groups of vertebrates. Many such innovations often occur repeatedly within, as well as between, clades. One example is the evolution of the prehensile tail, which has arisen in primates, nonprimate mammals, seahorses, amphibians, and several groups of lizard, allowing its possessors to move through the environment in novel ways (2–6). Although the acquisition of such innovations is often implicated in both diversification and ecological specialization, much less is known about the causes and consequences of their secondary reduction and loss. If such novelties promote organismal diversity, then the evolutionary simplification of such innovations might commonly occur only when their diminution becomes even more advantageous. Generally, across the tree of life, we might expect that many of the most extraordinary examples of adaptive divergence should coincide not only with the origin and retention of innovations but also with their simplification, and ultimately their loss, in association with adaptation to new situations.
Much of what is known about the secondary simplification of locomotor structures relates to limb loss/reduction in tetrapods (7–11), such as in snakes and lizards. The increase in the rate of evolutionary change in these situations suggests that the origin of an elongate body likely led not only to relaxed selection for the retention of limbs, but also to rapid adaptive selection, favoring both their loss and other associated morphological changes. In these cases, it is expected that both the rate of morphological evolution and the area occupied in phylomorphospace would increase (12). As the mode of locomotion changes, the loss of an innovation would be functionally advantageous and thus favored. However, to determine if this pattern is generalizable, it would be appropriate to investigate the resulting changes and trade-offs that occur during evolutionary reduction and functional loss within a clade that displays a spectrum of changes in a highly functional anatomical complex.
The gekkotan adhesive system has been instrumental in enabling these lizards to occupy otherwise inaccessible regions of the locomotor habitat by enhancing climbing effectiveness through the ability to cling to surfaces. The system is inherently hierarchical, and includes the integration of setae (microscopic beta keratin hair-like structures), scansors (expanded digital scales), modified skeletal elements of the foot, as well as other proximal parts of the limb, and associated locomotor kinematics that permit the system to be deployed. As such, this system is a well-documented example of a complex adaptation (13–16) and is thought to have originated independently at least 11 times within the Gekkota, and to have been lost a minimum of 9 times (17). The lability of the gecko adhesive complex makes it a promising system for examining the evolutionary consequences of repeated adaptive reduction of a locomotor novelty.
The adhesive interaction between gecko digits and the substratum is associated with finely controlled processes that promote both attachment and release. The deployment and disengagement of the adhesive system of geckos is highly choreographed between autopodia (18) and occupies a specific period within the step cycle. This process ultimately places a constraint on how fast a gecko can run when using its adhesive system (19). When running on a level surface, pad-bearing geckos maintain the distal portions of the digits in a hyperextended position (15, 19), thus eliminating the deployment and detachment phases of the adhesive system from the step cycle. However, in comparison with lizards that primitively lack an adhesive system, this precludes the distal parts of the digits from engagement with the substratum and thus reduces the length of the out lever of the foot, reducing the speed at which a gecko can run. For example, Tarentola mauritanica suffers a 37% decrease in running speed when using its adhesive system on a 10° incline compared with a level surface, where the adhesive system is not used (19). However, for the primitively padless gecko Eublepharis macularius, there was no significant change in speed on the incline compared with a level substrate (19).
Adhesion is thought to have evolved to enhance the effectiveness of climbing. However, some lineages of geckos that are nested within clades with a fully expressed adhesive apparatus have reverted to a terrestrial lifestyle and forsaken a functional adhesive system (20, 21). For example, the Pachydactylus radiation of southern Africa contains a majority of species that are rock-dwelling climbers, but several species have evolved to occupy flat ground (20, 22–25). This clade is arguably among the best documented of gekkotans, from a variety of standpoints [evolutionary patterns, morphology, biogeography, locomotion, physiology (17, 22, 24–31)] and provides an excellent vehicle for the investigation of the biomechanics of locomotion that are associated with reduction and, ultimately, effective loss of the adhesive complex.
We examined the functional and morphological consequences of reduction and simplification of the adhesive apparatus within the Pachydactylus radiation. Using a comparative framework, we predict that species that have reduced or have lost adhesive capabilities: (i) will show different limb kinematics than those that use adhesion, (ii) will exhibit accelerated rates of kinematic and morphological evolution, (iii) will display different kinematic responses to changes in substrate incline, and (iv) will run faster or more slowly than those with a fully expressed adhesive system. To address these predictions, we examined 14 species within the Pachydactylus radiation that contains at least two species displaying unambiguous and evolutionarily independent loss of the adhesive system (Chondrodactylus angulifer and Pachydactylus rangei), and two taxa that exhibit substantial reductions (Rhoptropus afer and Colopus wahlbergii). All four species exploit a range of novel ecological circumstances and locomotor substrata.
Results
Morphology.
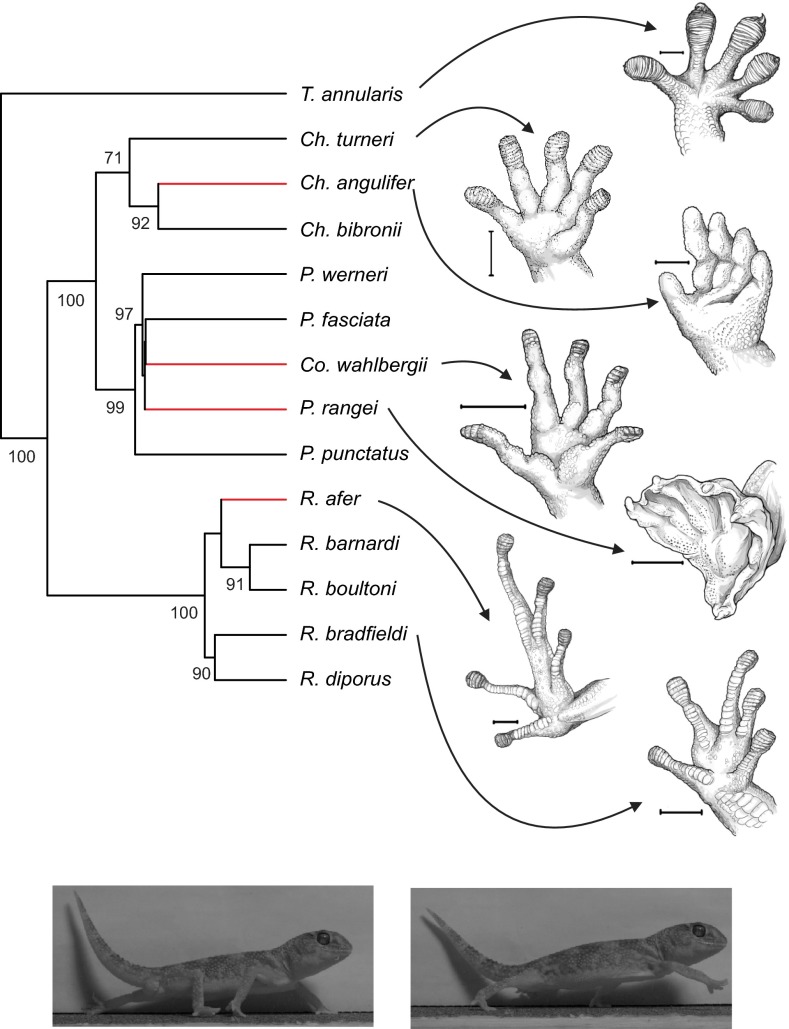
Species of gecko that have simplified or lost the adhesive system (Asimp) tend to have either longer or shorter limb segments compared with species with fully functional (Afunc) adhesive systems (Fig. 1 and Table S1). For example, C. angulifer and C. wahlbergii both exhibit decreased digit 3 lengths, whereas R. afer exhibits an increase relative to Afunc species (Fig. 1). P. rangei exhibits an intermediate morphology, falling within the range of the Afunc species. Although the length of the crus is evolving only 1.2-times faster in the Asimp species, both the thigh and digit 3 exhibit a greater rate of evolution in the Asimp species compared with the Afunc species, with the former evolving 5.1-times faster in the Asimp species, and the latter 6.6-times faster.
Fig. 1.
Phylogenetic relationships among the 14 species of gecko examined in this study, with illustrations of the ventral side of the right hindfoot of 7 of these. (Scale bars: 2 mm.) Tarentola annularis was included as the outgroup of the Pachydactylus radiation. Numbers indicate posterior probabilities behind the nodes that have over 50% posterior support. The branches in red indicate those species that either exhibit a substantial simplification or complete loss of the adhesive apparatus (see Methods for details). The images of gecko feet to the right of the phylogeny highlight several of the morphological changes that accompany loss of adhesion. The example still images at the bottom show Chondrodactylus angulifer at footfall (Left) and end stance (Right) of the right pes in the 30° incline treatment.
Kinematics.
For all species combined, speed ranged from ∼0.5 ms−1 to ∼2.6 ms−1 for all conditions combined. For phylogenetically uncorrected values, there was a strong positive correlation between stride frequency and speed (on both level and incline) and between stride length and speed (Fig. S1). In addition, all species exhibited a decrease in running speed with a transition from locomotion on the level to that on the incline. This decrease ranged from 0.07 ms−1 (Rhoptropus barnardi) to 0.91 ms−1 (C. wahlbergii), resulting in an average decrease of 26% (Fig. S1 and Table S2). This decrease in speed on average resulted from a combination of 10.4% decrease in stride frequency and an 18.4% decrease in stride length.
Using the scores from the phylogenetic principal component analysis (PCA), which included 10 kinematic variables, there were striking differences between the groups. On PC1, the kinematics were different between the Afunc and Asimp groups on the incline, but not on the level substrate (Table S3). For PC2, the kinematics were markedly different between the Afunc and Asimp groups on the level, but not on the incline (Table S3). This finding indicates that two orthogonal axes of kinematic divergence separate the Afunc and Asimp clusters.
The key variables driving PC1 (loadings ≥ 0.3) on the incline were femur depression at footfall, knee angle at the end of stance, minimum knee angle, minimum ankle angle, duty factor, stride frequency, and speed (Table S3). For example, femur depression was much less at footfall for the Asimp species compared with the Afunc species (Fig. 2). The key variables driving PC2 on the level were femur depression at footfall, femur rotation, minimum femur depression, stride length (relative to intergirdle length), and speed (Table S3). It is also clear that the Asimp and Afunc groups occupy different regions of phylokinematic space (Fig. 3).
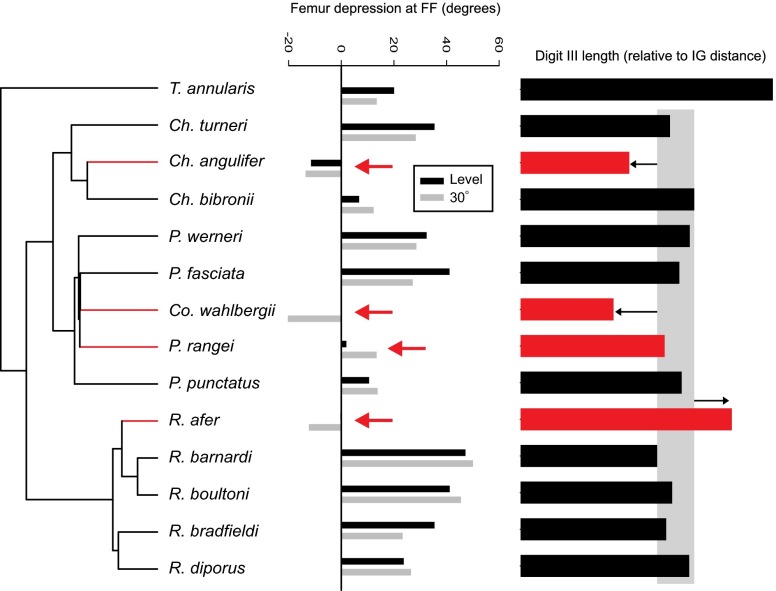
Fig. 2.
Femur depression at footfall and the relative length of pedal digit 3 mapped onto the phylogeny of the members of the Pachydactylus radiation that we examined. Each value represents the average of multiple individuals, and for femur depression, both level (black) and incline (gray) are shown. The red arrows indicate the values for species within the Asimp group. Note that values are significantly lower for the Asimp species, reflecting a sprawled posture. In addition, the rate of evolution of femur depression at footfall was more than nine-times greater in the Asimp group than the Afunc group. The relative lengths of pedal digit 3 are shown to the right. To highlight one striking instance of morphological conservation, the gray bar encompasses the range of pedal digit 3 lengths in Afunc members of the Pachydacytus radiation that we examined. The arrows indicate the reduction or elongation of Asimp species relative to the range delineated by the Afunc group.
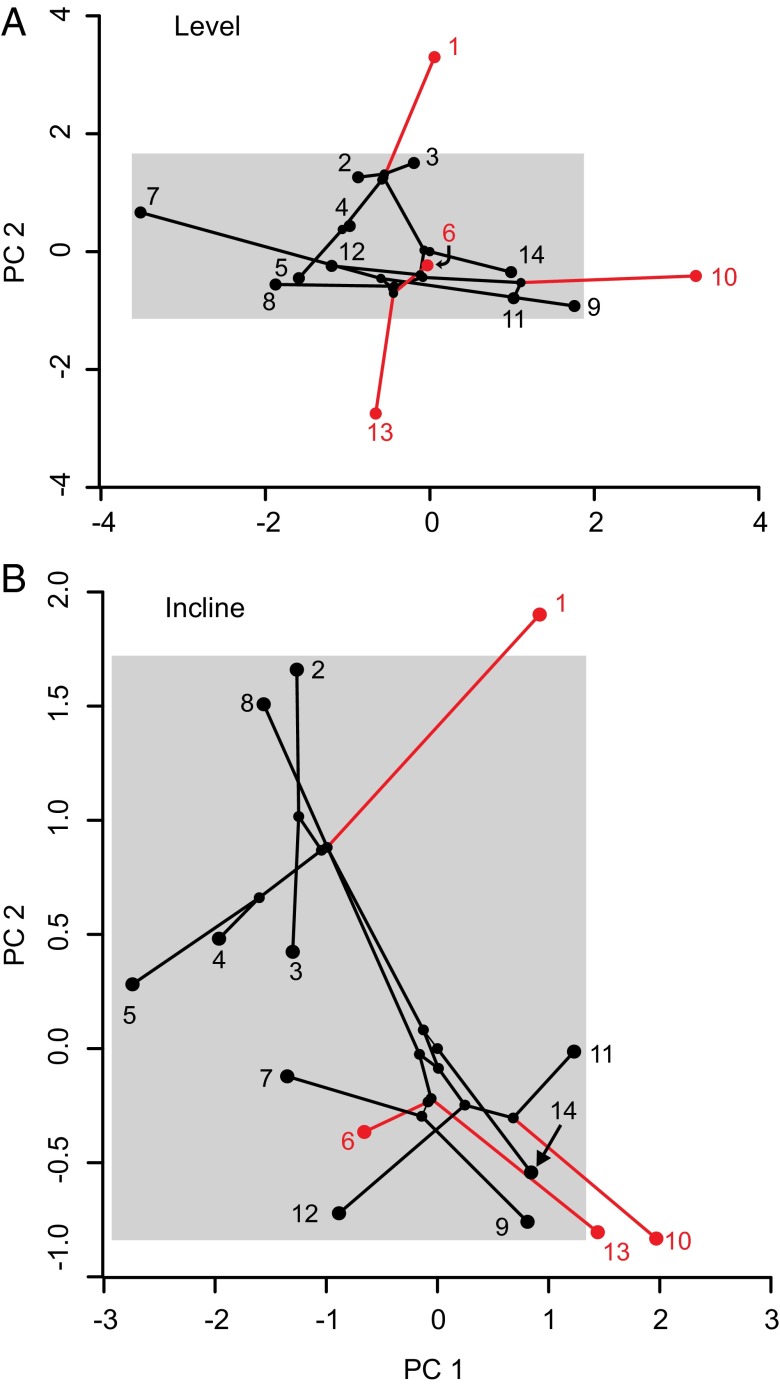
Fig. 3.
Phylogenetic PCAs were performed for 10 kinematic variables measured on all of the species on the level (A) and inclined (B) locomotor substrates. The lines connecting the values for the species in this phylokinematic space depict the evolutionary relationships among the species, with the Asimp species being shown in red and the Afunc species in black. The range of values for the Afunc taxa is partitioned in gray to highlight observed differences in PC scores between the two adhesive categories and where the Asimp groups diverged outside of the range of Afunc species. On the incline, the kinematics on PC1 were found to evolve significantly differently in the Afunc and Asimp groups, although PC1 did not differentiate the groups on the level substrate. However, PC2 kinematics were highly different between the Afunc and Asimp groups on the level substrate but not the incline. The difference in locomotor substrate uncovered two evolutionary orthogonal axes that separated the kinematic divergence between the two adhesive categories of geckos and differed based on the orientation of the substrate. 1: R. afer; 2: Rhoptropus bradfieldi; 3: Rhoptropus diporus; 4: R. boultoni; 5: R. barnardi; 6: P. rangei; 7: Pachydactylus werneri; 8: Pachydactylus punctatus; 9: Pachydactylus fasciatus; 10: C. angulifer; 11: Chondrodactylus bibronii; 12: Chondrodactylus turneri; 13: Colopus wahlbergii; 14: T. annularis.
Rates of Kinematic Evolution.
Overall analysis of the PCA results (from PCA scores) reveals that the rates of kinematic evolution between the Afunc and Asimp groups are effectively identical on the level surface. For example, the two-rate model shows that the kinematics of the Asimp species are evolving with a σ2 of 4.84 and the kinematics of the Afunc species are evolving with a σ2 of 4.97.
Alternatively, on the incline kinematics of the Asimp species appear to evolve 2.7-times faster than the Afunc species. Notably, locomotor speed in the Asimp species is evolving ∼1.5-times faster than the Afunc species. Additionally, the height of the shoulder at midstance in the Asimp species is evolving 4.1-times faster than in the Afunc species (Table S4), indicating differential rates of behavioral evolution. Other variables that differed strongly in rate between the two adhesive groups were duty factor and femur depression at footfall. In the Asimp species these two traits were inferred to be evolving almost 10-times faster than for the Afunc species (Table S4).
Discussion
Markedly reducing or simplifying the adhesive system significantly alters how geckos run. In addition to changes in speed and kinematics, the rate of morphological evolution is greater in those species that exhibit loss or simplification of their adhesive apparatus, suggesting that the constraints associated with adhesion have been circumvented. The association between a terrestrial lifestyle and the loss/reduction of adhesion suggests a direct link between morphology, biomechanics, and ecology. Based on previous work showing a trade-off between climbing and sprinting ability among geckos (19), it is not surprising that terrestrial geckos would undergo evolutionary reduction or simplification of the adhesive system. In fact, reduction or simplification could remove constraints on locomotion that would otherwise preclude cursorial geckos from obtaining high speeds (32) or effectively burrowing and moving on loose sand that can clog their setae (33).
Evolution of Morphology.
Geckos that exhibit reduction or simplification of the adhesive apparatus undergo an increased rate of morphological evolution, at least for the upper hindlimb (thigh) and hind foot. We propose two explanations for these accelerated rates. The first explanation relates to the constraints placed upon limb function and structure by the presence of the adhesive system. The acquisition of pads within geckos likely imposed constraints on the digits to enable adhesive attachment (34). Thus, mechanical demands can limit the way in which different limb segments are altered. The second explanation relates to use of inclined habitats. Those species that occupy relatively horizontal habitats, and that have reduced or essentially lost the adhesive system, use their limbs for functions other than climbing. These new functions result in changes within the limbs. For example, R. afer is able to run very rapidly, and does so over long distances. This species exhibits an increase in leg and toe length (24, 28), which translate to an increase in out lever lengths for propulsion, which, in turn, results in greater speed.
In contrast to the increases in toe length noted above, C. angulifer and C. wahlbergii exhibit decreases in toe length relative to species that maintain an adhesive system (Fig. 1). These species also exhibit behaviors that differ from pad-bearing species. They both dig burrows and exhibit spinous scales on swollen plantar surfaces (33). This aspect likely enhances the exclusion of sand while digging, and avoids clogging of the plantar surfaces. The reduction in toe length documented here may aid in force generation during digging because the length of the out lever is reduced, elevating mechanical advantage. Interestingly, P. rangei, which does not have an adhesive system, exhibits moderate levels of both femur depression and digit 3 length (Fig. 2). This species excavates burrows and has webbed feet, which are important for moving on top of soft, fine-grained sand (33, 35) and for shifting excavated material. The existence of webbing may have thus constrained the morphology and kinematics of this species in different ways, yielding intermediate values relative to other Asimp species.
We recovered varying rates of evolution among external morphological characteristics of the hindlimbs, but it is clear that internal morphology varies considerably among geckos, even when external morphology appears similar (34, 36). The complexity of the adhesive control mechanism may drive some of this internal variation. It is noteworthy that the elongation of the digits in R. afer is achieved by marked elongation of the proximal phalanges, whereas the distal phalanges, which are associated with the carriage of the adhesive system, become greatly shortened (28). The reduction in size of the adhesive system in this species (31) and the elongation of the proximal phalanges clearly illustrates the two functional modalities of the digits, with their basal parts being used in running on the level [even in species with a fully expressed adhesive system (19)], and the distal portion carrying the adhesive system being deployed on inclines. With R. afer’s invasion of a largely horizontally structured habitat, the proximal parts of its digits have likely become increasingly important as force modified levers during locomotion, resulting in changes in limb proportions that have occurred over relatively short stretches of evolutionary time (24). It is evident that the reduction and loss of the adhesive system has impacted the rates of evolution of internal morphological features. Future investigations should integrate the structure of the forelimbs into this approach (37).
Evolution of Kinematics.
Hindlimb kinematics varied considerably among the 14 species of gecko in our study. Speed differed between species, and both stride frequency and length were greater for the faster species (Fig. S1). Speed also decreased for every species when moving on the incline relative to moving on the level. This is a common finding for terrestrial vertebrates (38), and likely reflects the increase in energetic demand associated with moving uphill. In addition, the modulation of both stride frequency and length appear to be important for changing speed with changes in incline, although adjustment of stride length appears to be slightly more important. This finding contrasts with a recent report of the absence of differences in stride-length of terrestrial animals on level and inclined surfaces (38). Why geckos exhibit a decrease in stride length is likely related to the large decrease in speed that they exhibit when moving on a 30° incline.
Interestingly, some species that exhibited a relatively modest change in speed with changes in incline actually underwent a shift in the patterns of locomotor movements responsible for speed. For example, Rhoptropus boultoni exhibited a decrease in running speed of only 4.4% with an increase in incline, this being accomplished by a 6.4% increase in stride frequency and an 8.6% decrease in stride length. A shift in this control may be beneficial for geckos that maintain a relatively constant speed. For example, taking shorter and more frequent steps on inclined surfaces may enhance stability.
A key difference in kinematics between the Afunc and Asimp species was femur depression (Fig. 2). The values for Afunc species are greater and fall within a fairly narrow range (Fig. 2). Thus, geckos with a well-developed adhesive system adopt a more upright limb posture, which is also supported by greater knee angles at the end of stance and larger minimum ankle angles. This finding is somewhat surprising given that terrestrial desert-dwelling geckos have been noted to adopt a more upright posture to enhance their field of view (39). However, other selective pressures may override this benefit. One possibility is that the more sprawling posture adopted by the Asimp group reflects the need for stability. A more sprawled posture enhances stability in lizards by increasing the base of support (40). Perhaps those species that have a well-developed adhesive system need not rely on posture for stability because the adhesive system itself may enhance it. In addition, the increased femur depression exhibited by the Afunc group may allow the adhesive system to stay in contact with the substratum longer and make contact earlier. Future studies exploring the impact of an adhesive system on stability would be interesting.
We found that the kinematics of the Afunc species were significantly different from those of the Asimp species on both inclined and level surfaces, but each orientation was represented by a different phylogenetic PC axis (Fig. 3). Thus, the kinematic differences on the inclined and level surfaces that separate geckos in the two adhesive categories are completely evolutionarily independent. Because they separate along PC1 only on the incline, this finding suggests that inclination is more important for driving kinematic differences between the groups. The fact that the incline treatment explained more variation in the data suggests that it is the more demanding of the two circumstances, which has been confirmed for other terrestrial vertebrates. The differences on the inclines also make sense given that the species with an adhesive system are predominantly climbers, and rarely (if ever) move on level surfaces. However, the kinematic PC2 clearly differentiates the groups when on a level substrate. Thus, the differential response to incline is likely because of the fact that the adhesive system is critical during climbing, but not during level locomotion. Overall, we show that the two groups not only exhibit different rates of kinematic evolution, but also occupy different regions of phylokinematic space (Fig. 3). Importantly, elevated rates of evolution do not necessarily imply the exploration of novel phenotypes (12). However, our phylokinematic space of locomotion in the Pachydactylus radiation revealed both elevated rates and new kinematics in Asimp species that were outside the range adopted by the Afunc group.
Adaptive Secondary Simplification.
Morphological innovations like the adhesive system of geckos are complexly integrated modifications of a phenotype that are often associated with diversification (41, 42) as well as the invasion of previously unavailable niches (43). However, using an adhesive system requires additional active attachment and detachment actions related to the adhesive setae. During running, digits must also be flattened from the hyperextended state and adpressed against the substratum subsequent to footfall, as well as hyperextended before the end of the stance phase (16). This added engagement-detachment cycle related to the deployment of the adhesive system necessarily slows a gecko down, as evidenced in situations in which the adhesive system may or may not be deployed (19). If increased competition or the formation of a structurally novel environment (e.g., desertification) results in geckos occupying flatter, more open terrain, then selection likely favored the reduction or loss of the adhesive system (44, 45). Furthermore, the origin of locomotory abilities not associated with climbing, such as burrowing in nocturnal species and greater running speeds in diurnal geckos, could lead to the rapid evolution of both morphology and kinematics. We suggest that significantly reducing or simplifying the adhesive system in secondarily terrestrial geckos exemplifies an adaptation that promoted increased rates of morphological and kinematic evolution and is likely related to their effective exploitation of previously unavailable habitats.
Unlike exaptations, which result from the co-option of a structure from an original function to a “new” one (46), evolution can also favor the reduction or simplification of a previous complex adaptation, especially when the adaptive complex compromises functional abilities in a new environment. This type of adaptive change, resulting in reduction or loss, has been documented for a number of other taxa, such as eye loss in cave fishes (47), in which the energetic cost of maintaining vision is likely great enough to be detrimental to survival in a cave environment. The reduction and simplification of the adhesive apparatus in geckos is not the only such case exhibited by the locomotor system. Almost every tetrapod group includes cases of limb reduction or loss, and associated body elongation. Following a shift to lateral undulation, the girdles are spaced farther apart and reduced, and sinuous displacement of the body axis lessens the effectiveness of the limbs as propulsive devices or anchors, and the limbs likely become a hindrance to forward progression (10). Thus, limb reduction or loss in vertebrates is analogous to the loss of the adhesive system in geckos in that both represent responses to locomotor demands imposed by exploitation of new sectors of the environment. Such interactions through new synergs (44) lead to selective pressures that countermand the maintenance of the original adaptive complex in certain ecological situations.
A recent study of different populations of R. afer found that the morphology of the adhesive apparatus was related to the use of inclines in the habitat (22). Those populations that occupy habitats dominated by shallower inclines exhibited a greater degree of reduction of the adhesive apparatus. Thus, it is clear that there is a measurable relationship between the structure of the habitat in which a gecko lives and the morphology of the adhesive system. It is currently unclear how these morphological differences among populations translate into functional differences, but because of the macroevolutionary patterns uncovered here this system clearly warrants further study.
Conclusions
When geckos simplified or lost their adhesive capabilities their evolutionary rates of change in morphology and 3D locomotor kinematics were elevated. However, our study examining the movements of the limbs would be nicely complemented by dynamic force measurements that would help to more fully link changes in evolutionary biomechanics of gecko locomotion (48).
Methods
Animals and Field Studies.
Fourteen species of gecko were used in this study (Fig. 1). Apart from two species (Tarentola annularis and Chondrodactylus bibronii), which were obtained through the pet trade, two to four individuals of each species were collected from coastal areas around Swakopmund and inland areas around Gobabeb, Brandberg, and Spitzkoppe in Namibia, Africa. At these sites, the geckos typically occupied sheet rock, hard-packed sand, or rocky outcrops. The lizards were caught by hand or noose. Lizards were immediately placed in a breathable cotton bag and transported to a laboratory in Swakopmund. Animals were handled humanely under University of California, Riverside Institutional Animal Care and Use Committee AUP# A20110038E and a Ministry of Environment and Tourism research permit (1706/2012) from Namibia.
Morphology.
Morphological measurements [body mass, snouth-vent length, thigh length, crus length, intergirdle distance (distance along the dorsal midline between the center of the pectoral and pelvic girdles), and length of the third toe] were taken using a Pesola spring balance (error = 0.1 g) and digital calipers (error = 0.001 mm). Linear morphological variables were scaled to intergirdle length.
Experimental Procedures.
Two high-speed cameras (Phantom Miro M110) operating at 1,000 Hz were used to obtain dorsal and lateral views of each gecko running along a level or inclined (30°) surface. The trackway was ∼1-m long and lined with 60-grit sandpaper to mimic a natural surface. All geckos were run at a comparable body temperature of 30 °C. Only those trials in which the gecko ran steadily across the field of view were used for further analyses.
Biomechanical Analyses.
Three-dimensional coordinates of markers on the dorsal midline (five to seven markers), between the two girdles, as well as limb markers on the left and right hip, right knee, right ankle, and right hindlimb digit tips were digitized using DLTdv3 (49) in Matlab (release R2012b, The Mathworks). The x, y, and z axes represent the fore-aft, vertical, and medio-lateral planes, respectively. Following digitization, the coordinates were processed using custom written script in Matlab (R2013b, The Mathworks) to obtain a proxy for center of mass (CoM, by averaging the dorsal markers along the spine), and joint angles and spatiotemporal characteristics for the hindlimb. The CoM proxy was then used to determine speed. Stride length (fore-aft distance traveled from footfall to subsequent footfall of the same leg) and step length (fore-aft distance traveled during the stance phase) were calculated based on the mid dorsal spine marker. For details regarding the calculation of the 3D joint angles, see SI Methods.
Comparative Analyses.
Phylogenetic relationships among the 14 species in this study were determined using genetic information available from GenBank for cytb and 12S mitochondrial sequences, and RAG1 nuclear sequences (see SI Methods for details). Using the 100 phylogenies, we generated a mean and SE for all comparisons between geckos exhibiting a fully functional adhesive system (Afunc) and those with a significantly simplified, reduced, or fully lost adhesive system (Asimp). These categories were based on previous studies that found a significantly reduced adhesive system relative to other closely related species, or the complete loss of the system. Four species were included in the Asimp group, P. rangei, C. wahlbergii, C. angulifer, and R. afer. All other species were assigned to the Afunc category. Although we acknowledge that some variation may occur between those species with simplified (reduced) adhesive systems and those that have completely lost the system, we grouped them to retain sufficient statistical power.
We tested for different rates (σ2) of Brownian motion phenotypic evolution of both morphological and kinematic traits between groups of species in our two adhesive categories using the program OUwie (50) implemented in R (51). Additionally, we examined support for differences in a constant rate for all of the geckos examined and for different rates in the two adhesive categories. Models of Brownian motion (one rate vs. two rates) showing the lowest Akaike Information Criterion value are discussed. The rates of morphological evolution were determined, and the changes from Afunc to Asimp were assessed.
To reduce the evolutionary variation in kinematics to two major evolutionary axes, we performed phylogenetic PCA on the 10 kinematic variables using functions available in phytools (52, 53). The scores from these PCAs were then examined using a phylogenetic ANOVA (54) with adhesive category (Afunc or Asimp) as the grouping variable. Each combination of phylogenetic PCA axis and incline was assessed separately. Finally, using OUwie (50) the Brownian motion rate on the incline treatment was determined for each kinematic variable individually to determine which variables showed the greatest differences in rate between the two adhesive categories. We depict PCA divergence in the locomotion of the geckos on both the incline and level surfaces using a “phylokinematic space” approach. Divergence in the phylokinematic space is depicted with respect to the phylogenetic relationships among species, directionality of change, and amount of divergence (branch lengths) in the kinematic principal components (12).
Supplementary Material
Acknowledgments
We thank the Namibia Ministry of Fisheries and Marine Resources (Swakopmund) and Gobabeb Research and Training Centre for providing valuable laboratory space, accommodation, and advice, and Amy Cheu for providing the artwork for Fig. 1. This research was supported by National Science Foundation Grant IOS-1147043 (to T.E.H.) and Natural Sciences and Engineering Research Council Grant 9745-2008 (to A.P.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1418979112/-/DCSupplemental.
References
- 1.Frazzetta TH. Complex Adaptations in evolving Populations. Sinauer Associates; Sunderland, MA: 1975. pp 267. [Google Scholar]
- 2.Garber PA, Rehg JA. The ecological role of the prehensile tail in white-faced capuchins (Cebus capucinus) Am J Phys Anthropol. 1999;110(3):325–339. doi: 10.1002/(SICI)1096-8644(199911)110:3<325::AID-AJPA5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 3.German RZ. The functional morphology of caudal vertebrae in New World monkeys. Am J Phys Anthropol. 1982;58(4):453–459. doi: 10.1002/ajpa.1330580414. [DOI] [PubMed] [Google Scholar]
- 4.Hale ME. Functional morphology of ventral tail bending and prehensile abilities of the seahorse, Hippocampus kuda. J Morphol. 1996;227(1):51–65. [Google Scholar]
- 5.Meldrum DJ. Tail-assisted hind limb suspension as a transitional behavior in the evolution of the platyrrhine prehensile tail. In: Strasser E, Fleagle J, Rosenberger A, McHenry H, editors. Primate Locomotion: Recent Advances. Plenum Press; New York: 1998. pp. 145–156. [Google Scholar]
- 6.Zippel KC, Glor RE, Bertram JEA. On caudal prehensility and phylogenetic constraint in lizards: The influence of ancestral anatomy on function in Corucia and Furcifer. J Morphol. 1999;239(2):143–155. doi: 10.1002/(SICI)1097-4687(199902)239:2<143::AID-JMOR3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 7.Bejder L, Hall BK. Limbs in whales and limblessness in other vertebrates: Mechanisms of evolutionary and developmental transformation and loss. Evol Dev. 2002;4(6):445–458. doi: 10.1046/j.1525-142x.2002.02033.x. [DOI] [PubMed] [Google Scholar]
- 8.Wiens JJ, Slingluff JL. How lizards turn into snakes: A phylogenetic analysis of body-form evolution in anguid lizards. Evolution. 2001;55(11):2303–2318. doi: 10.1111/j.0014-3820.2001.tb00744.x. [DOI] [PubMed] [Google Scholar]
- 9.Lande R. Evolutionary mechanisms of limb loss in tetrapods. Evolution. 1978;32(1):73–92. doi: 10.1111/j.1558-5646.1978.tb01099.x. [DOI] [PubMed] [Google Scholar]
- 10.Gans C. Tetrapod limblessness: Evolution and functional corollaries. Am Zool. 1975;15(2):455–467. [Google Scholar]
- 11.Skinner A, Lee MSY, Hutchinson MN. Rapid and repeated limb loss in a clade of scincid lizards. BMC Evol Biol. 2008;8:310. doi: 10.1186/1471-2148-8-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sidlauskas B. Continuous and arrested morphological diversification in sister clades of characiform fishes: A phylomorphospace approach. Evolution. 2008;62(12):3135–3156. doi: 10.1111/j.1558-5646.2008.00519.x. [DOI] [PubMed] [Google Scholar]
- 13.Autumn K, et al. Adhesive force of a single gecko foot-hair. Nature. 2000;405(6787):681–684. doi: 10.1038/35015073. [DOI] [PubMed] [Google Scholar]
- 14.Autumn K, Peattie AM. Mechanisms of adhesion in geckos. Integr Comp Biol. 2002;42(6):1081–1090. doi: 10.1093/icb/42.6.1081. [DOI] [PubMed] [Google Scholar]
- 15.Russell AP. A contribution to the functional analysis of the foot of the Tokay, Gekko gecko (Reptilia: Gekkonidae) J Zool. 1975;176(4):437–476. [Google Scholar]
- 16.Russell AP. Integrative functional morphology of the gekkotan adhesive system (Reptilia: Gekkota) Integr Comp Biol. 2002;42(6):1154–1163. doi: 10.1093/icb/42.6.1154. [DOI] [PubMed] [Google Scholar]
- 17.Gamble T, Greenbaum E, Jackman TR, Russell AP, Bauer AM. Repeated origin and loss of adhesive toepads in geckos. PLoS ONE. 2012;7(6):e39429. doi: 10.1371/journal.pone.0039429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Autumn K, et al. Dynamics of geckos running vertically. J Exp Biol. 2006;209(Pt 2):260–272. doi: 10.1242/jeb.01980. [DOI] [PubMed] [Google Scholar]
- 19.Russell AP, Higham TE. A new angle on clinging in geckos: Incline, not substrate, triggers the deployment of the adhesive system. Proc Biol Sci. 2009;276(1673):3705–3709. doi: 10.1098/rspb.2009.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haacke WD. The burrowing geckos of Southern Africa, 5 (Reptilia: Gekkonidae) Ann Transvaal Mus. 1976;30(5):71–89. [Google Scholar]
- 21.Russell AP. Some comments concerning interrelationships amongst gekkonine geckos. In: Bellairs AD, Cox CB, editors. Morphology and Biology of Reptiles, Linnean Society Symposium Series. Academic; London: 1976. pp. 217–244. [Google Scholar]
- 22.Collins CE, Russell AP, Higham TE. Subdigital adhesive pad morphology varies in relation to structural habitat use in the Namib Day Gecko, Rhoptropus afer. Funct Ecol. 2014 doi: 10.1111/1365-2435.12312. [DOI] [Google Scholar]
- 23.Haacke WD, Odendaal FJ. The distribution of the genus Rhoptropus (Reptilia, Gekkonidae) in the central Namib Desert. Madoqua. 1981;12(4):199–215. [Google Scholar]
- 24.Higham TE, Russell AP. Divergence in locomotor performance, ecology, and morphology between two sympatric sister species of desert-dwelling gecko. Biol J Linn Soc Lond. 2010;101(4):860–869. [Google Scholar]
- 25.Lamb T, Bauer AM. Footprints in the sand: Independent reduction of subdigital lamellae in the Namib-Kalahari burrowing geckos. Proc Biol Sci. 2006;273(1588):855–864. doi: 10.1098/rspb.2005.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauer AM. Phylogeny and biogeography of the geckos of southern Africa and the islands of the western Indian Ocean: A preliminary analysis. In: Peters G, Hutterer R, editors. Vertebrates in the Tropics. Museum Alexander Koenig; Bonn: 1990. pp. 275–284. [Google Scholar]
- 27.Bauer AM, Good DA. Phylogenetic systematics of the day geckos, genus Rhoptropus (Reptilia: Gekkonidae), of south-western Africa. J Zool (Lond) 1996;238(4):635–663. [Google Scholar]
- 28.Bauer AM, Russell AP, Powell GL. The evolution of locomotor morphology in Rhoptropus (Squamata: Gekkonidae): Functional and phylogenetic considerations. Afr J Herpetol. 1996;45(1):8–30. [Google Scholar]
- 29.Autumn K. Secondarily diurnal geckos return to cost of locomotion typical of diurnal lizards. Physiol Biochem Zool. 1999;72(3):339–351. doi: 10.1086/316666. [DOI] [PubMed] [Google Scholar]
- 30.Lamb T, Bauer AM. Mitochondrial phylogeny of Namib day geckos (Rhoptropus) based on cytochrome b and 16S rRNA sequences. Copeia. 2001;2001(3):775–780. [Google Scholar]
- 31.Johnson MK, Russell AP. Configuration of the setal fields of Rhoptropus (Gekkota: Gekkonidae): Functional, evolutionary, ecological and phylogenetic implications of observed pattern. J Anat. 2009;214(6):937–955. doi: 10.1111/j.1469-7580.2009.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higham TE, Irschick DJ. Springs, steroids, and slingshots: The roles of enhancers and constraints in animal movement. J Comp Physiol B. 2013;183(5):583–595. doi: 10.1007/s00360-012-0734-z. [DOI] [PubMed] [Google Scholar]
- 33.Bauer AM, Russell AP. Pedal specializations in dune-dwelling geckos. J Arid Environ. 1991;20(1):43–62. [Google Scholar]
- 34.Russell AP. Parallelism and integrated design in the foot structure of Gekkonine and Diplodactyline geckos. Copeia. 1979;1979(1):1–21. [Google Scholar]
- 35.Russell AP, Bauer AM. Substrate excavation in the Namibian web-footed gecko, Palmatogecko rangei Andersson 1908, and its ecological significance. Trop Zool. 1990;3(2):197–207. [Google Scholar]
- 36.Johnson MK, Russell AP, Bauer AM. Locomotor morphometry of the Pachydactylus radiation of lizards (Gekkota: Gekkonidae): A phylogenetically and ecologically informed analysis. Can J Zool. 2005;83(12):1511–1524. [Google Scholar]
- 37.Foster KL, Higham TE. How forelimb and hindlimb function changes with incline and perch diameter in the green anole, Anolis carolinensis. J Exp Biol. 2012;215(Pt 13):2288–2300. doi: 10.1242/jeb.069856. [DOI] [PubMed] [Google Scholar]
- 38.Birn-Jeffery AV, Higham TE. The scaling of uphill and downhill locomotion in legged animals. Integr Comp Biol. 2014;54(6):1159–1172. doi: 10.1093/icb/icu015. [DOI] [PubMed] [Google Scholar]
- 39.Werner YL, Broza M. Hypothetical function of elevated locomotory postures in geckos (Reptilia: Gekkonidae) Isr J Zool. 1969;18:349–355. [Google Scholar]
- 40.Russell AP, Bels V. Biomechanics and kinematics of limb-based locomotion in lizards: Review, synthesis and prospectus. Comp Biochem Physiol A Mol Integr Physiol. 2001;131(1):89–112. doi: 10.1016/s1095-6433(01)00469-x. [DOI] [PubMed] [Google Scholar]
- 41.Hunter JP. Key innovations and the ecology of macroevolution. Trends Ecol Evol. 1998;13(1):31–36. doi: 10.1016/s0169-5347(97)01273-1. [DOI] [PubMed] [Google Scholar]
- 42.Hodges SA, Arnold ML. Spurring plant diversification: Are floral nectar spurs a key innovation? Proc Biol Sci. 1995;262(1365):343–348. [Google Scholar]
- 43.Wainwright PC. Functional versus morphological diversity in macroevolution. Annu Rev Ecol Evol Syst. 2007;38(2007):381–401. [Google Scholar]
- 44.Bock WJ, von Wahlert G. Adaptation and the form-function complex. Evolution. 1965;19(3):269–299. [Google Scholar]
- 45.Russell AP, Johnson MK, Delannoy SM. Insights from studies of gecko-inspired adhesion and their impact on our understanding of the evolution of the gekkotan adhesive system. J Adhes Sci Technol. 2007;21(12-13):1119–1143. [Google Scholar]
- 46.Gould SJ, Vrba ES. Exaptation—A missing term in the science of form. Paleobiol. 1982;8(1):4–15. [Google Scholar]
- 47.Protas M, Conrad M, Gross JB, Tabin C, Borowsky R. Regressive evolution in the Mexican cave tetra, Astyanax mexicanus. Curr Biol. 2007;17(5):452–454. doi: 10.1016/j.cub.2007.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dickinson MH, et al. How animals move: An integrative view. Science. 2000;288(5463):100–106. doi: 10.1126/science.288.5463.100. [DOI] [PubMed] [Google Scholar]
- 49.Hedrick TL. Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspir Biomim. 2008;3(3):034001. doi: 10.1088/1748-3182/3/3/034001. [DOI] [PubMed] [Google Scholar]
- 50.Beaulieu JM, Jhwueng D-C, Boettiger C, O’Meara BC. Modeling stabilizing selection: Expanding the Ornstein-Uhlenbeck model of adaptive evolution. Evolution. 2012;66(8):2369–2383. doi: 10.1111/j.1558-5646.2012.01619.x. [DOI] [PubMed] [Google Scholar]
- 51.Team RDC. 2012. R: A Language and Environment for Statistical Computing. (R Foundation for Statstical Computing, Vienna, Austria) Available at www.R-project.org. Accessed June, 2014.
- 52.Revell LJ. Size-correction and principal components for interspecific comparative studies. Evolution. 2009;63(12):3258–3268. doi: 10.1111/j.1558-5646.2009.00804.x. [DOI] [PubMed] [Google Scholar]
- 53.Revell LJ. phytools: An R package for phylogenetic comparative biology (and other things) Method Ecol Evol. 2012;3(2):217–223. [Google Scholar]
- 54.Garland T, Jr, Dickerman AW, Janis CM, Jones JA. Phylogenetic analysis of covariance by computer simulation. Syst Biol. 1993;42(3):265–292. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.