Abstract
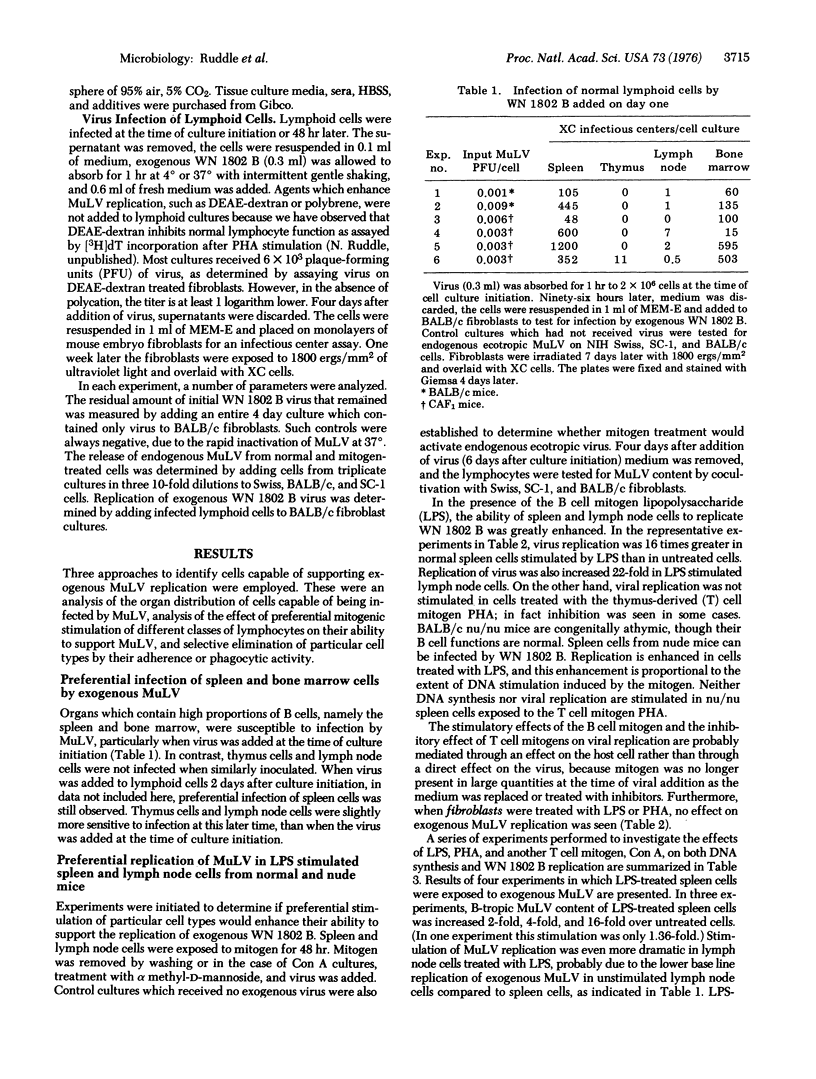
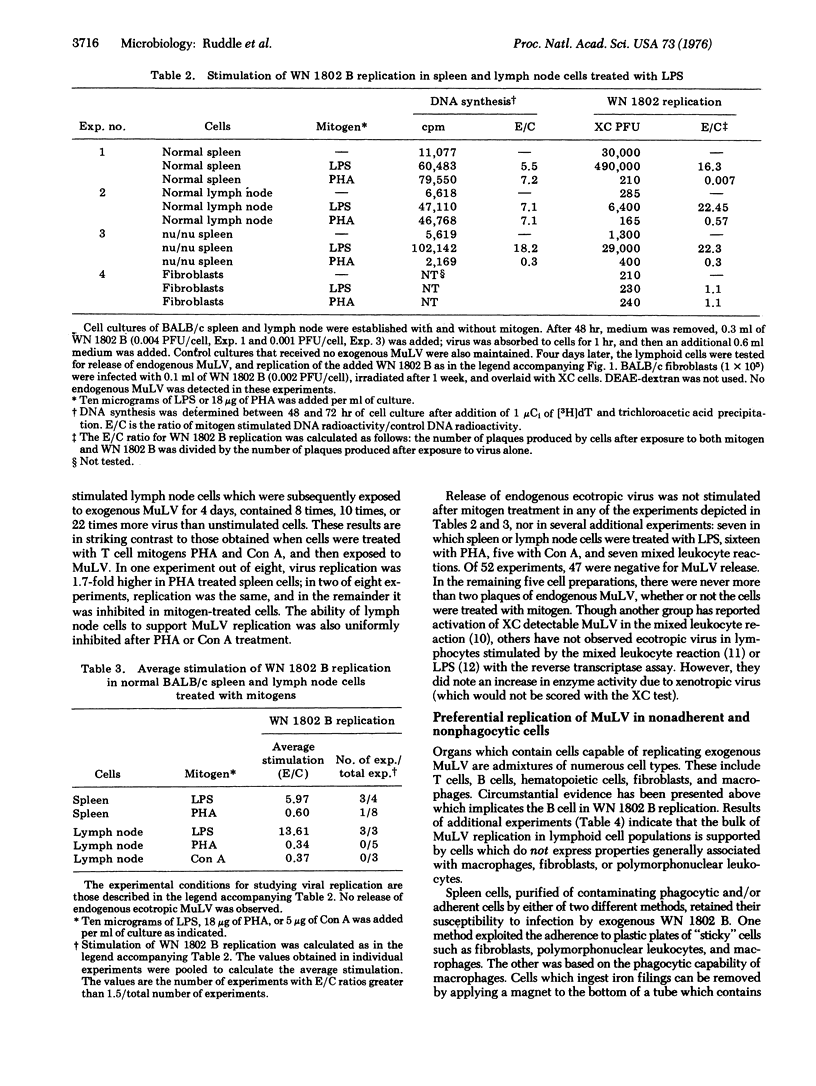
Murine lymphoid cells were infected in vitro with WN 1802 B, a naturally occurring murine leukemia virus isolated from the spleen of an 18-month-old BALB/c mouse. Normal spleen and bone marrow cells were more susceptible to infection than were cells prepared from thymus and lymph node. Spleen cells from athymic nu/nu mice also could be readily infected with virus. Permissive cells did not ingest iron readily infected with virus. Permissive cells did not ingest iron filings and did not adhere to plastic. Exogenous replication of murine leukemia virus was enhanced in spleen and lymph node cells treated with lipopolysaccharide, a bone marrow-derived lymphocyte mitogen. Conversely, cells treated with the thymus-derived lymphocyte cell mitogens, phytohemagglutinin and concanavalin A, were less capable of supporting murine leukemia virus replication. These studies suggest that the natural host for WN 1802 B is the bone marrow-derived lymphocyte.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong M. Y., Ruddle N. H., Lipman M. B., Richards F. F. Tumor induction by immunologically activated murine leukemia virus. J Exp Med. 1973 May 1;137(5):1163–1179. doi: 10.1084/jem.137.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerny J., Hensgen P. A., Fistel S. H., Demler L. M. Interactions of murine leukemia virus (MuLV) with isolated lymphocytes. II. Infections of B and T cells with Friend virus complex indiffusion chambers and in vitro: effect of polyclonal mitogens. Int J Cancer. 1976 Aug 15;18(2):189–196. doi: 10.1002/ijc.2910180208. [DOI] [PubMed] [Google Scholar]
- Cerny J., Waner E. B. Specific susceptibility of sensitized (memory) B cells to suppression and antigenic alteration by murine leukemia virus. J Immunol. 1975 Feb;114(2 Pt 1):571–580. [PubMed] [Google Scholar]
- Datta S. K., Melief C. J., Schwartz R. S. Lymphocytes and leukemia viruses: tropism and transtropism of murine leukemia virus. J Natl Cancer Inst. 1975 Aug;55(2):425–432. [PubMed] [Google Scholar]
- Epstein L. B., Kreth H. W., Herzenberg L. A. Fluorescence-activated cell sorting of human T and B lymphocytes. II. Identification of the cell type responsible for interferon production and cell proliferation in response to mitogens. Cell Immunol. 1974 Jun;12(3):407–421. doi: 10.1016/0008-8749(74)90097-5. [DOI] [PubMed] [Google Scholar]
- GROSS L. Viral etiology of spontaneous mouse leukemia; a review. Cancer Res. 1958 May;18(4):371–381. [PubMed] [Google Scholar]
- Haas M., Hilgers J. In vitro infection of lymphoid cells by thymotropic radiation leukemia virus grown in vitro. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3546–3550. doi: 10.1073/pnas.72.9.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. S., Phillips S. M., Solnik C., Black P. H., Schwartz R. S., Carpenter C. B. Activation of leukemia viruses by graft-versus-host and mixed lymphocyte reactions in vitro. Proc Natl Acad Sci U S A. 1972 May;69(5):1069–1072. doi: 10.1073/pnas.69.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R., Fraumeni J. F., Jr Risk of cancer in renal-transplant recipients. Lancet. 1973 Jul 14;2(7820):55–57. doi: 10.1016/s0140-6736(73)93256-x. [DOI] [PubMed] [Google Scholar]
- Ishizaki R., Bolognesi D. P. Reactivity of avian RNA tumor viruses with lectins. J Virol. 1975 Jan;17(1):132–139. doi: 10.1128/jvi.17.1.132-139.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Klein G. Surface markers on human B and T lymphocytes. II. Presence of Epstein-Barr virus receptors on B lymphocytes. J Exp Med. 1973 Dec 1;138(6):1365–1378. doi: 10.1084/jem.138.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano S., Bloom B. R., Howe M. L. Enumeration of activated thymus-derived lymphocytes by the virus plaque assay. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2299–2303. doi: 10.1073/pnas.70.8.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni C., Schumann G. Lipopolysaccharide induces C-type virus in short term cultures of BALB/c spleen cells. Nature. 1975 Mar 6;254(5495):60–61. doi: 10.1038/254060a0. [DOI] [PubMed] [Google Scholar]
- Olding L. B., Jensen F. C., Oldstone M. B. Pathogenesis of of cytomegalovirus infection. I. Activation of virus from bone marrow-derived lymphocytes by in vitro allogenic reaction. J Exp Med. 1975 Mar 1;141(3):561–572. doi: 10.1084/jem.141.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D., Scher C. D. In vitro transformation of lymphoid cells by Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1975 May;72(5):1932–1936. doi: 10.1073/pnas.72.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Ruddle N. H., Armstrong M. Y., Richards F. F. Tumor induction by immunologically activated murine leukemia virus: cellular immunity early in the graft-vs-host reaction. J Immunol. 1974 Feb;112(2):706–715. [PubMed] [Google Scholar]
- Temin H. M., Baltimore D. RNA-directed DNA synthesis and RNA tumor viruses. Adv Virus Res. 1972;17:129–186. doi: 10.1016/s0065-3527(08)60749-6. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Sherr C. J., Benveniste R. E., Lieber M. M., Melnick J. L. Type C viruses of baboons: isolation from normal cell cultures. Cell. 1974 May;2(1):55–61. doi: 10.1016/0092-8674(74)90008-7. [DOI] [PubMed] [Google Scholar]
- Wallen W. C., Neubauer R. H., Rabin H., Cicmanec J. L. Nonimmune rosette formation by lymphoma and leukemia cells from Herpesvirus saimiri-infected owl monkeys. J Natl Cancer Inst. 1973 Sep;51(3):967–975. doi: 10.1093/jnci/51.3.967. [DOI] [PubMed] [Google Scholar]


