Significance
This paper reports the first visualization of calcium dynamics in Drosophila eggs in vivo and in vitro, demonstrating that a calcium wave is a conserved feature of egg activation (the process by which a mature egg becomes able to initiate embryo development). In vertebrates and echinoderms, the fertilizing sperm triggers egg activation by inducing calcium release from the egg’s internal stores, causing wave(s) of increased calcium to sweep across the egg. However, insect eggs activate without fertilization. We show that a wave of increased calcium occurs during activation of Drosophila eggs. The wave is induced during ovulation by influx of calcium into the egg through mechanosensitive ion channels. Release of calcium from intracellular stores is required for wave propagation.
Keywords: egg activation, oocyte, fertilization, calcium, mechanosensitive ion channels
Abstract
Egg activation is the process by which a mature oocyte becomes capable of supporting embryo development. In vertebrates and echinoderms, activation is induced by fertilization. Molecules introduced into the egg by the sperm trigger progressive release of intracellular calcium stores in the oocyte. Calcium wave(s) spread through the oocyte and induce completion of meiosis, new macromolecular synthesis, and modification of the vitelline envelope to prevent polyspermy. However, arthropod eggs activate without fertilization: in the insects examined, eggs activate as they move through the female’s reproductive tract. Here, we show that a calcium wave is, nevertheless, characteristic of egg activation in Drosophila. This calcium rise requires influx of calcium from the external environment and is induced as the egg is ovulated. Pressure on the oocyte (or swelling by the oocyte) can induce a calcium rise through the action of mechanosensitive ion channels. Visualization of calcium fluxes in activating eggs in oviducts shows a wave of increased calcium initiating at one or both oocyte poles and spreading across the oocyte. In vitro, waves also spread inward from oocyte pole(s). Wave propagation requires the IP3 system. Thus, although a fertilizing sperm is not necessary for egg activation in Drosophila, the characteristic of increased cytosolic calcium levels spreading through the egg is conserved. Because many downstream signaling effectors are conserved in Drosophila, this system offers the unique perspective of egg activation events due solely to maternal components.
The oocyte is unlike any cell type in the body. This highly specialized cell, once fertilized, will become totipotent, yet in many organisms the oocyte must remain developmentally arrested for years. The resting oocyte within the ovary is filled with maternally provided stores of mRNAs and proteins that will drive embryogenesis before zygotic genome activation, its cell cycle is stalled in a species-specific stage of meiosis (typically metaphase I or II), and its vitelline membrane is amenable to penetration by sperm and small molecules (1, 2). The mature oocyte remains in such a state until it is ready to be ovulated and fertilized. This quiescence period ranges from days in fruit flies to decades in humans.
The series of events that leads to actualization of the oocyte’s developmental potential is collectively termed “egg activation.” In the vertebrates and echinoderms in which it has been most intensively studied, activation is sparked by a rise in cytosolic Ca2+ levels that is triggered by the fertilizing sperm (3–5). Depending on the organism, this Ca2+ rise sweeps through the egg in a single wave or in multiple oscillations, but in all cases the outcome is the same: meiosis resumes, the vitelline envelope is restructured, and mRNA and protein pools undergo modifications and turnover (6, 7). Evidence from other organisms such as mice and frogs indicates that the rise in Ca2+ likely starts a signaling cascade through calcium-dependent kinases and phosphatases such as CaMKII and calcineurin, which may have myriad effects on downstream pathway components to produce all of the hallmarks of egg activation (8, 9).
Ca2+ transients were originally identified in medaka, where it was initially hypothesized that free Ca2+ from the external environment enters the oocyte with the fertilizing sperm, and calcium-induced calcium release from endoplasmic reticulum (ER) stores propagates the wave across the egg (10, 11). In teleost fish, extracellular Ca2+ can be sufficient, but not always necessary, to induce egg activation in vitro (e.g., zebrafish) (12, 13), but in many cases activation in vivo requires fertilization. In organisms in which the fertilizing sperm induces egg activation, the sperm either introduces a specific isoform of phospholipase C (PLC) (as in mice) (14), or sperm binding activates a Src-family kinase and in turn activates PLC (as in echinoderms) (15). Active PLC induces phosphoinositide signaling and ultimately results in release of Ca2+ from stores in the egg’s ER (14, 16). However, in the arthropods that have been examined, egg activation occurs independently of fertilization: in Drosophila melanogaster fruit flies (17) and Pimpla turionellae wasps (18), eggs activate as they move through the female’s reproductive tract, and Sicyonia shrimp eggs activate upon spawning into sea water (19). In addition, parthenogenetically reproducing organisms do not require sperm for egg activation to occur.
That the insects tested thus far do not require fertilization for egg activation allows the study of egg activation events independent of the presence of sperm and thus of the initiation of zygote development. For example, this permitted experiments in Drosophila that showed that maternal functions that permit remodeling of the sperm nucleus into a male pronucleus are triggered by egg activation, not by fertilization (20). However, despite the difference in initial trigger, the downstream events of egg activation (resumption of meiosis, translation of new proteins from stored maternal RNAs, destabilization of selected maternal mRNAs, and changes in the egg’s coverings) occur in arthropods as well as in mammals (17, 21–24), and the changes that occur during Drosophila egg activation are signaled through many of the same pathways and components as in other organisms. For example, the requirement for calcineurin (20, 25–27), likely involved in the initial transmission of the calcium signal, characterizes egg activation in Xenopus as well as in Drosophila, and the activities of CDC20 (Drosophila cortex) in meiotic resumption (28), GLD-2 (Drosophila wispy) in cytoplasmic polyadenylation (29–32), and MAPK in signal transduction (33, 34) are conserved in Drosophila and other animals. Furthermore, phosphomodulation of proteins occurs during egg activation in the two organisms examined to date [sea urchin (4) and Drosophila (35)] and ∼80% of the phosphomodulated proteins in Drosophila are conserved across species, which may indicate a conserved phenomenon at egg activation (35). This conservation of “downstream” egg activation events raises the question of what triggers this process in organisms like Drosophila where sperm are not needed for egg activation and how this compares to events in sperm-activated systems. Ultimately, we aim to use the powerful tools available for Drosophila to genetically define the pathways governing this process.
We previously showed that activation of Drosophila oocytes is triggered during the process of ovulation (passage of an oocyte from the ovary into the oviducts) (17) and could be accelerated by application of pressure. However, the immediate consequence of this on the oocyte was unknown. Our finding that calcineurin regulation is essential for egg activation (20, 25–27) and that Drosophila egg activation requires Ca2+ in the extracellular environment as well as the activity of mechanosensitive ion channels (36) suggests that a calcium flux might occur during activation of Drosophila eggs despite the different trigger.
Here, using transgenic lines carrying a genetically encoded Ca2+ sensor (GCaMP3) expressed in the female germline, we discovered that intracellular calcium levels increase in the Drosophila oocyte as it is ovulated. The rise could be best visualized in vitro, where the calcium level increase initiates at one or both poles of the egg and sweeps through the egg cytoplasm. In vitro experiments show that the rise is triggered by mechanical pressure and requires the activity of ion channels in the egg’s plasma membrane. Although initiation of the Ca2+ wave requires external calcium [like some aspects of egg activation in mammals (3)], its propagation requires IP3-mediated release of internal Ca2+ stores.
Results
Ca2+ Levels Increase in Drosophila Oocytes as They Are Ovulated.
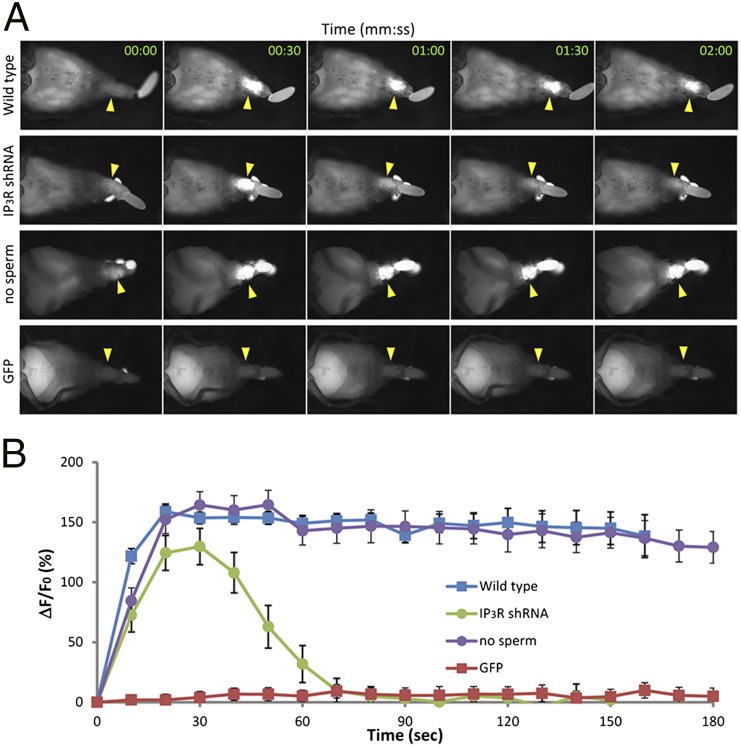
Previous physiological studies indicated that oocytes were triggered to activate by the process of ovulation (17). Oocytes in the ovary do not complete meiosis or modify their vitelline envelopes. In contrast, in oviducts before fertilization, meiosis has resumed and vitelline envelopes have begun to cross-link. To test whether, and when, the process of ovulation induces a calcium rise in oocytes, we examined the calcium flux in oocytes during ovulation in vivo. Females that expressed the calcium sensor GCaMP3 in their oocytes were anesthetized, and ovulation was induced using triethylamine. Abdomens were imaged as ovulation occurred. Under dissecting microscopy (Fig. 1 and Movies S1 and S2) and at higher magnification (Fig. S1 and Movies S3 and S4), a rise in calcium was observed in oocytes as they entered and moved through the oviducts (Fig. 1A, top row, and Movie S1; Fig. S1A, and Movie S3). Calcium levels remain high in the oocyte cytoplasm for many minutes following the original local increase (Fig. 1B, blue tracing). As is the case for egg activation, and consistent with ovulation inducing the calcium rise, fertilization was not required for the calcium rise (Fig. 1A, third row; Fig. 1B, purple tracing). No change in GFP fluorescence was seen during ovulation and egg movement in control females expressing a non–calcium-responsive GFP (Fig. 1A, bottom row, and Movie S2; Fig. 1B, red tracing; Fig. S1B, and Movie S4). Thus, ovulation induces a rise in calcium levels in the oocyte that is fertilization-independent and is sustained for several minutes.
Fig. 1.
Ca2+ rises in oocytes during ovulation in vivo. Calcium rises were detected by GCaMP fluorescence in oocytes as they moved through live females. Ca2+ imaging was performed with a Leica MZ16F fluorescence stereo microscope. (A) An intense fluorescence increase was seen in ovulating oocytes (arrowheads) in the abdomens of transgenic female flies expressing GCaMP3 in their oocytes (top row; matα4-GAL-VP16 > UASp-GCaMP3) but not in control females expressing a GFP that is not calcium-responsive (aequorin–GFP in the absence of coelenterazine, bottom row; details are in SI Materials and Methods). The wave initiated from one pole 90% of the time. Ten percent of the time, waves initiated from both poles (e.g., Fig. S1); this was most commonly seen in oocytes that failed to move apace through the reproductive tract and thus likely came under pressure along their entire length at once. The second row shows an ovulating oocyte of a female whose germline expressed GCaMP3 but also was knocked down for the IP3 receptor; the calcium rise is transient. The third row shows fluorescence of an oocyte from a matα4-GAL-VP16 > UASp-GCaMP3 female who was mated to a spermless (XO) male (instead of a normal male as was used in the other rows in this figure). The fluorescence increase was like that seen in control matings, indicating that fertilization is not needed for the calcium rise in oocytes. Time after release from the ovary is indicated in each panel. Images in rows 1, 2, and 4 are taken from Movies S1, S10, and S2, respectively; images for the spermless mating are also from a movie, which is available upon request. Anterior is to the left. Brightness under the rear of the female in row 2 is excreta, which fluoresce under these conditions. (B) Quantitative analysis of GCaMP3 fluorescence intensity (∆F/F0). Blue is wild type (n = 6), green is IP3R-shRNA oocytes (n = 6), purple is unfertilized (n = 12), and red is control (n = 9).
Ca2+ Waves Traverse the Drosophila Oocyte During Egg Activation.
Optical and physical limitations, as well as the speed of the calcium rise, prevented clear visualization of the dynamics of the calcium flux in vivo. To visualize the nature of the calcium flux in detail, we therefore imaged oocytes as they activated in vitro. Drosophila oocytes can be activated ex vivo by incubation in a hypotonic Ca2+-containing solution (37). Oocytes containing maternally expressed GCaMP3 were dissected in isolation buffer (IB), a solution hypertonic to the egg that maintains the egg in a dehydrated, inactivated state, and were placed on the microscope stage. For imaging, the dish was then flooded with activation buffer (AB), a solution hypotonic to the egg that causes egg hydration, swelling, and activation. After the egg began to swell, indeed usually after swelling appeared complete, we observed an increase in its cytoplasmic calcium levels. This increase appeared as single or converging waves of increased cytosolic Ca2+ concentrations that initiated from the egg pole(s) and moved toward the center of the oocyte. Fluorescence peaked, maintained a brief plateau, and then decreased in the same direction as the original wave’s movement (Fig. 2 and Movie S5). Total time from wave initiation to peak fluorescence was ∼15 min (Fig. S2). Waves typically began within 10 min of adding AB, although they occasionally took longer to begin. If a wave was slow to start, we were often able to initiate a wave by adding 1–5 drops of additional AB or deionized H2O. We hypothesize that the addition of water to the activating solution causes greater hypotonicity of the solution to the egg, resulting in further egg swelling, which may be critical for egg activation as further discussed below (36). The optimal in vitro system (37) cannot fully recapitulate in vivo conditions; in vitro oocytes are in buffer, whereas in vivo oocytes are held tightly within the oviduct, which likely subjects them to additional mechanical pressure. We believe that for these reasons the speed of the calcium increase in vitro does not match the rapid speed seen in vivo. Relatedly, given that mechanical forces trigger the wave, as discussed below, we believe that stochastic differences between the sensing of the membrane’s stretching caused the difference between the proportion of oocytes with single vs. bipolar initiation of the wave in vivo vs. in vitro.
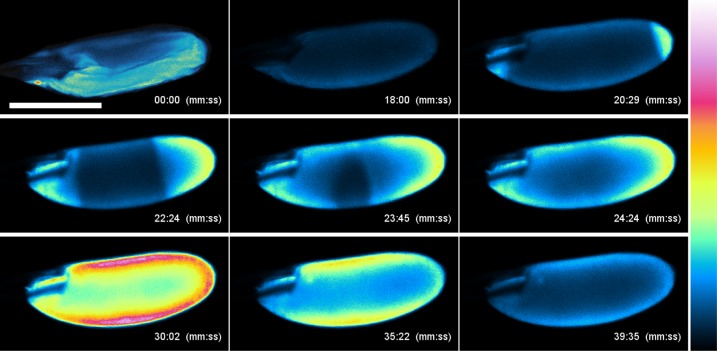
Fig. 2.
In vitro activation of oocytes causes converging Ca2+ waves. Ca2+ flux in oocytes detected by GCaMP3. Oocytes containing GCaMP3 were dissected in IB and activated by replacing IB with AB. Time (in minutes and seconds) after replacement with AB is indicated in each panel. Oocytes gradually swelled from 00:00 to 18:00. A Ca2+ rise was detected at both poles (20:29), extended toward the center of the oocyte, and spread throughout the oocyte at 24:24. The signal intensity was maximum at 30:02, gradually decreased (35:22), and then returned to the basal level (39:35). Calcium rises were seen from both poles in 90% of in vitro-incubated oocytes; the remainder showed a rise from one pole only. We postulate that the difference in relative percentage of bipolar and monopolar rises in vitro vs. in vivo reflects the difference in nature and location of mechanical stress experienced by the oocyte membrane under these two conditions. Fluorescence intensities are presented using a false-color scale, shown on the right. (Scale bar, 200 μm.) n = 14. See Movie S5.
To confirm that the observed signal was due to a rise in cytosolic Ca2+ and was not an artifact of imaging parameters such as autofluorescence, we examined Ca2+ dynamics in activating oocytes using a different sensor: a GFP–aequorin fusion (38). This sensor does not require the use of an excitation laser; rather, in the presence of the aequorin substrate coelenterazine, the GFP is induced to fluoresce by bioluminescent resonant energy transfer (BRET). Any signal that is detected under these experimental conditions is due directly to a rise in Ca2+ and cannot be attributed to autofluorescence or artifact. Using this system, we again observed a calcium rise that initiated at the oocyte poles and spread through the cytoplasm (Fig. S3 and Movie S6), confirming the existence and dynamics of a calcium flux in Drosophila oocytes during egg activation in vitro.
Production of the Ca2+ Wave Requires Extracellular Calcium.
Drosophila oocytes cannot undergo in vitro activation in buffers depleted of Ca2+ (36), suggesting that calcium from external sources is necessary to activate the eggs. We therefore examined whether influx of calcium is needed to initiate the calcium rise. We performed in vitro activation experiments using buffers depleted of Ca2+ using the Ca2+ chelator BAPTA [1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrapotassium salt]. Cytosolic Ca2+ levels did not increase in oocytes incubated in BAPTA-treated buffers, even though egg swelling did occur (Fig. 3); these oocytes did not activate, as assessed by bleach resistance (32) (Table S1). Oocytes that were incubated in parallel in control buffers showed a normal Ca2+ rise and activation (Fig. 3, Table S1, Movie S7). Thus, the Ca2+ rise in activating Drosophila oocytes requires calcium in the extracellular medium.
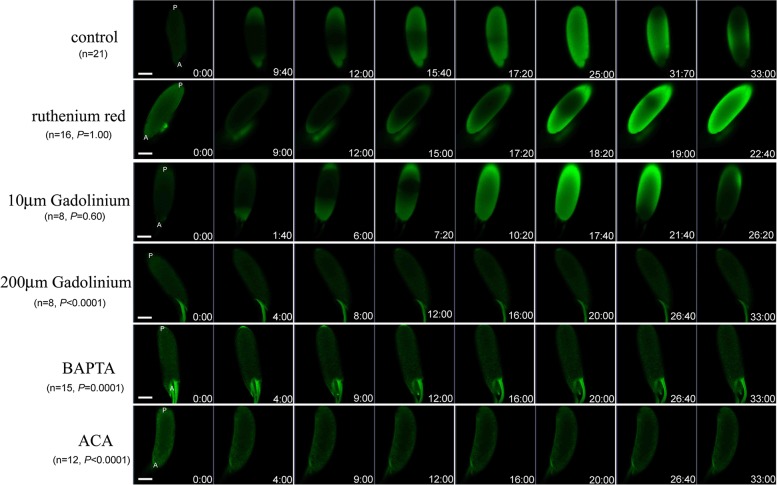
Fig. 3.
Perturbation of Ca2+ wave in vitro through treatment with pharmacological agents. We incubated oocytes from GCaMP3 females in buffers containing various inhibitors of Ca2+ channels and pathways, as described in the text, and tested their ability to produce the Ca2+ wave like that shown in Fig. 2 and in the control (no inhibitor) row at the top of this figure. Incubation with ruthenium red and low concentrations (10 µM) of GdCl3 did not perturb production of a normal-appearing Ca2+ flux. However, incubating oocytes in buffers containing BAPTA (e.g., Ca2+-depleted IB and AB), high concentrations (>100 µM) of GdCl3, or ACA abolished the Ca2+ flux that is normally seen in vitro. Time is displayed as minutes and seconds after addition of AB. A, anterior; P, posterior. Movies S7, S9, and S11 show examples of control, 10 µM of GdCl3, and ruthenium red incubations, respectively. (Scale bar, 100 μm.) Sample sizes and P values relative to control are shown.
Mechanical Pressure Is Sufficient to Elicit a Ca2+ Wave.
Previous studies indicated that mechanical stress may trigger or enhance egg activation in Drosophila and some other insects. For example, pulling on the dorsal appendages of Drosophila eggs, or squeezing wasp eggs through a capillary with the same diameter as a wasp ovipositor, could activate those eggs (18, 36, 39). Consistent with the idea that Drosophila egg activation might be triggered by mechanical cues, Drosophila egg activation can be accelerated in vitro by applying pressure to the oocyte (36), and blocking mechanosensitive ion channels on the Drosophila oocyte with gadolinium inhibits egg activation (36). To test whether the Ca2+ flux that we observed in Fig. 2 was affected by mechanical pressure, we performed in vitro imaging while gentle pressure was applied to the oocyte under the weight of a coverslip. We found that even without bathing the oocyte in hypotonic solution, the pressure exerted by the coverslip was sufficient to elicit a Ca2+ wave like that observed during incubation in AB (Fig. 4 and Movie S8). This finding demonstrates that mechanical stimulation, which ultimately results in activation (36), is sufficient to cause a Ca2+ flux in Drosophila oocytes.
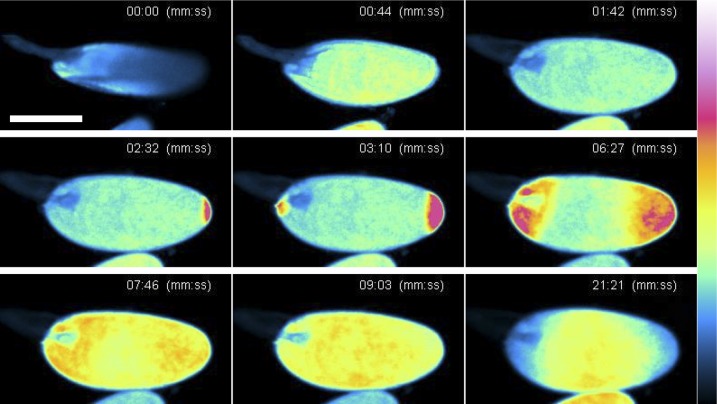
Fig. 4.
Mechanical pressure induces converging Ca2+ waves in oocytes. Sequential images of Ca2+ flux in an oocyte upon mechanical pressure. Oocytes were placed in a drop of the hypertonic buffer (IB), and a coverslip was gently placed on the drop. Excess IB was removed using filter paper to subject eggs to a mild pressure (00:00–01:42). Ca2+ increase was detected at both poles (02:32–03:10), and the waves moved toward the center of the oocyte (06:27–07:46). Unlike the above method of activation, the fluorescent signal pattern remained spread throughout the oocyte (09:03). We believe that this is due to the flattened shape of the oocyte under mechanical pressure. Fluorescence intensities are presented using a false-color scale shown on the right. (Scale bar, 200 μm.) n = 8. Frames are taken from Movie S8.
Extracellular Ca2+ Enters the Oocyte Through Mechanosensitive Channels.
The finding that mechanical pressure (or egg swelling) triggers the calcium wave, and that external calcium is needed for the wave to occur, led us to test whether mechanosensitive ion channels were needed for the calcium wave, as they are required to trigger the downstream events of egg activation (36). To examine this, we incubated dissected mature GCaMP3-containing oocytes in concentrations of GdCl3 ranging from 10 to 400 µM in IB before imaging in AB containing GdCl3. Oocytes treated with 100–400 µM GdCl3 did not show an increase in cytosolic Ca2+ during egg activation (Fig. 3 and Table S1), whereas control samples tested in parallel showed the stereotypical Ca2+ flux. This result indicates that Ca2+ likely enters the oocyte through mechanosensitive ion channels. Interestingly, treatment of oocytes with 10 µM GdCl3 was unable to inhibit the Ca2+ flux during egg activation (Fig. 3, Table S1, and Movie S9). Some Ca2+ channels are more sensitive to gadolinium than others; for example, TRP-L channels are insensitive to low concentrations (10 µM) of gadolinium but sensitive to higher concentrations (100 µM). In our experiments, concentrations of GdCl3 that inhibited the wave also inhibited egg activation (Table S1).
Based on RNAseq data (40), there are several mechanosensitive channels that may be present in the Drosophila oocyte plasma membrane (for a review see ref. 41). Three genes of the transient receptor potential (TRP) family encoding mechanosensitive ion channels are expressed in the ovary, but their role has not been assessed during Drosophila egg activation. We tested a broad-spectrum blocker of TRP-type channels, N-(p-amylcinnamoyl)anthranilic acid (ACA), for effects on the dynamics of the calcium wave during egg activation. Oocytes were incubated with 10 µM ACA in IB for 30 min before activating in AB containing ACA. ACA-treated oocytes could not produce the Ca2+ flux during in vitro egg activation, although egg swelling did occur (Fig. 3), whereas untreated controls were able to produce the wave as previously observed. Although caution is needed in interpreting results with a single chemical inhibitor, our results suggest that calcium may enter the oocyte through mechanosensitive TRP channels during egg activation.
Propagation of the Ca2+ Wave Requires IP3-Mediated Release from Internal Stores.
In most organisms studied to date, the first calcium wave that occurs at egg activation occurs through sequential release of Ca2+ from ER stores within the egg, often triggered through phosphoinositide signaling. To test whether this was also the case for propagation of the calcium wave in Drosophila oocytes, we examined calcium waves when the IP3 receptor was depleted. Females with germline clones of null or altered sensitivity IP3R alleles failed to ovulate under the conditions for in vivo imaging, preventing their use for these studies. Although we could dissect oocytes from such females, those eggs were fragile, making them impossible to image consistently. This precluded our use of germline clones to examine the details of calcium dynamics in oocytes.
Therefore, we used RNAi knockdown to test for a role of phosphoinositide-based release of calcium from internal stores in the calcium wave. In GCaMP3-containing oocytes knocked down for the IP3 receptor, a calcium increase occurred initially, but was not sustained (Fig. 1A, second row, and Movie S10; Fig. 1B, green tracing). Taken together, our data indicate that the calcium rise initiates independently of IP3R, but that propagation of the calcium rise requires IP3R.
The germline clone and knockdown oocytes used in our in vivo tests had developed throughout oogenesis with little or no IP3R. Thus, it was possible that their phenotype reflected developmental issues due to a need for this activity during oogenesis. To test whether phosphoinositide-based signaling is needed only in mature oocytes for the wave, we performed imaging of in vitro activation in oocytes treated with LY294002, which inhibits PI3K (42). Over a range of concentrations (43–45), this inhibitor significantly prevented the calcium wave, and the effect was dose-responsive (Fig. S4; P < 0.03 for all concentrations tested). These results further support a role for phosphoinositide-mediated calcium release in oocytes for the propagation of the calcium wave.
In vertebrates, Ca2+ can also be released from ER stores through activation of the ryanodine receptor on the ER plasma membrane. However, we do not believe that this process is involved in the Drosophila calcium wave propagation. High-throughput studies show no expression of ryanodine receptor in the adult ovary of virgin and mated females (46). Moreover, we tested directly for the role of the ryanodine receptor in Drosophila egg activation in vitro by using ruthenium red, an inhibitor of the ryanodine receptor (47). Ruthenium red showed no effect on the Ca2+ flux and no inhibition of egg activation; thus ryanodine receptor activity is not required for either process (Fig. 3, Table S1, and Movie S11).
Discussion
Egg activation is a conserved phenomenon that prepares an animal oocyte for successful embryogenesis through completion of meiosis, restructuring of the vitelline membrane, and changes to the existing protein and mRNA pools within the egg. The trigger for Drosophila (and other arthropod) egg activation differs from the better known cases of vertebrate and echinoderm egg activation in that it is decoupled from fertilization. Despite this critical difference in egg activation trigger mechanisms, we report that a calcium wave occurs during egg activation in Drosophila, as in other animals. In vivo imaging of oocyte calcium levels indicates that the intracellular calcium rise is triggered by ovulation. In vitro imaging shows that this rise takes the form of wave(s) that initiate from egg pole(s) and move across the egg; the rise in cytosolic calcium is then followed by a decrease. We propose that this dynamic rise and fall in cytosolic calcium triggers the events of egg activation in Drosophila, as suggested for the calcium transients in other organisms such as mouse (8, 9).
The calcium rise during Drosophila egg activation can occur only in the presence of calcium in the extracellular environment. We propose that the wave initiates when Ca2+ enters the oocyte through activation of mechanosensitive ion channels on the oocyte cell surface. These channels are proposed to be activated by either or both of the following mechanisms. First, the oocyte swells as it passes through the oviducts, presumably by taking up fluid: mature oocytes in the ovary are shriveled in appearance, but laid eggs are swollen and taut. In our in vitro experiments, we noted that the calcium wave does not initiate until after the egg has begun to swell. Additionally, we were able to increase the speed of initiation by adding a few drops of water to the activating medium during imaging, thus increasing hypotonicity and causing faster egg swelling. We postulate that swelling exerts a stretch tension force on the membrane, which triggers the opening of mechanosensitive Ca2+ channels. Second, we show that, independently of oocyte swelling, mechanical pressure exerted on the oocyte is capable of initiating the wave. We propose that oocytes may be subjected to both triggers during ovulation: pressure from the outside as they move out of the ovary and into the oviducts and swelling as they encounter the oviductal fluid. As a result, mechanosensitive ion channels open, and calcium levels rise in the oocyte. In vivo imaging of oocytes as they are ovulating supports our pressure hypothesis: the movement of the oocyte into the oviduct is not smooth and fluid; instead, the oocyte moves slowly at first and then rather suddenly pushes into the oviduct, as if it meets some resistance force as it begins ovulation (Movies S3 and S4).
Recent evidence from mice indicates that a requirement for external Ca2+ for egg activation is not unique to insects like Drosophila, although a requirement for calcium influx to initiate the first wave is. In mice, after the initial Ca2+ rise induced by sperm PLC, further calcium oscillations require Ca2+ uptake from the extracellular environment through a store-operated Ca2+ entry mechanism; when intracellular ER Ca2+ stores are depleted, plasma membrane channels open to allow Ca2+ back into the cell (3).
How could a wave be triggered from the egg pole(s)? It is possible that the mechanosensitive ion channels that mediate the calcium rise are localized at the poles, analogous to the localization of some of the embryo-polarity machinery including a terminal-group signaling cascade that marks the two ends of the embryo as similar to one another but different from the interior (48). In this model, mechanical cues applied to the egg would activate those channels, and because they are at the poles, the wave would initiate at the poles. It will be intriguing to test this hypothesis by determining which mechanosensitive ion channels are needed to trigger the calcium wave (and egg activation) and whether they show polar localization. Toward this end, our finding that the wave is inhibited by gadolinium and ACA suggests that the relevant channels might be members of the TRP family of calcium channels (49). The best candidates are three TRP family channels that are expressed in the ovary [painless (TRPA1), trpm (TRPM3), and trpml (TRPP1/Pkd2)] (reviewed in ref. 41). Further experiments will be needed to determine the particular channel(s) that is needed to initiate the wave and its localization in the oocyte membrane. Alternatively, it is possible that the required channels are not localized but rather that the egg cytoskeleton is less rigid on the poles of the egg. Normally, uniform swelling of a prolate spheroid (such as a Drosophila oocyte) would exert greater tension along the center or waistline (50). However, different cytoskeletal makeup at the poles may cause tension to be experienced differently there, and in this way channels spread uniformly throughout the plasma membrane may open first at the poles. Further experiments will be required to determine why the wave initiates at the poles.
In other organisms in which the signaling pathway has been studied downstream of the Ca2+ influx, an increase in intracellular Ca2+ is thought to be the ultimate cause of the meiosis resumption that permits subsequent embryonic mitosis, and of changes in macromolecular synthesis or stability. However, the way in which these events are connected to the calcium wave is still unclear in any system. Here, we have shown that a calcium wave occurs during Drosophila egg activation and that the sensitivity of this wave to manipulations (pressure, swelling, chemical inhibitors) mirrors that for egg activation events. Given this finding, and the fact that many signaling pathways and events downstream of the calcium signal appear to be conserved between Drosophila other species, Drosophila will offer the unique perspective of isolating egg activation events from fertilization events, as well the possibility of genetic manipulation and larger-scale “omics” studies that will help to link a Ca2+ flux to downstream egg activation events.
Materials and Methods
DNA Constructs and Transgenic Flies.
To generate transgenic flies expressing GCaMP3 (51) in oocytes, we constructed pUASp-GCaMP3-attB and nos-GCaMP3-attB and introduced them into the fly genome using a phiC31-based integration system (52). pUASp-GCaMP3-attB was generated from pUAST-attB (52) by replacing the UAS region with a EcoRV and PstI fragment of pUASp (53) and GCaMP3 ORF (51). To construct nos-GCaMP3-attB, the GCaMP3 ORF was inserted between 1.5 kb upstream sequences and 1 kb downstream sequences of the nos coding sequence using the In-Fusion HD cloning kit (Clontech). Details of the construction procedure are available upon request. The construct was integrated into the attP site at P{y[+t7.7]=CaryIP}su(Hw)attP5 or P{y[+t7.7]=CaryIP}su(Hw)attP2 (54) in the y w genetic background, and transgenic lines were stabilized by standard procedures. Females with germline knockdown of IP3R were matα4-GAL-VP16 > UASp-GCaMP3; P{TRiP.GLC01786}attP40. The shRNA line from the TRiP collection (55) was obtained from the Bloomington Stock Center. Spermless XO males for the experiment shown in Fig. 1 were obtained from a cross between Canton S females and attached XY [C(1;Y)1, y1 w1 f1] males; we confirmed that these XO males do not make sperm. Details of IP3R germline clone generation are in SI Materials and Methods.
Ca2+ Imaging of Oocytes.
In vivo.
Female flies carrying matα4-GAL-VP16 > UASp-GCaMP3 (56) were anesthetized with triethylamine (57). Anesthetized female flies were affixed to a microscope slide with their ventral sides up using double-sided tape and examined with a Leica MZ16F Fluorescence Stereo Microscope equipped with a CCD camera (Leica DFC300 FX). Fluorescence images of the abdomen were taken continuously. Control flies were matα4-GAL-VP16 > UASp-GFP::aequorin. This GFP–aequorin fusion does not respond to calcium in the absence of coelenterazine (the aequorin substrate, which was not present in these experiments); in these situations, this protein’s fluorescence behavior is like that for unmodified GFP (see SI Materials and Methods for details). To measure fluorescence intensity (ΔF/F0) at a given time point, an oval area at the center of the oocyte (476 pixels), corresponding to ∼60% of total oocyte area, was selected, and fluorescence intensity was measured. The time that the oocyte entered the uterus was defined as time 0.
In vitro.
Oocytes were imaged using a Zeiss 710 inverted LSM with ZEN software or a Nikon C1si LSM with a water immersion lens. Oocytes were dissected from transgenic females expressing GCaMP3 and induced to activate in vitro following methods similar to those that were previously described (36, 37). For imaging, a single oocyte was placed in a drop of IB (37) in a glass-bottomed dish (MAT-TEK). Once imaging parameters were set, the dish and egg were flooded with ∼1 mL of piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES)-based AB (36) to induce egg activation in vitro. Images were taken for 20–60 min after the addition of AB. If no change was seen in intracellular Ca2+ levels within 10 min, an additional 0.5 mL of AB or a few drops of ddH2O were added to the dish to accelerate egg activation.
To measure the effects of mechanical pressure on the formation of the calcium wave, 10–20 stage-14 oocytes were dissected in IB and transferred to a 100-µL drop of IB on a microscope slide. A coverslip (18 × 18 mm) was placed over the drop. The preparation was set on the stage of a Nikon C1si laser confocal microscope equipped with a 10× lens. To apply a mild mechanical pressure to the oocytes, IB was wicked from the edge of the coverslip using filter paper. Images were captured for a period of 20–60 min at the rate of 1 frame per second.
Drug and Inhibitor Treatment.
Removal of external Ca2+.
BAPTA (Sigma-Aldrich) was used to chelate calcium from IB and AB. IB and AB were prepared without the addition of CaCl2, and sucrose was added to these buffers to match the osmolarity of control buffers, which were prepared normally. BAPTA was added to IB and AB as previously described (36) to buffer Ca2+ to levels below 50 nM.
Inhibitors of Ca2+ entry or release.
Stock solutions of BAPTA, GdCl3, ACA, ruthenium red, and LY294002 (all from Sigma-Aldrich) were prepared and added to IB and AB on the day of the experiment. Oocytes were dissected and incubated in drug-treated IB or control IB for 30 min before beginning the experiment. Inhibitor-treated oocytes were then activated in AB also containing the inhibitor, and oocytes incubated in control IB were activated in control AB lacking the inhibitor. Final concentrations of inhibitors used were based on effective concentrations given in the literature. The concentrations were the following: GdCl3, 10, 100, 200, or 400 µM (36, 58); ACA, 10 or 20 µM (59); LY294002, 50, 100, 175, 200, and 250 µM (42); and ruthenium red, 10 or 20 µM. Control solutions were prepared in parallel without additional reagents.
Statistics.
Fisher’s exact test was used to compare the effects of the drug and inhibitor treatments, relative to control; P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank R. Akiyoshi for helping us with the bioluminescence imaging; J. Fetcho and C. Bayles (Cornell Microscopy and Imaging Facility) for advice and equipment access for some of the confocal imaging; K. Muramatsu for generating transgenic flies; H. Kato for assisting with fly dissection; J.-R. Martin for plasmids; P. Krasicky for assistance with modeling tension on a prolate spheroid; M. Okajima for assistance with assays of bleach resistance in the presence of inhibitors; the Drosophila Genetic Resource Center (Kyoto, Japan) and the Bloomington Stock Center for fly stocks; J. Brill for advice; J. Sitnik for assistance with graphics; and J. Liu, M. Goldberg, J. Brill, and two anonymous reviewers for helpful comments on the manuscript. T.A. and M.F.W. thank the Binational Agriculture Research and Development Fund and Y. Heifetz for the 2012 Insect Reproductive Molecules meeting at which this collaboration was initiated. We also thank the Julian Wicker Fund from the Cornell Graduate School for a grant to C.V.S., which made it possible for C.V.S. to visit T.A.’s laboratory to carry out some of this research. This work was supported by a grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (KAKENHI 22019029) to T.A. and by the National Institutes of Health Grants R01-GM044659 and R21-HD072714 (to M.F.W.), as well as Training Grant T32-GM07617, which provided partial support to C.V.S. and V.L.H.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1420589112/-/DCSupplemental.
References
- 1.Horner VL, Wolfner MF. Transitioning from egg to embryo: Triggers and mechanisms of egg activation. Dev Dyn. 2008;237(3):527–544. doi: 10.1002/dvdy.21454. [DOI] [PubMed] [Google Scholar]
- 2.Raz T, Shalgi R. Early events in mammalian egg activation. Hum Reprod. 1998;13(Suppl 4):133–145. doi: 10.1093/humrep/13.suppl_4.133. [DOI] [PubMed] [Google Scholar]
- 3.Miao YL, Stein P, Jefferson WN, Padilla-Banks E, Williams CJ. Calcium influx-mediated signaling is required for complete mouse egg activation. Proc Natl Acad Sci USA. 2012;109(11):4169–4174. doi: 10.1073/pnas.1112333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roux MM, et al. A functional genomic and proteomic perspective of sea urchin calcium signaling and egg activation. Dev Biol. 2006;300(1):416–433. doi: 10.1016/j.ydbio.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Xu Z, Kopf GS, Schultz RM. Involvement of inositol 1,4,5-trisphosphate-mediated Ca2+ release in early and late events of mouse egg activation. Development. 1994;120(7):1851–1859. doi: 10.1242/dev.120.7.1851. [DOI] [PubMed] [Google Scholar]
- 6.Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211(2):157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- 7.Whitaker M. Calcium at fertilization and in early development. Physiol Rev. 2006;86(1):25–88. doi: 10.1152/physrev.00023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ducibella T, Schultz RM, Ozil JP. Role of calcium signals in early development. Semin Cell Dev Biol. 2006;17(2):324–332. doi: 10.1016/j.semcdb.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Markoulaki S, Matson S, Abbott AL, Ducibella T. Oscillatory CaMKII activity in mouse egg activation. Dev Biol. 2003;258(2):464–474. doi: 10.1016/s0012-1606(03)00133-7. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto T. Physiological studies on fertilization and activation of fish eggs. V. The role of calcium ions in activation of Oryzias eggs. Exp Cell Res. 1954;6(1):56–68. doi: 10.1016/0014-4827(54)90147-0. [DOI] [PubMed] [Google Scholar]
- 11.Gilkey JC, Jaffe LF, Ridgway EB, Reynolds GT. A free calcium wave traverses the activating egg of the medaka, Oryzias latipes. J Cell Biol. 1978;76(2):448–466. doi: 10.1083/jcb.76.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee KW, et al. Ionophore-induced calcium waves activate unfertilized zebrafish (Danio rerio) eggs. Biol Bull. 1996;191(2):265–267. doi: 10.1086/BBLv191n2p265. [DOI] [PubMed] [Google Scholar]
- 13.Lee KW, Webb SE, Miller AL. A wave of free cytosolic calcium traverses zebrafish eggs on activation. Dev Biol. 1999;214(1):168–180. doi: 10.1006/dbio.1999.9396. [DOI] [PubMed] [Google Scholar]
- 14.Saunders CM, et al. PLC zeta: A sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129(15):3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- 15.Giusti AF, et al. Requirement of a Src family kinase for initiating calcium release at fertilization in starfish eggs. J Biol Chem. 1999;274(41):29318–29322. doi: 10.1074/jbc.274.41.29318. [DOI] [PubMed] [Google Scholar]
- 16.Swann K, Saunders CM, Rogers NT, Lai FA. PLCzeta(zeta): A sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Semin Cell Dev Biol. 2006;17(2):264–273. doi: 10.1016/j.semcdb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Heifetz Y, Yu J, Wolfner MF. Ovulation triggers activation of Drosophila oocytes. Dev Biol. 2001;234(2):416–424. doi: 10.1006/dbio.2001.0246. [DOI] [PubMed] [Google Scholar]
- 18.Went DF, Krause G. Egg activation in Pimpla turionellae (Hym.) Naturwissenschaften. 1974;61(9):407–408. doi: 10.1007/BF00622633. [DOI] [PubMed] [Google Scholar]
- 19.Lindsay LL, Hertzler PL, Clark WH., Jr Extracellular Mg2+ induces an intracellular Ca2+ wave during oocyte activation in the marine shrimp Sicyonia ingentis. Dev Biol. 1992;152(1):94–102. doi: 10.1016/0012-1606(92)90159-e. [DOI] [PubMed] [Google Scholar]
- 20.Horner VL, et al. The Drosophila calcipressin sarah is required for several aspects of egg activation. Curr Biol. 2006;16(14):1441–1446. doi: 10.1016/j.cub.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Doane WW. Completion of meiosis in uninseminated eggs of Drosophila melanogaster. Science. 1960;132(3428):677–678. doi: 10.1126/science.132.3428.677. [DOI] [PubMed] [Google Scholar]
- 22.Macdonald PM, Struhl G. A molecular gradient in early Drosophila embryos and its role in specifying the body pattern. Nature. 1986;324(6097):537–545. doi: 10.1038/324537a0. [DOI] [PubMed] [Google Scholar]
- 23.Tadros W, et al. SMAUG is a major regulator of maternal mRNA destabilization in Drosophila and its translation is activated by the PAN GU kinase. Dev Cell. 2007;12(1):143–155. doi: 10.1016/j.devcel.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Malcuit C, Kurokawa M, Fissore RA. Calcium oscillations and mammalian egg activation. J Cell Physiol. 2006;206(3):565–573. doi: 10.1002/jcp.20471. [DOI] [PubMed] [Google Scholar]
- 25.Takeo S, Tsuda M, Akahori S, Matsuo T, Aigaki T. The calcineurin regulator sra plays an essential role in female meiosis in Drosophila. Curr Biol. 2006;16(14):1435–1440. doi: 10.1016/j.cub.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 26.Takeo S, Hawley RS, Aigaki T. Calcineurin and its regulation by Sra/RCAN is required for completion of meiosis in Drosophila. Dev Biol. 2010;344(2):957–967. doi: 10.1016/j.ydbio.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Takeo S, et al. Shaggy/glycogen synthase kinase 3β and phosphorylation of Sarah/regulator of calcineurin are essential for completion of Drosophila female meiosis. Proc Natl Acad Sci USA. 2012;109(17):6382–6389. doi: 10.1073/pnas.1120367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swan A, Schüpbach T. The Cdc20 (Fzy)/Cdh1-related protein, Cort, cooperates with Fzy in cyclin destruction and anaphase progression in meiosis I and II in Drosophila. Development. 2007;134(5):891–899. doi: 10.1242/dev.02784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnard DC, Ryan K, Manley JL, Richter JD. Symplekin and xGLD-2 are required for CPEB-mediated cytoplasmic polyadenylation. Cell. 2004;119(5):641–651. doi: 10.1016/j.cell.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 30.Benoit P, Papin C, Kwak JE, Wickens M, Simonelig M. PAP- and GLD-2-type poly(A) polymerases are required sequentially in cytoplasmic polyadenylation and oogenesis in Drosophila. Development. 2008;135(11):1969–1979. doi: 10.1242/dev.021444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui J, Sackton KL, Horner VL, Kumar KE, Wolfner MF. Wispy, the Drosophila homolog of GLD-2, is required during oogenesis and egg activation. Genetics. 2008;178(4):2017–2029. doi: 10.1534/genetics.107.084558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kadyk LC, Kimble J. Genetic regulation of entry into meiosis in Caenorhabditis elegans. Development. 1998;125(10):1803–1813. doi: 10.1242/dev.125.10.1803. [DOI] [PubMed] [Google Scholar]
- 33.Choi T, et al. Mos/mitogen-activated protein kinase can induce early meiotic phenotypes in the absence of maturation-promoting factor: A novel system for analyzing spindle formation during meiosis I. Proc Natl Acad Sci USA. 1996;93(10):4730–4735. doi: 10.1073/pnas.93.10.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sackton KL, Buehner NA, Wolfner MF. Modulation of MAPK activities during egg activation in Drosophila. Fly (Austin) 2007;1(4):222–227. doi: 10.4161/fly.5200. [DOI] [PubMed] [Google Scholar]
- 35.Krauchunas AR, Horner VL, Wolfner MF. Protein phosphorylation changes reveal new candidates in the regulation of egg activation and early embryogenesis in D. melanogaster. Dev Biol. 2012;370(1):125–134. doi: 10.1016/j.ydbio.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horner VL, Wolfner MF. Mechanical stimulation by osmotic and hydrostatic pressure activates Drosophila oocytes in vitro in a calcium-dependent manner. Dev Biol. 2008;316(1):100–109. doi: 10.1016/j.ydbio.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page AW, Orr-Weaver TL. Activation of the meiotic divisions in Drosophila oocytes. Dev Biol. 1997;183(2):195–207. doi: 10.1006/dbio.1997.8506. [DOI] [PubMed] [Google Scholar]
- 38.Baubet V, et al. Chimeric green fluorescent protein-aequorin as bioluminescent Ca2+ reporters at the single-cell level. Proc Natl Acad Sci USA. 2000;97(13):7260–7265. doi: 10.1073/pnas.97.13.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Endow SA, Komma DJ. Spindle dynamics during meiosis in Drosophila oocytes. J Cell Biol. 1997;137:1321–36. doi: 10.1083/jcb.137.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graveley BR, et al. The developmental transcriptome of Drosophila melanogaster. Nature. 2011;471(7339):473–479. doi: 10.1038/nature09715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sartain CV, Wolfner MF. Calcium and egg activation in Drosophila. Cell Calcium. 2013;53(1):10–15. doi: 10.1016/j.ceca.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269(7):5241–5248. [PubMed] [Google Scholar]
- 43.Saul D, Fabian L, Forer A, Brill JA. Continuous phosphatidylinositol metabolism is required for cleavage of crane fly spermatocytes. J Cell Sci. 2004;117(Pt 17):3887–3896. doi: 10.1242/jcs.01236. [DOI] [PubMed] [Google Scholar]
- 44.Wong R, et al. PIP2 hydrolysis and calcium release are required for cytokinesis in Drosophila spermatocytes. Curr Biol. 2005;15(15):1401–1406. doi: 10.1016/j.cub.2005.06.060. [DOI] [PubMed] [Google Scholar]
- 45.Wong R, Fabian L, Forer A, Brill JA. Phospholipase C and myosin light chain kinase inhibition define a common step in actin regulation during cytokinesis. BMC Cell Biol. 2007;8:15. doi: 10.1186/1471-2121-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.St Pierre SE, Ponting L, Stefancsik R, McQuilton P. FlyBase Consortium FlyBase 102: Advanced approaches to interrogating FlyBase. Nucleic Acids Res. 2014;42(Database issue) D1:D780–D788. doi: 10.1093/nar/gkt1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vázquez-Martínez O, Cañedo-Merino R, Díaz-Muñoz M, Riesgo-Escovar JR. Biochemical characterization, distribution and phylogenetic analysis of Drosophila melanogaster ryanodine and IP3 receptors, and thapsigargin-sensitive Ca2+ ATPase. J Cell Sci. 2003;116(Pt 12):2483–2494. doi: 10.1242/jcs.00455. [DOI] [PubMed] [Google Scholar]
- 48.Deng WM, Bownes M. Patterning and morphogenesis of the follicle cell epithelium during Drosophila oogenesis. Int J Dev Biol. 1998;42(4):541–552. [PubMed] [Google Scholar]
- 49.Harteneck C, Klose C, Krautwurst D. Synthetic modulators of TRP channel activity. In: Islam MS, editor. Transient Receptor Potential Channels, Advances in Experimental Medicine and Biology. Springer; Cham, Switzerland: 2011. [DOI] [PubMed] [Google Scholar]
- 50.Regen DM. Tensions and stresses of ellipsoidal chambers. Ann Biomed Eng. 1996;24(3):400–417. doi: 10.1007/BF02660889. [DOI] [PubMed] [Google Scholar]
- 51.Tian L, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6(12):875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci USA. 2007;104(9):3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rørth P. Gal4 in the Drosophila female germline. Mech Dev. 1998;78(1-2):113–118. doi: 10.1016/s0925-4773(98)00157-9. [DOI] [PubMed] [Google Scholar]
- 54.Pfeiffer BD, et al. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186(2):735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ni JQ, et al. Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat Methods. 2008;5(1):49–51. doi: 10.1038/nmeth1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bossing T, Barros CS, Brand AH. Rapid tissue-specific expression assay in living embryos. Genesis. 2002;34(1-2):123–126. doi: 10.1002/gene.10145. [DOI] [PubMed] [Google Scholar]
- 57.Fuyama Y. Triethylamine: An anesthetic for Drosophila with prolonged effect. Drosoph Inf Serv. 1977;52:173. [Google Scholar]
- 58.Hardie RC. TRP channels and lipids: From Drosophila to mammalian physiology. J Physiol. 2007;578(Pt 1):9–24. doi: 10.1113/jphysiol.2006.118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harteneck C, Frenzel H, Kraft R. N-(p-amylcinnamoyl)anthranilic acid (ACA): A phospholipase A(2) inhibitor and TRP channel blocker. Cardiovasc Drug Rev. 2007;25(1):61–75. doi: 10.1111/j.1527-3466.2007.00005.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.