Significance
Evolution of malaria parasite drug resistance has thwarted efforts to control this deadly disease. Use of drug combinations has been proposed to slow that evolution. Artemisinin is a favorite drug in the global war on malaria and is frequently used in combination therapies. Here we show that using the whole plant (Artemisia annua) from which artemisinin is derived can overcome parasite resistance and is actually more resilient to evolution of parasite resistance; i.e., parasites take longer to evolve resistance, thus increasing the effective life span of the therapy.
Keywords: malaria, drug resistance, artemisinin, Plasmodium, evolution
Abstract
Pharmaceutical monotherapies against human malaria have proven effective, although ephemeral, owing to the inevitable evolution of resistant parasites. Resistance to two or more drugs delivered in combination will evolve more slowly; hence combination therapies have become the preferred norm in the fight against malaria. At the forefront of these efforts has been the promotion of Artemisinin Combination Therapy, but despite these efforts, resistance to artemisinin has begun to emerge. In 2012, we demonstrated the efficacy of the whole plant (WP)—not a tea, not an infusion—as a malaria therapy and found it to be more effective than a comparable dose of pure artemisinin in a rodent malaria model. Here we show that WP overcomes existing resistance to pure artemisinin in the rodent malaria Plasmodium yoelii. Moreover, in a long-term artificial selection for resistance in Plasmodium chabaudi, we tested resilience of WP against drug resistance in comparison with pure artemisinin (AN). Stable resistance to WP was achieved three times more slowly than stable resistance to AN. WP treatment proved even more resilient than the double dose of AN. The resilience of WP may be attributable to the evolutionary refinement of the plant’s secondary metabolic products into a redundant, multicomponent defense system. Efficacy and resilience of WP treatment against rodent malaria provides compelling reasons to further explore the role of nonpharmaceutical forms of AN to treat human malaria.
The fight against malaria predates the discovery of its causative agent, and for centuries malaria-associated fever was treated using herbal remedies. In the West, quinine (Cinchona bark extract) was the only affordable treatment against malaria until Paul Ehrlich’s magic bullet concept was adopted and thousands of synthetic compounds were tested against malaria parasites. Very few of these compounds were effective and/or safe for human use, but in the 1930s chloroquine rose to ascendancy as a miracle cure for malaria (1). In the late 1950s, chloroquine was the main weapon used by the World Health Organization (WHO) in its Global Malaria Eradication Program (GMEP). Sadly, development of drug-resistant parasites and concomitant failure of chloroquine as the drug of choice led to the eventual demise of GMEP by the close of the 1960s. Following chloroquine’s failure, various antimalarial compounds were serially deployed, and each in its turn failed as parasites evolved resistance, thus leaving millions of malaria patients without affordable treatment.
In the 1970s, artemisinin was discovered as a pure drug extracted from the plant Artemisia annua. In wide-scale clinical trials, pure artemisinin showed poor pharmacokinetic properties but nonetheless demonstrated potent antimalarial activity with a high safety profile (2). It was determined that artemisinin when modified to artesunate or artemether improved bioavailability and was more effective when used in combination with other antimalarial drugs, mainly mefloquine, which became known as Artemisinin Combination Therapy (ACT) (3). It was hoped that use of ACTs would minimize risk of drug resistance. However, in 2005 the earliest evidence of P. falciparum resistance to ACTs arose in Southeast Asia (4–8). The fight against malaria became critical once again when it became apparent that ACT might be following chloroquine’s path toward obsolescence with no affordable replacement in sight.
We demonstrated the efficacy of the whole A. annua plant as a malaria therapy and found it to be more effective than a comparable dose of pure artemisinin in a rodent malaria model (9). WHO has cautioned against use of nonpharmaceutical sources of artemisinin because of the risk of delivering subtherapeutic doses that could exacerbate the resistance problem (10). This warning is valid given the low artemisinin content of juice extractions, teas, and infusion preparations of plant material used for most nonpharmaceutical plant-based therapies. However, the Whole Plant (WP) A. annua therapy that we have tested is not an extraction, a tea, or an infusion, but is based on oral consumption of the dried leaves of the whole plant. Based on our proof of principle in a rodent model, we postulate that with further development WP might provide a more abundant and affordable source of artemisinin-based therapy by eliminating the need for artemisinin extraction during manufacture.
WP may be more effective than monotherapeutic artemisinin because WP may constitute a naturally occurring combination therapy that augments artemisinin delivery and synergizes the drug’s activity. This plant Artemisinin Combination Therapy (pACT) is the result of evolutionary refinement of the plant’s secondary metabolic products into a resilient and multicomponent defense system. As was demonstrated for other combination therapies, we hypothesized that a WP-based pACT would (i) overcome existing resistance to monotherapeutic pure artemisinin and (ii) increase the longevity of this therapy by delaying the onset of parasite resistance among wild types. Here, we tested these two hypotheses in two mouse malaria models, artemisinin-resistant Plasmodium yoelii (strain: ART) and artemisinin-sensitive Plasmodium chabaudi (strain: ASS).
Results and Discussion
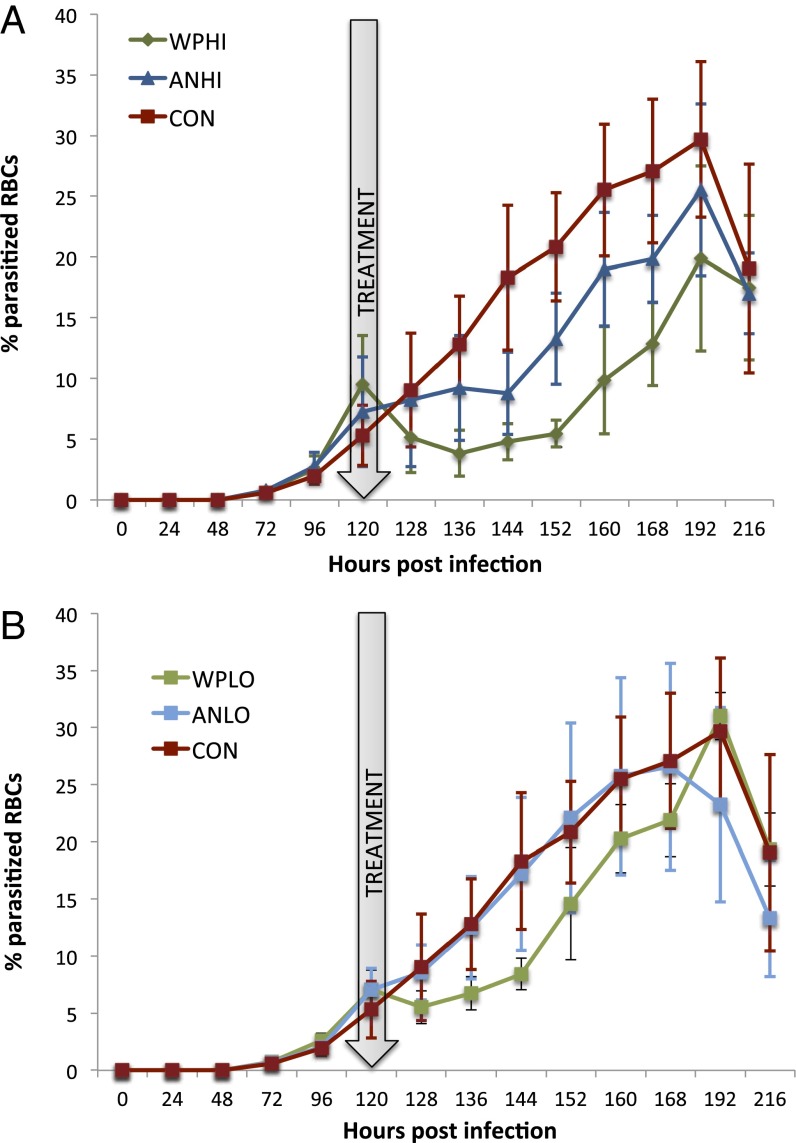
Previously, we found that dried leaves of whole-plant A. annua killed P. chabaudi malaria parasites more effectively than a comparable dose of pure artemisinin (AN) (9). In that experiment, malaria-infected mice received one of two doses of oral artemisinin—low (LO) (24 mg/kg) or high (HI) (120 mg/kg)—by one of two delivery modes—AN (pure artemisinin) or WP (whole plant). Thus, the factorial arrangement of four treatment groups was created: ANHI, ANLO, WPHI, and WPLO. Control group (CON) mice received only mouse chow. Here we used a similar experimental design to show that dried whole-plant A. annua effectively kills rodent malaria parasites that are resistant to artemisinin. Mice given a high, single dose (WPHI) showed significantly greater reduction in parasitemia than those in the ANHI group for each measurement point from 16 to 48 h posttreatment (Fig. 1). Parasitemias of mice treated with ANLO did not differ from control at any point, whereas treatment with WPLO (24 mg AN/kg) was as effective as ANHI, despite the fact the ANHI dose contained five times more AN/kg (Fig. 1). In these single-dose studies, all parasite populations recrudesced around day 5 posttreatment, which is typical of this self-limiting parasite model.
Fig. 1.
AN-resistant P. yoelii (ART) single-dose WP and AN treatments. Arrow indicates time of treatment. (A) Mean parasitemia (±SD) for (light green) WPHI-treated and (dark blue) ANHI-treated mice; both treatments correspond to a total artemisinin dose of 120 mg/kg, but WPHI is delivered as whole dried plant as opposed to ANHI, which is delivered as pure drug. (B) Mean parasitemia (±SD) for (light green) WPLO-treated and (light blue) ANLO-treated mice receiving the equivalent of 24 mg/kg of artemisinin. Placebo control (red) received only mouse chow. All treatements and control were run simultaneously, but the plots are presented separately by dosage to show comparisons between delivery methods.
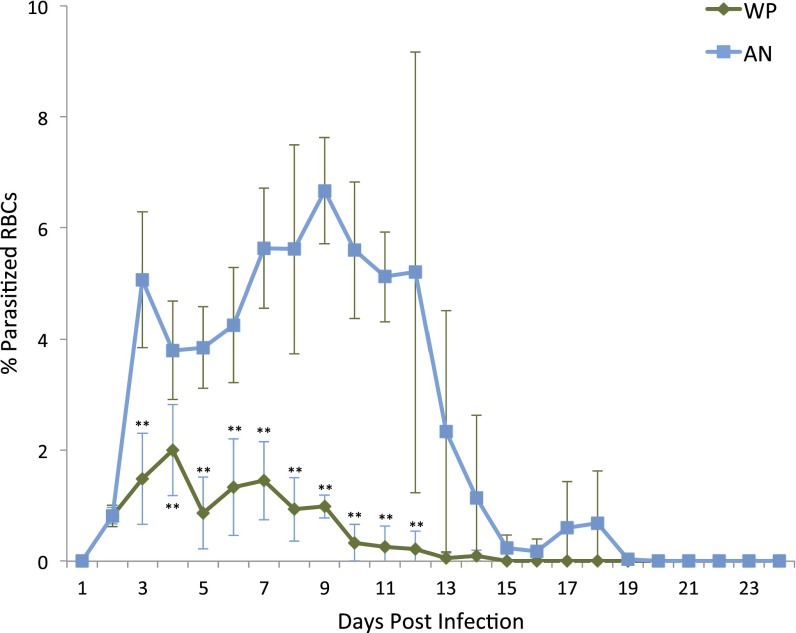
To test whether WP could completely clear infection with repeated dosing, WPHI (120 mg/kg/day) was administered daily for 9 consecutive days. This multidose treatment reduced P. yoelii (ART) parasitemia below 2% for 7 days post-infection (p.i.) and to less than 1% from day 8 until complete clearance of parasitemia on day 14 p.i. Mice from WP treatment groups appeared normal and showed no signs of sickness. Infected groups of mice treated with comparable multiple doses of pure ANHI (120 mg mg/kg/day) for 9 days had mean parasitemia of 6% through day 7. As expected, ANHI showed little effectiveness against these resistant parasites that followed the normal course of self-limiting infection and eventual clearance 18 d p.i. (Fig. 2). Cured mice in WP and AN groups were monitored by Giemsa-stained thin blood smears taken every 72 h from day 24 until mice were euthanized on day 42, and no recrudescence was observed.
Fig. 2.
AN-resistant P. yoelii (ART) curative treatment. Mice were infected with AN-resistant P. yoelii (ART) and treated with either ANHI or WPHI daily for 9 consecutive days starting on day 2 postinfection. Blue line indicates mean parasitemia of ANHI-treated mice (n = 6) and green line represents means parasitemia of WPHI with error bars representing SD. Days with significant difference in mean parasitemia are indicated with “**” (P < 0.01).
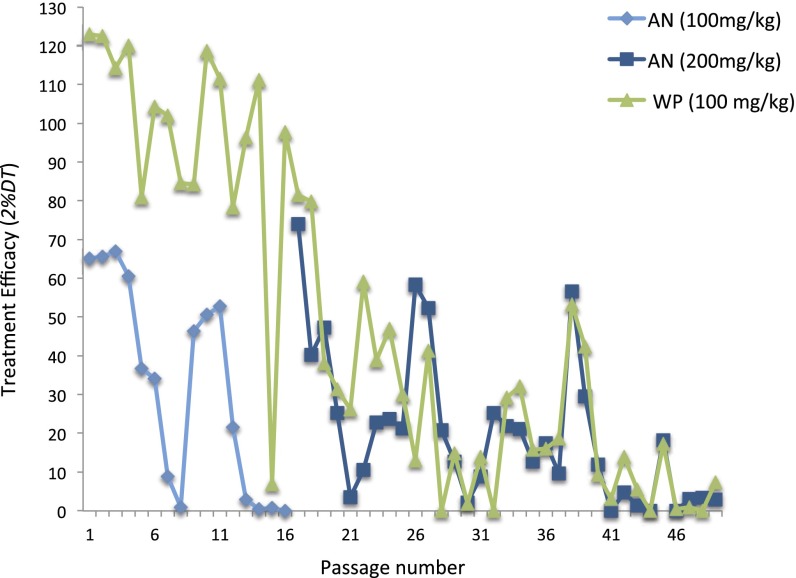
Having demonstrated that WP is effective against artemisinin-resistant rodent parasites, we conducted an artificial evolution experiment to compare rates at which resistance to these two treatment modalities arises among wild-type parasite lines. The long-term selection of drug resistance in Plasmodium chabaudi parasites was achieved by the 2%-relapse technique developed and used by Peters et al. to select for parasites that are resistant to a variety of antimalarials (11–13). The parameter 2% delay time (2%DT) measures treatment efficacy as the difference (in hours) required for treated and untreated hosts to reach parasitemia of 2% infected red blood cells. Parasites susceptible to treatment proliferate slowly (or not at all) and thus take longer to reach 2% parasitemia (i.e., high 2%DT), whereas resistant parasites reach 2% parasitemia at the same rate that untreated parasites do (i.e., 2%DT = 0). As reported by Peters et al., fluctuations in 2%DT are a general feature of the 2%-relapse technique (11–13). The initial delay times relative to control were 2%DT = 65 h and 2%DT = 122 h for AN and WP, respectively, with the starting dose of 100 mg/kg. Stable resistance to that dose of AN was achieved at passage 16, which was the third consecutive passage with 2%DT < 1 h. On passage number 17, the AN dose was increased to 200 mg/kg. Parasites acquired tolerance to this double dose after 40 passages with consistent 2%DT < 4 h, with the exception of passage number 45 when 2%DT spiked to 18 h and then returned to <4 (Fig. 3). The comparable dose of WP (100 mg/kg) was far more resilient, and although efficacy waned to 2%DT < 1 h after 45 passages, complete resistance to WP was never achieved (Fig. 3). Thus, WP treatment of 100 mg/kg was more resilient than the doubled dose of pure AN (200 mg/kg). P. chabaudi generally develops resistance to antimalarial drugs faster than other rodent malaria species, so these results are probably conservative (13). Our results are consistent with another selection study using a combination therapy of artesunate 30 mg/kg/day + mefloquine 5 mg/kg/day given over 3 days, wherein resistance was achieved in P. chabaudi after 27 passages (14). Just as ACT treatment is more resilient than pure AN alone (15), WP treatment is also more resilient than pure AN because WP treatment contains multiple and complementary compounds effective in killing malaria parasites, including those parasites resistant to pure AN.
Fig. 3.
Experimental evolution of drug resistance in P. chabaudi (ASS) following the 2% relapse technique. Selection for resistance in P. chabaudi required more passages in mice administered WP (green) than in mice receiving AN (blue). Drug efficacy (y axis) is measured as the difference in time to reach 2% infected red blood cells between treated and untreated animals. Consistent with evolution of resistance, drug efficacy (2%DT) waned over time for all groups. However, AN ceased impairing parasite replication by passage 16, whereas the equivalent dose of WP never resulted in total loss of efficacy. When 2%DT for “AN (100 mg/kg)” reached zero, the dose of AN was doubled (200 mg/kg), but this only temporarily restored drug efficacy as stable resistance to that dose was achieved by passage 46.
Emergence of artemisinin-resistant malaria parasites raises the urgent need for alternative treatment that is affordable, resilient, and effective against resistant parasites. In our previous study, we reported that antimalarial activity of whole-plant A. annua was at least five times more effective than an equivalent dose of pure AN (9). Here we also show that dried whole-plant material of A. annua is more effective in killing malaria parasites resistant to artemisinin and that WP is more resilient than pure AN and may actually delay the onset of resistance. Although the exact mechanisms still need to be identified, the antimalarial activity of WP against artemisinin-resistant parasites can be explained by increased bioavailability of artemisinin, synergism among artemisinin and other A. annua constituents, and/or the presence of other compounds in A. annua that may have antimalarial activity independent from artemisinin. The efficacy and resilience of A. annua-based WP treatment against rodent malaria provides compelling reasons to further evaluate the role of more holistic and nonpharmaceutical forms of artemisinin to treat human malaria.
A. annua has found a number of different practical uses in agriculture and public health. These include repellency against beetles (16), larvicidal activity against Anopheles stephensi (17), insecticidal activity against elm leaf beetle (18), and use as an acaricide (19). Moreover, AN and its derivatives have effects on a number of viruses (20), a variety of human cancer cell lines (20, 21), and several neglected tropical parasitic diseases including schistosomiasis (22), leishmaniasis (23, 24), New- and Old-World trypanosomiases (25), and some livestock diseases (26).
To better understand the reasons behind the efficacy of a plant in killing mammalian parasites, we must consider carefully plant biology and evolution. Similar to animals, plants have an adaptive and inducible defense system; however, instead of antibody production, plants will release or produce a plethora of small toxic molecules to repel or kill natural enemies including viruses, bacteria, fungus, parasites, insects, and animals. These small molecules may include terpenoids, alkaloids, and flavonoids (27). Evolutionarily successful plant species such as A. annua possess large arrays of bioactive compounds providing robust protection against micro and macro threats (28, 29). Many of the bioactive secondary metabolites of A. annua are synthesized and stored in glandular trichomes, which are small cusps of epidermal origin consisting of differentiated basal, stalk, and apical cells. The glandular trichomes of A. annua are specialized to synthesize, store, and secrete large amounts of phytochemicals including artemisinin, other terpenoids, and flavonoids (29–31).
The A. annua chemical defense system also includes allelopathic factors that may inhibit neighboring plants from germinating or growing. Artemisinin has potent herbicidal activity (32, 33) in the electron transport in chloroplast thylakoid membranes (30). That these defenses are also effective at killing a mammalian blood-borne parasite seems at first coincidental; however, there is a plausible evolutionary explanation for this cross-species protection. Malaria parasite cells contain apicoplasts, which were derived initially from the same ancestral cyanobacterial endosymbiont that gave rise to chloroplasts in plants (34), so it is reasonable that both are targets of artemisinin.
It is well known that the bacterial origin of the apicoplast renders malaria parasites susceptible to antibiotics such as fosmidomycin and doxycycline. Fosmidomycin inhibits deoxyxylulose 5-phosphate reductoisomerase, the second enzyme of the nonmevalonate isoprenoid biosynthetic pathway located in the plastids of plants and the apicoplast of Plasmodium sp (27, 35, 36). Doxycycline inhibits transcription and translation of prokaryotes by blocking its replication and malaria parasite cell death (34, 35). The presence of plant-like organelles and pathways in parasites that are absent in the human host present intriguing prospects for co-opting herbicidal compounds as medicaments (37). Various plant-extracted herbicides already have been found to have specific parasite-killing activity against apicomplexan parasites including P. falciparum, Cryptosporidium parvum, and Toxoplasma gondii (38). These herbicides may be synthetic or, as with the WP therapy discussed here, derived from natural products.
Among the phytochemical repertoire that is synthesized and located in the glandular trichomes are the terpenoids, the essential oil constituents of A. annua that give the plant its fragrance (39). Terpenoids are composed of 5-C isoprene units and/or their dimethylallyl isomers and may include extensive branching and cyclization. Plasmodium spp. possess their own terpenoid pathway within the apicoplast that is essential for the growth and replication of intra- and extraerythrocytic stages (35, 36). Some plant-derived terpenoids have been shown to disrupt apicoplast function, resulting in parasite death (40). For example, nerolidol inhibits biosynthesis of the isoprenic chain (41), and limonene inhibits protein isoprenylation in the erythrocytic stages of P. falciparum (42). The IC50 of nerolidol, linalool, limonene, and farnesol against P. falciparum are 760 nM, 0.28 mM, 1.22 mM, and 64 µM, respectively (43). Monoterpenes isolated from eucalyptus oil (80% 1,8-cineole) inhibit chloroquine-resistant and chloroquine-sensitive P. falciparum by mechanisms thought related to membrane disruption (44). Moreover, several other mono- and sesquiterpenes in A. annua have shown activity against malaria parasites (45, 46). The ability of plants to produce a virtually unlimited diversity of terpenoids by elongations, cyclizations, and secondary chemical transformations increases the potential for redundant and robust parasite-killing activity (27, 47).
As in the challenge experiments against malaria parasites, allelopathic activity is higher for the whole plant than a comparable dose of pure artemisinin when incorporated into soil (48). The higher allelopathic activity of whole-plant A. annua versus its pure extracted artemisinin is attributable to the synergism among A. annua constituents (48) and/or the presence of other constituents of A. annua that have potent herbicidal activity (49). This same synergism between A. annua constituents has been reported to potentiate the antimalarial activity against P. falciparum (9, 45, 50–52).
The improved antimalarial activity of WP over pure AN may possibly be explained by enhanced bioavailability of artemisinin due to inhibitory effects of some A. annua flavonoids on the hepatic and intestinal cytochrome P450 enzymes that metabolize artemisinin (53, 54). Plant material enhanced by >40-fold the amount of artemisinin that entered the blood stream (55). The presence of plant material even from mouse chow also significantly increased the amount of artemisinin that appeared in the serum (54).
Whole-plant A. annua may also have increased activity against malaria parasites due to the synergism among some key flavonoids and artemisinin (9, 50–52) or by the antimalarial activity of other A. annua constituents independent from artemisinin. A. annua flavonoids including artemetin, casticin, chrysosplenetin, chrysosplenol-D, cirsilineol, eupatorin, isovitexin, kaempferol, luteolin, myricetin, quercetin, and rutin showed antimalarial activity against chloroquine-sensitive (HB3 and 3D7) and chloroquine-insensitive (Dd2, 7G8, K1, and FCR3) strains of P. falciparum in the absence of artemisinin (45, 46, 56–58). Their IC50 values ranged from about 2.9 to 72.5 µM. There was no significant change in the IC50 based on chloroquine sensitivity for the tested flavonoids, suggesting that the flavonoids are acting on targets distinct from those of chloroquine.
The likelihood of developing resistance is greater for a single antimalarial compound than for a combination of compounds. Increased resilience of WP relative to a comparable dose of pure AN is consistent with this notion. Resilience of WP may be explained by the presence of a large phytochemical repertoire of small molecules that target many active enzymatic sites essential for malaria parasite survival and growth, making it more difficult for parasites to accumulate necessary resistance mutations in the ensemble of genes responsible for encoding those essential target sites.
The 19th-century conceptualization of “magic bullets” led to the serial use of monotherapies that inevitably selected for resistant micro- and macro-organisms throughout the 20th century. The benefits realized in roughly the first half of that period in medicine and agriculture were undone in the latter half by proliferation of resistance to antibiotics, antimalarials, insecticides, and herbicides. A renewed appreciation for evolution and the adaptive potential of the targeted organisms has led to more sustainable approaches using combinations of control agents. The success of the combinatorial approach is evidenced throughout nature, and A. annua is a prime example with its repertoire of several hundred compounds composing that plant’s defense system. If plants had followed the pharmaceutical model of serial production of single-component protection against its enemy, they would have become extinct long ago (52). The WP antimalarial therapy serves as a case study of how those resilient naturally occurring systems might be co-opted for use against animal pathogens. Although much work remains, the clear evidence of the efficacy of WP as a naturally occurring combination therapy pACT against rodent malaria models warrants its further consideration to explore how we might develop inexpensive, abundant, and resilient malaria therapies from a nonpharmaceutical product.
Materials and Methods
Rodent Malaria Parasites.
All experiments were performed using an appropriate rodent malaria model, obtained through the Malaria Research and Reference Reagent Resource Center (MR4) as a part of the BEI Resources Repository, National Institute of Allergy and Infectious Diseases, National Institutes of Health. For long-term selection experiments, we chose P. chabaudi (ASS; MRA-429) because of its known susceptibility to WP and artemisinin treatment. P. yoelii (ART; MRA-421) was chosen because it is an artemisinin-resistant strain (ART).
P. chabaudi (ASS).
Tubes of blood collected from infected mice were removed from liquid nitrogen storage and left at room temperature for 30 min. To activate parasite stocks, two C57BL/6 mice were injected intraperitoneally (i.p.) with 200 µL of infected blood. Percentage of parasitemia was determined in Giemsa-stained thin blood smears on days 3–7 p.i. Seven days after infection, one mouse was euthanized, and cardiac puncture was used to collect blood into lithium heparin tubes. Infected blood was volumetrically adjusted by dilution into Dulbecco’s PBS (DPBS) to create a 200-µL aliquot of 105 infected erythrocytes for infection into two additional mice for a second round of activation. The activated parasites were used in subsequent drug selection and resilience tests.
P. yoelii (ART).
To activate the parasite after long-term storage in liquid nitrogen, two C57BL/6 mice were injected i.p. with 200 µL of infected blood. Seven days after infection to build up parasitemia, one mouse was euthanized, and cardiac puncture was used to collect blood into lithium heparin tubes. Infected blood was volumetrically adjusted by dilution in DPBS to create a 200-µL aliquot of 107 infected erythrocytes for infection into four additional mice. Two mice were immediately treated with a dose of 150 mg AN/kg recommended by the provider to activate and maintain resistant phenotype, and the other two mice were left untreated as controls. Seven days postinoculation, the infected blood of one mouse that first reached 2% parasitemia from each group was used in the next passage. Activation for AN-resistant phenotype was repeated for 10 passages until the treated and untreated mice reached 2% parasitemia at the same time. The activated parasites were used in the subsequent challenge.
Antimalarial Treatments.
A. annua L. (SAM cultivar; voucher MASS 00317314) containing 1.48 ± 0.06% AN (dry weight) was used in this study. Detailed information about plant material and AN analysis was reported in our previous work (9, 54). To test dose-dependent effects, we used two doses of each treatment such that the amount of artemisinin was equivalent in the two low (WPLO and ANLO) and the two high (WPHI and ANHI) treatment groups. Artemisinin was obtained from Sigma-Aldrich Chemical.
Mouse Feeding and Drug Delivery Details.
All treatments were given in two dosages, LO and HI. Each mouse in the WPLO and WPHI groups received either 40 or 200 mg dried A. annua plant powder corresponding to 24 or 120 mg AN/kg live body weight, respectively. Plant powder was mixed with water to final volumes of 0.5 mL (WPLO) or 0.75 mL (WPHI). Each mouse in the artemisinin ANLO and ANHI groups received either 600 or 3,000 µg AN corresponding to 24 or 120 mg AN/kg, respectively, which was freshly dissolved in 60 µL DMSO mixed with water and 40 or 200 mg of powdered sieved mouse chow to final volumes of 0.5 mL (ANLO) or 0.75 mL (ANHI), respectively. Placebo control consisted of 60 µL DMSO mixed with water and 40 mg powdered sieved mouse chow to a final volume of 0.5 mL. Delivery of the appropriate treatment/control was performed immediately after dose preparation by oral-gastric gavage into each mouse using a feeding needle (18 G, curved, 2 in, and 2.25 pall diameter). Individual mice were identified by tail markings using a permanent marker. Percentage parasitemia was determined in Giemsa-stained thin blood smears from a drop of peripheral blood obtained from the tail. Mice were observed twice daily for signs of disease or stress.
Artemisinin-Resistant P. yoelii Single Dose–Response.
An aliquot of 106 P. yoelii (ART)-infected erythrocytes was inoculated i.p. into each of 30 C57BL6, 12-wk-old male mice weighing an average of 25 g; mice were randomly divided into five groups (WPHI, ANHI, WPLO, ANLO, and CON) with six mice per group. Food and water were provided ad libitum for the first 4 days. On day 4, food was withheld for 24 h, but water was freely available. Mice were treated on day 5 p.i. Percentage parasitemia was determined every 24 h in Giemsa-stained thin blood smears for the first 4 days p.i. and then every 8 h for 48 h postgavage and again in 24-h intervals for days 8–13 p.i. All mice were euthanized on day 13 p.i. via asphyxiation in a CO2 chamber followed by cervical dislocation.
Artemisinin-Resistant P. yoelii Curative Treatment.
An aliquot of 107 P. yoelii (ART)-infected erythrocytes was inoculated i.p. into each of 12 DBA/2, 12-wk-old male mice, which were randomly divided into two groups (WPHI, ANHI) with six mice per group. Beginning 2 days p.i., each mouse in the WPHI and ANHI groups received nine daily doses of 200 mg of plant material (corresponding to 120 mg AN/kg) or 3,000 µg pure AN (corresponding to 120 mg AN/kg), respectively. Percent parasitemia was determined every 24 h in Giemsa-stained thin blood smears from days 1–24 p.i. and then every 72 h for days 27–42 p.i. All mice were euthanized on day 42 p.i. via asphyxiation in a CO2 chamber followed by cervical dislocation.
Selection for Drug Resistance and Resilience Test.
Parasites were selected for resistance following the 2% relapse technique (11–13) in which a single treatment dose was given immediately after parasite infection of each mouse in a treatment group, and a control group was inoculated and left untreated. The degree of resistance to treatment is measured as the time differential to reach 2% parasitemia in the treated group compared with the control group. This critical parameter is the 2%DT (11, 13). In each selection passage, three groups of two mice were inoculated i.p. with 107 red blood cells infected with AN-sensitive P. chabaudi (ASS).
Resistance was selected at two doses corresponding to artemisinin content of 100 and 200 mg/kg. For AN resistance selection, mice received a single dose of 2,500 or 5,000 µg AN. For WP resistance selection, mice received 167 mg of dried whole-plant material (also corresponding to a comparable dose of 100 mg/kg of artemisinin but in this case delivered via the whole plant). Selection for the higher dose of WP was not done. Mice used as controls received placebo (mouse chow), and gavaged materials were prepared as previously mentioned.
Passage of infection was performed on day 7 or after all groups achieved >2% parasitemia. In early passages when parasites were highly susceptible to drug, it took as many as 9 d to achieve parasitemia sufficient for the next passage. Parasites from the first mouse to reach 2% parasitemia from each group were used in the subsequent parasite passage. Parasitemia was measured every 24 h during each passage in Giemsa-stained thin blood smears. The parasitemia estimate in each passage was the mean of two mice in each group. Time at which parasites reached 2% parasitemia was calculated by extrapolation of the hourly rate of change between the two parasitemia counts, i.e., on the first day parasitemia was >2% and the day immediately prior. The difference in time to reach 2% parasitemia in the treated group compared with the control group is the 2%DT. The AN-treated group reached 2%DT < 1 h for three successive passages on the 16th passage, after which the AN dose was doubled and selection continued for that double dose. Selection for WP resistance ended on passage number 49; selection for AN resistance at 100 and 200 mg/kg ended on passages 16 and 49, respectively.
Ethics Statement.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All efforts were made to minimize suffering of animals during experimental procedures.
Statistical Analysis.
We fit linear mixed models to estimate and compare the average parasitemia for each treatment group at each measured time point. Including a random intercept for individual mice allowed us to adjust for repeated observations on the same mouse. Single-dose analysis compared the CON, WPLO, WPHI, ANLO, and ANHI treatment groups at all measured time points.
For each model, 10,000 Markov chain Monte Carlo (MCMC) samples were drawn from the posterior distributions of the average parasitemia levels for each treatment group at each time point. Then, 95% confidence interval endpoints for a particular parasitemia level were established at the 2.5 and 97.5 quantiles of the MCMC samples for that parameter. An estimated difference between two groups was declared “significant” if the 95% confidence interval for the difference did not cover zero. Analyses were conducted using the statistical software R v2.15 (59). Fig. 1 was produced using the ggplot2 package (60).
Acknowledgments
We thank Ricardo Gazzinelli (University of Massachusetts Medical School), Doug Golenbock (University of Massachusetts Medical School), and Guang Xu (University of Massachusetts, Amherst) for assistance and advice in conducting experiments. We also thank the Worcester Polytechnic Institute and the University of Massachusetts Center for Clinical and Translational Science (CCTS-20110001) for funding this project.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Croft S. Antimalarial chemotherapy: Mechanisms of action, resistance and new directions in drug discovery. Drug Discov Today. 2001;6(22):1151. doi: 10.1016/s1359-6446(01)02035-9. [DOI] [PubMed] [Google Scholar]
- 2.Faurant C. From bark to weed: The history of artemisinin. Parasite. 2011;18(3):215–218. doi: 10.1051/parasite/2011183215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen I, Eastman R, Lanzer M. Drug-resistant malaria: Molecular mechanisms and implications for public health. FEBS Lett. 2011;585(11):1551–1562. doi: 10.1016/j.febslet.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 4.Cheeseman IH, et al. A major genome region underlying artemisinin resistance in malaria. Science. 2012;336(6077):79–82. doi: 10.1126/science.1215966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jambou R, et al. Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet. 2005;366(9501):1960–1963. doi: 10.1016/S0140-6736(05)67787-2. [DOI] [PubMed] [Google Scholar]
- 6.Dondorp AM, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361(5):455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denis MB, et al. Surveillance of the efficacy of artesunate and mefloquine combination for the treatment of uncomplicated falciparum malaria in Cambodia. Trop Med Int Health. 2006;11(9):1360–1366. doi: 10.1111/j.1365-3156.2006.01690.x. [DOI] [PubMed] [Google Scholar]
- 8.Alker AP, et al. Pfmdr1 and in vivo resistance to artesunate-mefloquine in falciparum malaria on the Cambodian-Thai border. Am J Trop Med Hyg. 2007;76(4):641–647. [PubMed] [Google Scholar]
- 9.Elfawal MA, et al. Dried whole plant Artemisia annua as an antimalarial therapy. PLoS ONE. 2012;7(12):e52746. doi: 10.1371/journal.pone.0052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO 2012 Effectiveness of non-pharmaceutical forms of Artemisia annua L. against malaria. Available at www.who.int/malaria/position_statement_herbal_remedy_artemisia_annua_l.pdf. Accessed December 16, 2014.
- 11.Peters W. The chemotherapy of rodent malaria. LVII. Drug combinations to impede the selection of drug resistance, Part 1: Which model is appropriate? Ann Trop Med Parasitol. 1999;93(6):569–587. doi: 10.1080/00034989958087. [DOI] [PubMed] [Google Scholar]
- 12.Peters W, Robinson BL. The chemotherapy of rodent malaria. LVIII. Drug combinations to impede the selection of drug resistance, Part. 2: The new generation—artemisinin or artesunate with long-acting blood schizontocides. Ann Trop Med Parasitol. 2000;94(1):23–35. doi: 10.1080/00034980057581. [DOI] [PubMed] [Google Scholar]
- 13.Peters W, Robinson BL, Stewart LB, Butcher GA. The chemotherapy of rodent malaria. LIX. Drug combinations to impede the selection of drug resistance, Part 3: Observations on cyproheptadine, an antihistaminic agent, with chloroquine. Ann Trop Med Parasitol. 2000;94(7):689–697. doi: 10.1080/00034983.2000.11813592. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues LA, et al. Experimental evolution of resistance to artemisinin combination therapy results in amplification of the mdr1 gene in a rodent malaria parasite. PLoS ONE. 2010;5(7):e11593. doi: 10.1371/journal.pone.0011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chawira AN, Warhurst DC, Peters W. Qinghaosu resistance in rodent malaria. Trans R Soc Trop Med Hyg. 1986;80(3):477–480. doi: 10.1016/0035-9203(86)90351-2. [DOI] [PubMed] [Google Scholar]
- 16.Tripathi AK, Prajapati V, Aggarwal KK, Khanuja SP, Kumar S. Repellency and toxicity of oil from Artemisia annua to certain stored-product beetles. J Econ Entomol. 2000;93(1):43–47. doi: 10.1603/0022-0493-93.1.43. [DOI] [PubMed] [Google Scholar]
- 17.Bartarya R, et al. Larvicidal activity of Artemisia annua L. callus culture against Anopheles stephensi larvae. J Environmental Biol. 2009;30(3):395–398. [PubMed] [Google Scholar]
- 18.Shekari M, Sendi JJ, Etebari K, Zibaee A, Shadparvar A. Effects of Artemisia annua L. (Asteracea) on nutritional physiology and enzyme activities of elm leaf beetle, Xanthogaleruca luteola Mull. (Coleoptera: Chrysomellidae) Pestic Biochem Physiol. 2008;91(1):66–74. [Google Scholar]
- 19.Zhang Y, Ding W, Zhao Z, Wu J, Fan Y. Studies on acaricidal bioactivities of Artemisia annua L. extracts against Tetranychus cinnabarinus Bois. (Acari: Tetranychidae) Agric Sci China. 2008;7(5):577–584. [Google Scholar]
- 20.Efferth T. Artemisinin: A versatile weapon from traditional Chinese medicine. In: Ramawat KG, editor. Herbal Drugs: Ethnomedicine to Modern Medicine. Springer; Heidelberg, Germany: 2009. pp. 179–194. [Google Scholar]
- 21.Firestone GL, Sundar SN. Minireview: Modulation of hormone receptor signaling by dietary anticancer indoles. Mol Endocrinol. 2009;23(12):1940–1947. doi: 10.1210/me.2009-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Utzinger J, et al. Current progress in the development and use of artemether for chemoprophylaxis of major human schistosome parasites. Curr Med Chem. 2001;8(15):1841–1860. doi: 10.2174/0929867013371581. [DOI] [PubMed] [Google Scholar]
- 23.Avery MA, et al. Structure-activity relationships of the antimalarial agent artemisinin. 8. Design, synthesis, and CoMFA studies toward the development of artemisinin-based drugs against leishmaniasis and malaria. J Med Chem. 2003;46(20):4244–4258. doi: 10.1021/jm030181q. [DOI] [PubMed] [Google Scholar]
- 24.Sen R, et al. Artemisinin triggers induction of cell-cycle arrest and apoptosis in Leishmania donovani promastigotes. J Med Microbiol. 2007;56(Pt 9):1213–1218. doi: 10.1099/jmm.0.47364-0. [DOI] [PubMed] [Google Scholar]
- 25.Mishina YV, Krishna S, Haynes RK, Meade JC. Artemisinins inhibit Trypanosoma cruzi and Trypanosoma brucei rhodesiense in vitro growth. Antimicrob Agents Chemother. 2007;51(5):1852–1854. doi: 10.1128/AAC.01544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreira JF, Peaden P, Keiser J. In vitro trematocidal effects of crude alcoholic extracts of Artemisia annua, A. absinthium, Asimina triloba, and Fumaria officinalis: Trematocidal plant alcoholic extracts. Parasitol Res. 2011;109(6):1585–1592. doi: 10.1007/s00436-011-2418-0. [DOI] [PubMed] [Google Scholar]
- 27.Croteau R, Kutchan TM, Lewis NG. Biochemistry and Molecular Biology of Plants. American Society of Plant Biologists; Rockville, MD: 2000. Natural products (secondary metabolites) pp. 1250–1268. [Google Scholar]
- 28.Inderjit , Wardle DA, Karban R, Callaway RM. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol Evol. 2011;26(12):655–662. doi: 10.1016/j.tree.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Brown GD. The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao) Molecules. 2010;15(11):7603–7698. doi: 10.3390/molecules15117603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bharati A, Kar M, Sabat SC. Artemisinin inhibits chloroplast electron transport activity: Mode of action. PLoS ONE. 2012;7(6):e38942. doi: 10.1371/journal.pone.0038942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White NJ. The treatment of malaria. N Engl J Med. 1996;335(11):800–806. doi: 10.1056/NEJM199609123351107. [DOI] [PubMed] [Google Scholar]
- 32.Chen PK, Leather GR. Plant growth regulatory activities of artemisinin and its related compounds. J Chem Ecol. 1990;16(6):1867–1876. doi: 10.1007/BF01020500. [DOI] [PubMed] [Google Scholar]
- 33.Duke SO, Vaughn KC, Croom EM, Elsohly HN. Artemisinin, a constituent of annual wormwood (Artemisia annua), is a selective phytotoxin. Weed Sci. 1987;35(4):499–505. [Google Scholar]
- 34.Lim L, McFadden GI. The evolution, metabolism and functions of the apicoplast. Philos Trans R Soc Lond B Biol Sci. 2010;365(1541):749–763. doi: 10.1098/rstb.2009.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeh E, DeRisi JL. Chemical rescue of malaria parasites lacking an apicoplast defines organelle function in blood-stage Plasmodium falciparum. PLoS Biol. 2011;9(8):e1001138. doi: 10.1371/journal.pbio.1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lange BM, Rujan T, Martin W, Croteau R. Isoprenoid biosynthesis: The evolution of two ancient and distinct pathways across genomes. Proc Natl Acad Sci USA. 2000;97(24):13172–13177. doi: 10.1073/pnas.240454797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts F, et al. Evidence for the shikimate pathway in apicomplexan parasites. Nature. 1998;393(6687):801–805. doi: 10.1038/31723. [DOI] [PubMed] [Google Scholar]
- 38.Duke SO. Herbicide and pharmaceutical relationships. Weed Sci. 2010;58(3):334–339. [Google Scholar]
- 39.Bhakuni RS, Jain DC, Sharma RP, Kumar S. Secondary metabolites of Artemisia annua and their biological activity. Curr Sci India. 2001;80(1):35–48. [Google Scholar]
- 40.Dewick PM. The biosynthesis of C5-C25 terpenoid compounds. Nat Prod Rep. 2002;19(2):181–222. doi: 10.1039/b002685i. [DOI] [PubMed] [Google Scholar]
- 41.de Macedo CS, Uhrig ML, Kimura EA, Katzin AM. Characterization of the isoprenoid chain of coenzyme Q in Plasmodium falciparum. FEMS Microbiol Lett. 2002;207(1):13–20. doi: 10.1111/j.1574-6968.2002.tb11021.x. [DOI] [PubMed] [Google Scholar]
- 42.Lopes NP, et al. Antimalarial use of volatile oil from leaves of Virola surinamensis (Rol.) Warb. by Waiãpi Amazon Indians. J Ethnopharmacol. 1999;67(3):313–319. doi: 10.1016/s0378-8741(99)00072-0. [DOI] [PubMed] [Google Scholar]
- 43.Rodrigues Goulart H, et al. Terpenes arrest parasite development and inhibit biosynthesis of isoprenoids in Plasmodium falciparum. Antimicrob Agents Chemother. 2004;48(7):2502–2509. doi: 10.1128/AAC.48.7.2502-2509.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su V, King D, Woodrow I, McFadden G, Gleadow R. Plasmodium falciparum growth is arrested by monoterpenes from eucalyptus oil. Flavour Fragrance J. 2008;23(5):315–318. [Google Scholar]
- 45.Suberu JO, et al. Anti-plasmodial polyvalent interactions in Artemisia annua L. aqueous extract: Possible synergistic and resistance mechanisms. PLoS ONE. 2013;8(11):e80790. doi: 10.1371/journal.pone.0080790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Zyl RL, Seatlholo ST, van Vuuren SF, Viljoen AM. The biological activities of 20 nature identical essential oil constituents. J Essent Oil Res. 2006;18:129–133. [Google Scholar]
- 47.Firn RD, Jones CG. The evolution of secondary metabolism: A unifying model. Mol Microbiol. 2000;37(5):989–994. doi: 10.1046/j.1365-2958.2000.02098.x. [DOI] [PubMed] [Google Scholar]
- 48.Lydon J, Teasdale JR, Chen PK. Allelopathic activity of annual wormwood (Artemisia annua) and the role of artemisinin. Weed Sci. 1997;45(6):807–811. [Google Scholar]
- 49.Duke SO, Paul RN, Lee SM. Terpenoids from the genus Artemisia as potential pesticides. In: Cutler HG, editor. Biologically Active Natural Products: Potential Use in Agriculture. Vol 380. American Chemical Society; Washington, DC: 1988. pp. 318–334. [Google Scholar]
- 50.Willcox M. Artemisia species: From traditional medicines to modern antimalarials—and back again. J Altern Complement Med. 2009;15(2):101–109. doi: 10.1089/acm.2008.0327. [DOI] [PubMed] [Google Scholar]
- 51.Ferreira JF, Luthria DL, Sasaki T, Heyerick A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules. 2010;15(5):3135–3170. doi: 10.3390/molecules15053135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rasoanaivo P, Wright CW, Willcox ML, Gilbert B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar J. 2011;10(Suppl 1):S4. doi: 10.1186/1475-2875-10-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Svensson US, Ashton M. Identification of the human cytochrome P450 enzymes involved in the in vitro metabolism of artemisinin. Br J Clin Pharmacol. 1999;48(4):528–535. doi: 10.1046/j.1365-2125.1999.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weathers PJ, Elfawal MA, Towler MJ, Acquaah-Mensah GK, Rich SM. Pharmacokinetics of artemisinin delivered by oral consumption of Artemisia annua dried leaves in healthy vs. Plasmodium chabaudi-infected mice. J Ethnopharmacol. 2014;153(3):732–736. doi: 10.1016/j.jep.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weathers PJ, et al. Artemisinin production in Artemisia annua: Studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochem Rev. 2011;10(2):173–183. doi: 10.1007/s11101-010-9166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu KCSC, Yang SL, Roberts MF, Elford BC, Phillipson JD. Antimalarial activity of Artemisia annua flavonoids from whole plants and cell cultures. Plant Cell Rep. 1992;11(12):637–640. doi: 10.1007/BF00236389. [DOI] [PubMed] [Google Scholar]
- 57.Lehane AM, Saliba KJ. Common dietary flavonoids inhibit the growth of the intraerythrocytic malaria parasite. BMC Res Notes. 2008;1:26. doi: 10.1186/1756-0500-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ganesh D, et al. Antiplasmodial activity of flavonol quercetin and its analogues in Plasmodium falciparum: Evidence from clinical isolates in Bangladesh and standardized parasite clones. Parasitol Res. 2012;110(6):2289–2295. doi: 10.1007/s00436-011-2763-z. [DOI] [PubMed] [Google Scholar]
- 59.Team RC. 2013 R: A language and environment for statistical computing. (R Foundation for Statistical Computing, Vienna). Available at www.R-project.org/. Accessed December 16, 2014.
- 60. Bates D, Maechler M, Bolker B, Walker S (2014) lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-6. Available at CRAN.R-project.org/package=lme4. Accessed December 16, 2014.