Abstract
Strategies that utilize controlled release of drugs and proteins for tissue engineering have enormous potential to regenerate damaged organs and tissues. The multiple advantages of controlled release strategies merit overcoming the significant challenges to translation, including high costs and long, difficult regulatory pathways. This review highlights the potential of controlled release of proteins for tissue engineering and regenerative medicine. We specifically discuss treatment modalities that have reached preclinical and clinical trials, with emphasis on controlled release systems for bone tissue engineering, the most advanced application with several products already in clinic. Possible strategies to address translational and regulatory concerns are also discussed.
Keywords: tissue engineering, scaffolds, protein delivery, translation, regulatory pathways
1. Introduction
With the large and constantly growing numbers of patients waiting for organ transplants, the need for technologies that can regenerate new tissues is greater than ever. The field is rapidly expanding, with over two hundred companies spending over $3.6 billion and generating over $3.4 billion in sales as of 2011 [1]. These numbers represent increases of 1.5× and 2.7× respectively compared to four years earlier, and are projected to continue rising [1]. It is likely that products with the capability for controlled release of drugs and proteins will constitute a significant portion of the projected increase in commercialization, since regulatory approval is easier than for traditional tissue-engineered products with a cell component. Indeed, a large portion of the annual revenue in the field is owed to Medtronic’s Infuse® ($750 million), which relies on the release of bone morphogenic protein-2 to regenerate bone tissue [1].
The traditional tissue-engineering paradigm involves cell culture in three-dimensional matrices or scaffolds; the tissue is implanted directly into the body or cultivated in vitro for some period of time before implantation. In both cases, the scaffold serves as a structural and logistic template for cell attachment, maintenance of differentiated function, and assembly of functional tissues.
While significant advances have been made in the design of scaffolds, it is likely that physical signals from the scaffolds alone are insufficient to achieve full tissue regeneration. The addition of bioactive factors - drugs and growth factors - to cell culture media can enhance tissue growth, but the short half-lives of drugs and proteins in the body, their rapid clearance from the target site, and potential cytotoxicity at high doses impede their utility in vivo. This is why tissue engineering scaffolds with systems in place for the controlled release (CR) of bioactive factors hold great potential for providing the molecular and biological cues necessary to achieve tissue regeneration in situ. Although combination products like drug-eluting scaffolds, even without a cellular component, face substantial translational and regulatory hurdles, their significant advantages tissue engineering merit the effort, and products already on the market demonstrate the feasibility and utility of these sophisticated approaches [2].
In this review, we highlight the main advantages to using tissue engineering scaffolds with ability for CR of various types of proteins. We will illustrate the advantages of CR systems using examples that have progressed to pre-clinical large animal models or clinical trials (summarized in Table 1). Many of these examples are geared toward bone tissue engineering, which boasts the most commercially advanced CR systems. Gene therapy approaches are outside the scope of this review, but are also promising for tissue engineering [3, 4]. We also discuss the translational concerns and the regulatory pathways involved in the design and scale-up of CR systems for tissue engineering. Finally, we discuss the current and future needs for facilitating clinical translation of CR systems for tissue engineering.
Table 1.
Preclinical and clinical studies of controlled release systems for tissue engineering
| Advantage of Controlled Delivery |
Example Technology |
Target Tissue | Animal Model | References |
|---|---|---|---|---|
| To extend bioactivity of drug | BMP2 and BMP7 from collagen sponges | Bone | Clinically applied for multiple bone defects | see Table 2 |
| PDGF-BB from β-tricalcium phosphate particles | Bone | Clinically applied for bone defects from periodontal disease | 171, 172 | |
| BMP2 and/or VEGF, PDGF or FGF from various hydrogels and scaffolds | Bone | Cranial defect in rats and pigs, femoral defects in rats, osteoporotic verterbral bodies in sheep | 46,47, 99a, 100a, 117–124 | |
| BMP7 from PLGA microspheres, carboxymethyl cellulose | Bone | Osteoporotic vertebral bodies in sheep | 125 | |
| VEGF and PDGF from brushite-chitosan scaffold | Bone | Intermedullary bone defects in rabbits | 126 | |
| bFGF from gelatin microspheres and collagen scaffolds | Bone | Alveolar bone defects in dogs | 127 | |
| IGF1 from fibrin | Cartilage | Cartilage defects in horses | 33–35 | |
| IGF1 from hyaluronan | Osteochondral tissue | Osteoarthritic temperomandibular joints in rabbits | 128 | |
| PDGF from collagen gel | Ligament | Collateral ligament injury in rats | 129 | |
| VEGF and IGF1 in alginate hydrogel | Muscle | Ischemic hindlimbs in mice | 36 | |
| BDNF in PNIPAAm-PEG hydrogels | Nerves | Spinal cord injuries in rats | 130 | |
| bFGF, FGF1, or FGF2 from gelatin or alginate microscpheres in collagen scaffold | Adipose tissue | Rat vascular pedicle/fat pad | 105b, 131, 132 | |
| To recruit cells | FGF2 from alginate microbeads to recruit endothelial cells | Angiogenesis | Clinical trials | 51, 52 |
| MCP1 from alginate microbeads in scaffolds to recruit monocytes | Angiogenesis | Subcutaneous in immunodeficient mice | 54 | |
| IGF1 from hydrogels to recruit chondrocytes | Cartilage | Subcutaneous in athymic mice | 62 | |
| TGFβ1 to recruit MSCs | Cartilage | Subperiosteally in rabbits | 133 | |
| SDF1α from PEGylated fibrinogen to recruit stem cells | Myocardial infarct | Myocardial infarction in mice | 134 | |
| SDF1α from PLGA scaffolds to recruit stem cells | Modulation of inflammatory response | Subcutaneous in mice | 135 | |
| VEGF, SDF1 and BMP-6 from microdelivery system for stem cell recruitment and differentiation | Bone | Subcutaneous in rats | 136 | |
| For temporal control | Sequential delivery of VEGF then PDGF from PLGA microspheres and scaffolds | Angiogenesis | Subcutaneous in rats; ischemic hindlimbs in mice; myocardial infarction in rats | 57, 68, 69, 137 |
| Sequential delivery of VEGF, PDGF, and TGFβ1 from alginate hydrogels | Angiogenesis | Subcutaneous in rats | 67 | |
| Sequential delivery of IGF1 then HGF from alginate hydrogel | Angiogenesis | Acute myocardial infarction in rats | 70 | |
| Sequential delivery of TGFβ1 then IGF1 | Cartilage | Osteochondral defects in rabbits | 96c, 138 | |
| Sequential delivery of VEGF and BMP2 (and vice versa) from PLGA microspheres in gelatin hydrogels and from alginate/PLA scaffolds | Bone | Subcutaneous implantation compared to bone defects in rats and dogs; segmental femur defects in mice | 25, 66a, 14 | |
| Sequential delivery of BMP2 then BMP7 from poly(ε-caprolactone) scaffolds | Bone | Iliac crest defects in rats | 139 | |
| For in situ differentiation of progenitor cells | Dexamethasone from PLGA microspheres | Cartilage | Subcutaneous in mice | 60 |
| TGFβ1 from gelatin microspheres in gelatin-HA-chondroitin scaffold | Cartilage | Osteochondral defects in rabbits | 75 | |
| bFGF from microspheres in collagen scaffold | Nerves | Cerebral ischemia/ stroke model in rats | 140 | |
| VEGF from PLGA microspheres | Angiogenesis | Ischemic hindlimbs in mice | 27 | |
| Potential for on-demand release kinetics | MMP-sensitive release of VEGF from PEG-based hydrogels | Angiogenesis | Subcutaneous in rats | 77, 79 |
| MMP-sensitive release of BMP2 from PEG-based hydrogels | Bone | Cranial defects in rats | 141 | |
| Inflammatory cell- and osteoclast-mediated release of BMP2 and VEGF | Bone | Subcutaneous in rats; cranial defects in mice | 83, 84d, 85, 142, 143 | |
| MMP-sensitive release of BDNF from HA hydrogels | Nerve | Spinal cord injuries in rats | 80 | |
| External control of VEGF then S1P via hollow fibers | Angiogenesis | Subcutaneous in mice | 144 | |
| Photolabile links to release RGD | Cartilage | in vitro | 87 |
These studies showed the importance of appropriate animal models (VEGF and BMP2 were synergistic ectopically but not orthotopically).
This study showed that different results can be obtained at different sites of implantation in the same animal model.
This study showed that in vitro results do not always translate to in vivo results.
This study showed that increasing the dose of BMP2 can impact the balance between bone formation and bone resorption.
2. Classes of CR systems for tissue engineering
Most tissue engineering approaches employ some type of three-dimensional structure to support the regeneration of new tissue (for review, [5, 6]). The addition of CR systems can render these biodegradable scaffolds bioactive and cell-instructive. The release of growth factors and cytokines can augment tissue growth or recapitulate aspects of developmental or repair processes. Proteins have been incorporated directly into porous scaffolds via surface modification or coatings. Polymeric microparticles and hydrogels can serve as injectable scaffolds.
2.1. Surface modification of scaffolds
Three dimensional, biodegradable scaffolds are often employed to guide tissue growth or regeneration. The surfaces of scaffolds can be modified with proteins through chemical conjugation or through non-covalent binding like surface adsorption, affinity binding, and ionic complexation. Modification of a biomaterial’s surface provides specific control over cell interactions without affecting its bulk properties. However, the bioactivity of the protein may be reduced during modification or as a result of immobilization [7]. An immobilized protein can retain its biological function for cells in direct contact with the scaffold, but it can only diffuse into the surrounding tissue if the scaffold is degraded. Nonspecific adsorption of protein on a scaffold via soaking often results in significant burst and rapid release [8, 9].
Proteins can be chemically conjugated to scaffolds via their primary amine groups using N-hydroxysuccinimide (NHS) or other crosslinking chemistry, or through the use of a linker molecule that is degradable or nondegradable. Incorporating heparin or heparin-rich proteoglycans into scaffolds extends the release profile of proteins with naturally high affinity for heparin such as vascular endothelial growth factor (VEGF) and bone morphogenic protein-2 (BMP2) [10], and this affinity can be augmented with the addition of heparin-binding domains [11]. Proteins have also been modified with titanium-binding motifs for attachment to titanium, one of the most widely used biomaterials [12].
Proteins can also be physically incorporated into the main structure of scaffolds if the scaffold fabrication methods are sufficiently gentle. In this case protein release is controlled by diffusion through pores in the polymer and degradation of the polymer. For example, BMP2 was incorporated into porous poly(lactic acid) scaffolds by plasticizing the dry polymer in the presence of the protein and foaming with supercritical CO2 [13]. Release of active protein was sustained for over 28 days in vitro and enhanced bone formation in a mouse segmental femur model [14].
Polymer film coatings are also used to achieve control over protein release. Coatings are formed by dipping scaffolds in aqueous polymer formulations containing proteins. Release of protein is controlled by diffusion and polymer degradation and depends on the type of polymer and the number and thickness of the layers [15]. Such coatings have been prepared from aqueous solutions of silk [16, 17] and water-in-oil emulsions with organic polymers like poly(caprolactone) and poly(lactic-co-glycolic acid) (PLGA) [18]. Coatings of calcium phosphate, in which protein release depends on electrostatic interactions with the mineral and its dissolution, are under investigation for bone tissue engineering [19, 20]. Disadvantages of polymer coatings include potential clogging of pores, inadequate adhesion to the substrate, and limitations in varying processing parameters [15, 21]. Some of these limitations can be overcome by using nanoscale thin films prepared using layer-by-layer (LbL) assembly of polyelectrolytes with alternating charges and with proteins in between [22]. The release of BMP2 was controlled over two weeks from layers of chondroitin sulfate, a negatively charged natural component of extracellular matrix, and a cationic poly(β-amino ester), and supported ectopic bone formation in an intramuscular rat model [23].
2.2. Microparticles
Encapsulation in polymeric microparticles, typically through water-in-oil-in-water double emulsions, can protect proteins from degradation and increase their stability and retention at the target site [24, 25]. Protein release, controlled primarily by diffusion through pores that form in the microparticles and degradation of the polymer, is affected by the composition and degradation profile of the polymer, the size of the particles, the dose of encapsulated protein, and polymer crosslinking. The high level of control over release profile via manipulation of fabrication parameters is a significant advantage of microparticles. Moreover, they can be engineered to release multiple proteins and they can be delivered in combination with cells [26, 27]. Protein-loaded microspheres have also been fused into scaffolds for tissue engineering [28, 29]. Microparticles prepared from poly(lactic-co-glycolic acid) (PLGA) are approved by the FDA for the CR of multiple drugs. However, protein bioactivity during microparticle fabrication can be diminished due to contact with harsh organic solvents [30].
2.3. Hydrogels
Hydrogels are 3D crosslinked hydrophilic polymers swollen in large amounts of water, making them useful as biomimetic tissue engineering scaffolds. Most hydrogels are prepared from aqueous solutions via gentle crosslinking conditions, so proteins can be directly incorporated with virtually 100% preservation of protein bioactivity. Hydrogels can also be loaded with protein subsequent to crosslinking by soaking in protein solution [31]. The release of proteins from hydrogels depends on interactions between the protein and the polymers that comprise the hydrogel, but is generally controlled by diffusion, so that release is typically characterized by a large burst release in the first 24hrs, and it rarely lasts longer than 2 weeks [32]. Nonetheless, this method is still more effective than bolus administration, and has been used to expose chondrocytes to insulin-like growth factor-1 (IGF1) via fibrin hydrogels in osteochondral defect repair in horse [33–35].
3. Advantages of CR systems for tissue engineering
3.1. Extended bioactivity
By far the most common goal of a CR system is to extend the amount of time that encapsulated cells or cells in the vicinity are exposed to the drug. In tissue engineering, the drugs of choice are typically growth factors and cytokines. Sustained release can ensure that the lowest therapeutic dose is continuously presented to cells, resulting in greater efficiency of drug presentation, greater efficacy of its action, and decreased risk of side effects compared to bolus administration [36]. For bone tissue engineering, long-term release (4 weeks) of bone morphogenic protein-2 (BMP2) resulted in higher bone formation than short-term release (3 days) at an equivalent dose [37].
Relatively fast release of bone morphogenic protein-2 (BMP2) and BMP7 (less than 2 weeks) has proved useful for bone tissue engineering, and is at this time the most extensively studied CR system clinically, with thousands of patients treated in more than 50 clinical trials (Table 2) [38, 39]. These BMP products are recommended as alternatives to autografts, which are a “gold standard” of care, in instances where large amounts of bone are required, to facilitate healing of non-unions in long bones, or spinal fusion [38].
Table 2.
Clinical trials of BMP2 and BMP7 release for bone tissue engineering.
| Protein | Carrier | Product | Company | Clinical target |
Outcome | References |
|---|---|---|---|---|---|---|
| BMP-2 | Collagen sponge | INFUSE® | Medtronic (Memphis, TN) | Long bone fractures | FDA approval for open tibial fractures | 41, 145–148 |
| BMP-2 | Collagen sponge | INFUSE® | Medtronic (Memphis, TN) | Oral and maxillofacial reconstruction | FDA approval for sinus and alveolar ridge augmentation | 40 |
| BMP-2 | Collagen sponge | INFUSE® | Medtronic (Memphis, TN) | Spinal fusion | FDA approval for interior lumbar interbody fusion | 43, 149–154 |
| BMP-2 | Collagen/calcium phosphate | Compression-resistant matrix (CRM); MASTERGRAFT; MBCP | Medtronic (Memphis, TN) | Spinal fusion | FDA approval for bone void filler | 155–159 |
| BMP-7 | Collagen sponge | OP-1, Osigraft | Stryker (Kalamazoo, MI) | Long bone fractures | FDA approval for long bone nonunions | 44, 160–164 |
| BMP-7 | Collagen sponge +/− paste-thickening agent | OP-1, OP-1 Putty | Stryker (Kalamazoo, MI) | Spinal fusion | FDA approval of OP-1 putty for posterolateral spinal fusion | 165–170 |
CR systems for the release of BMP2 or BMP7 are FDA-approved and available commercially as INFUSE® bone graft, a BMP2-soaked collagen sponge, from Medtronic (Memphis, TN) [40–43], and OP-1, a BMP7/collagen paste from Stryker Biotech (Hopkinton, MA) [44]. INFUSE® has become the subject of controversy after Medtronic’s other BMP-releasing product, AMPLIFY (a BMP2-releasing hyaluronic acid/ β-tricalcium phosphate scaffold) was not approved by the FDA because of adverse complications and elevated cancer risk [39] and a scathing review of Medtronic studies by prominent spine surgeons [45]. The elevated cancer risk was attributed to the high dose of BMP2, suggesting that the risk might be mitigated and efficacy increased with a CR system that sustains a lower dose for a longer period of time, which was probed experimentally in a rat model [46, 47]. Research on these more sophisticated CR systems is ongoing [38, 48, 49].
3.2. Cellular recruitment
While the main purpose of the studies of BMP2 and BMP7 was to induce growth and tissue production by cells in the surrounding area of implantation, another advantage of CR scaffolds could be the creation of a concentration gradient that can recruit the host cells to migrate into the matrix. The release of a chemotactic protein can in principle recruit cells from both the circulation and the surrounding tissue. The most common example of this strategy is the release of angiogenic factors from implanted biomaterials in order to recruit endothelial cells and supporting pericytes to enter the area and form new blood vessels. Such a strategy has been applied clinically to coax new blood vessels into ischemic areas of myocardium.
The sustained release (4–6 weeks) of 10µg or 100µg of fibroblast growth factor-2 (FGF2) from heparin-alginate microparticles implanted into ischemic myocardial tissue resulted in a reduction in size of the ischemic area, reduction in stress perfusion defects, and decreased incidence of angina in twenty-four patients in a Phase I trial [50–52]. This system has not been studied in larger clinical trials. However, a detailed pharmacokinetic analysis of the clearance of FGF2 from the infarcted myocardium suggested that sustained delivery of FGF2 alone is unlikely to achieve long-term clinical benefit [53]. Through analytical, ex vivo, and animal models, it was shown that relatively low molecular weight growth factors like FGF2 are rapidly cleared from tissue through microcapillaries, which increase in number with the action of the angiogenic factors, thereby increasing their own rate of clearance and limiting the therapeutic effect [53]. Using radiolabeled FGF1 incorporated into the sustained release heparin-alginate beads, it was shown that FGF penetrated the myocardium of rabbits at maximum level at 2 days, but drastically regressed over the next week, remaining low after 31 days, even with sustained release, as a direct result of the increased vascularization [53]. Neovascularization peaked at day 8, and was 56% greater in animals receiving FGF1 compared to control animals, but decreased over the next few weeks to settle at baseline levels. The authors suggest that therapeutic efficacy of angiogenic factors might be increased by engineering drugs with lower transendothelial permeability.
The molecular factors that regulate angiogenesis are complex. Perhaps a better approach to induce neovascularization might be through the recruitment of inflammatory cells, which would then release their own complex set of biological signals to effect angiogenesis. Alginate microparticles loaded with monocyte chemoattractant protein-1 (MCP1) were incorporated into poly(glycolic acid) (PGA) mesh tubes and implanted into the inferior vena cava of SCID/bg mice [54]. The release of MCP-1 over just three days recruited host monocytes, which in turn recruited endothelial cells and supportive smooth muscle cells, and resulted in functioning venous conduits after 10 weeks. The recruitment of monocytes to tissue engineered grafts appears to explain why the implantation of human bone marrow stromal cells into SCID mice results in vascularized tissue compose entirely of mouse cells [54], and sheds light on the mechanisms of efficacy observed in large-scale clinical trials of tissue-engineered vascular grafts formed from mesenchymal stem cells (MSCs) [55, 56].
3.3. Temporal control
To address the problem of unstable vasculature that regresses without constant stimulation by angiogenic growth factors, researchers have turned to the use of CR systems that rapidly release vascular endothelial growth factor (VEGF) followed by the delayed and sustained release of platelet-derived growth factor (PDGF) (a few weeks), a system that approximates the physiological profile observed in developmental or adult angiogenesis [57]. The VEGF is intended to recruit endothelial cells, and the PDGF is intended to recruit supporting pericytes or smooth muscle cells to stabilize newly forming blood vessels. The most common method of achieving this level of temporal control is through the use of polymeric microparticles. Because soluble proteins are hydrophilic, encapsulation in hydrophobic polymers like poly(lactic acid) (PLA) or poly(lactic-co-glycolic acid) (PLGA) limits their diffusion so that release is determined by degradation of the polymer [58]. Protein encapsulation in microparticles also increases their stability in physiological conditions, and processing parameters can be manipulated to control the rate of release [57, 59, 60]. Proteins can be encapsulated within the polymer microparticles for delayed and sustained release, or adsorbed to the surface of the microparticles for rapid release [57]. Such “sequential delivery” microspheres can be molded into scaffolds or embedded in scaffolds for hydrogels for even more control over release profile [28, 57, 61]. Embedding microparticles in hydrogels or scaffolds typically tempers the burst release and slows overall release because the drug interacts with the hydrogel matrix following its release from the microparticles [24, 62–64]. In addition, two different sets of proteins can be encapsulated in the two phases (hydrogel and microparticle), resulting in rapid release of the protein in the hydrogel and delayed, sustained release of the proteins from the microparticles [65, 66].
Freeman et al. extended the sequential delivery of growth factors to include a third protein [67]. By incorporating the proteins into alginate-sulfate hydrogel, which can bind multiple heparin-binding proteins, the timing of release of the three proteins was dependent on their equilibrium binding constants with heparin, so that VEGF was released first followed by PDGF and lastly by transforming growth factor-β1 (TGFβ1). After subcutaneous implantation in rats, the sequential release of the three factors over an eight-day period resulted in the formation of larger, more mature blood vessels than the simultaneous release of the three factors, prepared using non-sulfated alginate. This increase in vascularization persisted at 3 months for the sequential release scaffolds, while blood vessels regressed in groups treated with instantaneous release [67]. Despite the large numbers of publications regarding the sequential delivery of multiple growth factors for therapeutic angiogenesis [57, 67–70] and the failure of monotherapeutic strategies in multiple clinical trials [51, 52, 71–73], this strategy has not yet been studied in pre-clinical or clinical studies.
3.4. In situ differentiation of progenitor cells
For many applications, recruitment of cells from the surrounding tissue is inadequate for functional regeneration of the new tissue. The use of progenitor cells in combination with a biomaterial offers the possibility to regenerate tissues, in vitro and in vivo. Differentiation of progenitor cells requires biological signals, and can be induced by the addition of appropriate cytokines to cell culture media. However, for applications in which time is of the essence, like acute trauma, CR systems can provide the important differentiation factors to progenitor cells within biomaterials so that they can be implanted more quickly. Dramatic neovascularization was observed in a murine model of hindlimb ischemia when cord-blood derived endothelial progenitor cells were co-injected with a mixture of PLGA microspheres containing the angiogenic factors VEGF, hepatocyte growth factor, and angiopoietin-1 [27]. The level of perfusion was significantly higher when all three proteins were delivered than when cells were delivered alone or with just two of the proteins.
Allowing the new tissue to develop within the body may also enhance integration with surrounding tissue [74]. For example, rabbit MSCs were seeded onto porous gelatin-chondroitin-hyaluronate scaffolds containing TGFβ1-loaded gelatin microspheres and implanted into full thickness cartilage defects autologously in rabbits [75]. A comparison group of MSCs was pre-cultivated with TGFβ1 for 7 days and then seeded on scaffolds with blank microspheres prior to implantation. The release of TGFβ1 over 30 days in vivo resulted in enhanced chondrogenic differentiation and cartilage repair by the MSCs, with more integrated cartilage tissue than that produced by pre-differentiated MSCs.
3.5. Potential for “on demand” release kinetics
The significant level of control over protein release profile that can be achieved by CR systems allows the realization of more nuanced objectives, such as response to local microenvironmental stimuli, the coupling of scaffold degradation with new tissue formation or the modulation of protein delivery through the application of external stimuli. For example, pH-responsive hydrogels based on poly(N-isopropylacrylamide) and poly(acrylic acid) were designed to extend the release profile of angiogenic growth factors in the acidic environment of ischemic tissue and to dissolve upon restoration of physiological pH [76].
A central tenet of tissue engineering is that the newly forming tissue should replace the degrading biomaterial, preferably at the same rate. CR systems can be designed to achieve this goal by physically coupling the degradation of the scaffold and release of stimulatory factors with cellular ingrowth through cell-mediated degradation. Hydrogels were formed from a mixture of macromers containing cell-adhesive peptides, sites that were degradable by matrix metalloproteinases (MMPs), and VEGF, so that degradation and release of the protein occurred only when MMP-secreting cells were present [77–79]. This cell-mediated release of VEGF led to enhanced reperfusion of ischemic hind limbs in mice when the hydrogels were injected into the area and polymerized in situ [79]. The presence of the adhesive ligands were required for vascularization, showing the importance of the matrix composition, and the VEGF-containing hydrogels performed better than bolus administration of VEGF, indicating that sustained release can increase drug efficacy [79]. This method is also under investigation for the release of brain-derived neurotrophic factor (BDNF) for nerve regeneration [80] and the release of BMP2 for bone regeneration [81, 82].
Similarly, BMP2 was incorporated into biomimetic coatings of calcium phosphate (CaP) on porous scaffolds, so that its release was controlled by osteoclast degradation of the coating [83–85]. Titanium alloy discs were immersed in concentrated simulated body fluid to inhibit crystal growth, resulting in the formation of a thin layer of amorphous CaP that could serve as a seeding surface for the subsequent addition of a crystalline CaP layer containing BMP2 [83]. The release of BMP-2 in an ectopic ossification site (subcutanoues implantation) in rat was mediated by foreign body giant cells and then by osteoclasts after 3 weeks. The gradual, cell-mediated release of BMP2 over a few weeks using this method resulted in enhanced bone formation and integration with surrounding tissue, and reduced coverage by foreign body giant cells, compared to coated discs with adsorbed BMP2. After 5 weeks, the discs were almost completely surrounded by new woven bone. In contrast, discs coated with CaP but with only adsorbed BMP2 released the protein over a few days, resulting in limited bone formation after one week that was completely resorbed by the fifth week [83]. This coating technique was also used for cell-mediated liberation of BMP2 from porous titanium scaffolds implanted into the maxillae of adult miniature pigs [84]. Interestingly, in this orthotopic model, the presence of BMP2 caused an overall decrease in new bone volume, attributed to an overly high dose (13 and 23µg/implant) that stimulated bone resorption activities of osteoclasts. With a lower dose (5.8µg), the sustained release of BMP2 caused enhanced bone formation compared to burst release in a subcutaneous implantation model in rat, even in a mechanically unstable situation that normally inhibits osteogenesis [85].
External control over release from scaffolds and hydrogels has been achieved through the use of stimuli-responsive polymers that swell or degrade in response to externally introduced drugs or the application of light, ultrasound, or a magnetic or electric field [86]. Kloxin et al. prepared PEG-based hydrogels with photolabile groups that released the peptide RGDS upon application of light [87]. By using light to release the RGDS from the hydrogels at day 10, the temporal profile seen in developmental chondrogenesis, encapsulated hMSCs upregulated glycosaminoglycan production and cartilage-specific genes in vitro, compared to persistently expressed RGDS.
Externally controlled CR systems hold significant promise for advancing tissue engineering approaches, but none have yet passed the prototype stage, as logistical issues for implementing in a clinical setting are still being worked out. Methods to externally control protein release using noninvasive methods such as near-infrared light and ultrasound are under investigation [88–90]. Given that ultrasound can be used for imaging and has been shown to enhance tissue regeneration in animal models [91], its use in controlling protein delivery is particularly appealing.
4. Translation concerns
There are many design issues to consider in the clinical translation of all tissue engineering devices, such as the foreign body response, whether the mode of application is practical, and whether the device represents a substantial enough improvement over existing devices that clinicians will wish to adopt it. A few major challenges in the translation stage of product development that seem to be especially important for tissue engineering strategies that include a CR component are outlined below. At every stage of translation, a detailed analysis of the effects of the released growth factors must be conducted. In addition, clinically relevant cell sources must be studied to prevent age-, animal-, or disease-specific effects like the well-documented species-specificity of chondrocytes [92] or their decrease in responsiveness to IGF1 with age and disease [93].
4.1. Animal models
The first challenge comes in translating in vitro results to in vivo results. Despite numerous in vitro studies showing synergistic effects of IGF1 and TGFβ1 on chondrogenesis within hydrogels [31, 94, 95], this synergy did not translate into full-thickness cartilage defects in rabbits [96]. TGFβ1 loaded into the hydrogel phase was released rapidly, while IGF1 loaded into gelatin microspheres released more slowly, which approximates the physiological profile observed in chondrogenic differentiation of MSCs [97]. The authors suggested that the lack of agreement between in vitro and in vivo studies might be due to the complex environment seen in wound healing, where these growth factors exert a wide spectrum of effects.
Similarly, it is important to choose an appropriate, functional animal model for pre-clinical studies, especially considering that tissue engineering therapies will most likely be required in diseased tissues. The sequential delivery of VEGF and BMP2 showed improvements in ectopic bone formation in subcutaneous implantation, but VEGF had no beneficial effect compared to BMP2 alone in orthotopic bone defects in rats, dogs, and pigs [66, 98–100]. The fact that angiogenic factors have been shown to be beneficial for osteogenesis in vitro and in other rodent models [101–103] suggests that the interplay between angiogenic factors and BMP2 is complex and depends on the local environment. CR devices should thus be tested in multiple animal models at early stages of development [104].
In adipose tissue engineering, some studies showed that CR of angiogenic factors from biomaterials in the epigastric pedicle in rats caused substantial neovascularization that in turn drove the formation of large amounts of adipose tissue, while other studies showed that substantial neovascularization was not accompanied by adipose tissue formation when the CR systems were placed next to the femoral pedicle, which has less of a source of adipocytes [105]. Apparently conflicting results can be obtained using slightly different models, further emphasizing the need to evaluate CR devices in multiple animal models.
4.2. Clinical trials
In translating from large animal models to Phase I and larger clinical trials, the selection of the patient population can be the difference between successful CR devices and failure. The struggles of angiogenesis trials illustrate this point. Despite substantial preclinical evidence showing improvements in revascularization in several animal models of myocardial infarction or ischemic disease, as well as success in Phase I trials, no clinical improvement was achieved from angiogenic growth factor therapy in more than 25 Phase II and Phase III trials involving over 2500 patients [73].
It is likely that angiogenic therapy has not been proven efficacious in large clinical trials because they were not administered in a sustained release formulation, so that any newly formed blood vessels regressed after an initial improvement [53, 86]. However, other issues with clinical trial design are apparent. Many of the trials were restricted to the “no option” population of patients who have exhausted standard treatment strategies, and therefore might be inherently less responsive to attempts at neovascularization [106]. However, choosing a less severe population would face considerable challenges by institutional review boards and the U.S. Food and Drug Administration (FDA).
In addition, studies have shown considerable fluctuations in physiological measurements such as myocardial perfusion in patients with coronary artery disease, and ankle-brachial index and transcutaneous partial oxygen pressure in patients with peripheral artery disease, which further complicates measures of improvement [106, 107]. The identification of biomarkers of neovascularization would aid in measuring outcomes in clinical trials [106]. Angiogenesis trials in particular have been compounded by a considerable placebo effect, leading to the recommendation to conduct trials only in a double-blinded and randomized manner [73, 106].
Clinical trials of CR devices must also carefully evaluate potential side effects, especially cancer. Obviously proteins that are designed to promote growth might lead to the growth of tumors. Although there is no evidence that the release of BMP2 results in de novo tumor formation, Medtronic’s experience with AMPLIFY highlights the need to carefully screen patients for history of cancer or undiagnosed tumors.
4.3. Cost
Many experienced researchers agree that cost can be the main obstacle to translation. It has been estimated that the development of a CR device for tissue engineering could cost $50–$800 million over eight years from preclinical animal models to regulatory approval [104]. Good avenues of support to begin the translation stage include grants from the SBIR program of the NIH and from the Coulter Foundation, which provides funding for translational projects in biomedical engineering at 16 universities in the United States [108]. However, this funding is nowhere near that required to develop a tissue engineering product, so private investors are also required.
Finally, because patent protection is limited to 20 years from initial submission, it is important that new technologies are translated quickly and efficiently, in order to ensure favorable potential returns on investment [104]. Large companies are unlikely to buy new technologies directly from universities, so the greatest chance of success is held by small companies, usually university spin-offs, that can take the technology from the university through key animal studies to the start of clinical trials [109].
4.4. Regulatory approval
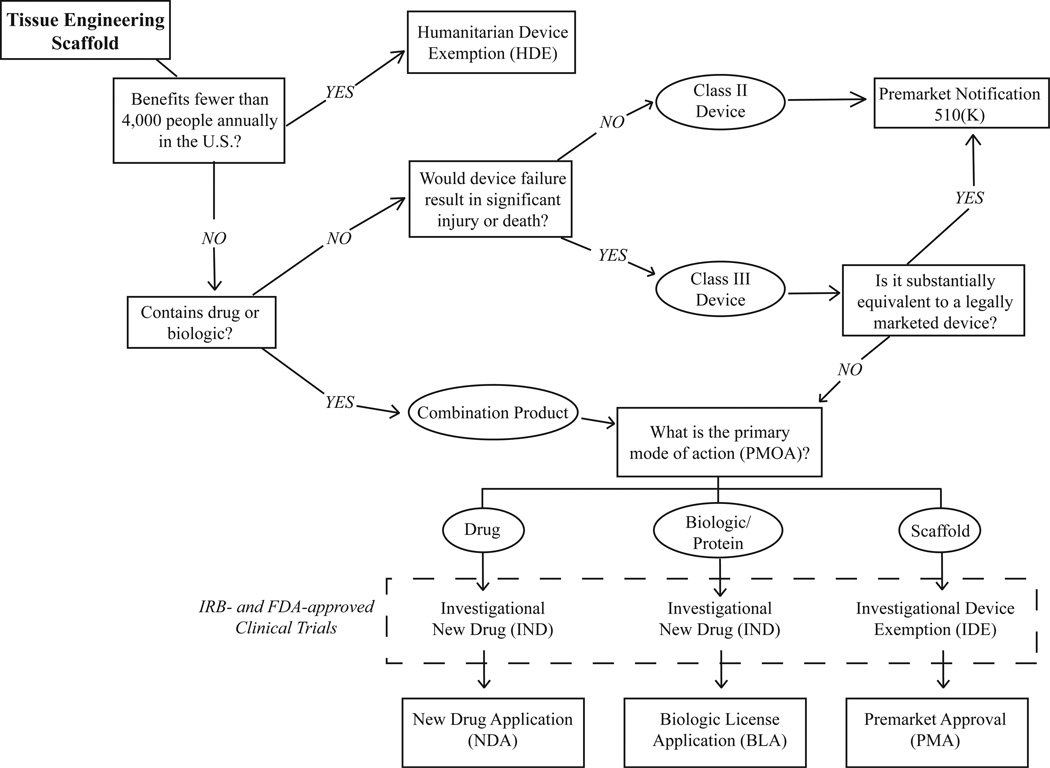
In order to market a medical device or drug in the U.S., it must be approved by the Food and Drug Administration (FDA). Classification, which determines its regulatory pathway, depends on its intended use, primary mode of action (PMOA), and level of risk (Fig. 1). Medical devices are classified as Class I (low risk, external to the body), Class II (more complicated, more risk), or Class III (substantial risk; implanted in the body or life-sustaining). Class I and II devices are either exempt from FDA approval or can achieve clearance through the premarket notification 510(k) pathway.
Figure 1.
FDA regulatory path for tissue-engineering devices in regenerative medicine
A tissue engineering scaffold is usually considered a Class II or III medical device if the primary intended purpose is not through chemical action, but the release of a drug or biologic or the presence of cells constitutes an additional designation as a “combination product” [110]. Tissue engineering scaffolds that do not contain a CR component are primarily reviewed by the FDA’s Center for Devices and Radiological Health (CDRH), while combination products are handled by the Office of Combination Products (OCP) and are referred for review by the Center for Biologic and Evaluation Research (CBER) or the Center for Drug Evaluation and Research (CDER) [111]. The OCP decides which pathway the product will follow for approval, which affects all aspects of product development including preclinical and clinical testing [112].
The major determinant of which of the largely parallel pathways is most appropriate is the biomaterial’s primary mode of action (PMOA): devices will eventually require a premarket approval (PMA), drugs will require a new drug application (NDA), and biologics (growth factors, cytokines, etc.) will require a biologic license application (BLA) (Fig. 1). The PMA/NDA/BLA is the most stringent level of market approval required by the FDA.
To facilitate FDA approval of a tissue engineering scaffold, with or without a CR system, preclinical animal studies and biocompatibility tests should be conducted and meticulously documented according to International Standard organization (ISO) 10993 guidelines, with short-term, intermediate-term, and long-term time points of evaluation [109]. The American Society for Testing and Materials (ASTM) International Committee is also in the process of developing standards for testing tissue-engineered products [113]. For a detailed description of regulatory considerations for tissue engineers at the preclinical stage, please see Lee et al. [111].
Clinical trials conducted under an investigational device exemption (IDE) or as an investigational new drug (IND) must be approved by an institutional review board (IRB) and the FDA [114]. An IDE/IND is also required to study products “off-label” in applications for which they are not FDA-approved. With these preliminary levels of approval, products are exempt from quality systems beyond normal design control or laws regulating shipping of the device.
To obtain a PMA/NDA/BLA, clinical trials must demonstrate that the device is safe and effective for its intended use. If clinical trials conducted outside the United States are to be used in support of a PMA/NDA/BLA, they must be conducted in accordance with IDE regulations and the ethical standards set by the Declaration of Helsinki [115]. Clinical trials should also be registered with the ClinicalTrials.gov data bank. In addition to successful completion of Phase III clinical trials, good manufacturing practices (GMP) must be in place to mass produce the biomaterials [109]. GMP documentation includes the Design History file with the detailed scaffold design, and the Device History File documenting the manufactured biomaterials [109].
It can be difficult to conduct studies of CR systems for tissue engineering with a large enough number of patients to garner the level of statistical significance that the FDA is accustomed to seeing in large drug trials [104]. It is recommended to involve regulatory bodies early in the design of preclinical and clinical trials to generate highly focused aims, to ensure that all requirements are met, and to avoid having to repeat studies [104, 111]. Some have recommended that the development of CR systems for tissue engineering follow a modular approach, in which approval for the scaffold is obtained separately from the biological component in order to reduce risk and increase the likelihood that a large company will pick up the technology and take it through a PMA [109]. A clear understanding of the relationship of the device to currently approved products will expedite the review process, especially if some components of the product can be cleared through a 510(k) pathway.
4.5. Scale-up and manufacturing
Scale-up and manufacturing practices are easier for tissue engineering products that do not contain a cellular component, but the presence of a system for the CR of proteins can make sterilization difficult. Proteins in a CR system are typically susceptible to damage during terminal sterilization methods like steam, dry heat, and ethylene oxide. Sterility must therefore be validated for all source materials and they must be processed using aseptic conditions that require special in-process controls and testing [111].
Tissue engineered medical products standards (TEMPS) are being prepared by subcommittees within Committee F04 of the American Society for Testing and Materials International (ASTM). FDA Guidances for Industry are available for Tissue Guidance to inform manufacturing processes [116]. Quality control during scale up will depend on a clear understanding of the biology and mechanisms of action of the product.
5. Current and Future Needs
CR systems hold enormous potential to stimulate tissue growth and stem cell differentiation in order to regenerate damaged tissues. To improve the success rate of clinical translation, several issues must be addressed. In terms of design of CR systems, it is vital that the dose and rate of release of drugs and proteins are carefully evaluated in multiple animal models. At this stage, it is no longer helpful to simply incorporate a protein in a scaffold and compare it to scaffolds without drug. Doses that are too high may cause unwanted side effects or change the balance of the catabolic and anabolic effects of the protein [84].
Similarly, the effects of release rate and duration must be carefully characterized, and, if possible, matched to the physiological profile seen in normal development or repair. The design of stimuli-responsive or bio-responsive CR systems may aid in this endeavor. There is a need for a simple way to monitor in vivo release kinetics [25]. Many studies analyze blood levels of radiolabelled proteins, which do not consider the local release profile and distribution.
Methods that visualize released and remaining drug can be combined with mathematical modeling to better describe the CR profile [53]. Finally, the complexity of physiological development indicate the need for more sophisticated CR systems that can control the delivery of multiple proteins in precisely defined spatiotemporal profiles. For this reason it may be more useful to recruit a cell type that can direct subsequent repair efforts, as was demonstrated by the recruitment of monocytes that then enhanced vascularization [54].
At the translation stage, it is important to seek advice early from clinicians, industry, and regulatory officials, to make sure that studies are carefully designed to make regulatory approval as smooth as possible. Careful selection of patient populations, methods of evaluation, and study endpoints is essential to achieving adequate levels of statistical significance and determining if there are any dangerous side effects. It is extremely time-consuming and costly to translate CR systems for tissue engineering to the market, but successful products already on the market (such as Medtronic’s Infuse®) show that it is possible and can be quite profitable.
6. Conclusions
CR systems for tissue engineering can be used to extend the bioactivity or presentation of drugs and proteins, recruit cells from the surrounding area, control the temporal presentation of drugs and proteins, induce in situ differentiation of stem cells, and physically couple scaffold degradation and new tissue growth. Many sophisticated systems are being studied at the preclinical and clinical levels, especially for bone and therapeutic angiogenesis. Challenges to translation include high cost, difficulties in recruiting appropriate and large enough patient populations for clinical trials, and complex regulatory pathways. More careful analysis of the technologies and more strategic business models may improve the successful translation of CR systems for tissue engineering [104].
References
- 1.Jaklenec A, et al. Progress in the tissue engineering and stem cell industry "are we there yet?". Tissue Eng Part B Rev. 2012;18(3):155–166. doi: 10.1089/ten.TEB.2011.0553. [DOI] [PubMed] [Google Scholar]
- 2.Medtronic. Medtronic INFUSE® Bone Graft + LT-CAGE® Lumbar Tapered Fusion Device Fact Sheet. 2012 http://wwwp.medtronic.com/Newsroom/LinkedItemDetails.do?itemId=1101769224707&itemType=fact_sheet&lang=en_US. [Google Scholar]
- 3.Betz VM, et al. Bone tissue engineering and repair by gene therapy. Front Biosci. 2008;13:833–841. doi: 10.2741/2724. [DOI] [PubMed] [Google Scholar]
- 4.Dang JM, Leong KW. Natural polymers for gene delivery and tissue engineering. Adv Drug Deliv Rev. 2006;58(4):487–499. doi: 10.1016/j.addr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nature Materials. 2009;8(6):457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 6.Tessmar JK, Gopferich AM. Matrices and scaffolds for protein delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59(4–5):274–291. doi: 10.1016/j.addr.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Puleo DA, Kissling RA, Sheu MS. A technique to immobilize bioactive proteins, including bone morphogenetic protein-4 (BMP-4), on titanium alloy. Biomaterials. 2002;23(9):2079–2087. doi: 10.1016/s0142-9612(01)00339-8. [DOI] [PubMed] [Google Scholar]
- 8.Lee M, et al. Modulation of protein delivery from modular polymer scaffolds. Biomaterials. 2007;28(10):1862–1870. doi: 10.1016/j.biomaterials.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Uludag H, et al. Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in the rat ectopic model. J Biomed Mater Res. 1999;46(2):193–202. doi: 10.1002/(sici)1097-4636(199908)46:2<193::aid-jbm8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Jeon O, et al. Enhancement of ectopic bone formation by bone morphogenetic protein-2 released from a heparin-conjugated poly(L-lactic-co-glycolic acid) scaffold. Biomaterials. 2007;28(17):2763–2771. doi: 10.1016/j.biomaterials.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Miller RE, et al. Intraarticular injection of heparin-binding insulin-like growth factor 1 sustains delivery of insulin-like growth factor 1 to cartilage through binding to chondroitin sulfate. Arthritis Rheum. 2010;62(12):3686–3694. doi: 10.1002/art.27709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vidal G, et al. Enhanced cellular adhesion on titanium by silk functionalized with titanium binding and RGD peptides. Acta Biomater. 2013;9(1):4935–4943. doi: 10.1016/j.actbio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanczler JM, et al. The effect of mesenchymal populations and vascular endothelial growth factor delivered from biodegradable polymer scaffolds on bone formation. Biomaterials. 2008;29(12):1892–1900. doi: 10.1016/j.biomaterials.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Kanczler JM, et al. The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials. 2010;31(6):1242–1250. doi: 10.1016/j.biomaterials.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 15.Siepmann F, et al. Polymer blends for controlled release coatings. J Control Release. 2008;125(1):1–15. doi: 10.1016/j.jconrel.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, et al. Controlled release from multilayer silk biomaterial coatings to modulate vascular cell responses. Biomaterials. 2008;29(7):894–903. doi: 10.1016/j.biomaterials.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, et al. Silk coatings on PLGA and alginate microspheres for protein delivery. Biomaterials. 2007;28(28):4161–4169. doi: 10.1016/j.biomaterials.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue W, Bandyopadhyay A, Bose S. Polycaprolactone coated porous tricalcium phosphate scaffolds for controlled release of protein for tissue engineering. J Biomed Mater Res B Appl Biomater. 2009;91(2):831–838. doi: 10.1002/jbm.b.31464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu G, et al. Biomimetic coating of organic polymers with a protein-functionalized layer of calcium phosphate: the surface properties of the carrier influence neither the coating characteristics nor the incorporation mechanism or release kinetics of the protein. Tissue Eng Part C Methods. 2010;16(6):1255–1265. doi: 10.1089/ten.TEC.2009.0588. [DOI] [PubMed] [Google Scholar]
- 20.Lee JS, Suarez-Gonzalez D, Murphy WL. Mineral coatings for temporally controlled delivery of multiple proteins. Adv Mater. 2011;23(37):4279–4284. doi: 10.1002/adma.201100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezwan K, et al. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18):3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 22.Ariga K, et al. Layer-by-layer assembly for drug delivery and related applications. Expert Opin Drug Deliv. 2011;8(5):633–644. doi: 10.1517/17425247.2011.566268. [DOI] [PubMed] [Google Scholar]
- 23.Macdonald ML, et al. Tissue integration of growth factor-eluting layer-by-layer polyelectrolyte multilayer coated implants. Biomaterials. 2011;32(5):1446–1453. doi: 10.1016/j.biomaterials.2010.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wenk E, et al. Microporous silk fibroin scaffolds embedding PLGA microparticles for controlled growth factor delivery in tissue engineering. Biomaterials. 2009;30(13):2571–2581. doi: 10.1016/j.biomaterials.2008.12.073. [DOI] [PubMed] [Google Scholar]
- 25.Kempen DH, et al. Retention of in vitro in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29(22):3245–3252. doi: 10.1016/j.biomaterials.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bian LM, et al. Enhanced MSC chondrogenesis following delivery of TGF-beta 3 from alginate microspheres within hyaluronic acid hydrogels in vitro in vivo. Biomaterials. 2011;32(27):6425–6434. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saif J, et al. Combination of injectable multiple growth factor-releasing scaffolds and cell therapy as an advanced modality to enhance tissue neovascularization. Arterioscler Thromb Vasc Biol. 2010;30(10):1897–1904. doi: 10.1161/ATVBAHA.110.207928. [DOI] [PubMed] [Google Scholar]
- 28.Jaklenec A, et al. Sequential release of bioactive IGF-I and TGF-beta 1 from PLGA microsphere-based scaffolds. Biomaterials. 2008;29(10):1518–1525. doi: 10.1016/j.biomaterials.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Jabbarzadeh E, et al. VEGF-incorporated biomimetic poly(lactide-co-glycolide) sintered microsphere scaffolds for bone tissue engineering. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2012;100B(8):2187–2196. doi: 10.1002/jbm.b.32787. [DOI] [PubMed] [Google Scholar]
- 30.Johnson PJ, et al. Maintaining bioactivity of NGF for controlled release from PLGA using PEG. J Biomed Mater Res A. 2008;86(2):420–427. doi: 10.1002/jbm.a.31635. [DOI] [PubMed] [Google Scholar]
- 31.Park H, et al. Delivery of TGF-beta1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials. 2005;26(34):7095–7103. doi: 10.1016/j.biomaterials.2005.05.083. [DOI] [PubMed] [Google Scholar]
- 32.Lowman A, Peppas N. Hydrogels. In: Mathiowitz E, editor. Encyclopedia of Controlled Drug Delivery. New York: John Wiley and Sons; 1999. [Google Scholar]
- 33.Fortier LA, et al. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84(2):276–288. doi: 10.1302/0301-620x.84b2.11167. [DOI] [PubMed] [Google Scholar]
- 34.Nixon AJ, et al. Enhanced repair of extensive articular defects by insulin-like growth factor-I-laden fibrin composites. Journal of Orthopaedic Research. 1999;17:475–487. doi: 10.1002/jor.1100170404. [DOI] [PubMed] [Google Scholar]
- 35.Gratz KR, et al. Biomechanical assessment of tissue retrieved after in vivo cartilage defect repair: tensile modulus of repair tissue and integration with host cartilage. J Biomech. 2006;39(1):138–146. doi: 10.1016/j.jbiomech.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Borselli C, et al. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci U S A. 2010;107(8):3287–3292. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeon O, et al. Long-term delivery enhances in vivo osteogenic efficacy of bone morphogenetic protein-2 compared to short-term delivery. Biochem Biophys Res Commun. 2008;369(2):774–780. doi: 10.1016/j.bbrc.2008.02.099. [DOI] [PubMed] [Google Scholar]
- 38.Lo KW, et al. Studies of bone morphogenetic protein based surgical repair. Adv Drug Deliv Rev. 2012 doi: 10.1016/j.addr.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orthopaedic and Rehabilitation Devices Advisory Panel, F, Executive Summary for P050036 Medtronic's AMPLIFY rhBMP-2 Matrix. 2010:69. [Google Scholar]
- 40.FDA. Approval letter of INFUSE Bone Graft- P050053. 2007 [Google Scholar]
- 41.FDA. Approval letter for INFUSE Bone Graft- P000054. 2004 [Google Scholar]
- 42.McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft) Int Orthop. 2007;31(6):729–734. doi: 10.1007/s00264-007-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glassman SD, et al. The efficacy of rhBMP-2 for posterolateral lumbar fusion in smokers. Spine (Phila Pa 1976) 2007;32(15):1693–1698. doi: 10.1097/BRS.0b013e318074c366. [DOI] [PubMed] [Google Scholar]
- 44.FDA. Approval letter for OP-1 IMplant- H010002. 2001 [Google Scholar]
- 45.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 46.Boerckel JD, et al. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials. 2011;32(22):5241–5251. doi: 10.1016/j.biomaterials.2011.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown KV, et al. Improving bone formation in a rat femur segmental defect by controlling bone morphogenetic protein-2 release. Tissue Eng Part A. 2011;17(13–14):1735–1746. doi: 10.1089/ten.TEA.2010.0446. [DOI] [PubMed] [Google Scholar]
- 48.Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) J Tissue Eng Regen Med. 2008;2(2–3):81–96. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- 49.Vo TN, Kasper FK, Mikos AG. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev. 2012 doi: 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edelman ER, et al. Controlled and modulated release of basic fibroblast growth factor. Biomaterials. 1991;12(7):619–626. doi: 10.1016/0142-9612(91)90107-l. [DOI] [PubMed] [Google Scholar]
- 51.Laham RJ, et al. Local perivascular delivery of basic fibroblast growth factor in patients undergoing coronary bypass surgery: results of a phase I randomized, double-blind, placebo-controlled trial. Circulation. 1999;100(18):1865–1871. doi: 10.1161/01.cir.100.18.1865. [DOI] [PubMed] [Google Scholar]
- 52.Ruel M, et al. Long-term effects of surgical angiogenic therapy with fibroblast growth factor 2 protein. J Thorac Cardiovasc Surg. 2002;124(1):28–34. doi: 10.1067/mtc.2002.121974. [DOI] [PubMed] [Google Scholar]
- 53.Le KN, et al. Vascular regeneration by local growth factor release is self-limited by microvascular clearance. Circulation. 2009;119(22):2928–2935. doi: 10.1161/CIRCULATIONAHA.108.823609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roh JD, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A. 2010;107(10):4669–4674. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsumura G, et al. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials. 2003;24(13):2303–2308. doi: 10.1016/s0142-9612(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 56.Shin'oka T, et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129(6):1330–1338. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 57.Richardson TP, Murphy WL, Mooney DJ. Polymeric delivery of proteins and plasmid DNA for tissue engineering and gene therapy. Crit Rev Eukaryot Gene Expr. 2001;11(1–3):47–58. [PubMed] [Google Scholar]
- 58.Panyam J, et al. Polymer degradation and in vitro release of a model protein from poly(D,L-lactide-co-glycolide) nano- and microparticles. J Control Release. 2003;92(1–2):173–187. doi: 10.1016/s0168-3659(03)00328-6. [DOI] [PubMed] [Google Scholar]
- 59.Cohen S, et al. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm Res. 1991;8(6):713–720. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- 60.Bae SE, et al. Effect of temporally controlled release of dexamethasone on in vivo chondrogenic differentiation of mesenchymal stromal cells. J Control Release. 2010;143(1):23–30. doi: 10.1016/j.jconrel.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 61.Shi X, et al. Sintered microsphere scaffolds for controlled release and tissue engineering. Pharm Res. 2011;28(5):1224–1228. doi: 10.1007/s11095-010-0359-4. [DOI] [PubMed] [Google Scholar]
- 62.Spiller KL, et al. A novel method for the direct fabrication of growth factor-loaded microspheres within porous nondegradable hydrogels: Controlled release for cartilage tissue engineering. J Control Release. 2012;157(1):39–45. doi: 10.1016/j.jconrel.2011.09.057. [DOI] [PubMed] [Google Scholar]
- 63.DeFail AJ, et al. Controlled release of bioactive TGF-beta 1 from microspheres embedded within biodegradable hydrogels. Biomaterials. 2006;27(8):1579–1585. doi: 10.1016/j.biomaterials.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 64.Holland TA, Tabata Y, Mikos AG. In vitro release of transforming growth factor-beta 1 from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol) fumarate) hydrogels. J Control Release. 2003;91(3):299–313. doi: 10.1016/s0168-3659(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 65.Holland TA, Tabata Y, Mikos AG. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release. 2005;101(1–3):111–125. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 66.Geuze R, et al. A differential effect of BMP-2 and VEGF release timing on osteogenesis at ectopic and orthotopic sites in a large animal model. Tissue Eng Part A. 2012 doi: 10.1089/ten.tea.2011.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freeman I, Cohen S. The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization. Biomaterials. 2009;30(11):2122–2131. doi: 10.1016/j.biomaterials.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 68.Sun Q, et al. Sustained release of multiple growth factors from injectable polymeric system as a novel therapeutic approach towards angiogenesis. Pharm Res. 2010;27(2):264–271. doi: 10.1007/s11095-009-0014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hao X, et al. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc Res. 2007;75(1):178–185. doi: 10.1016/j.cardiores.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 70.Ruvinov E, Leor J, Cohen S. The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials. 2011;32(2):565–578. doi: 10.1016/j.biomaterials.2010.08.097. [DOI] [PubMed] [Google Scholar]
- 71.Simons M, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation. 2002;105(7):788–793. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 72.Henry TD, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107(10):1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 73.Zachary I, Morgan RD. Therapeutic angiogenesis for cardiovascular disease: biological context, challenges, prospects. Heart. 2011;97(3):181–189. doi: 10.1136/hrt.2009.180414. [DOI] [PubMed] [Google Scholar]
- 74.Obradovic B, et al. Integration of engineered cartilage. J Orthop Res. 2001;19(6):1089–1097. doi: 10.1016/S0736-0266(01)00030-4. [DOI] [PubMed] [Google Scholar]
- 75.Fan H, et al. Porous gelatin-chondroitin-hyaluronate tri-copolymer scaffold containing microspheres loaded with TGF-beta1 induces differentiation of mesenchymal stem cells in vivo for enhancing cartilage repair. J Biomed Mater Res A. 2006;77(4):785–794. doi: 10.1002/jbm.a.30647. [DOI] [PubMed] [Google Scholar]
- 76.Garbern JC, et al. Delivery of basic fibroblast growth factor with a pH-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials. 2011;32(9):2407–2416. doi: 10.1016/j.biomaterials.2010.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zisch AH, et al. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17(15):2260–2262. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 78.Ehrbar M, et al. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ Res. 2004;94(8):1124–1132. doi: 10.1161/01.RES.0000126411.29641.08. [DOI] [PubMed] [Google Scholar]
- 79.Phelps EA, et al. Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci U S A. 2010;107(8):3323–3328. doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park J, et al. Nerve regeneration following spinal cord injury using matrix metalloproteinase-sensitive, hyaluronic acid-based biomimetic hydrogel scaffold containing brain-derived neurotrophic factor. J Biomed Mater Res A. 2010;93(3):1091–1099. doi: 10.1002/jbm.a.32519. [DOI] [PubMed] [Google Scholar]
- 81.Terella A, et al. Repair of a Calvarial Defect With Biofactor and Stem Cell-Embedded Polyethylene Glycol Scaffold. Archives of Facial Plastic Surgery. 2010;12(3):166–171. doi: 10.1001/archfacial.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim J, et al. In vivo evaluation of MMP sensitive high-molecular weight HA-based hydrogels for bone tissue engineering. J Biomed Mater Res A. 2010;95(3):673–681. doi: 10.1002/jbm.a.32884. [DOI] [PubMed] [Google Scholar]
- 83.Liu Y, de Groot K, Hunziker EB. BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone. 2005;36(5):745–757. doi: 10.1016/j.bone.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 84.Liu Y, et al. The influence of BMP-2 and its mode of delivery on the osteoconductivity of implant surfaces during the early phase of osseointegration. Biomaterials. 2007;28(16):2677–2686. doi: 10.1016/j.biomaterials.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 85.Hagi TT, et al. Cell-mediated BMP-2 liberation promotes bone formation in a mechanically unstable implant environment. Bone. 2010;46(5):1322–1327. doi: 10.1016/j.bone.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 86.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8(55):153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kloxin AM, et al. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324(5923):59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yavuz MS, et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nature Materials. 2009;8(12):935–939. doi: 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Uesugi Y, et al. An ultrasound-responsive nano delivery system of tissue-type plasminogen activator for thrombolytic therapy. Journal of Controlled Release. 2010;147(2):269–277. doi: 10.1016/j.jconrel.2010.07.127. [DOI] [PubMed] [Google Scholar]
- 90.Deckers R, Moonen CTW. Ultrasound triggered, image guided, local drug delivery. Journal of Controlled Release. 2010;148(1):25–33. doi: 10.1016/j.jconrel.2010.07.117. [DOI] [PubMed] [Google Scholar]
- 91.El-Bialy T, et al. In Vivo Ultrasound-Assisted Tissue-Engineered Mandibular Condyle: A Pilot Study in Rabbits. Tissue Engineering Part C-Methods. 2010;16(6):1315–1323. doi: 10.1089/ten.TEC.2009.0564. [DOI] [PubMed] [Google Scholar]
- 92.Giannoni P, et al. Species variability in the differentiation potential of in vitro-expanded articular chondrocytes restricts predictive studies on cartilage repair using animal models. Tissue Eng. 2005;11(1–2):237–248. doi: 10.1089/ten.2005.11.237. [DOI] [PubMed] [Google Scholar]
- 93.Loeser RF, et al. Reduction in the chondrocyte response to insulin-like growth factor 1 in aging and osteoarthritis: studies in a non-human primate model of naturally occurring disease. Arthritis Rheum. 2000;43(9):2110–2120. doi: 10.1002/1529-0131(200009)43:9<2110::AID-ANR23>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 94.Yasuda A, et al. In vitro culture of chondrocytes in a novel thermoreversible gelation polymer scaffold containing growth factors. Tissue Eng. 2006;12(5):1237–1245. doi: 10.1089/ten.2006.12.1237. [DOI] [PubMed] [Google Scholar]
- 95.Elisseeff J, et al. Controlled-release of IGF-I and TGF-beta1 in a photopolymerizing hydrogel for cartilage tissue engineering. J Orthop Res. 2001;19(6):1098–1104. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 96.Holland TA, et al. Degradable hydrogel scaffolds for in vivo delivery of single and dual growth factors in cartilage repair. Osteoarthritis Cartilage. 2007;15(2):187–197. doi: 10.1016/j.joca.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 97.Gadjanski I, Spiller K, Vunjak-Novakovic G. Time-dependent processes in stem cell-based tissue engineering of articular cartilage. Stem Cell Rev. 2012;8(3):863–881. doi: 10.1007/s12015-011-9328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kempen DH, et al. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30(14):2816–2825. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 99.Patel ZS, et al. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43(5):931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Young S, et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A. 2009;15(9):2347–2362. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Street J, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99(15):9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee JY, et al. Enhanced bone formation by controlled growth factor delivery from chitosan-based biomaterials. J Control Release. 2002;78(1–3):187–197. doi: 10.1016/s0168-3659(01)00498-9. [DOI] [PubMed] [Google Scholar]
- 103.Peng H, et al. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res. 2005;20(11):2017–2027. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- 104.Hunziker E, et al. Translation from research to applications. Tissue Eng. 2006;12(12):3341–3364. doi: 10.1089/ten.2006.12.3341. [DOI] [PubMed] [Google Scholar]
- 105.Moya ML, et al. The effect of FGF-1 loaded alginate microbeads on neovascularization and adipogenesis in a vascular pedicle model of adipose tissue engineering. Biomaterials. 2010;31(10):2816–2826. doi: 10.1016/j.biomaterials.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Simons M. Angiogenesis: where do we stand now? Circulation. 2005;111(12):1556–1566. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]
- 107.Burkhoff D, Jones JW, Becker LC. Variability of myocardial perfusion defects assessed by thallium-201 scintigraphy in patients with coronary artery disease not amenable to angioplasty or bypass surgery. J Am Coll Cardiol. 2001;38(4):1033–1039. doi: 10.1016/s0735-1097(01)01489-9. [DOI] [PubMed] [Google Scholar]
- 108.Wallace H. Coulter Foundation. 2012 http://www.whcf.org/. [Google Scholar]
- 109.Hollister SJ, Murphy WL. Scaffold translation: barriers between concept and clinic. Tissue Eng Part B Rev. 2011;17(6):459–474. doi: 10.1089/ten.teb.2011.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.FDA. Overview of Medical Devices and Their Regulatory Pathways. 2011 http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHTransparency/ucm203018.htm.
- 111.Lee MH, et al. Considerations for tissue-engineered and regenerative medicine product development prior to clinical trials in the United States. Tissue Eng Part B Rev. 2010;16(1):41–54. doi: 10.1089/ten.TEB.2009.0449. [DOI] [PubMed] [Google Scholar]
- 112.FDA. About Combination Products. 2009 http://www.fda.gov/CombinationProducts/AboutCombinationProducts/default.htm.
- 113.FDA. Tissue Engineered Medical Products Standards (TEMPS) 2009 http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Standards/ucm135369.htm.
- 114.FDA. Device advice: Investigation device exemption. 2012 [Google Scholar]
- 115.FDA. PMA Clinical studies. 2010 [Google Scholar]
- 116.FDA. [22 Dec 2012];Tissue Guidance Documents. 2012 http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/default.htm.
- 117.Ramazanoglu M, et al. The effect of combined delivery of recombinant human bone morphogenetic protein-2 and recombinant human vascular endothelial growth factor 165 from biomimetic calcium-phosphate-coated implants on osseointegration. Clin Oral Implants Res. 2011;22(12):1433–1439. doi: 10.1111/j.1600-0501.2010.02133.x. [DOI] [PubMed] [Google Scholar]
- 118.Schmitt CM, et al. Histological results after maxillary sinus augmentation with Straumann(R) BoneCeramic, Bio-Oss(R), Puros(R), and autologous bone. A randomized controlled clinical trial. Clin Oral Implants Res. 2012 doi: 10.1111/j.1600-0501.2012.02431.x. [DOI] [PubMed] [Google Scholar]
- 119.Li J, et al. Repair of rat cranial bone defects with nHAC/PLLA and BMP-2-related peptide or rhBMP-2. J Orthop Res. 2011;29(11):1745–1752. doi: 10.1002/jor.21439. [DOI] [PubMed] [Google Scholar]
- 120.Li M, et al. Calcium phosphate cement with BMP-2-loaded gelatin microspheres enhances bone healing in osteoporosis: a pilot study. Clin Orthop Relat Res. 2010;468(7):1978–1985. doi: 10.1007/s11999-010-1321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kolambkar YM, et al. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials. 2011;32(1):65–74. doi: 10.1016/j.biomaterials.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaipel M, et al. BMP-2 but not VEGF or PDGF in fibrin matrix supports bone healing in a delayed-union rat model. J Orthop Res. 2012 doi: 10.1002/jor.22132. [DOI] [PubMed] [Google Scholar]
- 123.Fujioka-Kobayashi M, et al. Cholesteryl group- and acryloyl group-bearing pullulan nanogel to deliver BMP2 and FGF18 for bone tissue engineering. Biomaterials. 2012 doi: 10.1016/j.biomaterials.2012.06.075. [DOI] [PubMed] [Google Scholar]
- 124.Yamamoto M, Takahashi Y, Tabata Y. Enhanced bone regeneration at a segmental bone defect by controlled release of bone morphogenetic protein-2 from a biodegradable hydrogel. Tissue Eng. 2006;12(5):1305–1311. doi: 10.1089/ten.2006.12.1305. [DOI] [PubMed] [Google Scholar]
- 125.Phillips FM, et al. In vivo BMP-7 (OP-1) enhancement of osteoporotic vertebral bodies in an ovine model. Spine J. 2006;6(5):500–506. doi: 10.1016/j.spinee.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 126.De la Riva B, et al. Local controlled release of VEGF and PDGF from a combined brushite-chitosan system enhances bone regeneration. J Control Release. 2010;143(1):45–52. doi: 10.1016/j.jconrel.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 127.Nakahara T, et al. In situ tissue engineering of periodontal tissues by seeding with periodontal ligament-derived cells. Tissue Eng. 2004;10(3–4):537–544. doi: 10.1089/107632704323061898. [DOI] [PubMed] [Google Scholar]
- 128.Liu XW, et al. Insulin-like growth factor-1 suspended in hyaluronan improves cartilage and subchondral cancellous bone repair in osteoarthritis of temporomandibular joint. Int J Oral Maxillofac Surg. 2011;40(2):184–190. doi: 10.1016/j.ijom.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 129.Batten ML, Hansen JC, Dahners LE. Influence of dosage and timing of application of platelet-derived growth factor on early healing of the rat medial collateral ligament. J Orthop Res. 1996;14(5):736–741. doi: 10.1002/jor.1100140509. [DOI] [PubMed] [Google Scholar]
- 130.Conova L, et al. A pilot study of poly(N-isopropylacrylamide)-g-polyethylene glycol and poly(N-isopropylacrylamide)-g-methylcellulose branched copolymers as injectable scaffolds for local delivery of neurotrophins and cellular transplants into the injured spinal cord. J Neurosurg Spine. 2011;15(6):594–604. doi: 10.3171/2011.7.SPINE11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hiraoka Y, et al. In situ regeneration of adipose tissue in rat fat pad by combining a collagen scaffold with gelatin microspheres containing basic fibroblast growth factor. Tissue Eng. 2006;12(6):1475–1487. doi: 10.1089/ten.2006.12.1475. [DOI] [PubMed] [Google Scholar]
- 132.Vashi AV, et al. Adipose tissue engineering based on the controlled release of fibroblast growth factor-2 in a collagen matrix. Tissue Eng. 2006;12(11):3035–3043. doi: 10.1089/ten.2006.12.3035. [DOI] [PubMed] [Google Scholar]
- 133.Huang Q, et al. In Vivo mesenchymal cell recruitment by a scaffold loaded with transforming growth factor b1 and the potential for in situ chondrogenesis. Tissue Eng. 2002;8(3):469–482. doi: 10.1089/107632702760184727. [DOI] [PubMed] [Google Scholar]
- 134.Zhang G, et al. Controlled release of stromal cell-derived factor-1 alpha in situ increases c-kit+ cell homing to the infarcted heart. Tissue Eng. 2007;13(8):2063–2071. doi: 10.1089/ten.2006.0013. [DOI] [PubMed] [Google Scholar]
- 135.Thevenot PT, et al. The pivotal role of fibrocytes and mast cells in mediating fibrotic reactions to biomaterials. Biomaterials. 2011;32(33):8394–8403. doi: 10.1016/j.biomaterials.2011.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schantz JT, Chim H, Whiteman M. Cell guidance in tissue engineering: SDF-1 mediates site-directed homing of mesenchymal stem cells within three-dimensional polycaprolactone scaffolds. Tissue Eng. 2007;13(11):2615–2624. doi: 10.1089/ten.2006.0438. [DOI] [PubMed] [Google Scholar]
- 137.Chen RR, et al. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res. 2007;24(2):258–264. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- 138.Holland TA, et al. Osteochondral repair in the rabbit model utilizing bilayered, degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds. J Biomed Mater Res A. 2005;75(1):156–167. doi: 10.1002/jbm.a.30379. [DOI] [PubMed] [Google Scholar]
- 139.Yilgor P, et al. An in vivo study on the effect of scaffold geometry and growth factor release on the healing of bone defects. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1456. [DOI] [PubMed] [Google Scholar]
- 140.Matsuse D, et al. Combined transplantation of bone marrow stromal cell-derived neural progenitor cells with a collagen sponge and basic fibroblast growth factor releasing microspheres enhances recovery after cerebral ischemia in rats. Tissue Eng Part A. 2011;17(15–16):1993–2004. doi: 10.1089/ten.TEA.2010.0585. [DOI] [PubMed] [Google Scholar]
- 141.Lutolf MP, et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21(5):513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 142.Wu G, et al. Functionalization of deproteinized bovine bone with a coating-incorporated depot of BMP-2 renders the material efficiently osteoinductive and suppresses foreign-body reactivity. Bone. 2011;49(6):1323–1330. doi: 10.1016/j.bone.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 143.Klenke FM, et al. Sustained release of VEGF from CaP ceramics promotes biomaterial vascularization in vivo. Journal of Bone and Joint Surgery: Orthopaedic Proceedings. 2010;92-B(SUPP IV):617. [Google Scholar]
- 144.Tengood JE, et al. Sequential delivery of vascular endothelial growth factor and sphingosine 1-phosphate for angiogenesis. Biomaterials. 2010;31(30):7805–7812. doi: 10.1016/j.biomaterials.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Govender S, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A(12):2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 146.Swiontkowski MF, et al. Recombinant human bone morphogenetic protein-2 in open tibial fractures. A subgroup analysis of data combined from two prospective randomized studies. J Bone Joint Surg Am. 2006;88(6):1258–1265. doi: 10.2106/JBJS.E.00499. [DOI] [PubMed] [Google Scholar]
- 147.Alt V, et al. A health economic analysis of the use of rhBMP-2 in Gustilo-Anderson grade III open tibial fractures for the UK, Germany, and France. Injury. 2009;40(12):1269–1275. doi: 10.1016/j.injury.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 148.Aro HT, et al. Recombinant human bone morphogenetic protein-2: a randomized trial in open tibial fractures treated with reamed nail fixation. J Bone Joint Surg Am. 2011;93(9):801–808. doi: 10.2106/JBJS.I.01763. [DOI] [PubMed] [Google Scholar]
- 149.Burkus JK, et al. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine (Phila Pa 1976) 2002;27(21):2396–2408. doi: 10.1097/00007632-200211010-00015. [DOI] [PubMed] [Google Scholar]
- 150.Baskin DS, et al. A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the CORNERSTONE-SR allograft ring and the ATLANTIS anterior cervical plate. Spine (Phila Pa 1976) 2003;28(12):1219–1224. doi: 10.1097/01.BRS.0000065486.22141.CA. discussion 1225. [DOI] [PubMed] [Google Scholar]
- 151.Shields LB, et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine (Phila Pa 1976) 2006;31(5):542–547. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 152.Burkus JK, et al. Use of rhBMP-2 in combination with structural cortical allografts: clinical and radiographic outcomes in anterior lumbar spinal surgery. J Bone Joint Surg Am. 2005;87(6):1205–1212. doi: 10.2106/JBJS.D.02532. [DOI] [PubMed] [Google Scholar]
- 153.Glassman SD, et al. RhBMP-2 versus iliac crest bone graft for lumbar spine fusion: a randomized, controlled trial in patients over sixty years of age. Spine (Phila Pa 1976) 2008;33(26):2843–2849. doi: 10.1097/BRS.0b013e318190705d. [DOI] [PubMed] [Google Scholar]
- 154.FDA. InFUSE™ Bone Graft/LT-CAGE™ Lumbar Tapered Fusion Devices - P000058. 2002 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfTopic/pma/pma.cfm?num=P000058.
- 155.Boden SD, et al. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976) 2002;27(23):2662–2673. doi: 10.1097/00007632-200212010-00005. [DOI] [PubMed] [Google Scholar]
- 156.Glassman SD, et al. Initial fusion rates with recombinant human bone morphogenetic protein-2/compression resistant matrix and a hydroxyapatite and tricalcium phosphate/collagen carrier in posterolateral spinal fusion. Spine (Phila Pa 1976) 2005;30(15):1694–1698. doi: 10.1097/01.brs.0000172157.39513.80. [DOI] [PubMed] [Google Scholar]
- 157.Dimar JR, et al. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine (Phila Pa 1976) 2006;31(22):2534–2539. doi: 10.1097/01.brs.0000240715.78657.81. discussion 2540. [DOI] [PubMed] [Google Scholar]
- 158.Mulconrey DS, et al. Bone morphogenetic protein (RhBMP-2) as a substitute for iliac crest bone graft in multilevel adult spinal deformity surgery: minimum two-year evaluation of fusion. Spine (Phila Pa 1976) 2008;33(20):2153–2189. doi: 10.1097/BRS.0b013e31817bd91e. [DOI] [PubMed] [Google Scholar]
- 159.Dawson E, et al. Recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge with an osteoconductive bulking agent in posterolateral arthrodesis with instrumentation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1604–1613. doi: 10.2106/JBJS.G.01157. [DOI] [PubMed] [Google Scholar]
- 160.Friedlaender GE. OP-1 clinical studies. J Bone Joint Surg Am. 2001;83-A(Suppl 1)(Pt 2):S160–S161. [PubMed] [Google Scholar]
- 161.Friedlaender GE, et al. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A(Suppl 1)(Pt 2):S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 162.Geesink RG, Hoefnagels NH, Bulstra SK. Osteogenic activity of OP-1 bone morphogenetic protein (BMP-7) in a human fibular defect. J Bone Joint Surg Br. 1999;81(4):710–718. doi: 10.1302/0301-620x.81b4.9311. [DOI] [PubMed] [Google Scholar]
- 163.Ristiniemi J, et al. RhBMP-7 accelerates the healing in distal tibial fractures treated by external fixation. J Bone Joint Surg Br. 2007;89(2):265–272. doi: 10.1302/0301-620X.89B2.18230. [DOI] [PubMed] [Google Scholar]
- 164.Calori GM, et al. Application of rhBMP-7 and platelet-rich plasma in the treatment of long bone non-unions: a prospective randomised clinical study on 120 patients. Injury. 2008;39(12):1391–1402. doi: 10.1016/j.injury.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 165.Johnsson R, Stromqvist B, Aspenberg P. Randomized radiostereometric study comparing osteogenic protein-1 (BMP-7) and autograft bone in human noninstrumented posterolateral lumbar fusion: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976) 2002;27(23):2654–2661. doi: 10.1097/00007632-200212010-00004. [DOI] [PubMed] [Google Scholar]
- 166.Vaccaro AR, et al. A pilot safety and efficacy study of OP-1 putty (rhBMP-7) as an adjunct to iliac crest autograft in posterolateral lumbar fusions. Eur Spine J. 2003;12(5):495–500. doi: 10.1007/s00586-003-0561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Furlan JC, et al. Use of osteogenic protein-1 in patients at high risk for spinal pseudarthrosis: a prospective cohort study assessing safety, health-related quality of life, and radiographic fusion. Invited submission from the Joint Section on Disorders of the Spine and Peripheral Nerves, March 2007. J Neurosurg Spine. 2007;7(5):486–495. doi: 10.3171/SPI-07/09/486. [DOI] [PubMed] [Google Scholar]
- 168.Leach J, Bittar RG. BMP-7 (OP-1) safety in anterior cervical fusion surgery. J Clin Neurosci. 2009;16(11):1417–1420. doi: 10.1016/j.jocn.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 169.Kanayama M, et al. A prospective randomized study of posterolateral lumbar fusion using osteogenic protein-1 (OP-1) versus local autograft with ceramic bone substitute: emphasis of surgical exploration and histologic assessment. Spine (Phila Pa 1976) 2006;31(10):1067–1074. doi: 10.1097/01.brs.0000216444.01888.21. [DOI] [PubMed] [Google Scholar]
- 170.FDA. OP-1 Putty - H020008. 2004 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cftopic/pma/pma.cfm?num=H020008.
- 171.Irokawa D, et al. Effect of beta tricalcium phosphate particle size on recombinant human platelet-derived growth factor-BB-induced regeneration of periodontal tissue in dog. Dent Mater J. 2010;29(6):721–730. doi: 10.4012/dmj.2010-033. [DOI] [PubMed] [Google Scholar]
- 172.FDA. Approval letter for GEM 21S (Growth-factor Enhanced Matrix) - P040013. 2005 [Google Scholar]