Abstract
Objectives
The present study evaluates the effects of a 6-month treatment with an ACE-inhibitor (ie, fosinopril) on serum concentrations of total IGF-1 and IGF binding protein (IGFBP)-3 in older adults at high risk for cardiovascular disease.
Design
Data are from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors (TRAIN) study, a double-blind, crossover, randomized, placebo-controlled trial.
Setting
Participants were recruited from the communities of Winston Salem, NC, and Greensboro, NC.
Participants
Subjects ≥55 years old with high cardiovascular disease risk profile.
Intervention
The intervention consisted of 6-month administration of fosinopril vs. placebo.
Measurements
Serum concentrations of total IGF-1 and IGFBP-3 were measured in 100 participants of the TRAIN study at baseline, 6-month and 12-month follow-up visits. Differences in total IGF-1 and IGFBP-3 concentrations were assessed using two-sided paired t-tests.
Results
The mean age of participants (47% women) was 66.5 (standard deviation 7.2) years. Serum concentrations of total IGF-1 were significantly higher after 6-month treatment with fosinopril compared to placebo (203.73 ng/mL vs 194.24 ng/mL; p=0.02): After ACE-inhibitor intervention, significantly higher serum IGFBP-3 concentrations compared to controls (4308.81 ng/mL vs 4086.93 ng/mL; p=0.03) were also reported.
Conclusions
A six-month treatment with fosinopril increases systemic levels of total IGF-1 and IGFBP-3 in older adults with high cardiovascular risk profile. This may represent a potential biological explanation to the beneficial effects of ACE-inhibition on stroke, ischemic heart disease and insulin resistance.
Keywords: Angiotensin Converting Enzyme inhibitor, Insulin like growth factor 1, Insulin like growth factor binding protein 3, older adults
Introduction
Angiotensin converting enzyme (ACE) inhibitors have demonstrated to protect against cardiovascular events (1) and reduce the incidence of type II diabetes (2). However, the magnitude of the therapeutic effects of ACE-inhibitors outweighs that expected on the basis of their anti-hypertensive action (3). In this context, observational studies have reported increased serum concentrations of insulin growth factor (IGF)-1 (4) and IGF binding protein (IGFBP)-3 (5) among individuals treated with ACE-inhibitors. Moreover, studies in experimental animals have demonstrated that the infusion of angiotensin II is able to reduce circulating concentrations of IGF-1 independently of blood pressure changes (6).
IGF-1 has been proposed as an important vascular protective factor, and its decline may be involved in the onset and progression of atherosclerosis (7;8). In fact, it has been shown that circulating IGF-1 levels are inversely associated with an atherogenic lipid profile (7) and increased arterial plaque stability in a population of older adults (8).
IGF-1 is predominantly synthesized by the liver in response to the growth hormone. In the circulation, 90-95% of IGF-1 is bound to specific high-affinity binding proteins, with IGF binding protein-3 (IGFBP-3) being the most abundant in the serum. IGFBP-3 not only modulates IGF-1 bioactivity, but may also have additional IGF-independent effects (e.g., inhibition of cell growth (9) and induction of apoptosis (10)).
Prospective studies have demonstrated that increased systemic IGF-1 concentrations are associated with reduced risk of incident ischemic heart disease (11). Furthermore, serum concentrations of both IGF-1 and IGFBP-3 have been inversely associated with increased risk of ischemic stroke (12). However, only a few clinical trials have explored the effects of ACE-inhibition on IGF-1(13;14), with no data on IGFBP-3.
To test the hypothesis that treatment with ACE-inhibitors increases serum concentration of IGF-1 and IGFBP-3, we evaluated the effects of a 6-month treatment with fosinopril on systemic concentration of those two variables in older adults with high cardiovascular risk profile.
Methods
Data are from a random sample of 100 participants enrolled in the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors (TRAIN). The TRAIN study design has been previously described (15;16). Briefly, this is a double-blind, cross-over, randomized, placebo-controlled trial aimed at assessing the biological mechanisms by which ACE inhibition may improve clinical outcomes in older persons with high cardiovascular risk profile. To be included in the TRAIN study, participants had to meet at least one of the following criteria: 1) coronary heart disease, 2) peripheral vascular disease, 3) history of stroke (>6 months), 4) diabetes along with more than one other cardiovascular risk factor [e.g. impaired glucose tolerance, hypertension, hyperlipidemia, current smoking, obesity, microalbuminuria, or any evidence of previous vascular disease], and 5) evidence of other clinical or subclinical cardiovascular disease (same as in point 4). Exclusion criteria were the current use or a known hypersensitivity to ACE-inhibitors, a diagnosis of specific cardiovascular diseases (including previous myocardial infarction, ejection fraction <40%, syncopal episodes, planned cardiac surgery or angioplasty within 3 months), conditions that would have affected results of the trial (e.g. significant renal disease, life-threatening illness, recent surgical procedure), simultaneous enrollment in another experimental drug trial, or plans to leave the area in the next 3 months.
Potential participants were first screened by a phone interview for eligibility. A clinical prescreening visit, aimed at reviewing medical history and records of potential participants was then arranged for those participants who successfully completed the phone interview. Subjects who successfully completed the prescreening visit were entered in a screening and single-blind run-in phase, in which compliance and tolerability of the ACE inhibitor were evaluated. The participants who successfully completed all of the preliminary phase interviews and visits were entered in the trial.
At the baseline visit, half of the eligible participants were randomized to receive fosinopril (10 mg once a day for one week, then 20 mg once a day for two to four weeks), and half to matching placebo. After the first follow-up visit (one month) the dose of study drug was increased to 40 mg once a day for additional 5 months. Participants who did not tolerate the 20 or 40 mg dosage received the higher tolerated dosage. Compliance with study intervention was measured by counting the pills left over in the container of prescription medicines and by asking the participants about the frequency of use. After 6 months and 3 mid-term visits, the cross-over occurred (those who received fosinopril received placebo, and vice versa). After further 6 months and 3 mid-term visits, participants made close-out visits.
Blood drawns by venipuncture were performed at three different time points: baseline, cross-over, and close-out visits. Blood drawns were performed in the morning, after having participants fasting for at least six hours to minimize the circadian fluctuation in the biomarkers concentrations. Blood samples were maintained in ice and processed within one hour from collection, aliquoted, and stored at −70°C until analysis. Serum concentrations of total IGF-1 and IGFBP-3 were measured in duplicate using a high-sensitivity enzyme-linked immunosorbent assay (ELISA) kits (Diagnostic Systems Laboratories Corporation, Webster, Texas). For total IGF-1, the minimum detection limit was 0.01 ng/ml; intra- and inter-assay coefficients of variation were 2.58% and 5.86% respectively. For IGFBP-3, the minimum detection limit was 0.04 ng/ml; intra- and inter-assay coefficients of variation were 2.63% and 2.58%, respectively.
Statistical analysis
Since all participants received both the study intervention and the placebo, each subject served as his/her own control, thus permitting to avoid adjusted analyses for potential confounders. Differences in total IGF-1 and IGFBP-3 serum concentrations according to the study intervention were assessed using two-sided paired t-tests. A p value <0.05 was considered as statistically significant. All analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, IL).
This trial is registered at www.clinicaltrials.gov with the identifier NCT00051389.
Results
The mean age of the sample population (n=100, women 47 %) was 66.5 (standard deviation, SD 7.2) years. The study sample was composed for 77% by Caucasians, for 22% by African-Americans, and for 1% by Asians. The prevalence (of the main clinical conditions in decreasing order) of the study sample was: hypertension (57%), diabetes (25%), history of cancer (17%), angina (10%), stroke (8%). Ten percent of the subjects were current smokers. Total IGF-1 concentrations ranged from 91.96 to 449.95 ng/ml [mean: 200.35, SD: 64.51] and IGFBP-3 concentrations ranged from 2151.72 to 6868.20 ng/ml [mean: 4150.78, SD: 1040.03].
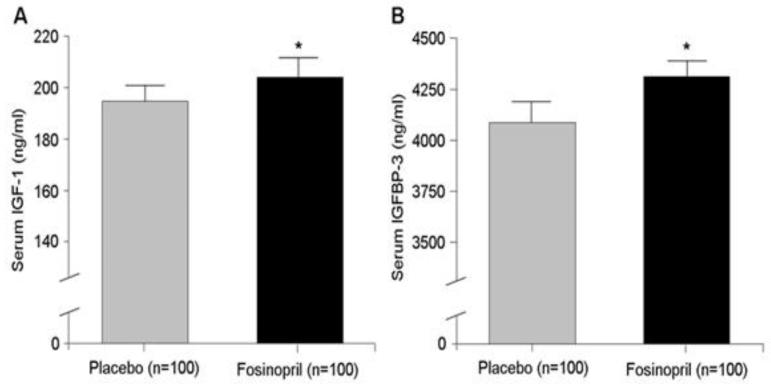
As shown in Figure 1, mean serum concentration of total IGF-1 was significantly higher after 6 months of treatment with fosinopril compared to the placebo (203.73 ng/ml vs. 194.24 ng/ml; p= 0.02). A significantly higher mean concentration of IGFBP-3 after fosinopril intervention compared to placebo (4308.81 ng/ml vs. 4086.93 ng/ml; p= 0.03) was also observed.
Figure 1.
A. Serum concentration of total IGF-1 is significantly higher after 6-month treatment with fosinopril (p=0.02) B. Serum concentration of IGFBP-3 is significantly higher after 6-month treatment
After restricting the study sample to participants with a minimum of 80% compliance to the intervention, consistent results were obtained for both total IGF-1 (205.50 ng/ml vs. 195.35 ng/ml; p= 0.02) and IGFBP-3 (4318.82 ng/ml vs. 4129.80 ng/ml; p= 0.07). Results were confirmed when analysis only considered participants receiving the highest dosage of fosinopril (≥ 40mg; total IGF-1 concentrations: fosinopril 205.51 ng/ml vs. placebo 195.57 ng/ml; p= 0.02; IGFBP-3 concentrations: fosinopril 4301.47 ng/ml vs. placebo 4145.42 ng/ml; p= 0.16).
Discussion
To our knowledge, this is the first clinical controlled trial evaluating the effects of an ACE inhibitor (i.e. fosinopril) on both serum IGF-1 and IGFBP-3 concentrations in a relatively large sample of community-dwelling older adults with high cardiovascular risk profile. Our findings show a statistically significant increase in total IGF-1 and IGFBP-3 serum concentrations following the ACE-inhibitor intervention.
Previous studies have reported a possible positive effect of ACE-inhibitors on the IGF-1 axis (4;5;13;17). For example, Corbalan and colleagues (13) in an uncontrolled trial conducted in nine older adults with chronic heart failure showed that an eight-week therapy with enalapril significantly increased plasma IGF-1 concentrations. Consistently, Anwar and colleagues (17) reported a positive association between ACE-inhibition and serum total IGF-1 concentrations in hospitalized patients with congestive heart failure. Moreover, cross-sectional studies also reported higher concentrations of total IGF-1(4) and IGFBP-3 (5) in community-dwelling older persons using ACE-inhibitors.
It is possible that the beneficial effects of ACE inhibition might, at least partly, be due to its influence on endothelial function. In fact, in vitro studies have demonstrated that IGF-1 is able to induce a dose-dependent increase of nitric oxide production through the activation of a constitutive form of nitric oxide synthase (18). However, this hypothesis needs further testing, also considering that a previous study of us showed no significant effect of fosinopril on two biomarkers of endothelial function (i.e. endothelin-1, vascular cell adhesion molecule-1) (16).
An important issue that should be considered is the magnitude of changes in serum levels of total IGF-1 and IGFBP-3 necessary to positively affect clinical outcomes. In our study, we documented a systemic total IGF-1 concentrations increase of 9.5 ng/ml and a systemic IGFBP-3 concentrations increase of 222 ng/ml after the ACE-inhibitor intervention. Previous studies have suggested that larger increases of total IGF-1 (19) and IGFBP-3 (20) compared to our results were associated to a significant reduction in the risk of adverse cardiovascular events. Nevertheless, we cannot exclude that the magnitude of those increments we found for the variables of interest may still be clinically relevant. In fact, it is possible that a marginal increase or even the simple maintenance of systemic IGF-1 and IGFBP-3 systemic concentrations in older adults may still be beneficial.
A limitation of our study resides in the selected sample population (older adults with a high cardiovascular risk profile), so that our results might be not applicable to other age groups, or individuals with different cardiovascular risk profiles. Furthermore, our trial tested the effects of a specific ACE-inhibitor (i.e. fosinopril). Therefore, different results might be obtained with different ACE-inhibitors, although their therapeutic benefits are commonly recognized as being due to a class effect. Finally, our study follow-up was limited to 6 months. Consequently, our findings should not be extended to longer periods of ACE-inhibition and we cannot rule that a longer exposure to fosinopril could result in greater increases in IGF-1 and IGFBP-3.
In conclusion, our study demonstrates a significant increase of serum IGF-1 and IGFBP-3 concentrations after an ACE-inhibitor intervention in a population of older adults with high cardiovascular risk profile. Future studies are needed to determine whether the biological effects we observed may be translated into clinically relevant improvements in older persons, and whether the chronic reduction of angiotensin II by ACE inhibitors is able to maintain overtime its positive effect on IGF-1 and IGFBP-3 systemic concentrations.
Acknowledgements
The TRAIN study is a National Institute of Health-funded project (NIH grant R01-HL68901). The TRAIN study was also (partially) supported by the University of Florida Claude D. Pepper Older Americans Independence Center (NIH grant 1P30-AG028740), Wake Forest University Claude D. Pepper Older Americans Independence Center (NIH grant 5P30-AG021332), and the Wake Forest University General Clinical Research Center (NIH grant M01-RR07122).
Reference List
- 1.Fox KM. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362(9386):782–788. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Gerstein H, Hoogwerf B, et al. Ramipril and the development of diabetes. JAMA. 2001;286(15):1882–1885. doi: 10.1001/jama.286.15.1882. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G, The Heart Outcomes Prevention Evaluation Study Investigators Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342(3):145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 4.Maggio M, Ceda GP, Lauretani F, et al. Relation of angiotensin-converting enzyme inhibitor treatment to insulin-like growth factor-1 serum levels in subjects >65 years of age (the InCHIANTI study) Am J Cardiol. 2006;97(10):1525–1529. doi: 10.1016/j.amjcard.2005.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onder G, Liperoti R, Russo A, et al. Use of ACE inhibitors is associated with elevated levels of IGFBP-3 among hypertensive older adults: results from the IlSIRENTE study. Eur J Clin Pharmacol. 2007;63(4):389–395. doi: 10.1007/s00228-007-0262-z. [DOI] [PubMed] [Google Scholar]
- 6.Brink M, Wellen J, Delafontaine P. Angiotensin II causes weight loss and decreases circulating insulin-like growth factor I in rats through a pressor-independent mechanism. J Clin Invest. 1996;97(11):2509–2516. doi: 10.1172/JCI118698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colao A, Spiezia S, Di SC, et al. Circulating insulin-like growth factor-I levels are correlated with the atherosclerotic profile in healthy subjects independently of age. J Endocrinol Invest. 2005;28(5):440–448. doi: 10.1007/BF03347225. [DOI] [PubMed] [Google Scholar]
- 8.Martin RM, Gunnell D, Whitley E, et al. Associations of insulin-like growth factor (IGF)-I, IGF-II, IGF binding protein (IGFBP)-2 and IGFBP-3 with ultrasound measures of atherosclerosis and plaque stability in an older adult population. J Clin Endocrinol Metab. 2008;93(4):1331–1338. doi: 10.1210/jc.2007-2295. [DOI] [PubMed] [Google Scholar]
- 9.Valentinis B, Bhala A, DeAngelis T, Baserga R, Cohen P. The human insulin-like growth factor (IGF) binding protein-3 inhibits the growth of fibroblasts with a targeted disruption of the IGF-I receptor gene. Mol Endocrinol. 1995;9(3):361–367. doi: 10.1210/mend.9.3.7539889. [DOI] [PubMed] [Google Scholar]
- 10.Rajah R, Valentinis B, Cohen P. Insulin-like growth factor (IGF)-binding protein-3 induces apoptosis and mediates the effects of transforming growth factor-beta1 on programmed cell death through a p53- and IGF-independent mechanism. J Biol Chem. 1997;272(18):12181–12188. doi: 10.1074/jbc.272.18.12181. [DOI] [PubMed] [Google Scholar]
- 11.Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation. 2002;106(8):939–944. doi: 10.1161/01.cir.0000027563.44593.cc. [DOI] [PubMed] [Google Scholar]
- 12.Johnsen SP, Hundborg HH, Sorensen HT, et al. Insulin-like growth factor (IGF) I, - II, and IGF binding protein-3 and risk of ischemic stroke. J Clin Endocrinol Metab. 2005;90(11):5937–5941. doi: 10.1210/jc.2004-2088. [DOI] [PubMed] [Google Scholar]
- 13.Corbalan R, Acevedo M, Godoy I, Jalil J, Campusano C, Klassen J. Enalapril restores depressed circulating insulin-like growth factor 1 in patients with chronic heart failure. J Card Fail. 1998;4(2):115–119. doi: 10.1016/s1071-9164(98)90251-2. [DOI] [PubMed] [Google Scholar]
- 14.Diez J, Laviades C. Insulin-like growth factor-1 and cardiac mass in essential hypertension: comparative effects of captopril, lisinopril and quinapril. J Hypertens Suppl. 1994;12(4):S31–S36. [PubMed] [Google Scholar]
- 15.Cesari M, Kritchevsky SB, Baumgartner RN, et al. Sarcopenia, obesity, and inflammation--results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr. 2005;82(2):428–434. doi: 10.1093/ajcn.82.2.428. [DOI] [PubMed] [Google Scholar]
- 16.Cesari M, Kritchevsky SB, Atkinson HH, et al. Angiotensin-converting enzyme inhibition and novel cardiovascular risk biomarkers: results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors (TRAIN) study. Am Heart J. 2009;157(2):334–338. doi: 10.1016/j.ahj.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anwar A, Gaspoz JM, Pampallona S, et al. Effect of congestive heart failure on the insulin-like growth factor-1 system. Am J Cardiol. 2002;90(12):1402–1405. doi: 10.1016/s0002-9149(02)02885-0. [DOI] [PubMed] [Google Scholar]
- 18.Tsukahara H, Gordienko DV, Tonshoff B, Gelato MC, Goligorsky MS. Direct demonstration of insulin-like growth factor-I-induced nitric oxide production by endothelial cells. Kidney Int. 1994;45(2):598–604. doi: 10.1038/ki.1994.78. [DOI] [PubMed] [Google Scholar]
- 19.Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89(1):114–120. doi: 10.1210/jc.2003-030967. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan RC, McGinn AP, Pollak MN, et al. Association of total insulin-like growth factor-I, insulin-like growth factor binding protein-1 (IGFBP-1), and IGFBP-3 levels with incident coronary events and ischemic stroke. J Clin Endocrinol Metab. 2007;92(4):1319–1325. doi: 10.1210/jc.2006-1631. [DOI] [PubMed] [Google Scholar]