Significance
Tic disorders, such as Tourette syndrome, are common but poorly understood. Postmortem studies have revealed the loss of a particular subset of neurons, the large cholinergic interneurons, in the caudate and putamen in severe disease. To test whether this neuronal loss leads to disease we turned to studies in mice, where questions of causality are more readily addressed. We developed a strategy for targeted ablation of these interneurons. Their disrupting in the dorsolateral striatum—roughly analogous to the putamen—produced abnormal tic-like movements after either acute stress or amphetamine treatment. This demonstrates, for the first time to our knowledge, that the loss of specific interneurons can cause behavioral changes in an animal model that resemble aspects of a movement disorder.
Keywords: Tourette sydrome, basal ganglia, interneurons, acetylcholine, animal models
Abstract
Gilles de la Tourette syndrome (TS) is characterized by tics, which are transiently worsened by stress, acute administration of dopaminergic drugs, and by subtle deficits in motor coordination and sensorimotor gating. It represents the most severe end of a spectrum of tic disorders that, in aggregate, affect ∼5% of the population. Available treatments are frequently inadequate, and the pathophysiology is poorly understood. Postmortem studies have revealed a reduction in specific striatal interneurons, including the large cholinergic interneurons, in severe disease. We tested the hypothesis that this deficit is sufficient to produce aspects of the phenomenology of TS, using a strategy for targeted, specific cell ablation in mice. We achieved ∼50% ablation of the cholinergic interneurons of the striatum, recapitulating the deficit observed in patients postmortem, without any effect on GABAergic markers or on parvalbumin-expressing fast-spiking interneurons. Interneuron ablation in the dorsolateral striatum (DLS), corresponding roughly to the human putamen, led to tic-like stereotypies after either acute stress or d-amphetamine challenge; ablation in the dorsomedial striatum, in contrast, did not. DLS interneuron ablation also led to a deficit in coordination on the rotorod, but not to any abnormalities in prepulse inhibition, a measure of sensorimotor gating. These results support the causal sufficiency of cholinergic interneuron deficits in the DLS to produce some, but not all, of the characteristic symptoms of TS.
Gilles de la Tourette syndrome (TS) represents the most severe end of a spectrum of tic disorders that, in aggregate, affect 5% of the population and produce substantial morbidity (1). Existing treatments are of limited efficacy in severe disease (2). The defining symptoms of TS are motor and phonic tics, defined as sudden, repetitive, nonrhythmic, involuntary or semiinvoluntary movements or utterances that involve discrete muscle groups. Tics fluctuate and are exacerbated by stress (3–5) and by acute challenge with prodopaminergic drugs (6). Individuals with TS also often have deficits in fine motor control (7, 8), procedural learning (9), and sensorimotor gating (10, 11).
Convergent evidence implicates the corticobasal ganglia circuitry in the pathophysiology of TS, although details remain poorly understood (12, 13). The input nucleus of the basal ganglia, the striatum, receives glutamatergic projections from the cortex and thalamus. The striatum is reduced in volume in individuals with TS (12–14), and this reduction predicts the severity and the persistence of symptoms (15). Whereas the cellular architecture of the dorsal striatum is fairly uniform throughout its medial–lateral extent, the topographic organization of cortical afferents leads to functional segregation among subregions (16–19). The dorsolateral striatum (DLS), which corresponds roughly to the human putamen, has been associated with sensorimotor habits (20) and with tic-like stereotypies in rodents (21, 22). Functional neuroimaging has correlated abnormalities in these lateral corticostriatal networks with tic characteristics in patients with TS, whereas abnormalities in medial networks correlated instead with comorbid obsessive-compulsive symptoms (23).
The principal cells of the striatum, the GABAergic medium spiny neurons (MSNs), are modulated by several types of interneuron (24). Recent postmortem work has revealed that specific interneuron populations are abnormal in patients with severe, refractory TS (25–27). Cholinergic interneurons, identified by their expression of choline acetyltransferase (ChAT), are critical regulators of striatal function, although they constitute only about 1% of all neurons in the striatum (24). They are reduced by ∼50% throughout the dorsal striatum in TS (26, 27). Abnormalities in ChAT interneurons in the ventral striatum have been reported in schizophrenia (28), but deficits in the dorsal striatum (the caudate and putamen) have only been described in TS (26).
However, the relationship of this interneuronal deficit to the etiopathophysiology of TS remains unclear. One possibility is that this deficit is causally related to symptoms; ChAT interneuron abnormalities in different striatal subregions may have dissociable contributions to symptomatology. Alternative possibilities include that the ChAT deficit observed in postmortem tissue is a compensation for the primary pathology, a consequence of treatment, or an epiphenomenon of no relevance to symptomatology. Finally, the ChAT interneuron deficit may be pathophysiologically important, but it may manifest only in the context of other pathology, such as the deficit in parvalbumin-expressing interneurons that has also been documented in postmortem tissue (25, 26), or only through its secondary effects on the functions of striatal circuits during development. Such questions cannot readily be addressed by observational studies in patients.
Therefore, to test the hypothesis that ChAT interneuron disruption in the dorsal striatum is sufficient to produce tic-like phenomenology, we turned to a mouse model. We found an immunotoxin that has been used previously to target striatal ChAT interneurons (29–32) to produce nonspecific effects (Fig. S1). We therefore developed an approach to induce targeted, regulated ablation of ChAT interneurons in mice. We anticipated that interneuronal abnormalities in different parts of the circuitry might contribute differentially to the TS phenotype. We targeted the ChAT interneurons of the DLS and dorsomedial striatum (DMS) in separate experiments, achieving a ∼50% reduction, which recapitulates the degree of abnormality found postmortem (26). We tested the effect of this targeted ablation on behavioral phenotypes that recapitulate core phenomenology of TS and that have been found to be abnormal in other animal models (10, 33). This ChAT ablation does not recapitulate all documented cellular abnormalities in TS (25, 26) or their developmental course, which is not well established. Rather, it tests sufficiency of an isolated ChAT interneuronal deficit in an otherwise normal adult brain to produce aspects of TS phenomenology.
Results
Viral Targeting of Interneurons.
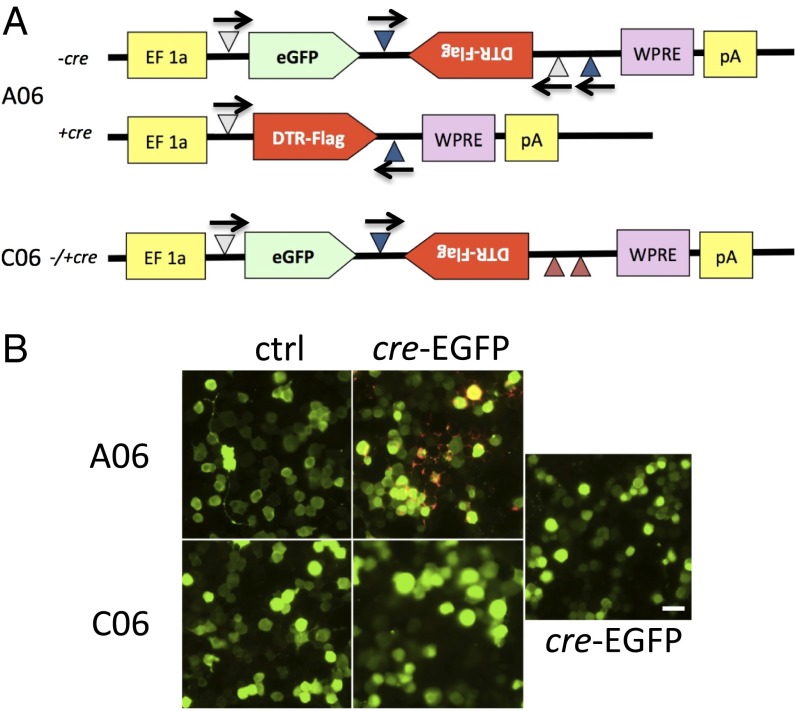
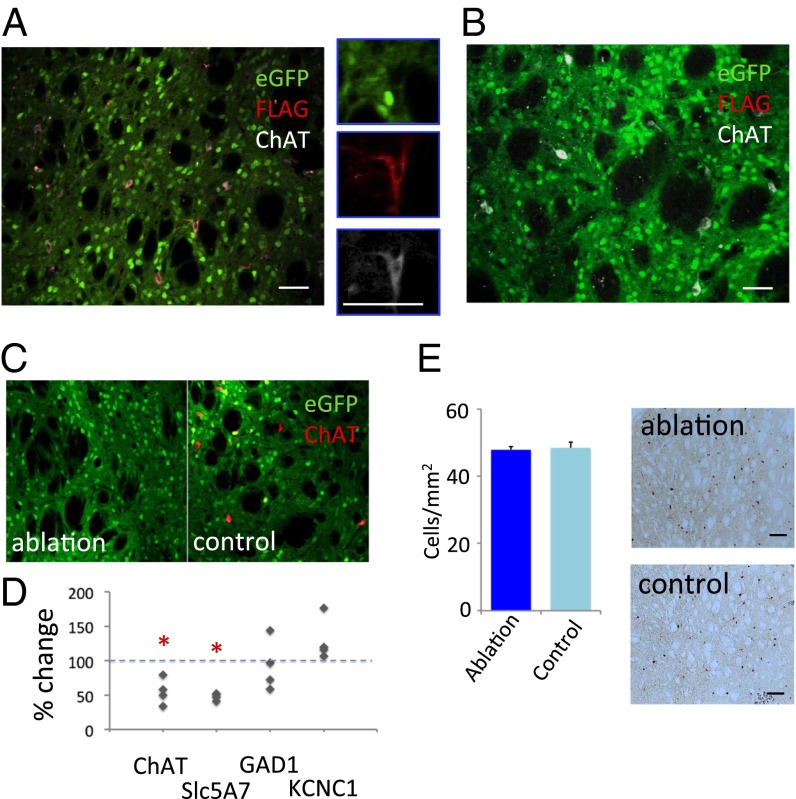
We designed a recombinant virus (A06) (Fig. 1A and Materials and Methods) to permit the specific expression of the simian diphtheria toxin receptor (DTR) in cre-expressing cells. This makes those cells specifically susceptible to ablation after systemic administration of diphtheria toxin (DT). This virus, and the negative control virus C06 (Materials and Methods), were injected into the dorsal striatum of ChAT-cre transgenic mice (www.jax.org: 006410); mice were euthanized 2 wk later. EGFP expression was seen throughout the striatum, confirming the spread of the rh10 viral serotype and the utility of eGFP as a marker of viral infection. FLAG immunoreactivity was seen only in cells also immunoreactive for ChAT (Fig. 2A). No FLAG immunoreactivity was ever seen after C06 injection (Fig. 2B) or when either virus was injected into wild-type mice.
Fig. 1.
A strategy for cre-inducible expression of DTR. (A) Map of AAVrh10 EF-FLEX-DTR-FLAG vector. EF, elongation factor 1a promoter; white triangles, lox 2722; blue triangles, Lox P; red triangles, mutated nonfunctional lox sites; eGFP, enhanced green fluorescent protein; DTR, diphtheria toxin receptor; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element. (B) FLAG expression in transiently transfected N2A cell cultures. GFP is expressed from A06 and C06 viruses as well as from cotransfected cre-eGFP. Only double transfection with A06 and cre-eGFP shows FLAG expression, as revealed by double immununostaining for eGFP (green) and FLAG (red). (Scale bar, 25 µm.)
Fig. 2.
Targeted interneuronal ablation in the ChAT-cre mouse striatum. (A) Triple immunostaining of striatal neurons after A06 virus infusion shows eGFP (green), FLAG (red), and ChAT (white); FLAG immunoreactivity, which corresponds to DTR expression, colocalizes specifically with ChAT, confirming the specificity of DTR expression using this system (compare with D). (B) Triple immunostaining of striatal neurons after C06 virus infusion, showing eGFP (green), FLAG (red; no staining is apparent, although conditions were identical to A), and ChAT (white). (C) Reduced ChAT-expressing interneurons after A06 infection and DT injection in the dorsal striatum, compared with control C06 virus (Fig. 3 B and E). (D) The specificity of cholinergic cell ablation was evaluated using quantitative PCR (qPCR) analysis of RNA isolated from A06-infected and contralateral C06-infected striatum. Expression of ChAT interneuron-related genes was reduced approximately twofold on the ablation side (n = 4 animals. Paired t test: ChAT, P = 0.03 (one tailed); Slc5A7, P = 0.01 (one tailed). GABA-related genes were not significantly reduced: GAD1, P = 0.58 (two tailed); KCNC1, P = 0.054 (two tailed). (E) Immunostaining for parvalbumin-positive interneurons revealed no qualitative or quantitative difference between experimental conditions, further confirming the specificity of our manipulation to the cholinergic interneurons. *P < 0.05. (Scale bar, 20 µm for A–C and 100 µm for E.)
To test interneuron ablation, we injected A06 virus into the dorsal striatum on one side and C06 into the contralateral dorsal striatum in ChAT-cre transgenic mice. Two weeks later, mice were injected i.p. with either DT (15 µg/kg body weight) or saline. One week after DT injection, ChAT immunoreactive cells were reduced on the A06-infected side relative to the C06 infected side (Fig. 2C), confirming interneuron ablation. The image shown here is taken from the center of the virus-infused area, where viral infection was highest and ablation was greatest; we achieved ∼50% reduction of the density of ChAT-expressing interneurons in the dorsal striatum more broadly (see below, Fig. 3 B and E), recapitulating the degree of deficit observed in postmortem material from patients (26).
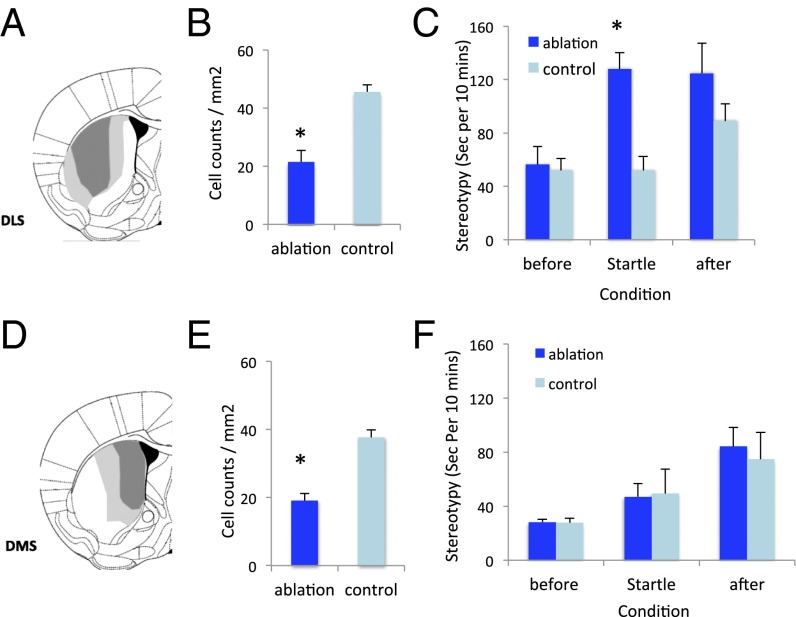
Fig. 3.
Stereotypy after stress. (A) Minimum and maximum A06 viral spread in dorsolateral striatum (DLS). (B) Reduced density ChAT-positive interneurons in the DLS in DLS-targeted mice after DT treatment, relative to C06-infected controls; see also Figs. S2 and S3. Student’s t test: t(9) = 4.8, P = 0.001. (C) Elevated fragmented/stereotypic grooming after startle stress in DLS ChAT-ablated mice. RM-ANOVA: main effect of block, F(2,18) = 4.85, P = 0.042; main effect of group, F(1,9) = 9.63, P = 0.013; group × block interaction, F(2,18) = 4.26, P = 0.055). (D) Viral spread in dorsomedial striatum (DMS)-targeted mice. (E) Reduced ChAT-positive interneurons, quantified over the entire striatum, in DMS-targeted mice after DT treatment, relative to C06-infected controls. Student’s t test: t(11) = 5.9, P < 0.001. (F) Normal grooming behavior after startle stress in DMS ChAT-ablated mice. RM-ANOVA: main effect of block, F(2,22) = 14, P = 0.004; main effect of group, F(1,11) = 0.034, P > 0.5; block × group interaction, F(2,22) = 0.20, P > 0.5).
The ChAT interneuron-specific genes ChAT and Slc5A7 (the choline transporter) showed a ∼50% reduction in A06-infected striatum, compared with C06-infected striatum, after DT (Fig. 2D and Table S1). Glutamic acid dehydrogenase (a marker of GABAergic cells) and the potassium channel KCNC1 (which is expressed in parvalbumin-containing interneurons) were not reduced by ChAT interneuron ablation (Fig. 2D). Immunostaining for parvalbumin-containing interneurons showed normal numbers of these interneurons (Fig. 2E).
Tic-Like Stereotypies After ChAT Interneuron Ablation.
We characterized behavioral phenotypes that parallel different domains of TS symptomatology (10, 33) following ∼50% ChAT interneuron ablation in the DLS (Fig. 3 A and B and Figs. S2 and S3).
ChAT-ablated mice did not exhibit detectable tic-like stereotypy at baseline. In this way they resemble a recently described genetic model, in which increased tic-like stereotypies only emerged after pharmacological challenge (10). To test the ability of an acute stressor to potentiate tic-like stereotypies (3–6, 34), we exposed ChAT-ablated animals (and C06-injected controls) to repeated unpredictable acoustic startle stimuli. Grooming, stereotypy, and other behaviors were observationally scored from video before, during, and after this block of startle stimuli. ChAT-ablated mice exhibited no behavioral abnormalities before the stressor, but increased grooming during and after the 11-min startle block (Fig. 3C). Grooming was fragmented, consisting primarily of repeated initiation of grooming of the face and whiskers, and progressing only infrequently to a full syntactic grooming chain encompassing the whole body; in this it qualitatively resembles the abnormal grooming seen (in the absence of an acute stressor) in mice with a deletion of the SAPAP3 gene (Movie S1) (35). These abnormal grooming bouts were not time-locked to startle stimuli but rather occurred continuously throughout the startle block and poststartle period.
To test the anatomical specificity of this phenomenon, we performed an otherwise identical ablation in the DMS, in a separate cohort of mice (Fig. 3 D and E and Figs. S2 and S3). The efficiency of ablation in the DMS was similar to that in the DLS. Mice with ChAT ablation in the DMS showed no alteration in acute stress-induced repetitive grooming (Fig. 3F).
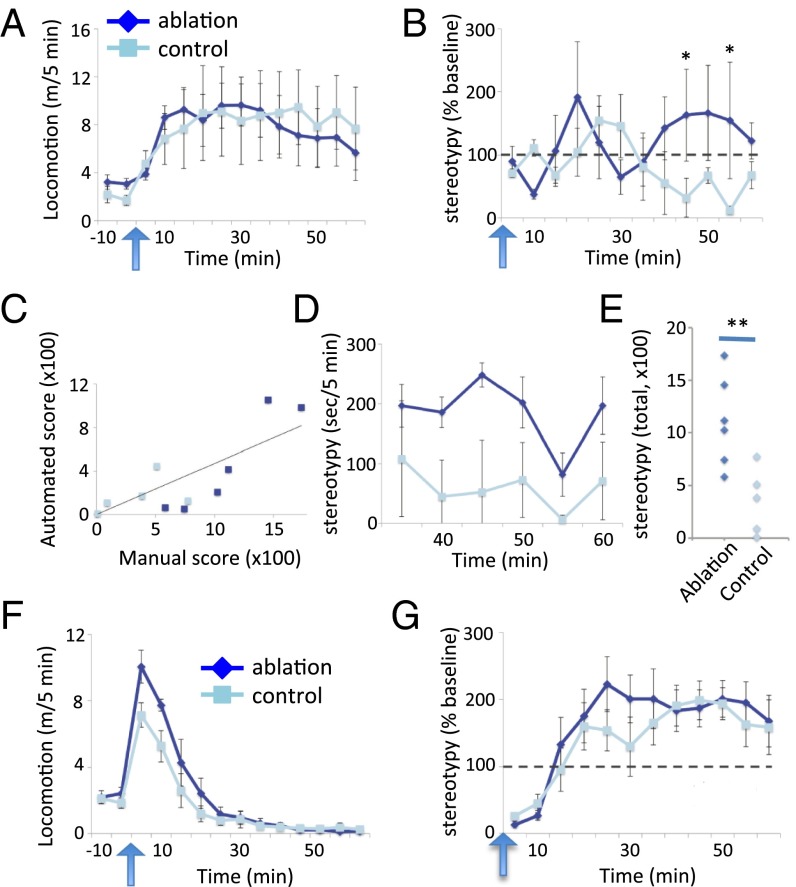
We also measured locomotor activity and stereotypy before and after i.p. injection of d-amphetamine (10). The dose (7 mg/kg) was empirically determined in pilot experiments to produce limited stereotypy in intact mice on this genetic background. Both DLS ablation and control groups showed locomotor activation following amphetamine challenge, with no difference between ChAT-ablated and control animals (Fig. 4A). To maximize throughput and objectivity in the initial analysis of stereotypy, we scored repetitive low-amplitude movements using an automated system (36). These movements, scored as “grooming” by the automated system, differed across groups, lasting longer and reaching higher levels following amphetamine administration in the ChAT-ablated animals [Fig. 4B; time × treatment interaction, F(11, 99) = 2.02, P = 0.034]. To confirm the phenotype, a rater blind to experimental condition manually scored stereotypy at all time points in the second half hour after amphetamine, where automated scoring indicated a separation between groups, for post hoc analysis. Manual and automated scoring correlated significantly, although imperfectly, across this time period (Fig. 4C). Manual scoring confirmed greatly increased stereotypies in ChAT-ablated mice, across all measured time points (Fig. 4 D and E). Qualitatively, these stereotypies consisted primarily of focused repetitive sniffing (Movie S2), similar to those observed in a recently described genetic animal model of TS, the histidine decarboxylase knockout mouse (10).
Fig. 4.
Stereotypy after amphetamine challege. (A) Normal locomotor activity after amphetamine challenge in DLS ChAT-ablated mice. RM-ANOVA: main effect of time, F(11,99) = 1.85, P = 0.056; main effect of group, F(1,9) = 0.014, P = 0.9; time × group interaction, F(11,99) = 0.537, P = 0.874. (B) Elevated motor stereotypies over time, as measured by an automated video-analysis system, after amphetamine challenge in DLS ChAT-ablated mice. RM-ANOVA: main effect of time, F(11,99) = 0.48, P = 0.9; main effect of group, F(1,9) = 0.25, P = 0.6; time × group interaction F(11,99) = 2.02, P = 0.034. Post hoc tests at specific time points are by two-tailed t test. (C) Automated stereotypy counts correlated with manual counts (measured 30–60 min after amphetamine) r = 0.813; P = 0.002. (D) Manual assessment of stereotypy, scored by a rater blind to experimental condition, performed post hoc 30–60 min after amphetamine (when automated scoring indicated a separation between groups), confirmed a significant increase in stereotypy after DLS ChAT ablation. RM-ANOVA: main effect of time, F(5,45) = 4.992; P = 0.051; main effect of group, F(1,9) = 10.60, P = 0.01; time × group interaction, F(5,45) = 1.57, P = 0.3. (E) Summed manually scored stereotypy across 30–60 min. T(9) = 3.255, P = 0.01. (F) Elevated locomotor activity after amphetamine challenge in DMS ChAT-ablated mice. RM-ANOVA: main effect of time, F(11,121) = 55.6, P < 0.001; main effect of group, F(1,11) = 3.2, P = 0.102; time × group interaction, F(11,121) = 2.16, P = 0.021. (G) Motor stereotypies after amphetamine challenge in DMS ChAT-ablated mice, scored by the automated system, did not differ between groups. RM-ANOVA: main effect of time, F(11,121) = 16, P < 0.001; main effect of group, F(1,11) = 0.047, P = 0.8; time × group interaction, F(11,121) = 0.7, P = 0.7. n = 6 ablated, 5 control for the DLS group and 7 ablated, 6 control for the DMS group; all values are mean ± SEM *P < 0.05, **P < 0.01.
After amphetamine, DMS ChAT-ablated mice showed slightly enhanced locomotor activation, relative to controls (Fig. 4F), and no change in stereotypy (Fig. 4G), confirming a dissociable role for DLS and DMS in the generation of tic-like phenomenology after ChAT interneuron ablation.
Other TS-Relevant Behavioral Phenotypes.
We tested DLS ChAT-ablated mice and found no prepulse inhibition (PPI) deficit (Fig. S4A). Excitotoxic DMS lesions impair PPI (37); however, we also found no PPI deficit after DMS ChAT ablation (Fig. S4B).
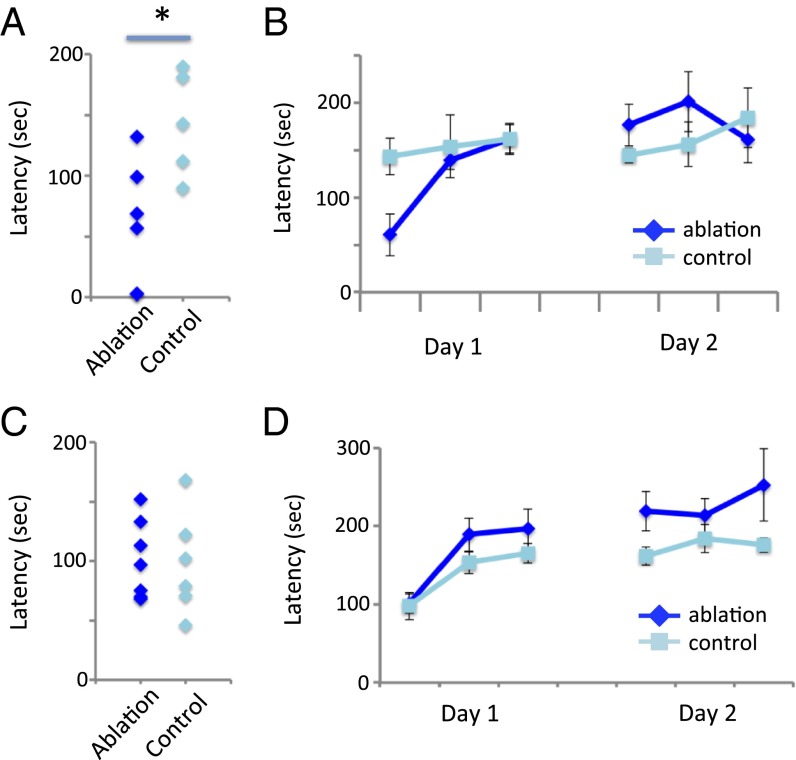
Patients with TS also exhibit deficits in certain fine motor skills (7, 8). We tested ChAT-ablated mice on the rotorod, which assays both baseline motor performance and improvement over time. Mice with a DLS ChAT lesion showed a marked deficit in baseline rotorod performance (Fig. 5A) but rapidly improved with training to a level of performance indistinguishable from controls (Fig. 5B). DMS ChAT-lesioned mice showed no deficits either at baseline or across trials (Fig. 5 C and D).
Fig. 5.
Motor coordination and learning after ChAT ablation. (A) Impaired rotorod performance at baseline in DLS ChAT-ablated mice. Student’s t test: t(9) = 2.9; P < 0.019. (B) Intact motor learning across repeated rotorod trials in DLS ChAT-ablated mice (2 d at 3 trials/d; note that T1 here is also shown in A). RM-ANOVA: main effect of trial, F(5,45) = 5.05, P = 0.001; main effect of group, F(1,9) = 0.24, P = 0.63; trial × group interaction, F(5,45) = 2.29, P = 0.06. (C) Intact initial rotorod performance in DMS ChAT-ablated mice. Student’s t test: t(11) = 0.15; P = 0.88. (D) Intact motor learning in DMS ChAT-ablated mice. RM-ANOVA: main effect of trial, F(5,55) = 9.53, P < 0.001; main effect of group, F(1,11) = 3.15, P = 0.10; trial × group interaction, F(5,55) = 0.946, P = 0.46. n = 6 ablated, 5 control for the DLS group and 7 ablated, 6 control for the DMS group; all values are mean ± SEM *P < 0.05.
ChAT-ablated mice and control mice showed similar activity in the open field and similar anxiety-like behaviors in both the open field and the elevated plus maze (Figs. S5 and S6).
Discussion
The etiology and pathophysiology of TS are not well understood (13). Postmortem studies show a reduction in ChAT-expressing striatal interneurons in the caudate and putamen in individuals with severe, refractory disease (26, 27). However, such correlational findings cannot elucidate the causal role of this cellular deficit: whether it is pathogenic, compensatory, epiphenomenomenal, or a marker of a more complex developmental disruption.
We have examined this question by producing a similar deficit in ChAT interneurons in the dorsal striatum of otherwise normal adult mice. Targeted cell ablation in the adult does not, of course, recapitulate the developmental consequences of a ChAT deficit and potential abnormal local connectivity that may accompany congenital pathology; rather, it examines the effects of a ChAT interneuron deficit in an otherwise normally developed striatal circuitry. Previous studies have examined effects of ChAT ablation in the nucleus accumbens (31, 32), or more broadly throughout the striatum (29), but they have not discriminated between DLS and DMS, have not systematically tested for TS-associated behavioral effects, and have relied on immunotoxins that we find to have nonspecific effects (Fig. S1). Our results establish the sufficiency of ChAT interneuron disruption in the dorsolateral striatum in the adult to recapitulate some aspects of TS, although not all. This argues in favor of a direct role for the ChAT deficit in pathophysiology, and against a compensatory or epiphenomenal one.
Some aspects of TS, such as the premonitory urges that often precede tics, are difficult or impossible to assess in an animal (3–5); but behaviors recapitulating aspects of the motor symptomatology are accessible in mice (10). Low-amplitude, stereotyped, repetitive movements that recapitulate aspects of tics can be quantified; these include excessive and fragmentary grooming (35, 38) and motor stereotypy (10, 39, 40). Other aspects of TS symptomatology can be captured using PPI and with tests of motor coordination and learning (33).
We see tic-like stereotypy only after DLS ChAT ablation, not after DMS ChAT ablation (16, 17). In postmortem material from patients with TS, the ChAT interneuron loss is prominent in dorsal striatum (both associative and sensorimotor territory) and not seen in the ventromedial striatum (limbic territory) (26). Our results suggest that tics may relate specifically to deficits in the lateral/sensorimotor striatum and not to those in the more medial, associative circuitry. This is consistent with the role of the DLS in stereotypy and in habit learning in other contexts (20–22, 41), and with findings of increased functional connectivity in the putamen to correlate with tic complexity (23). It is possible that dorsomedial pathology relates to other aspects of pathology, such as obsessive-compulsive symptoms (23), that are not readily captured by our behavioral assays in mice.
We observe manifest stereotypy only after stress or amphetamine challenge. This may be simply a matter of assay sensitivity; any quantification of stereotypy, automated or manual, is of limited sensitivity and may miss low-amplitude movements. Alternatively, it may be related to the fact that our ablation occurs in adulthood and thus incompletely recapitulates the relevant pathology. It may also be the case that ChAT deficiency is one of several insults that can destabilize the corticostriatal system, and that more than one such pathophysiological “hit,” such as pathology in other populations of interneuron (25, 26), developmental stress, or immune challenge (13), is required for spontaneous tic-like movements to emerge.
It should be noted that the individuals studied in the postmortem study on which our experiment is based (26) had particularly severe disease that persisted into adulthood and remained prominent despite attempts at treatment. It is possible that the ChAT deficit we are modeling here is specific to such refractory cases and would not be seen in treatment-responsive or developmentally remitting TS. The examination of such severe cases may nevertheless shed light on pathophysiological mechanisms that are shared with more typical disease. It might be, for example, that ChAT neurons are dysfunctional but not absent in milder disease and that compensatory optimization of their function is a mechanism for symptom improvement over the course of development.
We have described the repeated unpredictable presentation of a loud acoustic startle stimulus (Fig. 3) as an acute stressor. Both acute and chronic stressors can accentuate tic symptomatology in individuals with TS (3–5). We have not objectively measured other correlates of stress, such as corticosterone levels, and other interpretations of the mechanism by which the repeated startle stimuli produced enhanced and fragmented grooming stereotypies are possible. For example, startle stimuli can themselves in some cases trigger or potentiate tics (42, 43). However, the stereotypies we observe were not phasically entrained to the presentation of startle stimuli; rather, they occurred intermittently throughout and after the period of startle stimulus presentation (Movie S1 and Fig. 3). Irrespective of the specific underlying mechanism, this paradigm clearly illustrates increased tic-like stereotypies after ChAT interneuron ablation, in the absence of a pharmacological challenge.
The cause of the slight enhancement of amphetamine-induced locomotion after DMS ChAT ablation, and its relevance to TS, is unclear. It is noteworthy that the most medial portion of the rodent dorsal striatum is functionally related to the ventral striatum/nucleus accumbens, in which a ChAT deficit has been documented in schizophrenia (28) but not in TS (26).
The effects of ChAT ablation in the DLS on striatal neurochemistry and information processing remain to be explored in detail. Cholinergic interneurons, also termed “tonically active neurons,” have a role in the modulation of striatal dopamine dynamics (44, 45); disruption of dopamine (DA) may therefore underlie the observed effects (13). However, ChAT interneurons also regulate both other populations of striatal interneurons and the MSNs themselves; a ChAT deficit may therefore lead to disorganized striatal information processing, independent of any effect on DA (46). ChAT interneurons also encode task-relevant information during reward-motivated contingency learning (47–49) and mediate the integration of thalamic and cortical afferent information (48, 50). The disruption of these functions may also contribute to aspects of the symptomatology of TS.
The fact that we see behavioral abnormalities that recapitulate aspects of TS after ChAT interneuron ablation in the adult indicates that a cholinergic deficit in the adult is sufficient to produce TS-relevant consequences. A cholinergic deficit might be pharmacologically mitigated by using cholinesterase inhibitors, which extend the half-life of endogenously released acetylcholine. A few small studies and case reports have attempted this, with some reports of benefit but also with significant side effects (51, 52). An alternative is the use of muscarinic or nicotinic cholinergic agonists, although it remains unclear which receptors would optimally be targeted. A few studies suggest that nicotine gum or patch are of little benefit by themselves but can augment that efficacy of haloperidol treatment; side effects again have been limiting in many cases (51). It should be noted that TS symptoms fluctuate, and therefore that uncontrolled studies are notoriously unreliable; placebo-controlled trials of these therapeutic strategies are needed.
Our work does not address the cause of the cholinergic deficit. The ChAT interneurons may fail to develop or differentiate properly, fail to migrate to the striatum during development, migrate but die without integrating properly into the striatal circuitry, or develop and integrate normally but die (or dedifferentiate) at a later time. Inflammatory damage to them is an interesting possibility, given recent evidence for an elevated inflammatory state in the TS striatum (27, 53), but this remains speculative. The development of preventative or disease-modifying therapeutics targeting the cholinergic deficit will be facilitated by clarification of its etiology.
This work highlights the potential importance of cholinergic dysregulation to the pathophysiology of TS and establishes a system in which the consequences of this dysregulation, which recapitulates one aspect of the abnormalities documented postmortem (26), can be examined. These results are consistent with an emerging body of evidence suggesting that deep brain stimulation of the intralaminar nuclei of the thalamus, which project to ChAT-positive interneurons in the striatum (50), can be of benefit in severe, intractable TS (54). It is to be hoped that better understanding of this aspect of the pathophysiology of TS will lead to new treatment options.
Materials and Methods
All experiments were performed in accordance with the NIH’s Guide for the Care and Use of Laboratory Animals (55) and were approved by the Yale University Institutional Animal Care and Use Committee. More details are included in SI Materials and Methods.
Immunotoxins.
ChAT interneuron ablation in the striatum has been previously performed using proteinaceous toxins coupled to specific antibodies (29–32). Unfortunately, whereas ChAT interneuron ablation was apparent after ChAT-saporin (SAP), we also found reductions in ChAT immunoreactivity, qualitative changes in ChAT cell morphology, and patchy cell loss after treatment with IgG-SAP, even at low doses (Fig. S1). This motivated us to design a targeting system for more specific and controlled interneuronal ablation.
Vector Constructs and Validation of Cell Ablation.
DT induces apoptosis with extraordinary efficiency (56), but it cannot normally enter rodent cells. Expression of the simian diphtheria toxin receptor (sDTR) renders mouse cells sensitive to ablation after systemic DT (57). We developed a combinatorial approach to restrict sDTR, fused to a flu antigen (FLAG) epitope for ready immunohistochemical identification, to ChAT interneurons of the dorsal striatum. Construct A06 expresses eGFP in cre-negative cells but sDTR in cre-positive cells. The negative control construct, C06, expresses eGFP in all infected cells, irrespective of cre-expression (Fig. 1A). This was confirmed by transient transfection in cultured N2a neuroblastoma cells (Fig. 1B). The constructs were packaged into AAVrh10 serotype (58) and infused into the dorsal striatum, one on each side, to validate ChAT-specific DTR expression and cell ablation (Fig. 2).
Quantitative PCR Expression Analysis.
Mice were killed 15 d following DT injection (∼30 d following virus infusion), to correspond to the period when behavioral analysis was performed (7–27 d following DT). Brains were rapidly dissected and mRNA isolated; target genes were quantified by RT-PCR.
Behavioral Analysis.
For behavioral analysis, virus A06 was infused bilaterally into adult male ChAT-cre mice for ablation; genetically identical littermate control animals received a bilateral infusion of control virus C06. All mice received DT (15 µg/kg) 15 d after surgery; behavioral analysis began 1 wk later and continued for ∼3 wk.
Elevated plus maze, open field exploration, PPI, and rotorod testing were performed as previously described (10, 37, 59).
Stress-induced stereotypy was measured using an acute stress paradigm, which consisted of the repeated, unpredictable presentation of a loud startle stimulus in a sound-attenuating chamber. Grooming and stereotypies were scored from video by a rater blind to experimental condition.
For amphetamine-induced stereotypy, the amphetamine dose, 7.0 mg/kg, was empirically determined in pilot experiments to be sufficient to produce moderate stereotypies in animals on this genetic background. Stereotypy was initially scored using an automated system (HomeCageScan, www.cleversysinc.com; Fig. 4 B and G); confirmatory analysis was performed by manual scoring from video by a scorer blind to experimental condition, as previously described (10).
Supplementary Material
Acknowledgments
The authors thank Stacey Wilber for assistance with animal genotyping and husbandry, the staff of Charles River and the Yale Animal Resources Center for husbandry and veterinary support, Marina Picciotto for creative input during the development of the A06 viral system, and Thorsten Buch and Karl Deisseroth for plasmids used to construct the viral system. This work was supported by the Allison Family Foundation (C.P.), the Tourette Syndrome Association (M.X. and C.P.), and National Institute of Mental Health Grants R01MH091861 (to C.P.), K08MH081190 (to C.P.), and T32MH018268 (to J.L.; principal investigator: J. F. Leckman).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1419533112/-/DCSupplemental.
References
- 1.Scahill L, Tanner C, Dure L. The epidemiology of tics and Tourette syndrome in children and adolescents. Adv Neurol. 2001;85:261–271. [PubMed] [Google Scholar]
- 2.Bloch MH. Emerging treatments for Tourette’s disorder. Curr Psychiatry Rep. 2008;10(4):323–330. doi: 10.1007/s11920-008-0052-z. [DOI] [PubMed] [Google Scholar]
- 3.Du JC, et al. Tourette syndrome in children: An updated review. Pediatr Neonatol. 2010;51(5):255–264. doi: 10.1016/S1875-9572(10)60050-2. [DOI] [PubMed] [Google Scholar]
- 4.Kurlan R. Clinical practice. Tourette’s Syndrome. N Engl J Med. 2010;363(24):2332–2338. doi: 10.1056/NEJMcp1007805. [DOI] [PubMed] [Google Scholar]
- 5.Leckman JF. Tourette’s syndrome. Lancet. 2002;360(9345):1577–1586. doi: 10.1016/S0140-6736(02)11526-1. [DOI] [PubMed] [Google Scholar]
- 6.Denys D, et al. Dopaminergic activity in Tourette syndrome and obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2013;23(11):1423–1431. doi: 10.1016/j.euroneuro.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Bloch MH, Sukhodolsky DG, Leckman JF, Schultz RT. Fine-motor skill deficits in childhood predict adulthood tic severity and global psychosocial functioning in Tourette’s syndrome. J Child Psychol Psychiatry. 2006;47(6):551–559. doi: 10.1111/j.1469-7610.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- 8.Neuner I, et al. Fine motor skills in adult Tourette patients are task-dependent. BMC Neurol. 2012;12:120. doi: 10.1186/1471-2377-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh R, et al. Habit learning in Tourette syndrome: A translational neuroscience approach to a developmental psychopathology. Arch Gen Psychiatry. 2004;61(12):1259–1268. doi: 10.1001/archpsyc.61.12.1259. [DOI] [PubMed] [Google Scholar]
- 10.Castellan Baldan L, et al. Histidine decarboxylase deficiency causes Tourette syndrome: Parallel findings in humans and mice. Neuron. 2014;81(1):77–90. doi: 10.1016/j.neuron.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swerdlow NR, et al. Tactile prepuff inhibition of startle in children with Tourette’s syndrome: In search of an “fMRI-friendly” startle paradigm. Biol Psychiatry. 2001;50(8):578–585. doi: 10.1016/s0006-3223(01)01164-7. [DOI] [PubMed] [Google Scholar]
- 12.Leckman JF, Bloch MH, Smith ME, Larabi D, Hampson M. Neurobiological substrates of Tourette’s disorder. J Child Adolesc Psychopharmacol. 2010;20(4):237–247. doi: 10.1089/cap.2009.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams K, Bloch MH, State MW, Pittenger C. Tourette syndrome and tic disorders. In: Charney DS, Buxbaum JD, Sklar P, Nestler EJ, editors. Neurobiology of Mental Illness. 4th Ed Oxford Univ Press; New York: 2013. [Google Scholar]
- 14.Peterson BS, et al. Basal Ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry. 2003;60(4):415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- 15.Bloch MH, Leckman JF, Zhu H, Peterson BS. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65(8):1253–1258. doi: 10.1212/01.wnl.0000180957.98702.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 17.Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108(8):2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerfen CR. The neostriatal mosaic: Multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- 19.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20(6):2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 21.Baker DA, Specio SE, Tran-Nguyen LT, Neisewander JL. Amphetamine infused into the ventrolateral striatum produces oral stereotypies and conditioned place preference. Pharmacol Biochem Behav. 1998;61(1):107–111. doi: 10.1016/s0091-3057(98)00070-7. [DOI] [PubMed] [Google Scholar]
- 22.Taylor JR, et al. An animal model of Tourette’s syndrome. Am J Psychiatry. 2002;159(4):657–660. doi: 10.1176/appi.ajp.159.4.657. [DOI] [PubMed] [Google Scholar]
- 23.Worbe Y, et al. Distinct structural changes underpin clinical phenotypes in patients with Gilles de la Tourette syndrome. Brain. 2010;133(Pt 12):3649–3660. doi: 10.1093/brain/awq293. [DOI] [PubMed] [Google Scholar]
- 24.Kreitzer AC. Physiology and pharmacology of striatal neurons. Annu Rev Neurosci. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- 25.Kalanithi PS, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci USA. 2005;102(37):13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kataoka Y, et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518(3):277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lennington JB, et al. Transcriptome analysis of the human striatum in Tourette syndrome. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holt DJ, et al. Evidence for a deficit in cholinergic interneurons in the striatum in schizophrenia. Neuroscience. 1999;94(1):21–31. doi: 10.1016/s0306-4522(99)00279-1. [DOI] [PubMed] [Google Scholar]
- 29.Kitabatake Y, Hikida T, Watanabe D, Pastan I, Nakanishi S. Impairment of reward-related learning by cholinergic cell ablation in the striatum. Proc Natl Acad Sci USA. 2003;100(13):7965–7970. doi: 10.1073/pnas.1032899100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laplante F, Dufresne MM, Ouboudinar J, Ochoa-Sanchez R, Sullivan RM. Reduction in cholinergic interneuron density in the nucleus accumbens attenuates local extracellular dopamine release in response to stress or amphetamine. Synapse. 2013;67(1):21–29. doi: 10.1002/syn.21612. [DOI] [PubMed] [Google Scholar]
- 31.Laplante F, Lappi DA, Sullivan RM. Cholinergic depletion in the nucleus accumbens: Effects on amphetamine response and sensorimotor gating. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(2):501–509. doi: 10.1016/j.pnpbp.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Laplante F, et al. Cholinergic depletion in nucleus accumbens impairs mesocortical dopamine activation and cognitive function in rats. Neuropharmacology. 2012;63(6):1075–1084. doi: 10.1016/j.neuropharm.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 33.Pittenger C. Animal models of Tourette syndrome and obsessive-compulsive disorder. In: LeDoux ME, editor. Animal Models of Movement Disorders. Elsevier; New York: 2014. [Google Scholar]
- 34.Lin H, et al. Psychosocial stress predicts future symptom severities in children and adolescents with Tourette syndrome and/or obsessive-compulsive disorder. J Child Psychol Psychiatry. 2007;48(2):157–166. doi: 10.1111/j.1469-7610.2006.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welch JM, et al. Cortico-striatal synaptic deficits and OCD-like behaviours in SAPAP3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyzar E, et al. Towards high-throughput phenotyping of complex patterned behaviors in rodents: Focus on mouse self-grooming and its sequencing. Behav Brain Res. 2011;225(2):426–431. doi: 10.1016/j.bbr.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 37.Baldan Ramsey LC, Xu M, Wood N, Pittenger C. Lesions of the dorsomedial striatum disrupt prepulse inhibition. Neuroscience. 2011;180:222–228. doi: 10.1016/j.neuroscience.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shmelkov SV, et al. Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nat Med. 2010;16(5):598–602, 1p, 602. doi: 10.1038/nm.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graybiel AM, Canales JJ, Capper-Loup C. Levodopa-induced dyskinesias and dopamine-dependent stereotypies: a new hypothesis. Trends Neurosci. 2000;23(10) Suppl:S71–S77. doi: 10.1016/s1471-1931(00)00027-6. [DOI] [PubMed] [Google Scholar]
- 40.Kelley AE. 2001. Measurement of rodent stereotyped behavior. Curr Protoc Neurosci Chapter 8:Unit 8.8.
- 41.Quinn JJ, Pittenger C, Lee AS, Pierson JL, Taylor JR. Striatum-dependent habits are insensitive to both increases and decreases in reinforcer value in mice. Eur J Neurosci. 2013;37(6):1012–1021. doi: 10.1111/ejn.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eapen V, Moriarty J, Robertson MM. Stimulus induced behaviours in Tourette’s syndrome. J Neurol Neurosurg Psychiatry. 1994;57(7):853–855. doi: 10.1136/jnnp.57.7.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Commander M, Corbett J, Prendergast M, Ridley C. Reflex tics in two patients with Gilles de la Tourette syndrome. Br J Psychiatry. 1991;159:877–879. doi: 10.1192/bjp.159.6.877. [DOI] [PubMed] [Google Scholar]
- 44.Threlfell S, et al. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75(1):58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 45.Threlfell S, Cragg SJ. Dopamine signaling in dorsal versus ventral striatum: The dynamic role of cholinergic interneurons. Front Syst Neurosci. 2011;5:11. doi: 10.3389/fnsys.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leckman JF, Vaccarino FM, Kalanithi PS, Rothenberger A. Annotation: Tourette syndrome: A relentless drumbeat—driven by misguided brain oscillations. J Child Psychol Psychiatry. 2006;47(6):537–550. doi: 10.1111/j.1469-7610.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 47.Atallah HE, McCool AD, Howe MW, Graybiel AM. Neurons in the ventral striatum exhibit cell-type-specific representations of outcome during learning. Neuron. 2014;82(5):1145–1156. doi: 10.1016/j.neuron.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradfield LA, Bertran-Gonzalez J, Chieng B, Balleine BW. The thalamostriatal pathway and cholinergic control of goal-directed action: interlacing new with existing learning in the striatum. Neuron. 2013;79(1):153–166. doi: 10.1016/j.neuron.2013.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamada H, Matsumoto N, Kimura M. Tonically active neurons in the primate caudate nucleus and putamen differentially encode instructed motivational outcomes of action. J Neurosci. 2004;24(14):3500–3510. doi: 10.1523/JNEUROSCI.0068-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron. 2010;67(2):294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hartmann A. Clinical pharmacology of nondopaminergic drugs in Tourette syndrome. Int Rev Neurobiol. 2013;112:351–372. doi: 10.1016/B978-0-12-411546-0.00011-1. [DOI] [PubMed] [Google Scholar]
- 52.Cubo E, et al. Donepezil use in children and adolescents with tics and attention-deficit/hyperactivity disorder: An 18-week, single-center, dose-escalating, prospective, open-label study. Clin Ther. 2008;30(1):182–189. doi: 10.1016/j.clinthera.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 53.Kumar A, Williams MT, Chugani HT. 2014. Evaluation of basal ganglia and thalamic inflammation in children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection and Tourette syndrome: A positron emission tomographic (PET) study using 11C-[R]-PK11195. J Child Neurol. pii: 0883073814543303.
- 54.Visser-Vandewalle V, Kuhn J. Deep brain stimulation for Tourette syndrome. Handb Clin Neurol. 2013;116:251–258. doi: 10.1016/B978-0-444-53497-2.00020-6. [DOI] [PubMed] [Google Scholar]
- 55. Committee on Care and Use of Laboratory Animals (1996) Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
- 56.Yamaizumi M, Mekada E, Uchida T, Okada Y. One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell. 1978;15(1):245–250. doi: 10.1016/0092-8674(78)90099-5. [DOI] [PubMed] [Google Scholar]
- 57.Buch T, et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2(6):419–426. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- 58.Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther. 2006;13(3):528–537. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 59.Lee AS, Duman RS, Pittenger C. A double dissociation revealing bidirectional competition between striatum and hippocampus during learning. Proc Natl Acad Sci USA. 2008;105(44):17163–17168. doi: 10.1073/pnas.0807749105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.