Abstract
Since their discovery not long ago, microRNAs (miRNAs) have been extensively studied in hundreds of laboratories around the world. Initially thought of as merely cytoplasmic repressors of mRNA expression, it has since become more apparent that they also play regulatory roles in the nucleus. A recent study published in Nature introduces novel concepts in both miRNA regulation and function by showing that the let-7 miRNA regulates its own expression.
miRNAs are key posttranscriptional regulators of gene expression. Over a thousand eukaryotic miRNAs have been discovered, many of which are predicted to target hundreds of mRNAs.1,2 Binding of the mature ≈ 21-nucleotide miRNA to target mRNAs typically silences their expression, providing a complex layer of fine-tuned gene regulation. However, in the past few years, it has become clear that miRNAs are themselves subjected to sophisticated regulatory mechanisms. In a recent Nature article, Zisoulis et al3 reported the first example of a direct miRNA autoregulatory loop. They showed that the Caenorhabditis elegans (C. elegans) let-7 miRNA induces its own maturation in an Argonaute (ALG-1)-dependent manner. RNA immunoprecipitation assays revealed an ALG-1–binding site containing a let-7-complementary element near the 3′ end of the primary let-7 (pri-let-7) transcript. Northern blots showed that mutant worms with the pri-let-7–ALG-1 interaction disrupted had increased levels of the primary transcript and lower levels of the precursor and mature forms, suggesting a defect in pri-miRNA processing. Furthermore, the introduction of a wild-type let-7 gene into a let-7 mutant strain was able to upregulate levels of the mutant miRNA. Together, these data point to an intriguing new model whereby mature let-7 binds to a complementary element in the pri-let-7 transcript, recruiting ALG-1 and promoting its own downstream processing. The findings by Zisoulis et al3 introduce novel concepts in the regulation of let-7 expression and represent an important contribution to our overall understanding of miRNA regulatory pathways.
let-7 is one of the founding members of the miRNA family, first identified in C. elegans as a temporal regulator of cell fate decisions during development.4,5 let-7 promotes the transition from a larval to adult state by silencing genes that maintain stem-cell–like properties and inducing cell differentiation.4,5 Importantly, let-7 is highly conserved across animal species, both in sequence and function.4 It is present in numerous copies throughout the human genome and has been implicated in countless pathologies, including cancers and cardiac disease.1,4,6,7
Canonical miRNA biogenesis begins with the transcription of the primary miRNA, typically by RNA polymerase II.8–10 This transcript is 5′ capped and polyadenylated and cleaved by Drosha, an RNase III family member.8–10 The product of this cleavage is the ≈ 70-nucleotide precursor miRNA, which is exported out of the nucleus by exportin-5.8–10 In the cytoplasm, the precursor miRNA is further processed by Dicer, a Drosha-related RNase III that cleaves the precursor miRNA, producing an ≈ 21-bp miRNA duplex.8–12 One of these strands, the guide strand, is bound by Argonaute and incorporated into the miRNA-induced silencing complex (mRISC).8–11 Binding of this complex to target mRNAs via sequence complementarity with the guide strand usually results in transcript destabilization and translational repression.8–11
The findings from Zisoulis et al3 move away from the canonical miRNA pathway in many respects. First, they implicate a role for mature let-7, as well as Argonaute, in the nucleus. Argonaute proteins are classically known as key components of the miRNA-induced silencing complex, which regulates mRNA expression in the cytoplasm.8–11 However, using RNA immunopurification assays, Zisoulis et al3 showed that ALG-1 actually interacts with nuclear pri-let-7 in a let-7–dependent manner. They confirmed the nuclear localization of these factors by carrying their work from C. elegans into HeLa cells: ≈20% of total Argonaute and ≈50% of all mature let-7 were found to be in the nucleus. In addition, pri-let-7 co-purified with Argonaute from both whole cell and nuclear extracts, suggesting that this interaction observed in C. elegans was conserved in humans.
The authors also introduce a surprising noncanonical miRNA target: its own primary transcript. Furthermore, they show that let-7 does not silence, but instead directly activates, its own expression. This conclusion is based on 2 pieces of evidence. The first is that mature let-7 levels are reduced in worms missing the let-7–binding site in the primary transcripts. The second comes from elegant experiments using the n2853 C. elegans strain. These animals have a single G→A transition in the let-7 seed sequence, which is predicted to disrupt binding of the mature miRNA to the complementary element in its own primary transcript. Northern blot analyses of n2853 extracts revealed a defect in pri-let-7 processing, which correlated with a lack of ALG-1 binding. Importantly, mature n2853 let-7 levels were rescued by the introduction of wild-type let-7.
The findings by Zisoulis et al3 not only describe the first direct miRNA autoregulatory loop, but also add to a growing body of evidence, suggesting nuclear roles for both mature miRNAs and Argonaute proteins. For instance, promoter-directed small interfering RNAs introduced into human cells have previously been shown to inhibit transcription of endogenous genes through various epigenetic mechanisms.13,14 Studies in HeLa cells have revealed that a hexanucleotide element at the 3′ end of mature miRNAs may direct nuclear import15 and that small interfering RNAs can indeed mediate the specific degradation of nuclear RNAs.16 Just last year, Tang et al17 described the first example of a pri-miRNA transcript under direct miRNA regulation.
C. elegans research has been instrumental in elucidating RNA interference (RNAi) pathways as well. Recently discovered roles for C. elegans Argonaute proteins range from the nuclear transport of cytoplasmic small interfering RNAs18 to mediating the inheritability of RNAi phenotypes.19 Powerful genetic tools unique to C. elegans have facilitated the discovery of not only let-7, but also many of its regulatory targets.20 In turn, some of these factors can regulate various stages of let-7 expression, thus forming multiple feedback loops.4 The work by Zisoulis et al3 now adds an important positive auto-regulatory module to the let-7 biogenesis pathway (Figure). Positive autoregulation is a mechanism that can result in the integration of graded inputs (eg, intercellular signaling) to a binary output (eg, self-renewal versus differentiation).21 Given the pivotal role of let-7 in cell fate decisions, it is not surprising that positive feedback loops have evolved to generate a bistable system where mature let-7 levels are either very low or very high. Cells with low let-7 expression will self-renew, whereas those with high expression will differentiate. Such autoregulatory modules are common cellular mechanisms used to ensure the irreversibility of cell fate specifications.21
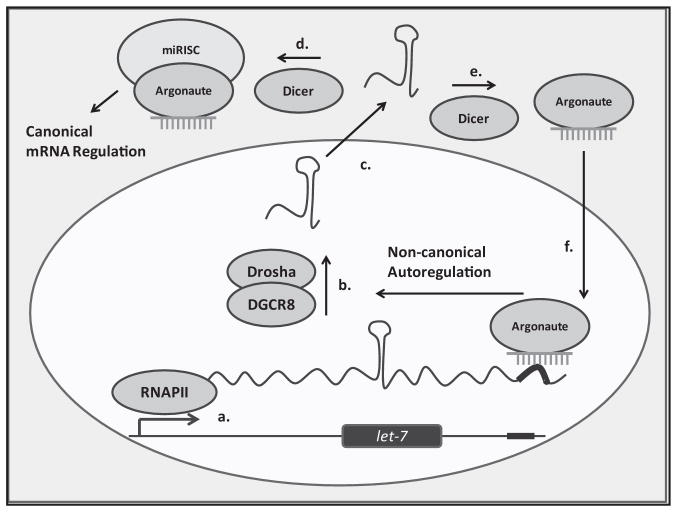
Figure. Positive autoregulation of let-7 biogenesis.
(a) Transcription of the primary microRNA (miRNA) by RNA polymerase II. (b) Processing and cleavage of the primary transcript by Drosha and DGCR8, producing the precursor miRNA. (c) Nuclear export of the precursor miRNA (dark green and light green represent the nucleus and cytoplasm, respectively). (d) The precursor miRNA undergoes the canonical pathway, processed by Dicer into the mature form and incorporated into the miRNA-induced silencing complex (miRISC). (e) and (f) A potential mode of mature miRNA nuclear transport by an Argonaute, after Dicer cleavage. The mature let-7-Argonaute complex binds to the complementary element (dark red) in the primary let-7 transcript, upregulating its own processing and completing the feedback loop.
In the mouse heart, let-7 accounts for ≈14% of all expressed miRNAs.1 If the autoregulatory loop described by Zisoulis et al3 is also true for human let-7, then it may be at least, in part, responsible for this high level of expression. Microarray analyses reveal that let-7 is significantly upregulated in many diseased heart tissues,1 suggesting that this autoregulatory loop may be a useful therapeutic target. It is intriguing that a common feature of many cardiac diseases involves the re-expression of a fetal gene program.21–23 In a simple model, we would have expected the downregulation of cardiac let-7 to help induce this fetal expression profile, given its role in C. elegans and human embryonic stem cells to promote cell differentiation.4,24 It is of course possible that in the context of certain cardiac expression profiles, let-7 upregulation may have different biological consequences than in development.
There are also many other questions that remain unanswered. Zisoulis et al3 have shown that mature let-7 can induce the maturation of its own primary transcripts by binding to a complementary element near their 3′ end and recruiting ALG-1. But what are the molecular events upstream and downstream of this binding? How is let-7 localized to the nucleus? Alternatively, is it possible that some miRNAs can mature without ever leaving the nucleus? How does ALG-1 promote let-7 processing? Given the diverse roles of Argonaute family members, identifying Argonaute-interacting factors by immunoprecipitation and mass spectrometry may provide important insights into the molecular mechanisms of nuclear miRNA functions.
Perhaps most importantly, a combination of computational and experimental approaches will be required to apply the concept of let-7 autoregulation to other miRNAs. Are such autoregulatory modules common in miRNA biogenesis pathways? How significant is the nuclear role of miRNAs in general? When assaying for nuclear levels of mature let-7, Zisoulis et al3 observed that some other miRNAs (miR-2, miR-244, miR-58) also displayed ≈ 20% nuclear localization. Could they be acting as an additional common layer of gene regulation? The answers to these questions will certainly lead the scientific community further away from the canonical view of miRNA biogenesis and function toward a more holistic understanding of miRNA-mediated gene regulation.
Footnotes
The opinions expressed in this Commentary are not necessarily those of the editors or of the American Heart Association.
Commentaries serve as a forum in which experts highlight and discuss articles (published here and elsewhere) that the editors of Circulation Research feel are of particular significance to cardiovascular medicine.
Commentaries are edited by Aruni Bhatnagar & Ali J. Marian.
Disclosures
None.
References
- 1.Small EM, Frost RJ, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation. 2010;121:1022–1032. doi: 10.1161/CIRCULATIONAHA.109.889048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 3.Zisoulis DG, Kai ZS, Chang RK, Pasquinelli AE. Autoregulation of microRNA biogenesis by let-7 and Argonaute. Nature. 2012;486:541–544. doi: 10.1038/nature11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008;18:505–516. doi: 10.1016/j.tcb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Johnson SM, Lin SY, Slack FJ. The time of appearance of the C. elegans let-7 microRNA is transcriptionally controlled utilizing a temporal regulatory element in its promoter. Dev Biol. 2003;259:364–379. doi: 10.1016/s0012-1606(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 6.Sun H, Wang Y. Restriction of big hearts by a small RNA. Circ Res. 2011;108:274–276. doi: 10.1161/CIRCRESAHA.110.239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thum T, Catalucci D, Bauersachs J. MicroRNAs: novel regulators in cardiac development and disease. Cardiovasc Res. 2008;79:562–570. doi: 10.1093/cvr/cvn137. [DOI] [PubMed] [Google Scholar]
- 8.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 9.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 10.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 11.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6:376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 13.Morris KV, Chan SW, Jacobsen SE, Looney DJ. Small interfering RNA-induced transcriptional gene silencing in human cells. Science. 2004;305:1289–1292. doi: 10.1126/science.1101372. [DOI] [PubMed] [Google Scholar]
- 14.Kawasaki H, Taira K. Induction of DNA methylation and gene silencing by short interfering RNAs in human cells. Nature. 2004;431:211–217. doi: 10.1038/nature02889. [DOI] [PubMed] [Google Scholar]
- 15.Hwang HW, Wentzel EA, Mendell JT. A hexanucleotide element directs microRNA nuclear import. Science. 2007;315:97–100. doi: 10.1126/science.1136235. [DOI] [PubMed] [Google Scholar]
- 16.Robb GB, Brown KM, Khurana J, Rana TM. Specific and potent RNAi in the nucleus of human cells. Nat Struct Mol Biol. 2005;12:133–137. doi: 10.1038/nsmb886. [DOI] [PubMed] [Google Scholar]
- 17.Tang R, Li L, Zhu D, Hou D, Cao T, Gu H, Zhang J, Chen J, Zhang CY, Zen K. Mouse miRNA-709 directly regulates miRNA-15a/16-1 biogenesis at the posttranscriptional level in the nucleus: evidence for a microRNA hierarchy system. Cell Res. 2012;22:504–515. doi: 10.1038/cr.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guang S, Bochner AF, Pavelec DM, Burkhart KB, Harding S, Lachowiec J, Kennedy S. An Argonaute transports siRNAs from the cytoplasm to the nucleus. Science. 2008;321:537–541. doi: 10.1126/science.1157647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckley BA, Burkhart KB, Gu SG, Spracklin G, Kershner A, Fritz H, Kimble J, Fire A, Kennedy S. A nuclear Argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature. 2012;489:447–451. doi: 10.1038/nature11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosshans H, Johnson T, Reinert KL, Gerstein M, Slack FJ. The temporal patterning microRNA let-7 regulates several transcription factors at the larval to adult transition in C. elegans. Dev Cell. 2005;8:321–330. doi: 10.1016/j.devcel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Ingolia NT, Murray AW. Positive-feedback loops as a flexible biological module. Curr Biol. 2007;17:668–677. doi: 10.1016/j.cub.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinugawa K, Minobe WA, Wood WM, Ridgway EC, Baxter JD, Ribeiro RC, Tawadrous MF, Lowes BA, Long CS, Bristow MR. Signaling pathways responsible for fetal gene induction in the failing human heart: evidence for altered thyroid hormone receptor gene expression. Circulation. 2001;103:1089–1094. doi: 10.1161/01.cir.103.8.1089. [DOI] [PubMed] [Google Scholar]
- 23.Chien KR, Knowlton KU, Zhu H, Chien S. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiologic response. FASEB J. 1991;5:3037–3046. doi: 10.1096/fasebj.5.15.1835945. [DOI] [PubMed] [Google Scholar]
- 24.Nimmo RA, Slack FJ. An elegant miRror: microRNAs in stem cells, developmental timing and cancer. Chromosoma. 2009;118:405–418. doi: 10.1007/s00412-009-0210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]