Summary
Acquisition and maintenance of vascular smooth muscle fate is essential for the morphogenesis and function of the circulatory system. Loss of contractile properties or changes in the identity of vascular smooth muscle cells (vSMC) can result in structural alterations associated with aneurysms and vascular wall calcification. Here we report that maturation of sclerotome-derived vSMC depends on a transcriptional switch between mouse embryonic days 13 and 14.5. At this time, Notch/Jag1-mediated repression of sclerotome transcription factors Pax1, Scx and Sox9 is necessary to fully enable vSMC maturation. Specifically, Notch signaling in vSMC antagonizes sclerotome and cartilage transcription factors, and promotes upregulation of contractile genes. In the absence of the Notch ligand Jag1, vSMC acquire a chondrocytic transcriptional repertoire that can lead to ossification. Importantly, our findings suggest that sustained Notch signaling is essential throughout vSMC life to maintain contractile function, prevent vSMC reprogramming and promote vascular wall integrity.
Introduction
Vascular smooth muscle cells (vSMC) provide essential mechanical and biological support to the circulatory system. During development vSMCs arise from distinct progenitors depending on their location (Majesky, 2007). This broad embryonic origin (somitic mesoderm, lateral mesoderm and neural crest) has helped to reconcile the intriguing anatomical specificity of vascular pathologies, particularly when most of the identified risk factors are systemic in nature (DeBakey and Glaeser, 2000). In fact, vSMC originating from different progenitor subtypes exhibit lineage-specific differences in growth, gene expression and functional properties (Gadson et al., 1997; Owens et al., 2010; Topouzis and Majesky, 1996).
Definitive vSMC in the descending aorta (DA) arise from the somatic mesoderm (Pouget et al., 2008; Wasteson et al., 2008). These cells migrate towards the DA and replace the first wave of primitive lateral mesodermal derivatives (Hoxb6+ cells) that surround the recently formed aorta early during development (Wasteson et al., 2008). Somitic progenitors from the sclerotome also give rise to tenocytes and cartilage of the axial skeleton (Brent and Tabin, 2002). These developmental links are of particular interest since several pathological conditions, such as osteochondrogenic lesions and calcification of the vascular wall might signify a reiteration of some of these previous fates. Therefore, a more concrete understanding of the molecular mechanisms that establish and maintain vSMC fate, as well as the operative molecular repertoire that represses alternative fates, holds developmental and clinical interest.
Progressive divergence of Pax1+ sclerotome progenitors occurs as they migrate from the somites and become specified by contextual signals (Brent and Tabin, 2002). For example, under the influence of Sonic Hedgehog (Shh) secreted by the notochord, sclerotome progenitors increase the expression of Sox9, a transcription factor critical for skeletal development (Bi et al., 1999; Zeng et al., 2002). Sox9 specifies sclerotome progenitors toward the chondrocyte lineage by inducing expression of Col2a1 (Bell et al., 1997). In parallel, scleraxis (Scx), which initially potentiates the activity of Sox9 for chondrogenesis, can eventually give rise to tenocytes if its expression is maintained (Furumatsu et al., 2010). Finally, Pax1+ progenitors that reach the DA progressively replace Hoxb6+ cells and differentiate into vSMC during mid- and late development (Pouget et al., 2008; Wasteson et al., 2008).
Major transcriptional regulators that drive vSMC specification include serum response factor (SRF) and myocardin (Miano et al., 2007; Wang et al., 2004; Yoshida et al., 2003). However, myocardin alone is not sufficient to activate the entire vSMC differentiation program in undifferentiated cells (Parmacek, 2004). Clearly additional, yet to be defined, combinations of transcriptional regulators are necessary for the expression of vSMC-selective genes.
Activation of the Notch pathway has been shown to be critical for recruitment and initial differentiation of vSMC from neural crest-derived progenitors and for patterning of the ductus arteriosus (Feng et al., 2010; High et al., 2007; Manderfield et al., 2012). Intermittent Notch signaling is also an important regulator of skeletogenesis (Mead and Yutzey, 2012). In fact, Notch is co-expressed along with Pax1, Sox9 and Scx in sclerotomal progenitors; these transcription factors shift in levels and activity, initiating fate divergence. However, full differentiation and maintenance of vSMC fate, relies on molecular pathways that are yet to be elucidated.
Using a combination of in vitro and in vivo models, as well as next generation RNA sequencing, we determined that constant Notch signaling is essential to suppress chondrogenic fate while enabling the acquisition of vSMC fate in the DA. This occurs through repression of osteochondrocytic transcription factors such as Sox9, Pax1 and Scx, which in the absence of Jag1 promotes the reprogramming of immature vSMC. Inactivation of Jag1 results in the loss of contractile properties and focal transdifferentiation of vSMC into chondrocytes which then undergo endochondral ossification.
Results
Cell Fate Specification of Sclerotomal Progenitors
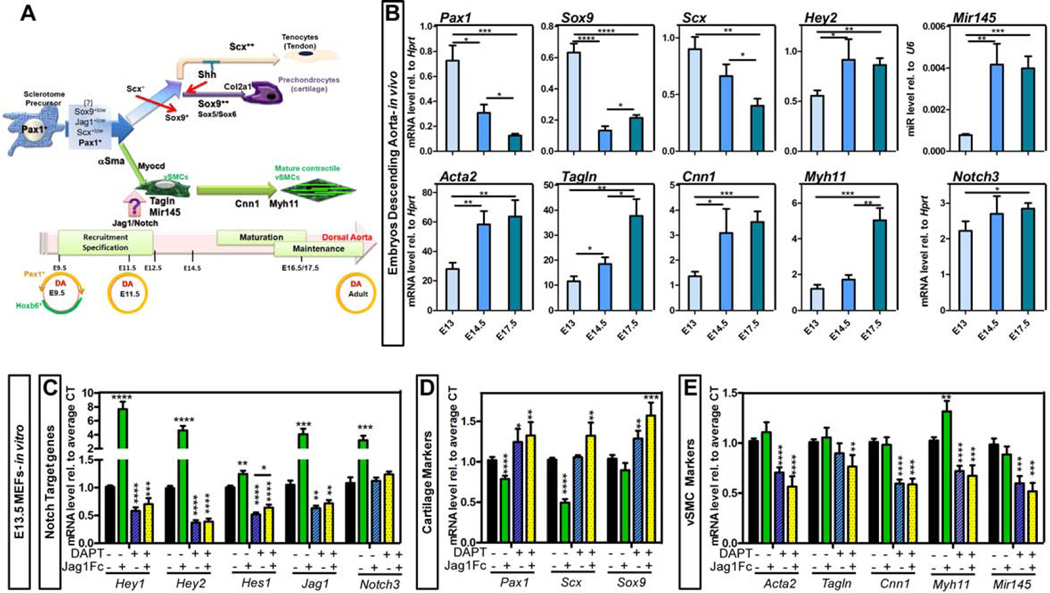
Pax1+ sclerotomal progenitors give rise to chondrocytes, tenocytes and vSMC (Figure 1A) but the emergence of one fate at the expense of the complete repression of others is not completely understood. In an effort to temporally characterize the molecular events leading to the acquisition and maintenance of contractile phenotype of vSMC, we first evaluated expression of key transcription factors in the descending aorta (DA) of wild type embryos (Figure 1B). By quantitative RT-PCR (qRT-PCR), the chondrocyte developmental markers Pax1 and Sox9 were still highly expressed at E13, a time when the DA is already completely invested with immature but specified vSMC, as per high expression of alpha smooth muscle actin and SM22. However a day later (E14.5), both Pax1 and Sox9 were significantly reduced and the cartilage and tenocyte transcription factor Scx followed a similar trend (Figure 1B). We also observed that Mir145, known to be essential for vSMC differentiation and contractile phenotype maintenance, was strongly upregulated between E13 and E14.5. In addition the Notch target gene Hey2 was increased during this tight window of development (Figure 1B). Together, the data identified E14.5 as a critical period in which differentiation and maturation of vSMC takes place and repression of other fates occurs. In fact, with the exception of more terminal markers, like Myh11, the timing at E14.5 also coincided with increased transcript level of Acta2 (encoding αSMA), Tagln (encoding Transgelin, also known as Sm22) and Cnn1 (encoding Calponin) (Figure 1B).
Figure 1. Vascular fate specification of sclerotomal precursors.
A- Schematic representation describing the embryonic origin of smooth muscle cells (vSMC) in the descending aorta (DA). B- Transcriptional analysis of total DA from WT E13 to E17.5 embryos. The relative expression of a subset of genes is represented as relative to Hprt housekeeping gene or U6 (for Mir145). n=4–9. C–E- Mouse embryonic fibroblasts (MEFs) isolated from E13.5 WT mice were exposed to immobilized Jag1Fc or Fc-control in the presence or absence of γ-secretase inhibitor (DAPT, 50µM) for 24h. Transcriptional level for Notch signaling molecules (C), sclerotome and cartilage transcription factors (D) and vSMC markers (E) was determined by qRT-PCR. Data are represented as mean +/− SEM. nFc-control and FcJag1=16; n+/− DAPT=6. B–E: *p<0.05; **p<0.001; ***p<0.0005; ****p<0.0001.
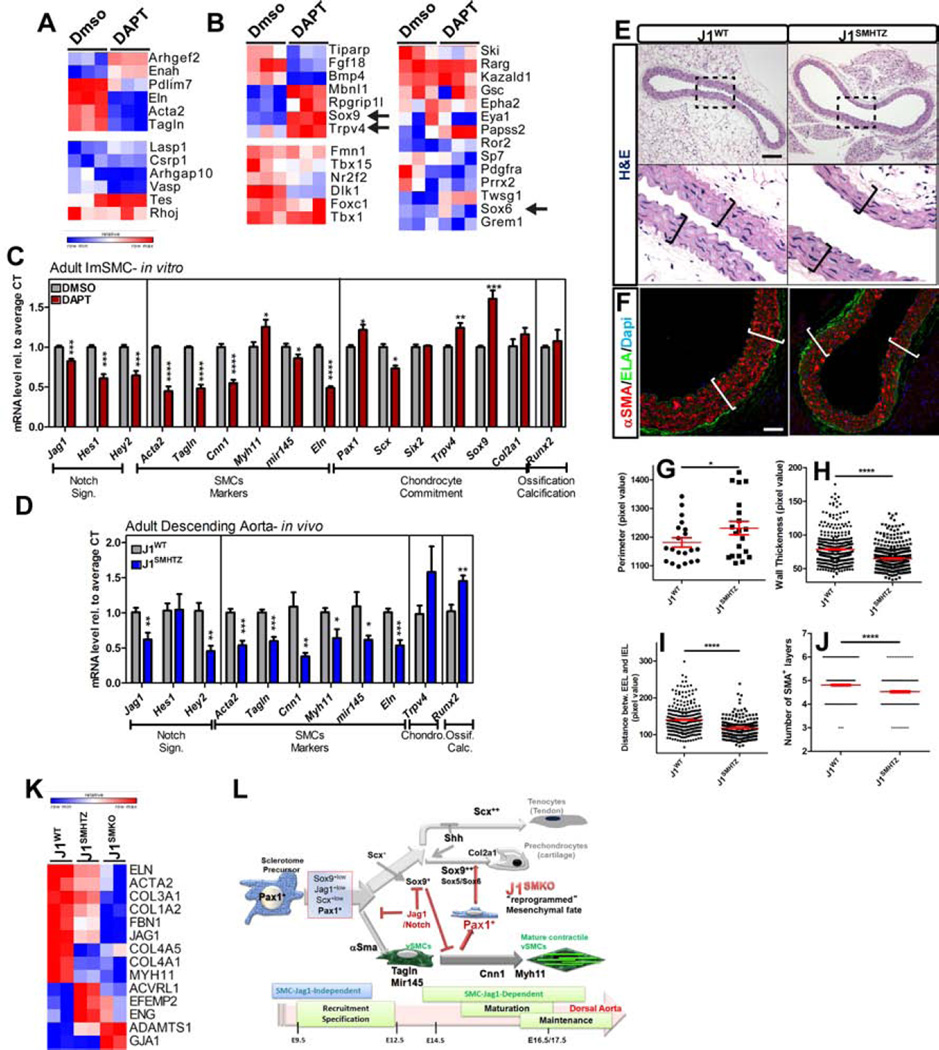
While a requirement for Notch signaling has been previously shown to be essential for the assembly of the vascular wall (Feng et al., 2010; High et al., 2007; Manderfield et al., 2012), the positive or negative effect of Notch signaling on full vSMC differentiation remains controversial (Doi et al., 2006; Fischer et al., 2004; Noseda et al., 2006; Proweller et al., 2005; Tang et al., 2008). To answer this question we examined the response of unspecified primary mesenchymal cells to the presence or absence of immobilized Notch ligand Jag1 (Figure 1C–E). Not surprisingly, Jag1Fc stimulation led to a significant upregulation of Notch targets: Hey1, Hey2, and Hes1, as well as Jag1 and Notch3 when compared to Fc-control treated cells (Figure 1C). In addition, Jag1Fc exposure resulted in a significant reduction of the sclerotome transcription factors Pax1 and Scx (Figure 1D). Furthermore, blocking Notch cleavage using a γ-secretase inhibitor (DAPT) led to a consistent decrease in vSMC contractile genes that was not rescued by addition of exogenous Jag1 (Figure 1E). These data support the notion that in the absence of a constant activation of Notch signaling, cells would allow to escape vSMC fate. Similarly, exposure to DAPT resulted in a decrease of Notch targets and ligands, ultimately resulting in muted responses to Jag1Fc (Figure 1C). Blocking Notch signaling was also associated with a slight increase in Pax1 level. In particular, we noted that by inhibiting Notch cleavage, the effect of Jag1Fc on Pax1 and Scx was abolished (Figure 1D). Finally treatment with DAPT increased the level of the cartilage transcription factor Sox9 (Figure 1D); indicating that basal activation of the Notch pathway is needed for restriction of Sox9 transcript level.
Nuclear Translocation of Notch Coincides with vSMC maturation
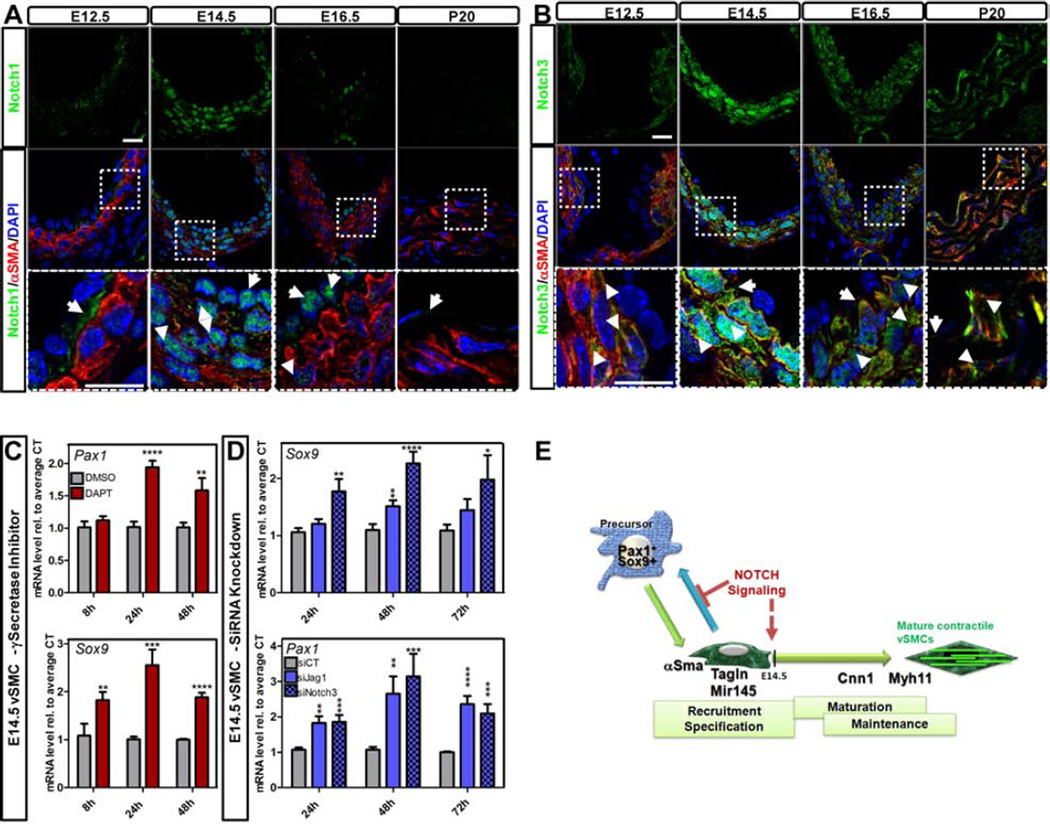
To further characterize the role of Notch signaling in the establishment of vSMC fate, we studied Notch expression pattern in embryos and young adult mice (postnatal day (P) 20) (Figure 2A–B; Figure S1A–B). While at E12.5, Notch1 (Figure 2A) was detected at the cell surface and particularly at the interface between the endothelium and αSMA+ vSMC layers, by E14.5 an impressive nuclear translocation was noted in both endothelial and vSMC. By E16.5, Notch1 was predominantly found in endothelial nuclei with conspicuous absence in vSMC. Notch3 followed the same trend but this receptor was exclusive to vSMC (Figure 2B). Furthermore, in contrast to Notch1, Notch3 persisted at later stages of development in the vSMC (Figure 2B). The expression levels of Notch receptors and ligands were further evaluated by qRT-PCR. Notch3 transcripts were significantly higher than the other receptors (Figure S1A) and Jag1 was by far the most prominent of the Notch ligands during development and after birth (Figure S1C). For receptors in the adult aorta, Notch1 was the most prominent in endothelial cells (EC; Figure S1B), while Notch3 was the highest of the Notch receptors in vSMC (vSMC; Figure S1B), in agreement with the immunodetection at P20 (Figure 2A–B). In the adult aorta, Jag1 and Dll1 levels were higher than Dll4 and Jag2 in EC while Jag1 was the most prominent Notch ligand for vSMC (Figure S1D). The continuous expression of Jag1 and Notch receptors in the adult vascular wall was suggestive of a requirement for this signaling pathway at later developmental stages and after birth.
Figure 2. Notch dynamics in the developing aorta impact sclerotome and cartilage transcription factors.
A-B- Immunofluorescence of Notch1 (A; green) and Notch3 (B; green) in combination with αSMA (A, B; red) on cross-sections of DA at indicated ages. Arrows indicate positive cells. Scale Bars: 10µm. C–D- Embryonic vSMC isolated at E14.5 from the DA of J1WT animals were treated with: 50µM of γ-secretase inhibitor (DAPT) or DMSO as vehicle control (C); siRNA scramble or siRNA targeting Jag1 or Notch3 (D). Transcriptional level of Pax1 and Sox9 was measured by qRT-PCR. Data are represented as mean +/− SEM. nDAPT/DMSO= 5–6, nsiCT/siJag1=8 nsiNotch3=6 *p<0.05; **p<0.001; ***p<0.0005; ****p<0.0001. E- Notch signaling is required to prevent immature (specified) vSMC from reprogramming toward sclerotome/cartilage fate by repressing Pax1 and Sox9 transcripts in vitro.
Loss of Jag1 in Immature vSMC Results in a Transcriptional Profile Switch towards the Chondrocytic Lineage
To determine the relevance of Notch signaling in immature vSMC, we isolated vSMC from WT E14.5 mice (Figure S1G–J), a time when both Notch1 and Notch3 are translocated to the nucleus (Figure 2A–B). Inhibition of Notch signaling (with DAPT) resulted in a significant increase of both Pax1 and Sox9 transcripts (Figure 2C). Similar results were observed when cells were transfected with siRNA targeting either Jag1 or Notch3 (Figure 2D; Figure S1L). The data suggests that Notch signaling is required to inhibit sclerotome and chondrocyte transcription factors in specified (immature) vSMC (Figure 2E).
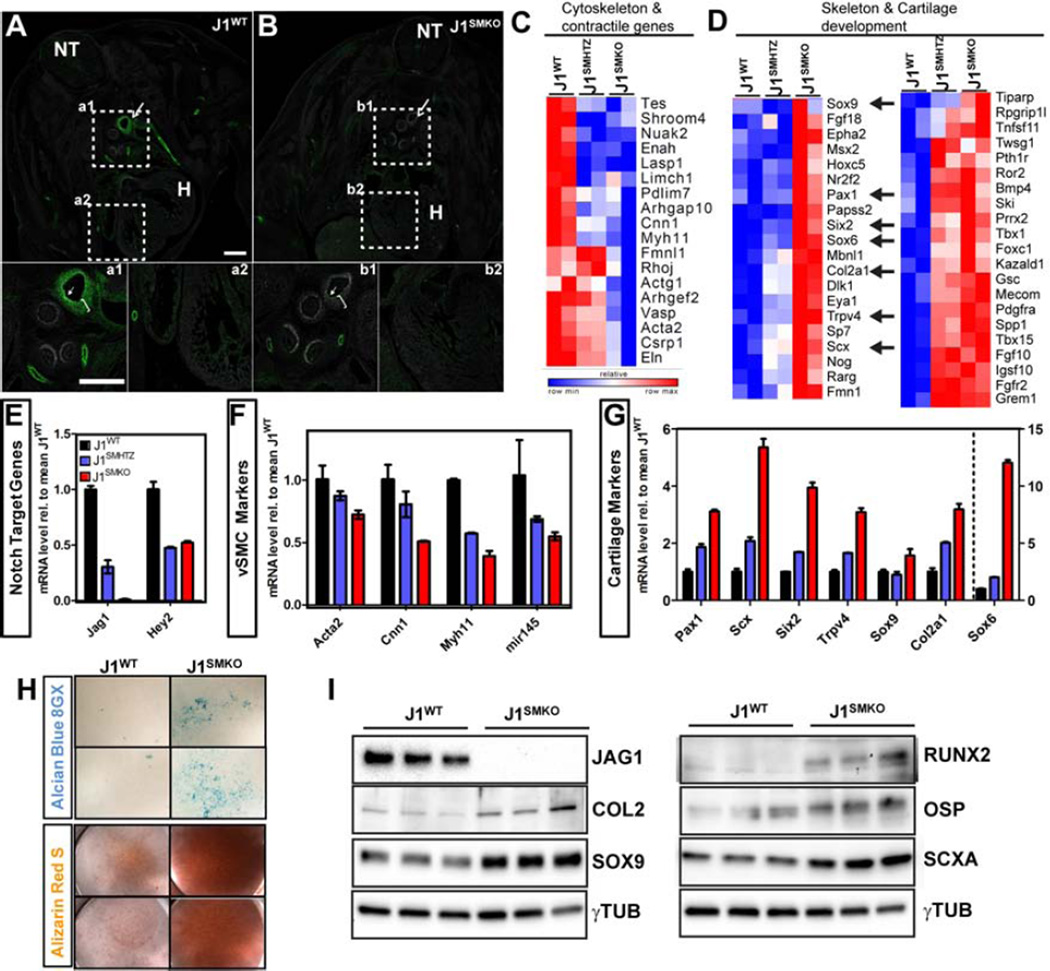
The impact of the loss of Jag1 from vSMC was then assessed by deep RNA sequencing of E14.5 vSMC isolated from Sm22-driven Cre negative (Jaglox/lox; J1WT), vSMC heterozygous (Jaglox/+ Cre+; J1SMHTZ) and Jag1-null (Jaglox/lox Cre+; J1SMKO) littermates (Figure 3; Figure S2) mice that we have previously used (Turlo et al., 2012). We confirmed excision of Jag1 at E12.5 (Figure 3A,B), before the subcellular translocation switch of Notch receptors has occurred (Figure 2), and at the time of isolation in J1SMKO DA compared to J1WT animals (E14.5; Figure S2A). High enrichment and purity of the eSMC was confirmed by Sm22-driven excision of Jag1 by about 99%. In addition, the full RNAseq profile and measurement of transcripts for various vascular cell types verify the purity of those vSMC populations (Figure S2B). Transcriptional profile analysis revealed that a number of the differentially expressed genes between J1WT and J1SMKO were potential targets of the transcriptional co-repressor CTBP2. Biological Pathway Gene Ontology (BP-GO) analysis further indicated that the top two categories affected were “skeletal system development” and “blood vessel development” (Figure S2C–E). In fact, in the absence of Jag1, the vSMC differentiation program was profoundly impacted as per the drastic decrease in contractile associated genes (18 genes; Figure 3C). Concurrently, a significant number of genes involved in skeletal development were increased upon Jag1 loss (41 genes; Figure 3D) supporting an intrinsic role for Notch signaling in the decision of fate between vSMC and bone during development.
Figure 3. Smooth muscle cell specific deletion of Jag1 in the developing descending aorta is associated with decrease in vSMC markers and increase in sclerotome/cartilage markers.
A- Co-immunofluorescence of Jag1 (green) and αSMA (white) at E12.5 demonstrated that Jag1 is highly expressed in arteries and aorta branches (a1; arrow) and faintly detected in the heart (a2). B- Specific loss of Jag1 was confirmed in vSMC in the J1SMKO (b1; bracket) but retained in endothelial cells (b1; arrowhead).H: heart; NT: neural tube; Scale Bars: 100µm. C–D- RNA deep sequencing analysis of J1WT, J1SMHTZ and J1SMKO vSMC isolated from the DA at E14.5 showed variation of transcripts classified in “cytoskeleton and contractile genes” (C) and “skeleton and cartilage development” (D). Relative row count for each transcript is represented following a pseudo-color scale. E–G- qRT-PCR on Notch target genes (E), vSMC markers (F) and cartilage markers (G) was performed on isolated vSMCs in vitro to confirm differentially expressed genes identified by RNA deep sequencing in the litter#1. Expression level is represented relative to mean of the J1WT samples +/− SEM. H–I- vSMC isolated from J1WT and J1SMKO DA were grown 10 days at high density and stained for alcian blue 8GX or alizarin red S (H). Protein level of JAG1 and chondro-osteogenic markers (COL2, SOX9, RUNX2, OSP and SCXA) was evaluated by Western blot. γTUBULIN was used as loading control (I).
The findings were validated by qRT-PCR and showed that decreased Jag1 expression resulted in downregulation of Hey2 (Figure 3E; Figure S2H) and vSMC specific genes: Acta2, Cnn1, Myh11 and Mir145 (Figure 3F, Figure S2I). Mirroring the RNAseq data, the decrease in Jag1 resulted in a significant increase in transcript level of genes involved in endochondral ossification. These included the sclerotome marker Pax1, as well as genes involved in cartilage development such as Scx, Six2, Trpv4, Sox9, its cofactor Sox6 and target gene Col2a1 (Figure 3G, Figure S2J). To further investigate this fate switch, we cultured J1WT and J1SMKO E14.5 vSMC at high density. Deposition of proteoglycan-rich matrix and calcification were assessed by alcian blue and alizarin red staining. J1SMKO cultures were positive for both, in contrast to J1WT cells (Figure 3H). In addition, chondro-osteogenic proteins (COL2, SOX9, RUNX2, OSP and SCXA) were increased in the J1SMKO cells (Figure 3I); further supporting the concept that absence of Jag1 in vSMC at midgestation predisposes cell fate changes toward the chondro-osteogenic lineage.
Notch Signaling Represses Sox9 to Support Acquisition and Maintenance of Contractile Genes in vSMC
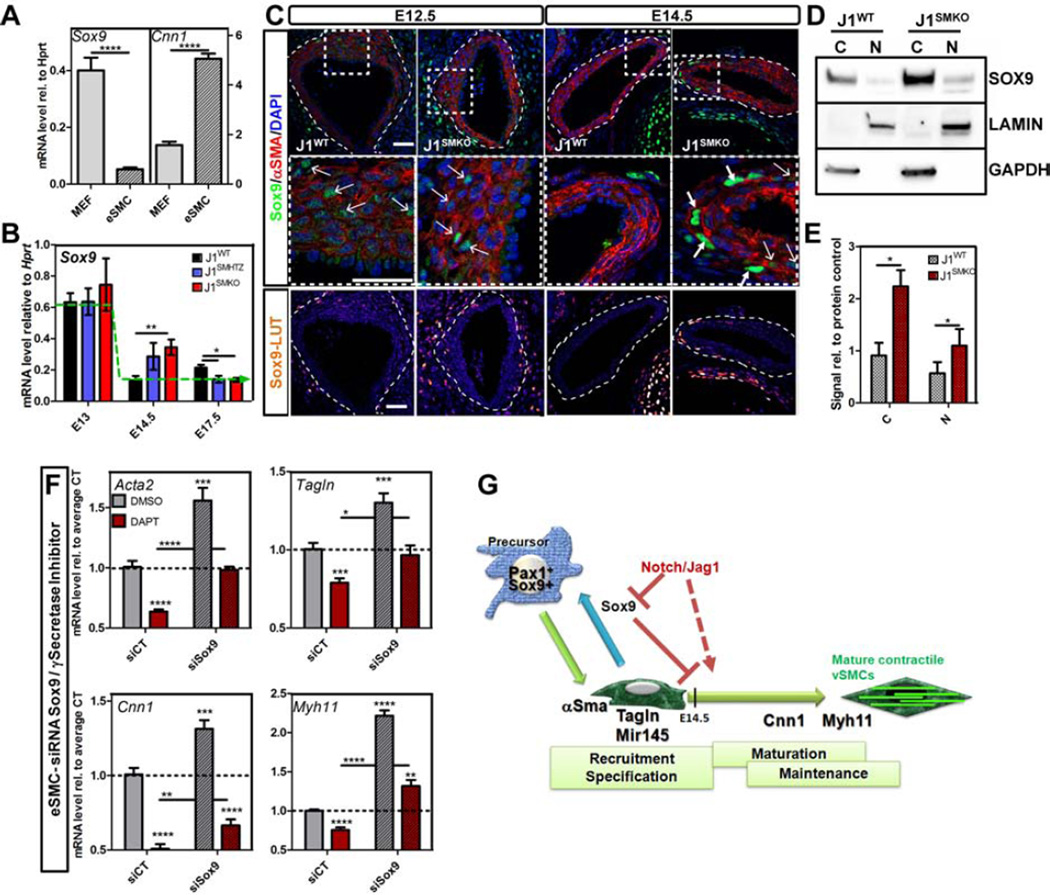
Our findings indicated that removal of Jag1 at E9.5 in SM22+ cells (already specified towards vSMC fate) was sufficient to skew their transcriptional program in the direction of a chondro–osteogenic fate. However, the molecular pathway(s) that led to such a change was unclear. Of interest, expression of Sox9, the master regulator of chondrocyte differentiation was elevated in J1SMKO cells compared to J1WT. Furthermore, we observed that Sox9 mRNA levels were inversely correlated with vSMC contractile genes (Figure 4A), a finding consistent with previous reports showing that an increase in Sox9 led to a decrease of vSMC markers expression in vitro (Xu et al., 2012).
Figure 4. Notch signaling represses Sox9 expression to allow acquisition and maintenance of vSMC contractile phenotype.
A- Transcriptional level of Sox9 and Cnn1 was determined by qRT-PCR in WT MEFs and E14.5 vSMC, normalized and represented relative to Hprt housekeeping gene. n=8–12; B-Sox9 transcript level was evaluated by qRT-PCR from dorsal aortae harvested at the indicated stages and represented relative to Hprt. J1WT (black bars), heterozygous (J1SMHTZ, blue bars) and J1SMKO (red bars) DA were analyzed. Green dotted line represents the trend of expression of J1WT over time. nJ1WT= 5–9; n J1SMHTZ =3–5; nJ1SMKO=3–5. C- Immunodetection of Sox9 (green; LUT) in combination with αSMA (red) in the DA at E12.5 and E14.5. Bottom panels are higher magnification view of respective top panels. Scale Bars: 25µm. D–E- Protein level of Sox9 in the cytoplasm (C) and in the nucleus (N) of J1WT and J1SMKO E14.5 vSMC was quantified by Western blot. GAPDH and LAMIN A/C were used as loading control for cytoplasmic and nuclear fraction respectively. n=3 (E). F- WT vSMC at E14.5 were treated with siRNA targeting Sox9 previous to DAPT treatment (50µM- 24h). Transcriptional analysis of Acta2, Tagln, Cnn1 and Myh11 was performed by qRT-PCR and represented as fold change compared to control treated with scramble siRNA and DMSO. Data are represented as mean +/− SEM n=7. A,B,E,F: *p<0.05; **p<0.001; ***p<0.0005; ****p<0.0001. E- Constant pressure of Notch signaling through Jag1 in the SM22+ compartment represses Sox9 expression in vivo. This prevents reprogramming of immature vSMC and allows maturation toward a contractile phenotype.
Quantification of Sox9 transcripts in the developing aorta in vivo revealed that while this transcription factor was switched off in J1WT between E13 and E14.5, a time where Notch receptors are translocated from the membrane to the nucleus, it was transiently maintained in J1SMHTZ and J1SMKO and only reduced by E17.5 in both genotypes (Figure 4B). In accordance with these data, immunodetection of Sox9 was similar in both J1WT and J1SMKO DA at E12.5 (Figure 4C), whereas Jag1 was already excised (Figure 3A). However by E14.5 differences in staining patters for Sox9 were evident. A subset of vSMCs in J1SMKO embryos showed strong staining for Sox9, in contrast to its complete absence in control littermates (Figure 4C; Figure S3A). Western blot analysis also confirmed that Sox9 protein was significantly increased in both the cytoplasm and the nucleus of J1SMKO vSMC compared to J1WT (Figure 4D–E). Similarly treatment of E14.5 WT vSMC with DAPT led to an increase of the transcription factor in the nuclear fraction (Figure S3B–C), supporting that Sox9 deregulation at the transcriptional and protein level was associated with absence of Notch/Jag1 signaling.
To further explore the relevance of Sox9 in vSMC maturation and maintenance, we superimposed siRNA for Sox9 with DAPT treatment (Figure 4F; Figure S3D–F). Supporting the central hypothesis, downregulation of Sox9 alone led to a significant increase in vSMC transcripts for contractile genes. Inhibition of Notch signaling in control siRNA transfected cells resulted in a marked increase of Sox9 levels and downregulation of Acta2, Tagln, Cnn1 and Myh11. Importantly, we could rescue this phenotype by knocking-down Sox9 upon suppression of Notch signaling by DAPT for Acta2, Tagln and Myh11 (Figure 4F). These findings indicate that Notch is upstream Sox9 and that Sox9 repression provides an appropriate transcriptional landscape for maturation of vSMCs (Figure 4G).
To further scrutinize our hypothesis, we explored the effect of retaining Sox9 in the DA using a transgenic mouse model. In these mice, Sox9 was maintained in αSMA+ cells of the tunica media at late developmental stages, whereas it was not detected in the control littermates (Figure 5A) as expected after E14.5 (Figure 4). As observed in the J1SMKO embryos, Sox9 detection was intense but restricted to a cohort of SMA+ cells in the tunica media of the transgenic animals (Figure 5A) resembling the pattern found upon Jag1 deletion (Figure 4C).
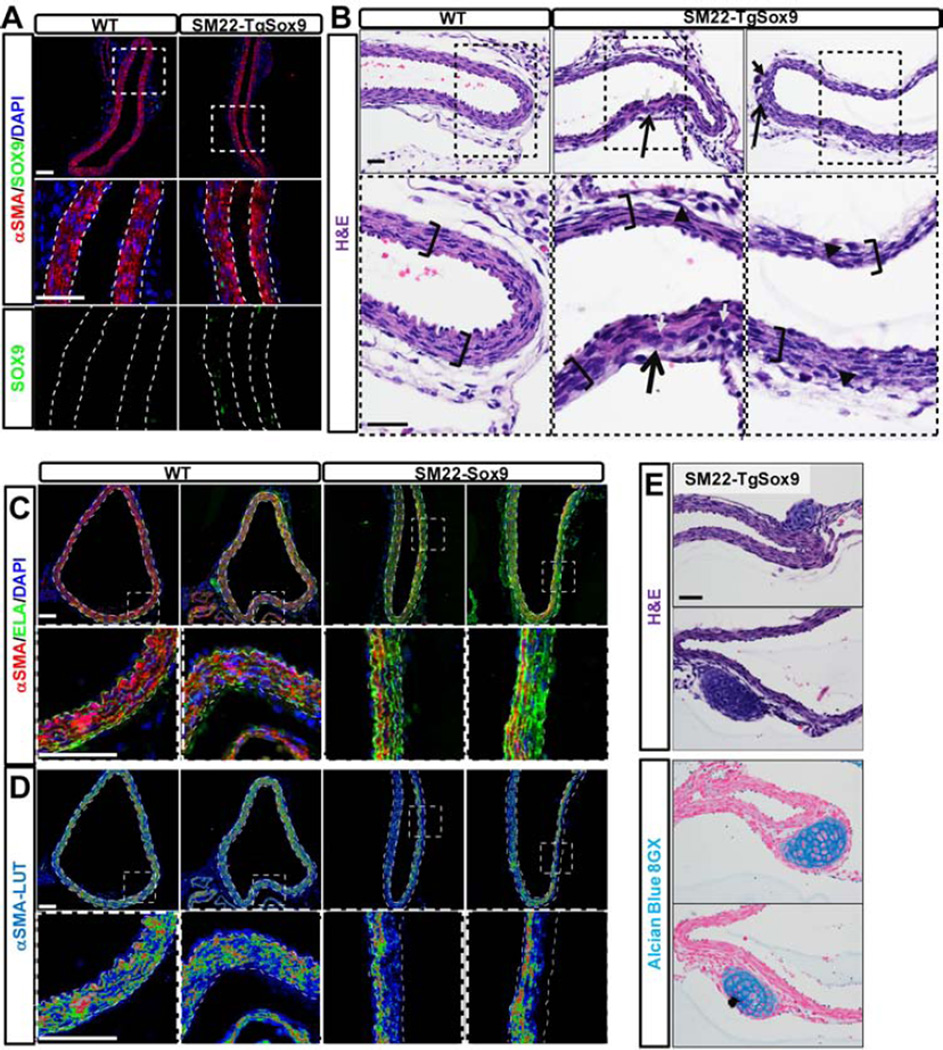
Figure 5. Misexpression of Sox9 in vSMCs prevents maturation of vSMC and results in the development of cartilage nodules.
A- Immunodetection of Sox9 (green) and αSMA (red) in the aorta of Sm22Cre-TgSox9 mice and control littermates at E18.5. B- H&E staining at E18.5 revealed anomalies in the DA of the Sm22Cre-TgSox9 mice: thinning of the tunica media (brackets), disorganization of the vSMC layers (opened arrows) presenting large nucleated cells (closed arrows) and cell detachment (arrowhead). C–D- Immunodetection of Elastin (ELA; green) and αSMA (red; LUT) in the DA of control and Sm22Cre-TgSox9 mice at E18.5. Dotted line delimits the internal and external elastic lamina. E- H&E and alcian blue 8GX staining at E18.5 in Sm22Cre-TgSox9 DA. Scale Bars: 40µm(A,C,D); 25µm(B,E).
Misexpression of Sox9 by vSMCs resulted in reduced thickness and disorganization of the tunica media (Figure 5B). While deposition of elastin was not affected, αSMA expression was spotty. In fact, αSMA was virtually absent from the cells that populated the third and fourth layer of the tunica media (Figure 5C–D). These findings are consistent with the notion that persistent expression of Sox9 affects maturation and/ or maintenance of vSMC phenotype. In addition retention of Sox9 resulted in the formation of ectopic cartilaginous nodules, positive for alcian blue, associated with the most external layers of the media (Figure 5E).
Inactivation of Jag1 in immature vSMC Results in Loss of vSMC Fate
The data presented so far is consistent with the notion that repression of chondrocyte transcription platform by Notch signaling is necessary for progression of vSMC fate. Thus, we evaluated the requirement for Jag1 in the maintenance of vSMC fate in vivo by first determining the transcriptional profile upon inactivation of Jag1. We found that Acta2, Tagln, Cnn1, Myh11, Notch3 and Mir145 in J1SMKO were significantly lower than in J1WT at E17.5 (Figure 6A). In contrast aortae from J1SMHTZ littermates were unaffected except for reduction in Notch3.
Figure 6. Jag1 is required for the acquisition and maintenance of a vSMC mature contractile phenotype in vivo.
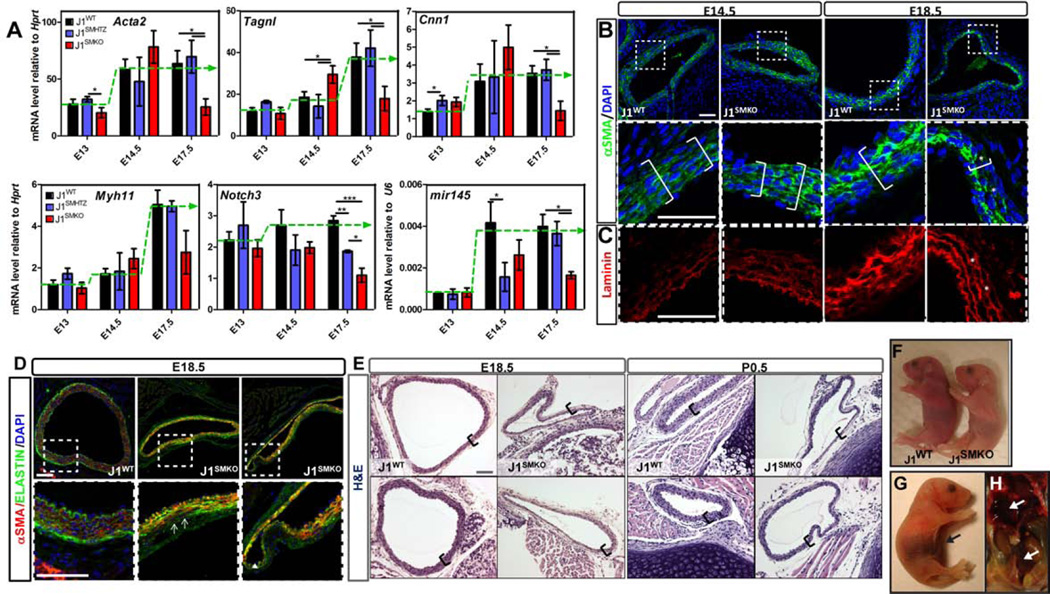
A- Transcriptional level of vSMC markers in the developing DA was evaluated by qRT-PCR and represented relative to Hprt or U6. J1WT (black bars), heterozygous (J1SMHTZ, blue bars) and J1SMKO (red bars) aortae were analyzed from E13 to E17.5. Green dotted line represents the trend of expression of J1WT over time. nJ1WT= 5–9; n J1SMHTZ =3–5; nJ1SMKO=3–5; *p<0.05; **p<0.001; ***p<0.0005. B–C- Immunofluorescence of αSMA (green) and Laminin (Red) in the developing DA at E14.5 and E18.5. Stars show the presence of αSMA-negative cells inside the vascular wall. D- Co-immunodetection of Elastin (ELA; green) and of αSMA (red) at E18.5. In the J1SMKO aorta αSMA-negative cells were detected where Elastin is still present (arrows). A consistent positive single layer of αSMA+ cells was found near the endothelium, that by virtue of expressing Jag1 consistently promotes activation of Notch in this first layer of vSMC. Bottom panels are a higher magnification view of dotted squares in upper panel (B–D). E-Histological evaluation (H&E) of DA showed a decrease of thickness in the aortic wall at E18.5 and P0.5 (brackets) of the J1SMKO compared to J1WT. Scale Bars: 25µm(B); 40µm(D); 50µm(E). F- Neonates J1SMKO were pale and exhibited jaundice when compared with J1WT littermates. G–H- Necropsy revealed the presence abdominal and thoracic hemorrhages at birth (G) or later in life (H; P21) (arrows).
Similarly, αSMA immunodetection did not show any remarkable differences at E14.5 supporting that Jag1 in vSMC is not required for recruitment and initial organization of the vascular wall in the DA. In contrast a drastic decrease in the number of αSMA+ cells was found in the J1SMKO by E18.5 (Figure 6B). Immunodetection of laminin (Figure 6C) revealed correct organization of ECM in both genotypes at E14.5. By E18.5, while laminin persisted, cells in the media of J1SMKO mice had already lost expression of αSMA (Figure 6C, stars). Similar findings were observed upon evaluation of elastin (Figure 6D); while matrix organization was present, SMA-negative cells populated much of the tunica media in J1SMKO mice.
The physiological consequence of vSMC fate loss was highlighted by their neonatal lethality. J1SMKO mice died during the first days of life (Figure S4B–C). J1SMKO neonates were clearly distinguishable from their J1WT littermates by their pale appearance (Figure 6F). Histological H&E cross-sections revealed thin walls and distortions in the diameter of the J1SMKO aorta (Figure 6E). These were likely sites of aorta aneurysms and dissections, as necropsy showed profuse abdominal hemorrhage in recently demised J1SMKO animals (Figure 6G–H).
Ectopic Endochondral Ossification Foci in J1SMKO mice Supports an Essential Requirement for Notch Signaling in Suppression of Chondrogenic Fate
The initial in vitro experiments highlighted a tight relationship between Notch activity and the repression of sclerotome and cartilage genes (Figure 1–4). This observation is of particular interest, as vascular calcification is a clinically prevalent pathological manifestation in large size arteries. To further ascertain the impact of Jag1 deletion on the regulation of cartilage genes in vivo, we first assessed transcriptional levels of early chondrocyte markers (Figure 7A). Pax1 was equally expressed in J1WT, J1SMHTZ and J1SMKO at E13.5. A day later, this sclerotome transcription factor was reduced in J1WT and J1SMHTZ but maintained in J1SMKO mice. Scx was increased in both J1SMHTZ and J1SMKO at E13.5, and subsequently downregulated in J1SMHTZ yet retained in the DA of J1SMKO mice (Figure 7A). The transient maintenance of Sox9 expression (Figure 4A) resulted in high expression of its target gene Col2a1 in J1SMKO DA and to a lesser extent in J1SMHTZ by E14.5 (Figure 7A). The difference in Col2a1 expression between the J1SMHTZ and J1SMKO indicates that in addition to the control of Sox9 transcript level, Jag1 is required to repress its activity on the promoter of target genes and other cofactors such as Scx and Sox6. These in vivo data are consistent with our in vitro observations indicating that Jag1 functions to repress cartilage genes in vSMC during development. Considering that Col2a1 is a typical marker for differentiating chondrocytes, its maintenance in the J1SMKO aorta strongly underlines the need for Notch signaling in the repression of the default cartilage fate of the vascular wall.
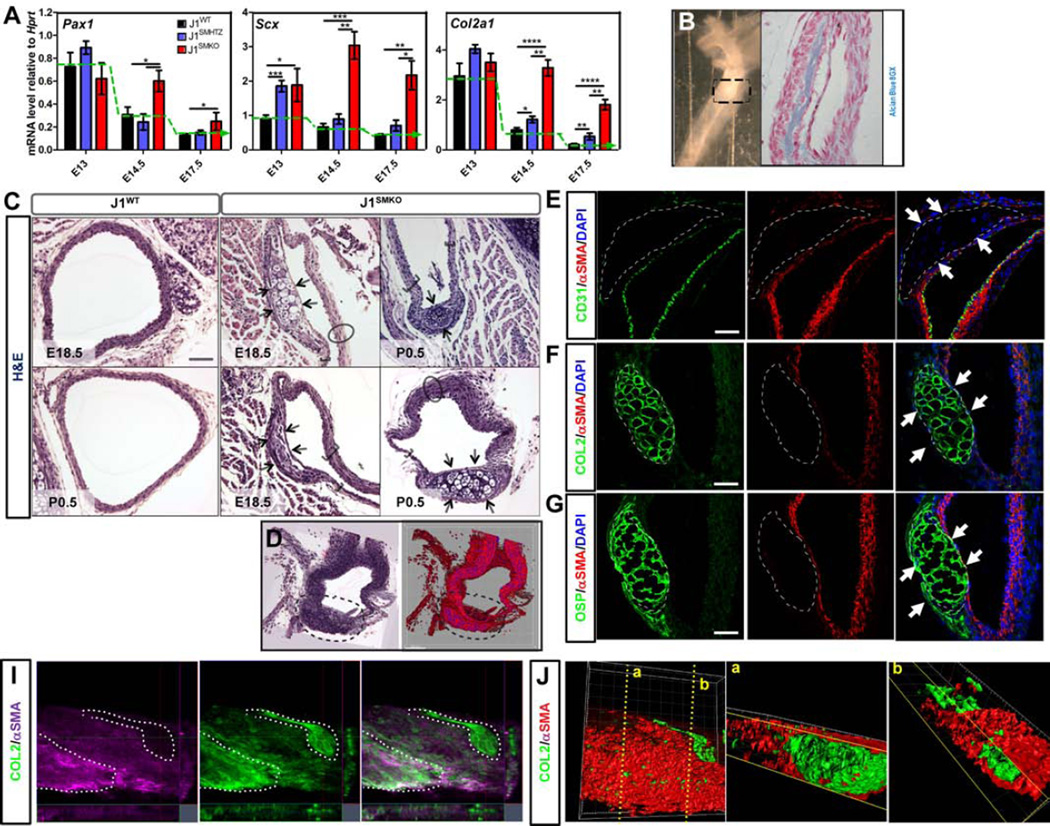
Figure 7. Loss of Jag1 in specified vSMC leads to cell fate switch and ectopic endochondral ossification.
A- qRT-PCR analyses of sclerotome and cartilage transcription factors (Pax1, Col2a1 and Scx) expression level in total DA from J1WT (black bars, n=5–9), J1SMHTZ (blue bars, n=3–5) and J1SMKO (red bars, n=3–5) embryos. Expression level was normalized and represented relative to Hprt housekeeping gene. Green dotted line represents the trend of expression of J1WT over time. Data are represented as mean +/− SEM. *p<0.05; **p<0.001; ***p<0.0005; ****p<0.0001. B- Whole mount picture of J1SMKO neonate DA shows a more dense region of the aorta (dotted scared) that revealed to be transdifferentiation of bone tissue (alcian blue 8GX positive) in the tunica media. C- H&E staining on DA from E18.5 and neonates (P0.5) reveals ectopic cartilaginous nodules that formed inside the tunica media of J1SMKO animals (arrows). D- 3D projection of cartilage nodule (dotted line) in the DA of J1SMKO neonate. E–G-Immunofluorescence analysis of P0.5 DA revealed that the cartilaginous nodules did not affect the CD31+ endothelium (E; green) but rather found in close proximity to αSMA+ cell layer (E; red). These nodules were positive for Collagen2 (F; COL2) and Osteopontin (G; OSP). Scale Bars: 50µm(C); 25µm(E–G). I–J- E14.5 DA explant were maintained 4 days in vitro. Immunodetection and 3D reconstitution shows presence of Collagen2 positive structures (green; I–J) in tight association with αSMA+ cells (purple, I; red, J).
In accordance with the lack of repression in cartilage genes between E13 and E14.5 (Figure 7A), ectopic cartilage nodules were found in the vascular wall of the DA of J1SMKO mice at E18.5 and after birth (Figure 7B–D, Figure S5A). The nodules did not affect the CD31 + endothelium, which still expresses Jag1, but were found within the tunica media as per adjacent αSMA+ cells (Figure 7E). The spotty cartilage nodules present in the tunica media of J1SMKO mice, resembles the phenotype of Sox9 transgene (Figure 5E). We confirmed full fate conversion of the nodules in J1SMKO mice by evaluation of the chondrocyte marker COL2 (Figure 7F) and osteoblast marker OSP (Figure 7G) indicating progression of ectopic endochondral ossification in the vascular wall of the J1SMKO DA. This phenotype was highly penetrant (83%-100% of mice between E18.5 and neonate). Cell fate tracing further indicated that the chondrocyte nodules resulted from the transdifferentiation of SM22+ vSMCs into cartilage (Figure S5B).
To rule out the intrinsic role of physical forces in the induction of cartilage nodules, we isolated and cultured E14.5 aorta explants from Jag1 null mice and control littermates (before any phenotypical anomaly was detected). After 4 days in culture, we found that absence of Jag1 in SM22 cells resulted in the formation of cartilaginous structures highly positive for COL2 (Figure 7I). 3D reconstitution revealed that these structures were tightly associated and intertwined with cells exhibiting αSMA+ staining (Figure 7J).
Decrease of Jag1 Expression in vSMC Predisposes to Aneurysms
J1SMKO neonates died shortly after birth whereas J1SMHTZ littermates appeared to develop and grow normally. However, the transcriptional profile of J1SMHTZ vSMCs in vitro was intermediate between J1WT and J1SMKO (Figure 3, Figure S2). These data suggest that partial, but prolonged deletion of Jag1 could potentially affect vSMC fate and function in vivo.
To determine whether a decrease in Notch signaling resulted in re-expression of sclerotome and early cartilage transcription factors in adult cells (or was an exclusive property of embryonic vSMC), we explored the outcome of Notch suppression in immortalized adult aortic vSMC (temperature sensitive- Immortalized SMC: ImSMC; Figure 8A–C). The logical analogous experiment (deletion of Jag1 in ImSMC) was not compatible with cell survival; therefore we used DAPT to impose loss of Notch signaling and performed full deep RNA sequencing analysis. Interestingly, short-term blockade of Notch signaling (3 days) in adult vSMC resulted in reduction of contractile-associated genes (Figure 8A, top panel). For sake of comparison, we focused (in Figure 8) on the subset of genes shown to change upon Jag1 deletion at E14.5 (Figure 3). Naturally given the distinct developmental stages (embryonic and adult) a few genes were excluded as they were below the acceptable range. Also considering the short-term Notch1 blockade (3 days), only the genes most sensitive to Notch suppression responded while others were unaffected. Even with these limitations, we observed increase in the levels of Sox9 , Trpv4, Mbnl1 and Rpgrip1l, genes involved in skeletal development (Figure 8B, Top left panel) and previously found increased in embryonic J1SMKO vSMC (Figure 3). Further validation of qRT-PCR confirmed that Eln (Elastin), Acta2 and Tagln were decreased and Cnn1, and mir145 were following the same trend when Notch signaling was inhibited by DAPT treatment. In addition to increasing Trpv4 and Sox9 mRNA level, DAPT treatment led to a modest but significant increase in the sclerotome transcription factor Pax1 (Figure 8C). These findings prompted us to ascertain the effect of prolonged reduction of Jag1 in vivo using heterozygous adult animals. Although adults J1SMHTZ (aged from 7.5–12months) exhibited no obvious phenotype, qRT-PCR revealed a significant decrease in vSMC markers compared to J1WT (Figure 8D). Among the early markers of chondrogenesis only Trpv4 was detected at a reliable level. In addition, the osteogenic transcription factor Runx2 was also increased in J1SMHTZ compared to J1WT, a phenotype consistent with a predisposition to chondro/osteoblastic transdifferentiation. Histological evaluation of DA from adult J1SMHTZ mice showed an increase in vascular perimeter (Figure 8E,G) and decrease in vascular wall thickness and EEL-IEL distance in J1SMHTZ compared to the J1WT (Figure 8E–I). Furthermore, the average number of vSMC was decreased in the J1SMHTZ compared to the J1WT (Figure 8J). In accordance with these observations, RNA sequencing analysis of Jag1 homo- and heterozygous deletion revealed a transcriptional profile previously associated with development of aneurysms (in mouse models and human) (Figure 8K).
Figure 8. Reduction of Jag1 in adult vSMC is associated with downregulation of vSMC contractile genes and increase of pro-ossification genes.
A-C Immortalized vSMC isolated from adult DA were treated with DAPT (50µM) or DMSO vehicle control for 3 days. Transcriptional analysis was performed by RNA deep sequencing (n=3) to measure differential expression of genes classified in “cytoskeleton and contractile genes” (A) and “skeleton and cartilage development” (B). Relative row count for each transcript (with a RPKM>1) is represented following a pseudo-color scale. C- qRT-PCR analysis confirmed a decrease in vSMC markers (Acta2, Tagln, Cnn1, mir145 and Eln) whereas Trpv4 and the cartilage transcription factors, Pax1 and Sox9, were induced in the presence of DAPT (nDMSO=5–6; nDAPT=6). D- mRNA isolated from adult DA from J1WT (n=6) and J1SMHTZ (n=5) mice was evaluated by qRT-PCR for the indicated genes. Hemizygote deletion of Jag1 in vSMC results in a decrease in vSMC markers mRNA level while Runx2 transcripts was increased. E–F H&E staining (E) and Elastin (ELA; green, F) of DA from adult mice showed a non circumferential decrease of the vascular wall thickness (brackets) in J1SMHTZ compared to J1WT. Scale Bars: 100µm(E); 40µm(F). G- Measurement of adult DA revealed a slight but significant increase in the perimeter in J1SMHTZ (n=3 mice, 20 sections) compared to J1WT (n=4 mice, 20 sections). H–J- Cross-sections of aortae were divided into quadrants of 20000 pixels scare and the thickness of the vascular wall (H), the distance between EEL and IEL (I) and he number of αSMA+ layers (J) was measured in each quadrant. J1WT (n=4 mice, 20 sections (H); 8 sections (I–J)); J1SMHTZ (n=3 mice, 20 sections (H); 6 sections (I–J)). Data are represented as mean +/− SEM. *p<0.05; **p<0.001; ***p<0.0005; ****p<0.0001. K- Differential expression analysis by RNA sequencing in E14.5 vSMC from J1WT, J1SMHTZ and J1SMKO mice showed that decrease in Jag1 expression led to deregulation of genes that have been associated and causative in aneurysm onset in humans and mouse models. L- Schema summarizing the role of Jag1/Notch signaling in the acquisition and maintenance of vSMC contractile phenotype in the descending aorta.
Discussion
It is well accepted that failure to acquire and/or maintain vSMC contractile properties results in life threatening vascular disorders. Among these defects, low expression of contractile proteins predisposes to aneurysms and aortic dissection (Ailawadi et al., 2009; Guo et al., 2007; Lindsay and Dietz, 2011; Pannu et al., 2007). In addition, several mouse models have shown that vascular calcification can be the outcome of vSMC transdifferentiation into cartilage and subsequent endochondral ossification (Galvin et al., 2000; Speer et al., 2009). Thus, the shared sclerotomal origin of cartilage and vSMC is particularly relevant when trying to clarify the etiology of vascular wall pathologies associated with the differentiation status of vSMC. Moreover, because vSMC have been shown to display phenotypical plasticity in adults (Owens et al., 2004), there is a critical need to elucidate how the transcriptional machinery in this cell type is regulated and potentially altered upon pathology. Here, we showed that Notch is an essential requirement for enabling maturation and maintenance of a functional vSMC phenotype through silencing the chondro-osteogenic fate of these sclerotome-originating cells. We demonstrated in vivo and in vitro that reduction of Jag1 alone is sufficient to impose a reprogramming cascade that predisposes the vascular wall to aneurysms and that, at least in the embryo, enables full transdifferentiation towards the chondrogenic lineage that could lead to vascular calcification.
The contribution of Notch signaling to vascular morphogenesis has been extensively evaluated in relation to endothelial sprouting and vSMC recruitment (Gridley, 2010). In fact, removal of Jag1 from endothelial cells prevents recruitment of vSMC to the vessel wall and results in demise at mid-gestation (Benedito et al., 2009; High et al., 2008). Notch is also required for assembly of the vascular wall through the propagation of pro-maturation signals in vSMC from the aortic arch, in the ductus arteriosus and adjacent DA (Feng et al., 2010; Manderfield et al., 2012). We also found similar results in our mouse model of Jag1 inactivation in specified vSMC. In the absence of Jag1 after E9.5 (a time where the Sm22-Cre is active), vSMC in the DA are recruited but failed to express and maintain sufficient levels of contractile proteins. The immediate conclusion is that Notch promotes differentiation of vSMC. However, Notch alone is unable to trigger differentiation of progenitors into vSMC, indicating the presence of an elaborate hierarchy of operative transcription factors.
Unraveling the multiple and often opposing function of Notch in vSMC differentiation has been, in fact, the focus of many studies in vitro, which have both supported (Doi et al., 2006; Fischer et al., 2004; Noseda et al., 2006; Wang et al., 2012) and negated (Morrow et al., 2005; Proweller et al., 2005; Tang et al., 2008) a presumed role for this signaling pathway in vSMC differentiation. The conflicting in vitro findings are likely due to possible differences in the selected cellular platform. Thus the epigenetic context and/or operational transcriptional machinery in the cell at the time of Notch activation were likely important culprits in the outcome (Borggrefe and Liefke, 2012).
By deleting the most prevalent ligand in the vascular wall in vivo (Jag1), our experimental model impacted all responsive Notch receptors that are likely to play a role in vSMC maturation and maintenance. This approach is important because the individual contribution of Notch receptors in vSMC biology is not completely understood, despite their impact to vascular disease. For example, dominant negative mutation of NOTCH3 leads to arteriopathy, characterized by progressive loss of vSMC that primarily affects the brain in patients with CADASIL syndrome (Ruchoux et al., 1995). In fact, Notch3 has been shown to play a critical role in arterial differentiation and postnatal maturation of vSMC (Wang et al., 2008). Although this receptor is predominant in the wall of the DA during development and after birth, CADASIL syndrome mainly affects small arteries and the Notch3−/− mice do not exhibit embryonic defects or morphological changes in the DA (Domenga et al., 2004). This suggests a role for other receptors that either compensate and/or contribute in alternative ways to the maturation and maintenance of the arterial wall in large arteries.
Even after complete differentiation, vSMC retain the ability to reversibly change their phenotype depending upon environmental, physiological and/or genetic cues (Alexander and Owens, 2012; Owens et al., 2004). This plasticity has been frequently associated with the contractile and synthetic phenotypes exhibited by this cell. However, vSMC have also been shown to transdifferentiate into chondrocytes, indicating that this plasticity extends lineage boundaries. For example, inactivation of Mgp or Madh6 (encoding Smad6) has resulted in the formation of cartilage in the vessel wall (Galvin et al., 2000; Speer et al., 2009). Intriguingly, when transdifferentiation occurs, the outcome is restricted to some specific lineages: chondrocytic, osteoblastic or fibroblastic-like (loss of contractile function). This choice in fates is particularly pertinent given that vSMC from the DA arise from sclerotomal progenitors (Pouget et al., 2008). Indicating that in fact, vSMC have the ability to revert back, or to reprogram into a progenitor state and, depending on the cues, select an alternative fate. Our findings indicate that sustained Notch signaling (likely Notch1 and Notch3) is required after E14 to prevent dedifferentiation and retain vSMC maturation and function.
Curiously, our data suggest that activation of Notch by Jag1 alone is not sufficient to trigger vSMC gene expression in the absence of additional transcription factors. In this regards, Notch signaling may act as “enabler” rather than inducer. Specifically we found that between E13 and E14.5 Notch permits the establishment of the vSMC phenotype by repressing other fates through the regulation of Sox9, Pax1 and Scx transcript level but also regulation of Sox9 activity on its target genes Col2a1 and Sox6. In fact in chondrocytes, RBP-J/NICD complex binds and represses Sox9 transcription (Chen et al., 2013). Furthermore, the Notch target genes Hey1 and Hes1 bind and repress the Col2a1 promoter through competition with Sox9 (Grogan et al., 2008). Thus the ability of Sox9 to induce chondrogenesis while in the presence of Notch signaling is likely limited.
Whereas the essential requirement of Sox9 for chondro-osteogenesis has been extensively studied, its role in vSMC biology remained to be clarified in vivo. It has been proposed that Sox9 can sequester myocardin and reduce SRF activity on target genes (Xu et al., 2012). This finding was also supported by the fact that in progenitors, expression of myocardin (Myocd) or SRF alone is unable to promote full differentiation of vSMCs (Alexander and Owens, 2012; Parmacek, 2004). Thus it appears that correct and temporally adequate combination of transcription factors, including Notch activation is required for vSMC specification, but its role is not restricted to development. In fact, it was recently reported that during healing of fractures, a population of αSMA+ progenitors increased expression of chondro-osteoblast markers while losing expression of components of Notch pathway. In this population, sustained activation of Notch signaling blocked their conversion toward the chondro-osteo lineage (Matthews et al., 2014). Based on others and our findings in the development of the aorta, we propose that repression of Sox9 by Notch signaling affects vSMC at two levels: 1- it enables the acquisition and maintenance of vSMC phenotype; 2- it blocks chondro-osteo fate emergence.
Although J1SMKO mice died shortly after birth, J1SMHTZ mice were viable. Because J1SMHTZ vSMC presented with an intermediate transcriptional profile, we suspected that prolonged hemizygous deletion of Jag1 would affect the contractile properties of the vascular wall. In fact, J1SMHTZ aged mice presented with a mild decrease in vascular wall thickness, but more importantly a decrease in contractile genes, indicating a profile that would favor aneurysms. In accordance with this finding RNA deep sequencing revealed that a great number of genes previously associated with aneurysms (in human and mouse) were deregulated in hemi- or homozygous deletion of Jag1. Furthermore, using the data from a whole genome expression profiling on human abdominal aortic aneurysm (AAA) generated by Kuivaniemi’s group (Lenk et al., 2007), we observed that Jag1 expression was consistently downregulated by 2.5 to 11 fold in AAA compared to aorta without aneurysms. Finally, partial deletion of Jag1 in adult J1SMHTZ showed an increase in the osteogenic factor Runx2 (Figure 8) that may reflect a higher susceptibility to vascular calcification. This is supported by studies showing that defect in Notch expression and signaling results in aortic valve calcification associated with chondro-osteogenic intermediate stage in adults animals (Hofmann et al., 2012; Nus et al., 2011).
Our attempts to completely remove Jag1 in adult vSMC in vitro resulted in cell demise, emphasizing the need for continuous Notch signaling in adult cells and consistent with the CADASIL phenotype where Notch3 is lost. Short-term suppression of Notch by DAPT, resulted in a decrease of contractile genes and increase in Sox9. Altogether these data suggest that, even in adult vSMC, temporal decreases in Notch signaling affect, albeit partially vSMC specification.
To summarize, we showed that Notch signaling through Jag1 in the descending aorta is primarily required to repress reprogramming of vSMCs toward other sclerotomal lineages. This switch in commitment happens between E13 and E14.5 and allows appropriate maturation of vSMC (Figure 8L). We can then propose that by imposing a constant pressure on vSMC fate maintenance, Notch signaling is not only a critical contributor in the prevention of aortic aneurysms, but also a potent repressor of osteoendochondral vascular calcification.
Experimental procedures
Mice and Cells
SM22-Cre Jag1lox/lox transgenic mice crossed to the reporter line Rosa26R LacZ where previously described (Hofmann et al., 2010). Please note that in the mixed phenotype these mice died 7–15 days post-natally exhibiting jaundice and a liver phenotype (Hofmann et al., 2010). In the C57Bl genotype, lethality occurred shortly after birth (1–2 days) from abdominal hemorrhage (this work). The liver phenotype described previously was still present in this mouse model (Figure S4F). Misexpression of Sox9 was obtained by crossing previously described Sm22-Cre males (Hofmann et al., 2010) with females homozygous for the Hprt<tm1(CAG-Sox9,-EGFP)Akis allele (synonym: HprtSox9lox/lox) expressing Hprt-Sox9 allele (Airik et al., 2010). Embryos were collected using timed mating.
Studies were conducted in accordance with UCLA Department of Laboratory Animal Medicine’s Animal Research Committee guidelines.
Embryonic vascular smooth muscle cells isolation and culture: Embryos were harvested by C-section at E14.5 and the DA was removed and plated in tissue culture dish with DMEM (GIBCO, Life technology, Grand Island, NY) containing 10%FBS and 1% antibiotics. Cells were allowed to grow out of the explants and after several days trypsinized for subculture (Figure S1G–J). Differential plating of 5 minutes was performed to separate fast attaching cells (mostly fibroblasts and few vSMC) from vSMC (suspension after 5min) that were plated in a separate dish at much lower density. The purity of the vSMC culture was confirmed by immunodetection of αSMA and specific Sm22-driven excision of Jag1 which was determined to be around 99% in the null cells (Figure S1I–J and Figure 3 and Figure S2 respectively). Further enrichment of the culture in vSMC was confirmed by quantification of markers from various vascular cell types (see RNA sequencing; Figure S2B)
For high density cultures, vSMC were cultured at 25 millions cells per ml in DMEM supplemented with 10%FBS and 1%antibiotics. After 10 days the cells were fixed and stained with 1% Alcian Blue 8GX or 2% Alizarin Red S solution, or harvested for Western Blot analysis.
Immortalized mouse smooth muscle cells (ImSMC) were isolated from Immortomice, CBA;B10-Tg(H2Kb-tsA58)6Kio/Crl, that were purchased from Charles River.
Transcriptional analysis
RNA, cDNA synthesis and transcriptional analysis of cells or aortae are detailed in Supplemental Experimental Procedures.
Jag1Fc stimulation and γ-secretase treatment
Chimeric Rat Jag1 -Human Fc (R&D system, Minneapolis, MN) or control Fc (Jackson Immunoresearch, West Grove, PA) were used to coat (20µg/ml in sterile PBS) 12-wells plates. Mouse embryonic fibroblast from E13 C57Bl/6 mice, grown in DMEM (GIBCO, Life Technology, Grand Island, NY) containing 10%FBS and 1% antibiotics, were then plated onto the recombinant protein in the absence or presence of DMSO (vehicle control) or γ-secretase inhibitor (DAPT, 50µM; Calbiochem, EMD Biosciences, San Diego, CA) for 24hours. J1WT E14.5 vSMCs were similarly treated with DMSO or DAPT for 8 to 48 hours.
Immunostaining and histological analysis
Tissue samples embedded in paraffin were used for histological examination. Primary antibodies used for immunostaining and detailed protocols are described in Supplemental Experimental Procedure.
Knock-down experiments
J1WT E14.5 vSMCs were transfected with 40pmol of siRNA mixture targeting Jag1 (sc-37203), Notch3 (sc-37136), Sox9 (sc-36534) or control scramble (sc-37007) (Santa-Cruz Biotechnology, Santa Cruz, CA) using HiPerfect transfection agent (QIAGEN, Valencia, CA). For efficient and more stable knock-down, two consecutive transfections were done. The first transfection was performed in suspension and the second the following day on plated cells. Total RNA was then isolated 24 to 72 hours after the second transfection for further analysis.
RNA sequencing and differential expression analysis
RNAseq libraries were created from total RNA from E14.5 purified vSMCs or ImSMC treated with DAPT (50µM, 3 days) respectively with the Truseq RNA sample prep kit (Illumina, San Diego, CA) and sequenced using the Illumina Hiseq 2000 platform. Analysis is detailed in Supplemental Experimental Procedure.
Western blot analysis
Cells were lysed in mRIPA buffer containing 1% Triton X-100 and 10% SDS or nuclear fraction was separated using the Nuclear Complex Co-IP kit (Active Motif, Carlsbad, CA). Western blot analysis and antibodies used are detailed in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
The authors wish to thank Michelle Steel, Marianne Ibrahim and Jun Woo Ha for technical assistance; the contribution of the Tissue Procurement Core Laboratory Shared Resource at UCLA; and Freddy Radke for kindly providing the Jag1lox mouse. This study was supported by funds from the Leducq Foundation (Artemis Grant # FLQ 09CVD02) and the NIH RO1 HL085618 and 114086 (to MLIA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Codes
The data sets for RNA sequencing analysis have been deposited in the NCBI Gene Expression Omnibus (GEO) under accession number GSE60643 for the SuperSeries and GSE60641 and GSE60642 for eSMC and ImSMC subseries respectively.
The authors have no conflicting financial interest.
References
- Ailawadi G, Moehle CW, Pei H, Walton SP, Yang Z, Kron IL, Lau CL, Owens GK. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. The Journal of thoracic and cardiovascular surgery. 2009;138:1392–1399. doi: 10.1016/j.jtcvs.2009.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airik R, Trowe MO, Foik A, Farin HF, Petry M, Schuster-Gossler K, Schweizer M, Scherer G, Kist R, Kispert A. Hydroureternephrosis due to loss of Sox9-regulated smooth muscle cell differentiation of the ureteric mesenchyme. Human molecular genetics. 2010;19:4918–4929. doi: 10.1093/hmg/ddq426. [DOI] [PubMed] [Google Scholar]
- Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annual review of physiology. 2012;74:13–40. doi: 10.1146/annurev-physiol-012110-142315. [DOI] [PubMed] [Google Scholar]
- Bell DM, Leung KK, Wheatley SC, Ng LJ, Zhou S, Ling KW, Sham MH, Koopman P, Tam PP, Cheah KS. SOX9 directly regulates the type-II collagen gene. Nature genetics. 1997;16:174–178. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nature genetics. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- Borggrefe T, Liefke R. Fine-tuning of the intracellular canonical Notch signaling pathway. Cell Cycle. 2012;11:264–276. doi: 10.4161/cc.11.2.18995. [DOI] [PubMed] [Google Scholar]
- Brent AE, Tabin CJ. Developmental regulation of somite derivatives: muscle, cartilage and tendon. Current opinion in genetics & development. 2002;12:548–557. doi: 10.1016/s0959-437x(02)00339-8. [DOI] [PubMed] [Google Scholar]
- Chen S, Tao J, Bae Y, Jiang MM, Bertin T, Chen Y, Yang T, Lee B. Notch gain of function inhibits chondrocyte differentiation via Rbpj-dependent suppression of Sox9. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013;28:649–659. doi: 10.1002/jbmr.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBakey ME, Glaeser DH. Patterns of atherosclerosis: effect of risk factors on recurrence and survival-analysis of 11,890 cases with more than 25-year follow-up. The American journal of cardiology. 2000;85:1045–1053. doi: 10.1016/s0002-9149(00)00694-9. [DOI] [PubMed] [Google Scholar]
- Doi H, Iso T, Sato H, Yamazaki M, Matsui H, Tanaka T, Manabe I, Arai M, Nagai R, Kurabayashi M. Jagged1-selective notch signaling induces smooth muscle differentiation via a RBP-Jkappa-dependent pathway. The Journal of biological chemistry. 2006;281:28555–28564. doi: 10.1074/jbc.M602749200. [DOI] [PubMed] [Google Scholar]
- Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, Klonjkowski B, Berrou E, Mericskay M, Li Z, et al. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes & development. 2004;18:2730–2735. doi: 10.1101/gad.308904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Krebs LT, Gridley T. Patent ductus arteriosus in mice with smooth muscle-specific Jag1 deletion. Development. 2010;137:4191–4199. doi: 10.1242/dev.052043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes & development. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumatsu T, Shukunami C, Amemiya-Kudo M, Shimano H, Ozaki T. Scleraxis and E47 cooperatively regulate the Sox9-dependent transcription. The international journal of biochemistry & cell biology. 2010;42:148–156. doi: 10.1016/j.biocel.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Gadson PF, Jr., Dalton ML, Patterson E, Svoboda DD, Hutchinson L, Schram D, Rosenquist TH. Differential response of mesoderm- and neural crest-derived smooth muscle to TGF-beta1: regulation of c-myb and alpha1 (I) procollagen genes. Experimental cell research. 1997;230:169–180. doi: 10.1006/excr.1996.3398. [DOI] [PubMed] [Google Scholar]
- Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA, Jr., et al. A role for smad6 in development and homeostasis of the cardiovascular system. Nature genetics. 2000;24:171–174. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- Gridley T. Notch signaling in the vasculature. Current topics in developmental biology. 2010;92:277–309. doi: 10.1016/S0070-2153(10)92009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan SP, Olee T, Hiraoka K, Lotz MK. Repression of chondrogenesis through binding of notch signaling proteins HES-1 and HEY-1 to N-box domains in the COL2A1 enhancer site. Arthritis and rheumatism. 2008;58:2754–2763. doi: 10.1002/art.23730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nature genetics. 2007;39:1488–1493. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- High FA, Lu MM, Pear WS, Loomes KM, Kaestner KH, Epstein JA. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development; Proceedings of the National Academy of Sciences of the United States of America; 2008. pp. 1955–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High FA, Zhang M, Proweller A, Tu L, Parmacek MS, Pear WS, Epstein JA. An essential role for Notch in neural crest during cardiovascular development and smooth muscle differentiation. The Journal of clinical investigation. 2007;117:353–363. doi: 10.1172/JCI30070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann JJ, Briot A, Enciso J, Zovein AC, Ren S, Zhang ZW, Radtke F, Simons M, Wang Y, Iruela-Arispe ML. Endothelial deletion of murine Jag1 leads to valve calcification and congenital heart defects associated with Alagille syndrome. Development. 2012;139:4449–4460. doi: 10.1242/dev.084871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann JJ, Zovein AC, Koh H, Radtke F, Weinmaster G, Iruela-Arispe ML. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development. 2010;137:4061–4072. doi: 10.1242/dev.052118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk GM, Tromp G, Weinsheimer S, Gatalica Z, Berguer R, Kuivaniemi H. Whole genome expression profiling reveals a significant role for immune function in human abdominal aortic aneurysms. BMC genomics. 2007;8:237. doi: 10.1186/1471-2164-8-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature. 2011;473:308–316. doi: 10.1038/nature10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky MW. Developmental basis of vascular smooth muscle diversity. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- Manderfield LJ, High FA, Engleka KA, Liu F, Li L, Rentschler S, Epstein JA. Notch activation of Jagged1 contributes to the assembly of the arterial wall. Circulation. 2012;125:314–323. doi: 10.1161/CIRCULATIONAHA.111.047159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews BG, Grcevic D, Wang L, Hagiwara Y, Roguljic H, Joshi P, Shin DG, Adams DJ, Kalajzic I. Analysis of alphaSMA-labeled progenitor cell commitment identifies notch signaling as an important pathway in fracture healing. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29:1283–1294. doi: 10.1002/jbmr.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead TJ, Yutzey KE. Notch signaling and the developing skeleton. Advances in experimental medicine and biology. 2012;727:114–130. doi: 10.1007/978-1-4614-0899-4_9. [DOI] [PubMed] [Google Scholar]
- Miano JM, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. American journal of physiology Cell physiology. 2007;292:C70–C81. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- Morrow D, Scheller A, Birney YA, Sweeney C, Guha S, Cummins PM, Murphy R, Walls D, Redmond EM, Cahill PA. Notch-mediated CBF-1/RBP-J{kappa}-dependent regulation of human vascular smooth muscle cell phenotype in vitro. American journal of physiology Cell physiology. 2005;289:C1188–C1196. doi: 10.1152/ajpcell.00198.2005. [DOI] [PubMed] [Google Scholar]
- Noseda M, Fu Y, Niessen K, Wong F, Chang L, McLean G, Karsan A. Smooth Muscle alpha-actin is a direct target of Notch/CSL. Circulation research. 2006;98:1468–1470. doi: 10.1161/01.RES.0000229683.81357.26. [DOI] [PubMed] [Google Scholar]
- Nus M, MacGrogan D, Martinez-Poveda B, Benito Y, Casanova JC, Fernandez-Aviles F, Bermejo J, de la Pompa JL. Diet-induced aortic valve disease in mice haploinsufficient for the Notch pathway effector RBPJK/CSL. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:1580–1588. doi: 10.1161/ATVBAHA.111.227561. [DOI] [PubMed] [Google Scholar]
- Owens AP, 3rd, Subramanian V, Moorleghen JJ, Guo Z, McNamara CA, Cassis LA, Daugherty A. Angiotensin II induces a region-specific hyperplasia of the ascending aorta through regulation of inhibitor of differentiation 3. Circulation research. 2010;106:611–619. doi: 10.1161/CIRCRESAHA.109.212837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiological reviews. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Pannu H, Tran-Fadulu V, Papke CL, Scherer S, Liu Y, Presley C, Guo D, Estrera AL, Safi HJ, Brasier AR, et al. MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Human molecular genetics. 2007;16:2453–2462. doi: 10.1093/hmg/ddm201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmacek MS. Myocardin--not quite MyoD. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1535–1537. doi: 10.1161/01.ATV.0000141044.03875.7f. [DOI] [PubMed] [Google Scholar]
- Pouget C, Pottin K, Jaffredo T. Sclerotomal origin of vascular smooth muscle cells and pericytes in the embryo. Developmental biology. 2008;315:437–447. doi: 10.1016/j.ydbio.2007.12.045. [DOI] [PubMed] [Google Scholar]
- Proweller A, Pear WS, Parmacek MS. Notch signaling represses myocardin-induced smooth muscle cell differentiation. The Journal of biological chemistry. 2005;280:8994–9004. doi: 10.1074/jbc.M413316200. [DOI] [PubMed] [Google Scholar]
- Ruchoux MM, Guerouaou D, Vandenhaute B, Pruvo JP, Vermersch P, Leys D. Systemic vascular smooth muscle cell impairment in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Acta neuropathologica. 1995;89:500–512. doi: 10.1007/BF00571504. [DOI] [PubMed] [Google Scholar]
- Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circulation research. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Urs S, Liaw L. Hairy-related transcription factors inhibit Notch-induced smooth muscle alpha-actin expression by interfering with Notch intracellular domain/CBF-1 complex interaction with the CBF-1-binding site. Circulation research. 2008;102:661–668. doi: 10.1161/CIRCRESAHA.107.165134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topouzis S, Majesky MW. Smooth muscle lineage diversity in the chick embryo. Two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-beta. Developmental biology. 1996;178:430–445. doi: 10.1006/dbio.1996.0229. [DOI] [PubMed] [Google Scholar]
- Turlo KA, Noel OD, Vora R, LaRussa M, Fassler R, Hall-Glenn F, Iruela-Arispe ML. An essential requirement for beta1 integrin in the assembly of extracellular matrix proteins within the vascular wall. Developmental biology. 2012;365:23–35. doi: 10.1016/j.ydbio.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhao N, Kennard S, Lilly B. Notch2 and Notch3 function together to regulate vascular smooth muscle development. PloS one. 2012;7:e37365. doi: 10.1371/journal.pone.0037365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Baron M, Trump D. An overview of Notch3 function in vascular smooth muscle cells. Progress in biophysics and molecular biology. 2008;96:499–509. doi: 10.1016/j.pbiomolbio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature. 2004;428:185–189. doi: 10.1038/nature02382. [DOI] [PubMed] [Google Scholar]
- Wasteson P, Johansson BR, Jukkola T, Breuer S, Akyurek LM, Partanen J, Lindahl P. Developmental origin of smooth muscle cells in the descending aorta in mice. Development. 2008;135:1823–1832. doi: 10.1242/dev.020958. [DOI] [PubMed] [Google Scholar]
- Xu Z, Ji G, Shen J, Wang X, Zhou J, Li L. SOX9 and myocardin counteract each other in regulating vascular smooth muscle cell differentiation. Biochemical and biophysical research communications. 2012;422:285–290. doi: 10.1016/j.bbrc.2012.04.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Sinha S, Dandre F, Wamhoff BR, Hoofnagle MH, Kremer BE, Wang DZ, Olson EN, Owens GK. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circulation research. 2003;92:856–864. doi: 10.1161/01.RES.0000068405.49081.09. [DOI] [PubMed] [Google Scholar]
- Zeng L, Kempf H, Murtaugh LC, Sato ME, Lassar AB. Shh establishes an Nkx3.2/Sox9 autoregulatory loop that is maintained by BMP signals to induce somitic chondrogenesis. Genes & development. 2002;16:1990–2005. doi: 10.1101/gad.1008002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.