Significance
Infant trauma induces preference learning about trauma-linked cues but negatively programs neurobehavioral development. Despite clinical evidence that trauma-linked cues remain powerful throughout life, the mechanisms underlying the interaction between infant trauma cues and the long-term effects of trauma are unknown. Using a rodent model of trauma bonding, which produces a life-long preferred odor and enduring effects that parallel the sequelae of child abuse, we show that the infant trauma odor rescues adult depressive-like behavior and amygdala dysfunction. Assessment of neural mechanism implicates amygdala serotonin (5-HT) and glucocorticoids (GCs). Our findings suggest that trauma-linked cues have an unexpected positive value in adulthood (i.e., antidepressant properties) and may provide insight as to why victims of childhood abuse are attracted to abuse-related cues.
Keywords: infant trauma, amygdala, serotonin, depression, safety signal
Abstract
Children form a strong attachment to their caregiver—even when that caretaker is abusive. Paradoxically, despite the trauma experienced within this relationship, the child develops a preference for trauma-linked cues—a phenomenon known as trauma bonding. Although infant trauma compromises neurobehavioral development, the mechanisms underlying the interaction between infant trauma bonding (i.e., learned preference for trauma cues) and the long-term effects of trauma (i.e., depressive-like behavior, amygdala dysfunction) are unknown. We modeled infant trauma bonding by using odor-shock conditioning in rat pups, which engages the attachment system and produces a life-long preference for the odor that was paired with shock. In adulthood, this trauma-linked odor rescues depressive-like behavior and amygdala dysfunction, reduces corticosterone (CORT) levels, and exerts repair-related changes at the molecular level. Amygdala microarray after rescue implicates serotonin (5-HT) and glucocorticoids (GCs), and a causal role was verified through microinfusions. Blocking amygdala 5-HT eliminates the rescue effect; increasing amygdala 5-HT and blocking systemic CORT mimics it. Our findings suggest that infant trauma cues share properties with antidepressants and safety signals and provide insight into mechanisms by which infant trauma memories remain powerful throughout life.
Early-life trauma is associated with compromised neurobehavioral development and vulnerability to later-life psychiatric disorders like depression (1–5). Long-lasting effects of infant trauma relevant to depression include disruptions in social behavior, alterations in the serotonin (5-HT) system, and amygdala dysfunction (2, 5–8), which have been replicated by animal models (9–15). Paradoxically, trauma-linked cues—even those associated with abusive attachment—can elicit strong attraction and feelings of comfort in humans (16, 17). Furthermore, a lifelong attraction to trauma-linked cues has also been demonstrated in rodent models of infant trauma, which suggests that sensory cues learned during infancy alter adult behaviors and amygdala activity (12, 18, 19).
To explore the enduring effects of infant trauma and the mechanisms underlying their modulation by infant trauma-linked cues, we used an odor-learning paradigm during the sensitive period for attachment learning in rat pups, postnatal (PN) days 8–12, which is characterized by rapid and robust odor-preference learning that extends to aversive stimuli (14, 20–22). Specifically, we used infant olfactory classical conditioning in which a novel peppermint odor is paired with a mild shock (0.5 mA, 1s) to produce a preferred odor that functions behaviorally as a maternal odor and results in neural activation comparable with that induced by natural maternal odor (20, 23–25). During the sensitive period, low endogenous levels of corticosterone (CORT) and attenuated shock-induced CORT release prevent the infant amygdala from exhibiting the learning-induced plasticity required for avoidance learning (14, 26). Thus, sensitive period odor-shock conditioning does not elevate CORT levels (27, 28). However, this paradigm produces later-life depressive-like behavior that is causally linked to amygdala dysfunction (12–14, 19). Although shock is painful to pups (12, 24), unpaired presentations of odor and shock do not result in learning or the adult depressive-like phenotype (12, 29), indicating that these effects are specific to infant paired odor shock.
Importantly, the infant-trauma odor alters neurobehavioral function throughout the life span and can rescue adult depressive-like behavior and modify amygdala activity (12, 18, 19). Intriguingly, these effects converge with the safety-signal literature. A safety signal is a learned sensory cue that can reduce stress and attenuate amygdala-dependent fear learning by being negatively correlated to an aversive event (30, 31) or positively correlated with an attachment formed in infancy or adulthood (32, 33). Indeed, safety signals attenuate fear learning and expression in humans, nonhuman primates, and rodents (18, 31, 34, 35) and reduce depressive-like behaviors in rodents (12, 19, 36). The amygdala is a principal target of safety signals (35, 37), which inhibit amygdala output to provide relief from fearful states (30, 34, 38).
One neurotransmitter that connects infant trauma and safety signals is 5-HT. First, increasing 5-HT in depressed patients decreases depression symptoms and its neural correlates (4, 39, 40). Second, 5-HT is implicated in initiating the developmental trajectory associated with later life depression vulnerability (41–43). Early postnatal life constitutes a critical period during which alterations in 5-HT impact adult emotional behavior, suggesting that 5-HT plays a key role in the development of brain circuits that modulate adult emotionality (43–47). Indeed, atypical infant increases in 5-HT induced by chronic fluoxetine (FLX) exposure, 5-HT transporter knockout, or disruptions in maternal care, result in long-term changes in 5-HT and contribute to later-life depression-like symptoms (11, 44–46, 48). Third, learned safety signals exert protective effects on animal models of depression comparable with those obtained by adult antidepressant treatment (36), suggesting that serotonergic mechanisms contribute to these repair effects. Collectively, these findings show that 5-HT is involved in the pathophysiology of depression, its treatment, and resolution (39–44). For these reasons, we provide a life span view of 5-HT’s role in the initiation and rescue of depression.
In sum, we used odor-shock conditioning as a model of infant trauma because it is a well-validated procedure that produces both an infant odor with strong positive value and adult depressive-like behavior (12, 19). The adult depressive-like phenotype was used as a behavioral assay with and without the infant trauma odor, to assess the safety-signal value of this odor. Our neural measurements focused on the amygdala because it is a critical brain area for both depression (13, 49, 50) and safety signals (31, 34, 35, 37) (see Fig. 1 for a schematic diagram of the experiments).
Fig. 1.
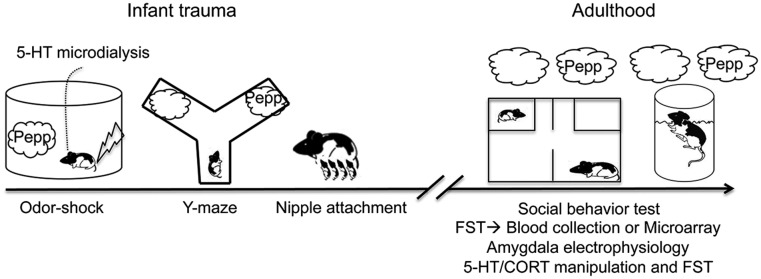
Timeline/experimental design. Infant rats underwent paired odor-shock conditioning from PN8- to -12. Amygdala 5-HT was assessed at PN12. We verified that odor-shock conditioning produces an odor that was preferred and comparable with the natural maternal odor by testing pups in the Y-maze and nipple attachment test during infancy. In adulthood, animals were given the social behavior test and the FST either in the absence or presence of the odor previously paired with shock (i.e., trauma cue). After the FST, trunk blood was collected or brains were removed, and the amygdala was dissected for microarray analysis. Additional animals were used to examine the effects of infant trauma odor exposure on amygdala electrophysiology. Finally, we assessed the role of 5-HT in mediating depressive-like behavior in the FST with and without odor by blocking amygdala 5-HT and increasing amygdala 5-HT and blocking CORT, respectively.
Results
Infant Trauma Induces Preference Learning of Trauma Cues and Amygdala 5-HT Efflux.
Infant trauma (i.e., paired odor shock) induced learning, as indicated by odor-induced behavioral activation of paired pups on the last day of conditioning (t = 25.23, P < 0.001) (Fig. 2A). These paired pups preferred the peppermint odor previously paired with shock as indicated by more odor choices in the Y-maze (t = 5.721, P < 0.001) (Fig. 2B). Moreover, this odor acquired comparable qualities to natural maternal odor and supported nipple attachment (t = 11.77, P < 0.001) (Fig. 2C). Previous work has shown that these effects are odor-specific and limited to paired odor-shock learning (12, 20, 23, 25). Furthermore, a one-way ANOVA revealed that infant trauma (i.e., odor-shock) increased amygdala 5-HT efflux (F(5,14)= 11.03, P < 0.001) at PN12, which was not observed in no-shock controls (Fig. 2D) and returned to control levels after conditioning.
Fig. 2.

Infant trauma, as modeled by paired odor-shock conditioning, induces preference learning and increases amygdala 5-HT. (A) At PN12, paired odor-shock animals exhibited increased odor-induced behavioral activation compared with no-shock controls (P < 0.001; n = 8 per group). Each data point represents the summation of behavior from two consecutive trials. (B) Paired odor-shock pups learned to prefer the peppermint odor paired with shock, as indicated by more conditioned odor choices in a Y-maze test (P < 0.001; n = 8 per group). (C) Paired odor-shock conditioning produced an odor that supports contact with the mother and guides nipple attachment (P < 0.001; n = 8 per group). (D) Paired odor-shock conditioning increased amygdala 5-HT efflux in PN12 pups compared with control (i.e., no shock) pups (P < 0.001). (E) Increase of 5-HT via systemic FLX administration during infancy produced later-life depressive-like behavior in the FST (n = 6–8 per group; P < 0.01). **P ≤ 0.01, ***P ≤ 0.001. Error bars represent SEM.
Infant Increases in 5-HT Produce Later-Life Depressive-Like Behavior.
Due to the shock-induced amygdala 5-HT increase, we assessed whether atypical infant increases in 5-HT contribute to later-life depressive-like behavior by giving pups daily injections of saline (SAL) or FLX (8 mg/kg, i.p/s.c.) over 10 days. Infant FLX administration produced later-life depressive-like behavior, as indicated by high levels of immobility in the forced swim test (FST) (t = 3.284, P < 0.01) (Fig. 2E)—a measure of behavioral despair in rodents used to screen for antidepressants (51, 52). These findings replicate previous results of early-life FLX treatment (48, 53) and are comparable with those obtained after infant trauma, as modeled by paired odor-shock conditioning (Fig. 3C) (12) or rearing with an abusive mother (13, 19). Although there was some variation within normal maternal behavior, the maternal behavior of dams with SAL/FLX-treated pups (i.e., half litter SAL; half litter FLX) did not differ from that of dams with undisturbed pups or dams with odor-shock pups (Fig. S1), suggesting that later-life depressive-like behavior resulting from infant FLX administration was not due to differences in mother–infant interactions.
Fig. 3.
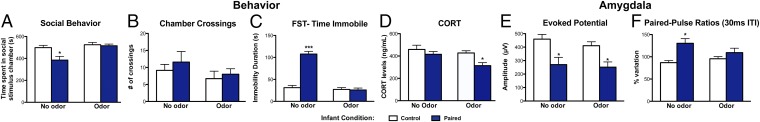
Long-term effects of infant trauma are reversed by the infant-trauma odor (i.e., odor previously paired with shock). (A) Infant trauma reduced adult social behavior, as indexed by less time in a social chamber compared with control (i.e., no shock) animals, which was normalized by presenting the infant-trauma odor (n = 7 per group; P < 0.05). (B) No significant differences in the number of crosses between chambers were found during the social behavior test (n = 7; P = 0.1948). (C) Infant trauma increased adult immobility in the FST, which was reversed to control levels by the infant-trauma odor (n = 6–9 per group; P < 0.001). (D) Infant-trauma odor exposure reduced CORT levels in paired animals during the FST (n = 8–10, P < 0.05). (E) Adults that underwent infant paired odor-shock conditioning exhibit a lower amplitude response within the BLA after a single-pulse electrical stimulation of the olfactory bulb compared with control no-shock animals (n = 6–7 per group; P < 0.05) that is not reversed by the infant-trauma odor. (F) As adults, infant paired odor-shock animals show paired-pulse facilitation (>100%) in response to paired-pulse (30 ms interpulse interval) electrical stimulation of the olfactory bulb, which was restored to control levels by the infant-trauma odor (n = 6–7 per group; P < 0.05). *P ≤ 0.05, ***P ≤ 0.001. Error bars represent SEM.
Infant-Trauma Odor Rescues Adult Behavioral Deficits.
Infant trauma impaired adult social interaction, as shown by less time in a chamber containing a younger, same-sex social stimulus animal. Presentation of the infant-trauma odor (i.e., odor previously paired with shock) during the test rescued this social behavior deficit and induced behavior similar to controls (Fig. 3A). Two-way ANOVA revealed a significant interaction between infant condition and odor presence [F(1,24)= 4.64, P < 0.05] and significant main effects of infant condition [F(1,24)= 6.57, P < 0.05] and odor presence [F(1,24)= 10.60, P < 0.01]. Post hoc Fisher tests revealed that infant trauma animals spent significantly less time in the social stimulus chamber compared with controls. No differences for any groups were found for chamber crossings [F(1,24)= 0.68, P = 0.4171], even with odor [F(1,24)= 1.78, P = 0.1948] or an interaction between the two [F(1,24)= 0.06, P = 0.8016] (Fig. 3B), indicating that these effects were not due to differences in locomotion.
Infant trauma induced increased immobility duration in the adult FST relative to no-shock controls (Fig. 3C). However, presentation of the infant-trauma odor during the FST rescued this index of depressive-like behavior. Two-way ANOVA revealed a significant interaction between infant condition and odor presence [F(1,26)= 61.89, P < 0.001]. Significant main effects of infant condition [F(1,26)= 58.02, P < 0.001] and odor presence [F(1,26)= 75.43, P < 0.001] were also detected. Post hoc Fisher tests revealed that the infant-trauma group was significantly different from controls in the absence of the odor, but not when the odor was present. Notably, this rescue effect is specific to the odor previously paired with shock during infancy (i.e., peppermint) because novel odor (i.e., citral) presentation has no effect (12).
Infant-Trauma Odor Reduces CORT Levels During the FST.
The FST is stressful, as indicated by animals exhibiting increased CORT levels (51, 54). GCs released in response to the FST are necessary for the incorporation of a behavioral response to the FST and modulate immobility behavior (55, 56). For these reasons, we assessed FST CORT levels both with and without the infant-trauma odor 30 min after the FST—when the peak FST-induced CORT response occurs (54). Although all animals exhibited similar CORT responses to the FST, the infant-trauma odor reduced CORT levels in trauma animals only (Fig. 3D). Two-way ANOVA revealed significant main effects of infant condition [F(1,33)= 1.43, P < 0.05] and odor presence [F(1,33)= 5.13, P < 0.05]. Post hoc Fisher tests indicated that the odor reduced CORT levels in paired animals, and this group was significantly different from all other groups.
Infant-Trauma Odor Alters Adult Amygdala Electrophysiology.
Because the infant-trauma odor repaired behavior, we questioned whether it also modulated amygdala electrophysiological properties. Indeed, the odor normalized amygdala paired-pulse effects that were modified by infant trauma (Fig. 3F). Repeated-measures two-way ANOVA showed a significant effect of infant condition [F(1,11)= 6.90, P < 0.05] and an interaction of infant condition × odor presence [F(1,11)= 9.15, P < 0.05]. Post hoc comparisons indicated that infant paired odor-shock animals show abnormal paired-pulse facilitation in adulthood that was abolished by the infant-trauma odor. In the presence of the odor, paired-pulse effects in odor-shock animals were comparable with controls (Fig. 3F). In addition to modifying paired-pulse effects, infant trauma also suppressed olfactory bulb-evoked potential amplitude recorded in the basolateral amygdala nucleus (BLA). However, in contrast to paired-pulse effects, the odor was ineffective at restoring this evoked potential, which differed between infant conditions [F(1,11)= 9.15, P < 0.05] (Fig. 3E). Post hoc comparisons showed that, in paired odor-shock animals, evoked potential amplitude was reduced compared with control no-shock animals. Similar analysis of responses in the olfactory cortex (Fig. S2A) and the cortical nucleus of the amygdala (Fig. S2B), which receives mainly olfactory inputs and relays them to other amygdalar nuclei, found no significant differences. These findings suggest that, whereas the transfer of olfactory information to other nuclei of the amygdala seems preserved, the processing within the BLA is altered.
Infant-Trauma Odor Changes Adult Amygdala Gene Expression.
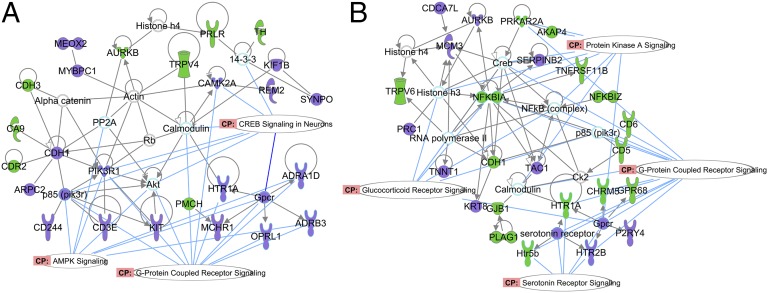
To examine molecular mechanisms contributing to the odor-mediated rescue effect, we used high-density oligonucleotide arrays to study gene expression changes in the amygdala, which mediates the effects of infant trauma (4, 7, 13) and is critically involved in the pathophysiology of depression (41, 49, 50). The Gene Functional Classification clustering algorithm in DAVID (Materials and Methods and SI Materials and Methods) defined three categories for the depressive phenotype (Fig. S3A) and two for the rescue phenotype (Fig. S3B). The major pathways for both conditions that were differentially regulated were solute transporters for cations and ion channels: i.e., voltage-gated sodium and potassium channels. Genes in the rescue phenotype also included cation transport and voltage-gated potassium channels. The major difference between the two phenotypes is that the rescue group, but not the depressive group, included G protein-coupled receptors (GPCRs), including 5-HT2B and 5-HT5B and the orphan GPCR88 (see Tables S1 and S2 for probes, fold change, and pfp values).
The Ingenuity Pathways Analysis (IPA) results complement those of the DAVID analysis. The primary networks linked to altered gene expression in the depressive phenotype were broad signaling paths such as cAMP response element-binding protein (CREB) and kinase paths, and GPCR signaling, (Fig. 4A). The latter included adenoreceptors, melanin, and the 5-HT1A receptor. Altered paths for the rescue phenotype included GC signaling, but also multiple paths representing cAMP and other GPCRs, and in particular 5-HT receptor coupling (genes associated with these paths are in Tables S3 and S4). The latter includes 5-HT2B and 5-HT5B receptors, as did the DAVID analysis, and the 5-HT1A receptor (Fig. 4B).
Fig. 4.
Network analysis of the depression and rescue phenotypes. The purple symbols are up-regulated genes, and the green symbols are down-regulated genes (as defined in Tables S1 and S2). The oblong white shapes show canonical pathways as determined by the Ingenuity curated database. For the depressive phenotype (A), the canonical paths, which were up-regulated, included GPCR signaling, AMP-activated protein kinase, and CREB signaling. For the rescue phenotype (B), the odor altered (up and down) a number of GPCR-related probes, protein kinase A (PKA), GCs, and, in particular, 5-HT receptor signaling (see Tables S3 and S4 for genes associated with these paths).
Amygdala 5-HT Mediates the Adult FST Rescue by the Infant-Trauma Odor.
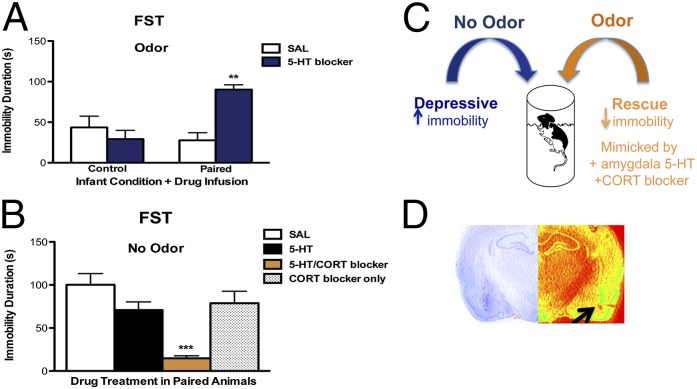
The amygdala is causally related to later-life depressive-like behavior resulting from infant trauma (13). Like the infant-trauma odor, selective serotonin reuptake inhibitors (SSRIs) also normalize depressive-like behavior in the FST (52). For these reasons, we assessed the role of amygdala 5-HT in the adult FST odor rescue. Temporary suppression of amygdala 5-HT via intraamygdala methysergide (5-HT receptor antagonist) infusion prevented the odor-mediated adult FST rescue (Fig. 5A). Two-way ANOVA revealed a significant interaction between drug and infant condition [F(1,20) = 14.54, P < 0.001], as well as significant effects of drug [F(1,20) = 5.67, P < 0.05] and infant condition [F(1,20) = 5.01, P < 0.05]. Post hoc tests revealed that infant-trauma animals receiving adult 5-HT blocker during the FST were significantly different from each of the other groups.
Fig. 5.
Behavioral rescue of adult depressive-like behavior after infant trauma is mediated by increasing amygdala 5-HT and CORT suppression. (A) Intraamygdala administration of 5-HT receptor antagonist (i.e., methysergide) blocked the adult behavioral rescue effect obtained by infant-trauma odor exposure in infant paired odor-shock animals, as indexed by increased immobility duration in the FST (n = 5–6 per group; P < 0.001). (B) Combination of intraamygdala 5-HT agonism and systemic CORT blockade mimicked the odor rescue effect in the adult FST after infant trauma, as indexed by a reduction in immobility duration (n = 6–7 per group; P < 0.001). (C) Rescue of adult depressive-like behavior by infant-trauma cues is mediated by an amygdala 5-HT/CORT interaction. (D) Cresyl and pseudocolored photomicrograph of representative cannula track location and tip placement in the BLA. Cannula tip placement is marked by an arrow. **P ≤ 0.01, ***P ≤ 0.001. Error bars represent SEM.
Adult Amygdala 5-HT Increase and CORT Blockade Mimics FST Odor Rescue Effect.
Since amygdala 5-HT was necessary for the behavioral repair elicited by the infant-trauma odor (Fig. 5A), we questioned whether increasing adult amygdala 5-HT was sufficient to rescue depressive-like behavior after infant trauma. In the odor’s absence, simply increasing amygdala 5-HT via intraamygdala 5-HT infusion was not sufficient to rescue FST immobility (Fig. 5B). However, we mimicked the adult odor-mediated rescue effect by combining amygdala 5-HT infusion and CORT inhibition via metyrapone (50 mg/kg i.p.; Tocris). One-way ANOVA followed by post hoc tests revealed that these animals were different from paired odor-shock animals receiving saline infusions, 5-HT infusions, or systemic metyrapone alone [F(3,21) = 11.22, P ≤ 0.001] (Fig. 5B; see Fig. S4 for cannula placements). Neither amygdala 5-HT nor CORT blockade alone produced rescue effects, suggesting that the mechanism underlying the antidepressant-like rescue effects of the trauma-linked odor involves a 5-HT/CORT interaction (Fig. 5C).
Discussion
Infant trauma, as modeled by paired odor-shock conditioning, has adverse and enduring effects in adulthood, including social-behavior disruptions, depressive-like behavior in the FST, and amygdala dysfunction (Fig. 3). Overall, these data are consistent with clinical and basic research of the neurobiological sequelae after early life stress (1, 2, 5, 9, 10, 13, 57). Our results corroborate prior findings showing that the infant-trauma odor rescues later life behavioral indices of depressive-like behavior and amygdala dysfunction (Fig. 3) (12, 19). Here, we expand on this knowledge and show that infant trauma produces an atypical infant amygdala 5-HT response (Fig. 2D) associated with later-life depressive-like behavior (Fig. 3 A and C), providing insight into possible mechanisms by which early-life FLX treatment produces detrimental effects (46, 48, 53, 58). These data demonstrate that 5-HT is developmentally sensitive to infant experiences and that alterations in 5-HT during critical periods in early life can be a predisposing factor for later-life affective dysfunction (41–43, 47, 57). Our findings may also provide insights into the mechanisms underlying the link between childhood abuse and later life depression, which is of clinical relevance given that childhood maltreatment and adult stressful life events interact to produce increased risk for depression in humans, and this relationship is moderated by 5-HT (3, 4, 8, 40, 59, 60).
Mechanisms Underlying Modulation of Adult Neurobehavioral Function by Infant-Trauma Cues.
Infant experiences result in long-term adaptations that program subsequent behavioral, endocrine, and neural function (3, 14, 57, 61). In addition, infant odors have a powerful modulatory influence on adult behaviors (62, 63). This phenomenon is not exclusive to odors associated with typical attachment experiences; it extends to trauma experienced within the context of attachment because the odor learned through our infant-trauma paradigm reduces adult fear learning, attenuates amygdala activity, rescues depressive-like behavior in the sucrose consumption test and FST, and decreases CORT (Fig. 3D) (12, 18, 19).
Here we show that the infant-trauma odor controls these behaviors by regulating amygdala activity. First, the odor normalized amygdala paired-pulse inhibition deficits induced by infant trauma (Fig. 3F). Because paired-pulse inhibition reflects the inhibitory feedback exerted by local gamma-aminobutyric (GABA)ergic interneurons onto glutamatergic projection neurons in the BLA (64), our data suggest that the odor influences amygdala circuit activity by modulating interneuron activity within the BLA. Moreover, paired odor-shock animals display an odor-specific enhancement of amygdala gamma oscillations, which reflect local circuit activity and are sensitive to GABAergic inhibitory interneuron function, in response to the infant-trauma odor (19), suggesting that infant trauma induces long-term changes in amygdala GABAergic function. Second, we show that the infant-trauma odor changes amygdala gene expression relating to GC and 5-HT receptor signaling (Fig. 4) compared with those seen in the depressive phenotype (i.e., infant paired odor-shock, no odor). Importantly, both GCs and 5-HT are implicated in clinical depression stemming from childhood abuse (3, 4, 8, 41, 59, 60). Animals with the depressive-like phenotype showed amygdala gene expression changes relating to CREB—an upstream transcriptional activator of brain-derived neurotrophic factor (BDNF). Both CREB and BDNF have also been implicated in depression and antidepressant response (39, 40, 65). Third, we show that amygdala 5-HT is necessary for the odor-mediated rescue effect because blocking amygdala 5-HT neurotransmission prevented the rescue effect (Fig. 5A). Fourth, we were able to pharmacologically mimic the odor’s rescue effect by simultaneously increasing amygdala 5-HT and suppressing CORT (Fig. 5B), which was further suggested by the CORT data (Fig. 3D) and the microarray data implicating GCs and 5-HT (Fig. 4 and Fig. S3). These results are compatible with clinical reports indicating that serotonergic compounds alleviate HPA-axis dysfunction associated with stress and affective disorders (40, 66). Preclinical studies have shown that 5-HT modulates excitatory inputs into the amygdala in a CORT-dependent manner via GABAergic interneurons (67, 68). Altogether, these findings implicate 5-HT/CORT interactions in amygdala-dependent states, such as the expression of depressive-like behavior in the FST (13).
Infant-Trauma Cues Are Reminiscent of Safety Signals and Antidepressant Actions.
The ability to identify events that provide relief from ongoing stress is crucial for the prevention of stress-induced psychopathologies like depression (31, 36). Our infant-trauma paradigm creates a learned sensory signal that prevents the expression of adverse neurobehavioral outcomes stemming from early life trauma (Fig. 3 A, C, and F), which overlaps with the effects of safety signals and antidepressant treatment (31). For example, safety signals exert antidepressant-like effects on the forced swim and sucrose consumption tests comparable with those obtained with SSRI/FLX treatment (36) and infant-trauma odor exposure (Fig. 3C) (12, 19). The infant-trauma odor also restored normal paired-pulse ratio in the BLA (Fig. 3F), the subregion in which distinct electrophysiological features of learned safety, including depression of conditioned stimulus-evoked activity, have been described (31, 34, 37, 38).
A Rodent Model for Socially Derived Safety Signals.
Infant rats are predisposed for attachment learning due to a unique neural circuitry that favors preference learning regardless of whether the learning is associated with pain or pleasure (14, 23, 32). Here, we used a rodent model of infant trauma experienced within attachment, which resulted in adult depressive-like behavior but also the infant acquisition of a safety signal that, although paired with trauma, was retained throughout the life span due to the unique developmental context in which the learning occurred (i.e., sensitive period) (14, 32). Moreover, our findings parallel the biological sequelae of infant trauma in clinical populations and may provide insight as to why victims of childhood abuse retain an attraction toward trauma-linked cues (i.e., trauma bonding).
Our rodent model safety signal conforms to safety-signal mechanisms involving modulation of BLA activity to reduce behavioral and physiological responses to stressors (30, 31, 34). Our findings suggest that the mechanism underlying the complex interplay between infant trauma-linked cues and later-life depressive-like behavior involves an amygdala 5-HT/CORT interaction that positively modulates the neurobehavioral dysregulation stemming from early-life trauma (Fig. 5C). In sum, trauma-linked cues play a critical role in modulating adult neurobehavioral function by controlling amygdala function (12, 18), thereby providing opportunities for intervention and possibly correction of maladaptive outcomes related to psychopathology resulting from infant trauma experienced within attachment (14).
Materials and Methods
All procedures were conducted in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committees at the Nathan Kline Institute, New York University Medical Center, University of Oklahoma, University Lyon1, and the Children's Hospital of Philadelphia.
Infant Odor-Shock Conditioning and Verification of Preference Learning.
Beginning at PN8, pups received 9–11 pairings (30 s peppermint odor, 0.5 mA shock during the last 1 s) daily for five consecutive days to induce a preferred odor with safety-signal value (12, 19). The control group did not receive shock. Subsets of pups were either permitted to grow up (males) or tested during infancy in a Y-maze or a nipple attachment test to assess preference learning (both males and females) (12, 25).
Infant Microdialysis/HPLC.
Extracellular amygdala 5-HT levels were measured by in vivo microdialysis during a PN12 odor-shock conditioning session, similar to that in ref. 69.
Infant FLX Administration.
Pups were injected (i.p./s.c.) with either FLX (8 mg/kg; Sigma; adapted from refs. 46 and 53) or SAL over a 10-d period in infancy.
Adult Behavioral Studies and Intraamygdala Drug Infusions.
Adult male rats (>PN75) were tested for social behavior or on the FST with or without the infant-trauma odor (i.e., peppermint), which was delivered using an olfactometer (2 L/min flow rate) at the same concentration used in infancy (12). For infusion experiments, animals underwent stereotactic surgery and were tested after a 7-d recovery period. All pharmacological compounds were dissolved in saline and administered at 0.1 μL/min for 5 min (0.5 μL per hemisphere) for a total infusion volume of 1 μL 15 min before testing.
RIA.
Trunk-blood samples were collected 30-min post FST, and plasma was used to analyze CORT levels (Rat Corticosterone Coat-A-Count Kit; Siemens).
Adult Microarray Analysis.
One hour after the FST, animals were decapitated, and the amygdala was dissected on ice. Biological replicates of bilateral amygdalas were assayed with the Affymetrix 230 2.0 chip.
Adult in Vivo Amygdala Electrophysiology.
Field potentials recorded in the amygdala in response to electrical stimulation of the olfactory bulb were obtained in adulthood after control or infant paired odor-shock experience, similar to ref. 12. Paired-pulse stimulation protocols were used to evaluate the level of amygdala inhibitory interneuron activity in the presence and absence of the infant odor previously paired with shock.
Statistical Analysis.
Statistical analyses included t tests, one- and two-way ANOVA, followed by Post hoc Fisher tests when appropriate. All differences were considered significant when P < 0.05.
Supplementary Material
Acknowledgments
We thank Shaoning Wang for technical assistance. This research was supported by National Science Foundation Graduate Research Fellowship Program Grant DGE-1137475 (to M.R.-C.), Grants NIH-MH091451 and NIH-DC009910 (to R.M.S.), and Grant NIH-MH80603 (to G.A.B. and R.M.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE58548).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1416065112/-/DCSupplemental.
References
- 1.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. Psychiatr Clin North Am. 2002;25(2):397–426, vii–viii. doi: 10.1016/s0193-953x(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 2.Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65(Suppl 1):18–28. [PubMed] [Google Scholar]
- 3.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Saveanu RV, Nemeroff CB. Etiology of depression: Genetic and environmental factors. Psychiatr Clin North Am. 2012;35(1):51–71. doi: 10.1016/j.psc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Pollak SD. Mechanisms linking early experience and the emergence of emotions: Illustrations from the study of maltreated children. Curr Dir Psychol Sci. 2008;17(6):370–375. doi: 10.1111/j.1467-8721.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Kolk BA. The neurobiology of childhood trauma and abuse. Child Adolesc Psychiatr Clin N Am. 2003;12(2):293–317, ix. doi: 10.1016/s1056-4993(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 7.Teicher MH, Samson JA. Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry. 2013;170(10):1114–1133. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caspi A, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 9.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 10.Sánchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev Psychopathol. 2001;13(3):419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, et al. Depressive behaviors and decreased expression of serotonin reuptake transporter in rats that experienced neonatal maternal separation. Neurosci Res. 2007;58(1):32–39. doi: 10.1016/j.neures.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Sevelinges Y, et al. Adult depression-like behavior, amygdala and olfactory cortex functions are restored by odor previously paired with shock during infant’s sensitive period attachment learning. Dev Cogn Neurosci. 2011;1(1):77–87. doi: 10.1016/j.dcn.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raineki C, Cortés MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: Infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. 2012;32(22):7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rincón-Cortés M, Sullivan RM. Early life trauma and attachment: Immediate and enduring effects on neurobehavioral and stress axis development. Front Endocrinol (Lausanne) 2014;5:33. doi: 10.3389/fendo.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coplan JD, et al. Early life stress and macaque amygdala hypertrophy: Preliminary evidence for a role for the serotonin transporter gene. Front Behav Neurosci. 2014;8:342. doi: 10.3389/fnbeh.2014.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Kolk BA. The compulsion to repeat the trauma: Re-enactment, revictimization, and masochism. Psychiatr Clin North Am. 1989;12(2):389–411. [PubMed] [Google Scholar]
- 17.Freud SS. A Child Is Being Beaten. Yale Univ Press; New Haven, CT: 1997. [Google Scholar]
- 18.Sevelinges Y, et al. Enduring effects of infant memories: Infant odor-shock conditioning attenuates amygdala activity and adult fear conditioning. Biol Psychiatry. 2007;62(10):1070–1079. doi: 10.1016/j.biopsych.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Raineki C, et al. 2014. Paradoxical neurobehavioral rescue by memories of early-life abuse: The safety signal value of odors learned during abusive attachment. Neuropsychopharmacology, 10.1038/npp.2014.266.
- 20.Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Dev Psychobiol. 1988;21(1):25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan RM, Hofer MA, Brake SC. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Dev Psychobiol. 1986;19(6):615–623. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan RM, Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Brain Res. 1986;392(1-2):278–282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407(6800):38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roth TL, Sullivan RM. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005;57(8):823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 25.Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biol Psychiatry. 2010;67(12):1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan RM, Holman PJ. Transitions in sensitive period attachment learning in infancy: The role of corticosterone. Neurosci Biobehav Rev. 2010;34(6):835–844. doi: 10.1016/j.neubiorev.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moriceau S, Raineki C, Holman JD, Holman JG, Sullivan RM. Enduring neurobehavioral effects of early life trauma mediated through learning and corticosterone suppression. Front Behav Neurosci. 2009;3:22. doi: 10.3389/neuro.08.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moriceau S, Shionoya K, Jakubs K, Sullivan RM. Early-life stress disrupts attachment learning: The role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. J Neurosci. 2009;29(50):15745–15755. doi: 10.1523/JNEUROSCI.4106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarro EC, Sullivan RM, Barr G. Unpredictable neonatal stress enhances adult anxiety and alters amygdala gene expression related to serotonin and GABA. Neuroscience. 2014;258:147–161. doi: 10.1016/j.neuroscience.2013.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christianson JP, et al. Inhibition of fear by learned safety signals: A mini-symposium review. J Neurosci. 2012;32(41):14118–14124. doi: 10.1523/JNEUROSCI.3340-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong E, Monje FJ, Hirsch J, Pollak DD. Learning not to fear: Neural correlates of learned safety. Neuropsychopharmacology. 2014;39(3):515–527. doi: 10.1038/npp.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan RM. Developing a sense of safety: The neurobiology of neonatal attachment. Ann N Y Acad Sci. 2003;1008:122–131. doi: 10.1196/annals.1301.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisenberger NI, et al. Attachment figures activate a safety signal-related neural region and reduce pain experience. Proc Natl Acad Sci USA. 2011;108(28):11721–11726. doi: 10.1073/pnas.1108239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogan MT, Leon KS, Perez DL, Kandel ER. Distinct neural signatures for safety and danger in the amygdala and striatum of the mouse. Neuron. 2005;46(2):309–320. doi: 10.1016/j.neuron.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Genud-Gabai R, Klavir O, Paz R. Safety signals in the primate amygdala. J Neurosci. 2013;33(46):17986–17994. doi: 10.1523/JNEUROSCI.1539-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollak DD, et al. An animal model of a behavioral intervention for depression. Neuron. 2008;60(1):149–161. doi: 10.1016/j.neuron.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sangha S, Chadick JZ, Janak PH. Safety encoding in the basal amygdala. J Neurosci. 2013;33(9):3744–3751. doi: 10.1523/JNEUROSCI.3302-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 2014;17(1):106–113. doi: 10.1038/nn.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castrén E. Is mood chemistry? Nat Rev Neurosci. 2005;6(3):241–246. doi: 10.1038/nrn1629. [DOI] [PubMed] [Google Scholar]
- 40.Willner P, Scheel-Krüger J, Belzung C. The neurobiology of depression and antidepressant action. Neurosci Biobehav Rev. 2013;37(10 Pt 1):2331–2371. doi: 10.1016/j.neubiorev.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Ansorge MS, Hen R, Gingrich JA. Neurodevelopmental origins of depressive disorders. Curr Opin Pharmacol. 2007;7(1):8–17. doi: 10.1016/j.coph.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Suri D, Teixeira CM, Cagliostro MK, Mahadevia D, Ansorge MS. 2015. Monoamine-sensitive developmental periods impacting adult emotional and cognitive behaviors. Neuropsychopharmacology 40(1):88–112. [DOI] [PMC free article] [PubMed]
- 44.Gingrich JA, Ansorge MS, Merker R, Weisstaub N, Zhou M. New lessons from knockout mice: The role of serotonin during development and its possible contribution to the origins of neuropsychiatric disorders. CNS Spectr. 2003;8(8):572–577. doi: 10.1017/s1092852900018848. [DOI] [PubMed] [Google Scholar]
- 45.Lira A, et al. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54(10):960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- 46.Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306(5697):879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 47.Lesch KP, Waider J. Serotonin in the modulation of neural plasticity and networks: Implications for neurodevelopmental disorders. Neuron. 2012;76(1):175–191. doi: 10.1016/j.neuron.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 48.Rebello TJ, et al. Postnatal day 2 to 11 constitutes a 5-HT-sensitive period impacting adult mPFC function. J Neurosci. 2014;34(37):12379–12393. doi: 10.1523/JNEUROSCI.1020-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sibille E, et al. A molecular signature of depression in the amygdala. Am J Psychiatry. 2009;166(9):1011–1024. doi: 10.1176/appi.ajp.2009.08121760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whalen PJ, Shin LM, Somerville LH, McLean AA, Kim H. Functional neuroimaging studies of the amygdala in depression. Semin Clin Neuropsychiatry. 2002;7(4):234–242. doi: 10.1053/scnp.2002.35219. [DOI] [PubMed] [Google Scholar]
- 51.Castagné V, Moser P, Roux S, Porsolt RD. 2011. Rodent models of depression: Forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci 55(8.10A):8.10A.1–8.10A.14. [DOI] [PubMed]
- 52.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29(4-5):547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 53.Karpova NN, Lindholm J, Pruunsild P, Timmusk T, Castrén E. Long-lasting behavioural and molecular alterations induced by early postnatal fluoxetine exposure are restored by chronic fluoxetine treatment in adult mice. Eur Neuropsychopharmacol. 2009;19(2):97–108. doi: 10.1016/j.euroneuro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Connor TJ, Kelly JP, Leonard BE. Forced swim test-induced neurochemical endocrine, and immune changes in the rat. Pharmacol Biochem Behav. 1997;58(4):961–967. doi: 10.1016/s0091-3057(97)00028-2. [DOI] [PubMed] [Google Scholar]
- 55.Mitchell JB, Meaney MJ. Effects of corticosterone on response consolidation and retrieval in the forced swim test. Behav Neurosci. 1991;105(6):798–803. doi: 10.1037//0735-7044.105.6.798. [DOI] [PubMed] [Google Scholar]
- 56.Veldhuis HD, De Korte CC, De Kloet ER. Glucocorticoids facilitate the retention of acquired immobility during forced swimming. Eur J Pharmacol. 1985;115(2-3):211–217. doi: 10.1016/0014-2999(85)90693-4. [DOI] [PubMed] [Google Scholar]
- 57.McEwen BS. Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev. 2003;9(3):149–154. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- 58.Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28(1):199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Power RA, et al. The interaction between child maltreatment, adult stressful life events and the 5-HTTLPR in major depression. J Psychiatr Res. 2013;47(8):1032–1035. doi: 10.1016/j.jpsychires.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 60.Kaufman J, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci USA. 2004;101(49):17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strüber N, Strüber D, Roth G. Impact of early adversity on glucocorticoid regulation and later mental disorders. Neurosci Biobehav Rev. 2014;38:17–37. doi: 10.1016/j.neubiorev.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 62.Leon M, Galef BG, Behse JH. Establishment of pheromonal bonds and diet choice in young rats by odor pre-exposure. Physiol Behav. 1977;18:387–391. [Google Scholar]
- 63.Fillion TJ, Blass EM. Infantile experience with suckling odors determines adult sexual behavior in male rats. Science. 1986;231(4739):729–731. doi: 10.1126/science.3945807. [DOI] [PubMed] [Google Scholar]
- 64.Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: Anatomy and physiology. Physiol Rev. 2003;83(3):803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- 65.Lee BH, Kim YK. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig. 2010;7(4):231–235. doi: 10.4306/pi.2010.7.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barden N, Reul JM, Holsboer F. Do antidepressants stabilize mood through actions on the hypothalamic-pituitary-adrenocortical system? Trends Neurosci. 1995;18(1):6–11. doi: 10.1016/0166-2236(95)93942-q. [DOI] [PubMed] [Google Scholar]
- 67.Stutzmann GE, McEwen BS, LeDoux JE. Serotonin modulation of sensory inputs to the lateral amygdala: Dependency on corticosterone. J Neurosci. 1998;18(22):9529–9538. doi: 10.1523/JNEUROSCI.18-22-09529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stutzmann GE, LeDoux JE. GABAergic antagonists block the inhibitory effects of serotonin in the lateral amygdala: A mechanism for modulation of sensory inputs related to fear conditioning. J Neurosci. 1999;19(11):RC8. doi: 10.1523/JNEUROSCI.19-11-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barr GA, et al. Transitions in infant learning are modulated by dopamine in the amygdala. Nat Neurosci. 2009;12(11):1367–1369. doi: 10.1038/nn.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.