ABSTRACT
Human norovirus (NoV) is the most frequent cause of epidemic nonbacterial acute gastroenteritis worldwide. We investigated the impact of nonthermal or cold atmospheric pressure plasma (CAPP) on the inactivation of a clinical human outbreak NoV, GII.4. Three different dilutions of a NoV-positive stool sample were prepared and subsequently treated with CAPP for various lengths of time, up to 15 min. NoV viral loads were quantified by quantitative real-time reverse transcription PCR (RT-qPCR). Increased CAPP treatment time led to increased NoV reduction; samples treated for the longest time had the lowest viral load. From the initial starting quantity of 2.36 × 104 genomic equivalents/ml, sample exposure to CAPP reduced this value by 1.23 log10 and 1.69 log10 genomic equivalents/ml after 10 and 15 min, respectively (P < 0.01). CAPP treatment of surfaces carrying a lower viral load reduced NoV by at least 1 log10 after CAPP exposure for 2 min (P < 0.05) and 1 min (P < 0.05), respectively. Our results suggest that NoV can be inactivated by CAPP treatment. The lack of cell culture assays prevents our ability to estimate infectivity. It is possible that some detectable, intact virus particles were rendered noninfectious. We conclude that CAPP treatment of surfaces may be a useful strategy to reduce the risk of NoV transmission in crowded environments.
Importance Human gastroenteritis is most frequently caused by noroviruses, which are spread person to person and via surfaces, often in facilities with crowds of people. Disinfection of surfaces that come into contact with infected humans is critical for the prevention of cross-contamination and further transmission of the virus. However, effective disinfection cannot be done easily in mass catering environments or health care facilities. We evaluated the efficacy of cold atmospheric pressure plasma, an innovative airborne disinfection method, on surfaces inoculated with norovirus. We used a clinically relevant strain of norovirus from an outbreak in Germany. Cold plasma was able to inactivate the virus on the tested surfaces, suggesting that this method could be used for continuous disinfection of contaminated surfaces. The use of a clinical strain of norovirus strengthens the reliability of our results as it is a strain relevant to outbreaks in humans.
Importance
Human gastroenteritis is most frequently caused by noroviruses, which are spread person to person and via surfaces, often in facilities with crowds of people. Disinfection of surfaces that come into contact with infected humans is critical for the prevention of cross-contamination and further transmission of the virus. However, effective disinfection cannot be done easily in mass catering environments or health care facilities. We evaluated the efficacy of cold atmospheric pressure plasma, an innovative airborne disinfection method, on surfaces inoculated with norovirus. We used a clinically relevant strain of norovirus from an outbreak in Germany. Cold plasma was able to inactivate the virus on the tested surfaces, suggesting that this method could be used for continuous disinfection of contaminated surfaces. The use of a clinical strain of norovirus strengthens the reliability of our results as it is a strain relevant to outbreaks in humans.
Observation
Recently, human norovirus (NoV) infection has been the most commonly identified cause for nonbacterial epidemic gastroenteritis outbreaks in Germany (1). Epidemic outbreaks of NoV occur in communities, military barracks, cruise ships, hospitals, and assisted living communities (2). Over 19 million cases of illness in the United States (3) and 110,000 infections in Germany (1) are verified by laboratory diagnosis each year. The estimated number of unreported cases is considerably higher. Annually, the costs of health care and lost productivity due to foodborne illness caused by NoV account for approximately $2 billion in the United States (3).
Norovirus, a member of the Caliciviridae family, is a single-stranded nonenveloped (positive)-stranded icosahedral RNA virus with a diameter of approximately 35 to 39 nm and a high variability of structural proteins in and around the receptor-binding domain. To date, five NoV genogroups (GI to GV) have been described; viruses of genogroups GI, GII, and GIV are known to infect humans. Of those three genogroups, GII most frequently causes human infections. Currently, genogroup GII contains 17 genotypes. GII.4 is the most widespread genotype and predominates in pandemics and outbreaks (2).
Person-to-person transmission is of great importance during NoV outbreaks and can lead to a vast number of infected persons. The infective dose of NoV is very low (4). Furthermore, NoV is extremely stable in the environment, showing resistance to detergent-based cleaning and disinfection with chlorine (5) and heating to 65°C, freezing, and acidification (6, 7). In addition, the matrix of NoV-containing samples may have protective effects on virus survival in inactivation studies (8, 9). Currently, clinical NoV strains can be obtained only using vomitus or feces. Hence, the search for effective disinfectants is highly complicated by these natural substrates.
As an innovative decontamination technology, cold or nonthermal atmospheric pressure plasma (CAPP) can be applied. Plasma is the fourth state of matter, defined as a partially or completely ionized gas that can be generated by applying an electrical field to an initially electrically neutral gas (10). The inactivation of the virus particles functions through synergy effects of the cold plasma-initiated air chemistry, which consists of nitric oxide (NO) (including its intermediates, NO radicals, NO−, and NO+, and adducts, NO2, NO2−, NO3−, N2O3, N2O4, and ONOO−) and reactive oxygen species (including ozone, atomic oxygen, singlet oxygen, and oxygen ions, which can have antimicrobial effects). The impact of CAPP on bacteria, some fungi, and yeast was already described by Fridman et al. (11). However, the effects of CAPP on viruses are relatively unexplored (12). In the present study, we used a cold atmosphere pressure plasma device (FlatPlaSter 2.0) to investigate the impact of CAPP on a clinically relevant NoV strain.
To our knowledge, this study represents the first direct treatment of a human NoV, GII.4 from stool suspension, with CAPP. Quantitative real-time reverse transcription PCR (RT-qPCR) of serial dilutions of the samples revealed that initial viral load (VL) was approximately 2.4 × 107 virus particles per ml (Fig. 1). In order to determine the effectiveness of the inactivation of NoV GII.4 by exposure to CAPP, we prepared three different stool dilutions. The quantities of NoV were determined as cycle threshold (CT) values using RT-qPCR (13) and subsequently converted into genomic equivalents.
FIG 1 .
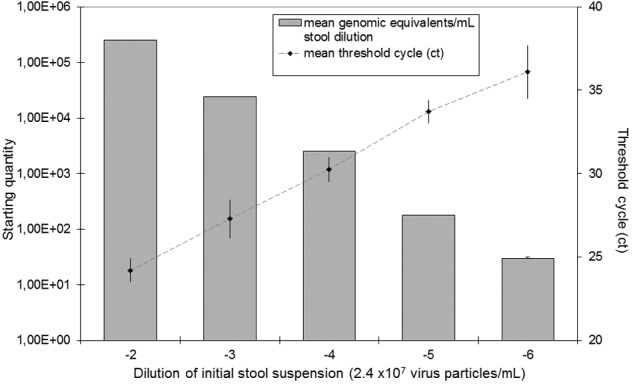
Standard curve of the norovirus quantification assay.
We found that increased plasma treatment times led to decreased copy numbers of NoV. In this case, we show log reduction compared to the nontreated sample (Fig. 2; see also Table S2 in the supplemental material). Depending upon the initial viral load, we observed a reduction of up to 1.69 log10 after CAPP treatment (P < 0.05). Lower dilutions of the initial stool suspension led to initial starting quantities of 2.36 × 104 genomic equivalents/ml. Here, a statistically significant reduction of virus particles was shown only after exposure to CAPP for 10 and 15 min, which caused 1.23 log10 and 1.69 log10 decreases compared with the control, respectively (P < 0.01). In contrast, CAPP treatment of lower starting quantities (subsequent dilutions of the stool suspension) of 1.1 × 103 genomic equivalents/ml and 3.67 × 101 genomic equivalents/ml reduced viral load by at least 10-fold after CAPP exposure for 2 min and 1 min, respectively (P < 0.05). Hence, viral load and matrix effects (feces and phosphate-buffered saline [PBS] solution) may influence the effect that CAPP treatment has on NoV particles. Similarly to this study, Li et al. (14) used PBS-diluted fecal suspension to test heat inactivation of noroviruses. The authors indicated that the stool could have complex components which may have protective effects during heat treatment of NoV. It is already known that the matrix of NoV suspension in principle could have protective effects on virus survival, as seen in other inactivation experiments for bacteria and viruses (8, 9). However, another study reports that the presence of added serum and stool from a NoV-positive sample did not adversely affect the reduction of feline calicivirus (FCV) by treatment with ozone gas (15). This leads to the conclusion that effective NoV inactivation is affected by matrix effects as well as the disinfection method used.
FIG 2 .
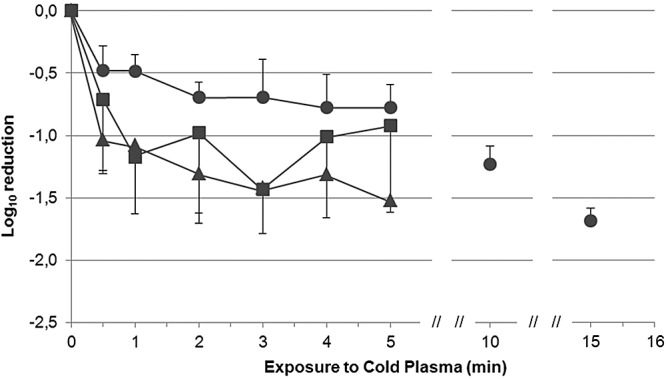
Effect of treatment time and sample dilution on norovirus viral load after CAPP treatment. The viral load (VL) after exposure to CAPP was defined as VL = log10(Nt/N0), where Nt represents the noroviral load after a session of CAPP treatment and N0 is the initial viral load. Results for the inactivation of norovirus are expressed in logarithmic units (log10 cycle): ●, dilution −2, initial starting quantity of 2.36 × 104 genomic equivalents/ml dilution; ▲, dilution −3, initial starting quantity of 1.10 × 103 genomic equivalents/ml dilution; ■, dilution −4, initial starting quantity of 3.67 × 101 genomic equivalents/ml.
Pooling of all samples treated with the same treatment time regardless of the previous dilution showed a statistically significant reduction for all treatment times compared to the untreated control (P < 0.01). Hence, the significance was not always shown for the unpooled samples. The reduction of approximately 10-fold occurred after CAPP application for at least 2 min (P < 0.001). Similar results were obtained by Hudson et al. (15), who evaluated the ability of ozone gas to inactivate NoV and FCV. These authors found that ozone treatment reduced the viral load by approximately 1.2 log10 steps for human NoV. The authors reported even greater reduction for FCV, as estimated by RT-qPCR (approximately 1.6-log10 step reduction) and plaque assays (approximately 1.8-log10 step reduction). Sánchez et al. (16) tested the ability of high-hydrostatic-pressure processing (HPP) to inactivate murine NoV-1 (MNV) and NoV GII.4. HPP treatment with 450 MPa for 15 min was sufficient to inactivate 6.5 log10 steps of MNV determined in culture medium. However, HPP treatments reduced numbers of NoV by <0.5 log10 units detected by RT-qPCR, regardless of the length of exposure time. Taken together, the results from our study and others suggest that NoV and related viruses vary in their susceptibility to disinfectant methods, but the CAPP treatment reduced the viral load by at least 10-fold.
It is obvious that this RT-qPCR assay has limitations, as it does not give any indication with respect to the viability and infectivity of the residual viral RNA (5). The lack of cell culture methods or suitable laboratory animals, and consequently the inability to replicate the virus in vitro for infectivity assays, is a limitation. RT-qPCR does not measure infectivity per se but rather a defined short sequence of the viral genome which could be more resistant to inactivation trials than infectivity. Thus, the decline of infectivity observed using an RT-qPCR assay could be underestimated due to possible alterations in capsid function that remain RNase resistant but have lost infectivity (15).
Attempts to replicate human NoV have been made (17), but with no success to date. RT-qPCR is the most sensitive and specific method for the detection of NoV (13), but evaluation of infectivity can be made only with volunteer studies (18). The heating of samples for 10 min at 95°C as negative controls led to only modest inactivation rates when RNase digestion was not carried out prior to elution. The digestion of free RNA after heat treatment led to undetectable amounts of NoV (see Table S1 in the supplemental material). The digestion of free RNA after treatment was shown to be a suitable model to study the inactivation of virus particles without cell culture (8) and therefore enables a cautious but robust estimation for the comparison of different genogroups and genotypes. The RNase assay used in our study was validated using dilutions of a NoV GII.4-positive fecal sample after long-term storage showing good differentiation between total viral RNA and disrupted viral particles after treatment, such as heating (see Table S1). This tool is essential because of high antigenic variability of certain strains, which leads to their persistence in human populations (19).
The majority of chemical disinfection studies and studies on processing interventions have been carried out using FCV and murine NoV-1 (MNV), which are cultivable viruses that belong to the family Caliciviridae with single-stranded RNA (ssRNA), as surrogates for NoV (15, 16). However, the susceptibility of FCV and MNV to different treatments varies considerably. Furthermore, NoV strains or genogroups differ from each other in their susceptibility to disinfecting treatments (20). Therefore, we agree with Richards (18) that FCV and MCV are poor surrogates for human NoV, particularly with regard to inactivation studies. For outbreak control, a rapid reaction is essential, to avoid spreading and cross-contamination by the virus. Regular cleaning and disinfection are effective but need time and preparation of the surfaces. To date, only the use of sodium hypochlorite as a chemical disinfectant allows a significant inactivation of human NoV strain GII on stainless steel (9). Because sodium hypochlorite treatment may leave residual chemicals, the effective inactivation of NoV using CAPP seems to be an attractive alternative, particularly in food production and health care settings. With CAPP application, an immediate decontamination can be achieved. The reduction of the NoV load by 1- to 2-log10 genomic equivalents/ml decreases the probability of infection of humans significantly (4). In addition, the application of CAPP as a preventive measure (continuous application on high-risk surfaces) can help to avoid outbreaks in mass catering facilities. Therefore, even a reduction lower than the virus safety step defined as a 4-log reduction has its value in immediate outbreak control or as a preventive measure.
The use of native outbreak samples in this study simulates the complicated in vivo conditions and the challenges of hygiene and disinfection that are necessary to interrupt the chain of infection and prevent outbreaks. The first studies on the use of CAPP showed an effect not only on surfaces but also on tissues. Despite a concern for potential mutagenicity of human tissues after plasma treatment, excised human skin showed no significant increase in the number of DNA double-strand breaks subsequent to CAPP treatment (21). Thus, CAPP treatment not only might overcome food matrix effects but also might enable an additional approach for preventive measures in outbreak situations. In practical application, a handheld device that can be used to disinfect different surfaces (worktop, handle, and tablet) as well as a plasma box for hands and requisites is conceivable. In future studies, we will test the disinfection properties on additional surfaces and NoV genotypes and examine the virus capsid structure before and after CAPP treatment.
Conclusions.
In summary, this is a comprehensive study on the impact of CAPP treatment on human norovirus GII.4. The results showed that CAPP treatment reduced significantly the viral load of NoV; however, the initial viral load may influence the efficacy of CAPP treatment. Thus, regardless of previous dilutions, a significant reduction of approximately 1 log10 step after CAPP treatment of at least 2 min was possible. The RT-qPCR assay has limitations. For example, this assay only detects and quantifies the amount of viral RNA; it does not estimate the viability or infectivity of intact viral particles. The use of an RNase pretreatment helps to control for disrupted viral particles and improves the estimations. CAPP treatment effectively reduced the amount of a clinically relevant outbreak strain, NoV II.4, without any chemical residues.
Strain and samples.
The fecal sample used as a NoV source was derived from a NoV outbreak in a German military facility. During October 2011, 27 soldiers fell ill in a suspected foodborne outbreak with gastrointestinal symptoms (nausea, diarrhea, and circulatory disorders). The microbiological investigation of served meals did not detect bacterial pathogens or toxins responsible for the outbreak. NoV genotype GII.4 was detected from stool samples using quantitative real-time reverse transcription PCR (RT-qPCR) (13) and confirmed by sequencing and typing (22). Sequence typing confirmed that NoV GII.4, a variant first discovered in New Orleans in 2009, was the causative agent of disease. This NoV strain was very similar to an outbreak strain from 2011 which was detected during a nosocomial outbreak in a military hospital. Further, GII.4 is the most common strain in Europe. Other relevant enteropathogens (Salmonella, Shigella, Yersinia, Campylobacter, enterohemorrhagic Escherichia coli [EHEC], Clostridium perfringens, Bacillus cereus, rotavirus [RotaV], and adenovirus [AdenoV]) as well as staphylococcal enterotoxins were excluded based on cultural or molecular methods. Viral load was estimated according to most probable number (MPN). MPN is a statistical tool that determines the most probable number of microorganisms based on the proportion of virus-positive samples to the total number of samples after serial decimal dilutions. For this study, the concentration of viruses was calculated from the positive/negative results of RT-qPCR. This method is used widely in some fields of microbiological research, including those on norovirus (23). Briefly, the NoV-positive sample was diluted with Dulbecco’s PBS without Ca2+ (Biochrom AG, Berlin, Germany) by a series of decimal dilutions. After extraction of nucleic acids, each dilution was subjected to RT-qPCR in triplicate as described below. RT-qPCR was performed on subsequent dilutions until all three tubes were virus negative. The number of viral particles was calculated due to CT values after RT-qPCR, including the adjustment of the volume used for detection (23).
For exposure experiments, the stool sample was diluted with Dulbecco’s phosphate-buffered saline (PBS) without Ca2+ (Biochrom AG, Berlin, Germany), such that approximately 102, 103, and 104 virus particles were spotted in a 100-µl total volume on a sterile petri dish. Samples were dried for 30 to 45 min at ambient temperature (23 to 24°C). Once dry, the samples were exposed to cold plasma for 0.5, 1, 2, 3, 4, 5, 10, or 15 min. Each run included four controls as follows: the RNase-untreated, unheated sample representing the initial viral load showed total NoV RNA; the RNase-untreated, heated sample showed detectable free RNA after virus destruction; the RNase-treated, unheated sample (as positive control); and the RNase-treated, heated sample confirmed effective RNase activity after complete virus destruction. Heat treatment was performed at 95°C for 10 min on each dilution prior to spotting on a sterile petri dish. CAPP-treated samples were subsequently handled using the same protocol as that described for the controls. Norovirus counts below the detection limit (cycle threshold [CT], 40) were integrated with 0.1 log10 in the calculation. Generally, the viral load (VL) after exposure to CAPP was defined as VL = log10(Nt/N0), where Nt represents the noroviral load after a session of CAPP treatment and N0 is the initial viral load. Results for the inactivation of norovirus are expressed in logarithmic units (log10 cycle). Each experiment was performed in triplicate.
Plasma dispenser.
In this study, a cold atmosphere pressure plasma device (FlatPlaSter 2.0) based on a surface microdischarge (SMD) technology was used. Details of the device can be found in the publication by Klämpfl et al. (24). The plasma dispenser which was used for this experiment is presented in Fig. S1a in the supplemental material. The housing box (polyoxymethylene copolymer; Goodfellow) of the dispenser has dimensions of 15 cm by 9 cm by 20 cm, and it is designed to confine the plasma gas for laboratory tests only. The SMD electrode consisted of a three-layer structure: a copper plate as the high-voltage (HV) electrode, a stainless steel mesh (6 per inch) with a wire diameter of 0.5 mm as the grounded electrode, and an 0.5-mm Teflon sheet in between as the dielectric material. The SMD electrode was mounted at the upper part of the housing box (see Fig. S1a). For plasma generation, the sinusoidal signal from a Voltcraft 8202 function generator was amplified by a Trek PM4015 HV amplifier before application to the HV electrode of the plasma dispenser. The peak-to-peak voltage and frequency were measured at 8.5 kV and 1 kHz, respectively, using a Tektronix TDS 2024B oscilloscope. The power consumption of the plasma discharge was approximately 30 mW/cm2, which was estimated using the Lissajous figure method. Plasma, which can be seen as a purple glow when the plasma dispenser is operated in an ambient-air environment (see Fig. S1b), was produced on the mesh electrode side. For a practical application onto a surface, the SMD electrode can be used without the housing box, so that it can be held by hand to apply the surface treatment. In that case, the electric power supply unit can be integrated into the SMD electrode. This concept for application is shown in Fig. S1c in the supplemental material.
Prepared samples were placed inside the plasma dispenser. The distance from the mesh electrode, where the plasma was produced, to the sample surface was approximately 3 mm. Plasma treatment was conducted under ambient conditions; the room temperature and relative humidity were 23.5°C and 40%, respectively.
Virus recovery, RNA extraction, and detection.
After exposure to cold plasma, the samples were processed according to the method of Scherer et al. (25). Briefly, the samples were moistened using nylon-flocked swabs (Copan Flock Technologies, Brescia, Italy). The virus-containing swabs were incubated at 56°C in 550 µl ATL buffer (Qiagen, Hilden, Germany) for 15 min at 1,000 rpm in a stirrer (Thermomixer; Eppendorf, Wesseling-Berzdorf, Germany). In order to digest free RNA from destroyed virus particles, 0.035 µl RNase A (concentrated solution, 7,000 U/ml, added in working dilution; Qiagen, Hilden, Germany) was added and incubated for 60 min at 37°C at 500 rpm. RNase digestion was subsequently stopped with 0.33 µl RNase inhibitor (2,000 U/ml; Qiagen) for 30 min at room temperature. RNA from intact virus particles was eluted automatically on the QIASymphony (protocol Complex_200_default_IC, virus/bacterium kit; Qiagen), according to the instructions of the manufacturer with addition of carrier RNA. The eluates were evaluated for the presence of NoV GII using RT-qPCR (13).
Statistical analysis.
The aim of this study was to explore correlation between the CAPP treatment time and the NoV concentration. Based on the initial viral concentration estimates, the CT values produced by RT-qPCR were converted to the corresponding genomic equivalents for further calculations. Samples with CT values less than 41 and a sigmoidal amplification plot were considered suitable for analysis. The level of significance of the study was set at α = 0.05. The level of P was adjusted using the Bonferroni correction to account for multiple comparisons. Genomic equivalents were transformed into log values. Means and standard deviations were calculated with the PROC UNIVARIATE function. Analysis of variance for repeated measurement was performed to determine variations in genomic equivalents among control samples and samples exposed to plasma with different treatment times Student’s t test was used to determine significance. Data were processed with Excel (Microsoft) and analyzed statistically with SAS Enterprise guide version 4.3 (Statistic Analyzing Systems; SAS Institute Inc., Cary, NC, USA). Furthermore, to boost the statistical power of the tests, we pooled all samples treated with identical treatment times regardless of previous dilution steps.
SUPPLEMENTAL MATERIAL
Plasma dispenser used for the norovirus treatment. The electric connections are in the back of the housing box, and NoV samples were put into the plasma box from the front opening. The lid was closed during the plasma treatment. Download
Image of the plasma box with cold plasma switched on. The purple glow is the emission from cold plasma. Download
Concept for practical application: the SMD electrode held by hand. In practical application, the cold plasma electrode can be designed as a handheld device so that it can be easily applied to different surfaces. Download
RNase digestion of free RNA for discrimination between destroyed and intact norovirus after heat treatment (data given as log10 values); n.d., not done; -, negative.
Effect of treatment time and sample dilution on norovirus viral load after CAPP treatment (data given as log10 values); n.d., not done.
ACKNOWLEDGMENTS
We give special thanks to Karin Lübbert, member of the Central Institute of the Bundeswehr Medical Service, for excellent support in performing the RT-qPCR assays.
G. Morfill, Y. Li, and J. L. Zimmermann participated in this research while employed by the Max Planck Society. All three are now founder members of a private company (terraplasma GmbH) which is engaged in cold plasma developments. There is no conflict of interest in connection with this publication.
Footnotes
Citation Ahlfeld B, Li Y, Boulaaba A, Binder A, Schotte U, Zimmermann JL, Morfill G, Klein G. 2015. Inactivation of a foodborne norovirus outbreak strain with nonthermal atmospheric pressure plasma. mBio 6(1):e02300-14. doi:10.1128/mBio.02300-14.
REFERENCES
- 1.Robert Koch Institute 2013. Epidemiological yearbook of notifiable infectious diseases for 2012. Robert Koch Institute, Berlin, Germany: (In German.) [Google Scholar]
- 2.Donaldson EF, Lindesmith LC, Lobue AD, Baric RS. 2008. Norovirus pathogenesis: mechanisms of persistence and immune evasion in human populations. Immunol Rev 225:190–211. doi: 10.1111/j.1600-065X.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention 2014. Burden of norovirus illness and outbreaks. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 4.Teunis PF, Moe CL, Liu P, Miller SE, Lindesmith L, Baric RS, Le Pendu J, Calderon RL. 2008. Norwalk virus: how infectious is it? J Med Virol 80:1468–1476. doi: 10.1002/jmv.21237. [DOI] [PubMed] [Google Scholar]
- 5.Barker J, Vipond IB, Bloomfield SF. 2004. Effects of cleaning and disinfection in reducing the spread of norovirus contamination via environmental surfaces. J Hosp Infect 58:42–49. doi: 10.1016/j.jhin.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 6.Baert L, Uyttendaele M, Van Coillie E, Debevere J. 2008. The reduction of murine norovirus 1, B. fragilis HSP40 infecting phage B40-8 and E. coli after a mild thermal pasteurization process of raspberry puree. Food Microbiol 25:871–874. doi: 10.1016/j.fm.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Baert L, Wobus CE, Van Coillie E, Thackray LB, Debevere J, Uyttendaele M. 2008. Detection of murine norovirus 1 by using plaque assay, transfection assay, and real-time reverse transcription-PCR before and after heat exposure. Appl Environ Microbiol 74:543–546. doi: 10.1128/AEM.01039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mormann S, Dabisch M, Becker B. 2010. Effects of technological processes on the tenacity and inactivation of norovirus genogroup II in experimentally contaminated foods. Appl Environ Microbiol 76:536–545. doi: 10.1128/AEM.01797-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowak P, Topping JR, Bellamy K, Fotheringham V, Gray JJ, Golding JP, Wiseman G, Knight AI. 2011. Virolysis of feline calicivirus and human GII.4 norovirus following chlorine exposure under standardized light soil disinfection conditions. J Food Prot 74:2113–2118. doi: 10.4315/0362-028X.JFP-11-087. [DOI] [PubMed] [Google Scholar]
- 10.Wan J, Coventry J, Swiergon P, Sanguansri P, Versteeg C. 2009. Advances in innovative processing technologies for microbial inactivation and enhancement of food safety—pulsed electric field and low-temperature plasma. Trends Food Sci Technol 20:414–424. doi: 10.1016/j.tifs.2009.01.050. [DOI] [Google Scholar]
- 11.Fridman G, Brooks AD, Balasubramanian M, Fridman A, Gutsol A, Vasilets VN, Ayan H, Friedman G. 2007. Comparison of direct and indirect effects of non-thermal atmospheric-pressure plasma on bacteria. Plasma Process Polym 4:370–375. doi: 10.1002/ppap.200600217. [DOI] [Google Scholar]
- 12.Zimmermann JL, Dumler K, Shimizu T, Morfill GE, Wolf A, Boxhammer V, Schlegel J, Gansbacher B, Anton M. 2011. Effects of cold atmospheric plasmas on adenoviruses in solution. J Phys D Appl Phys 44:505201. doi: 10.1088/0022-3727/44/50/505201. [DOI] [Google Scholar]
- 13.Höhne M, Schreier E. 2004. Detection and characterization of norovirus outbreaks in Germany: application of a one-tube RT-PCR using a fluorogenic real-time detection system. J Med Virol 72:312–319. doi: 10.1002/jmv.10573. [DOI] [PubMed] [Google Scholar]
- 14.Li D, Baert L, Xia M, Zhong W, Van Coillie E, Jiang X, Uyttendaele M. 2012. Evaluation of methods measuring the capsid integrity and/or functions of noroviruses by heat inactivation. J Virol Methods 181:1–5. doi: 10.1016/j.jviromet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Hudson JB, Sharma M, Petric M. 2007. Inactivation of norovirus by ozone gas in conditions relevant to healthcare. J Hosp Infect 66:40–45. doi: 10.1016/j.jhin.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez G, Aznar R, Martínez A, Rodrigo D. 2011. Inactivation of human and murine norovirus by high-pressure processing. Foodborne Pathog Dis 8:249–253. doi: 10.1089/fpd.2010.0667. [DOI] [PubMed] [Google Scholar]
- 17.Herbst-Kralovetz MM, Radtke AL, Lay MK, Hjelm BE, Bolick AN, Sarker SS, Atmar RL, Kingsley DH, Arntzen CJ, Estes MK, Nickerson CA. 2013. Lack of norovirus replication and histo-blood group antigen expression in 3-dimensional intestinal epithelial cells. Emerg Infect Dis 19:431–438. doi: 10.3201/eid1903.121029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richards GP. 2012. Critical review of norovirus surrogates in food safety research: rationale for considering volunteer studies. Food Environ Virol 4:6–13. doi: 10.1007/s12560-011-9072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donaldson EF, Lindesmith LC, Lobue AD, Baric RS. 2010. Viral shape-shifting: norovirus evasion of the human immune system. Nat Rev Microbiol 8:231–241. doi: 10.1038/nrmicro2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maalouf H, Schaeffer J, Parnaudeau S, Le Pendu J, Atmar RL, Crawford SE, Le Guyader FS. 2011. Strain-dependent norovirus bioaccumulation in oysters. Appl Environ Microbiol 77:3189–3196. doi: 10.1128/AEM.03010-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isbary G, Köritzer J, Mitra A, Li Y-, Shimizu T, Schroeder J, Schlegel J, Morfill GE, Stolz W, Zimmermann JL. 2013. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med 1:36–44. doi: 10.1016/j.cpme.2012.10.001. [DOI] [Google Scholar]
- 22.Richards GP, Watson MA, Fankhauser RL, Monroe SS. 2004. Genogroup I and II noroviruses detected in stool samples by real-time reverse transcription-PCR using highly degenerate universal primers. Appl Environ Microbiol 70:7179–7184. doi: 10.1128/AEM.70.12.7179-7184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katayama H, Haramoto E, Oguma K, Yamashita H, Tajima A, Nakajima H, Ohgaki S. 2008. One-year monthly quantitative survey of noroviruses, enteroviruses, and adenoviruses in wastewater collected from six plants in Japan. Water Res 42:1441–1448. doi: 10.1016/j.watres.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 24.Klämpfl TG, Isbary G, Shimizu T, Li YF, Zimmermann JL, Stolz W, Schlegel J, Morfill GE, Schmidt HU. 2012. Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl Environ Microbiol 78:5077–5082. doi: 10.1128/AEM.00583-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scherer K, Made D, Ellerbroek L, Schulenburg J, Johne R, Klein G. 2009. Application of a swab sampling method for the detection of norovirus and rotavirus on artificially contaminated food and environmental surfaces. Food Environ Virol 1:42–49. doi: 10.1007/s12560-008-9007-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plasma dispenser used for the norovirus treatment. The electric connections are in the back of the housing box, and NoV samples were put into the plasma box from the front opening. The lid was closed during the plasma treatment. Download
Image of the plasma box with cold plasma switched on. The purple glow is the emission from cold plasma. Download
Concept for practical application: the SMD electrode held by hand. In practical application, the cold plasma electrode can be designed as a handheld device so that it can be easily applied to different surfaces. Download
RNase digestion of free RNA for discrimination between destroyed and intact norovirus after heat treatment (data given as log10 values); n.d., not done; -, negative.
Effect of treatment time and sample dilution on norovirus viral load after CAPP treatment (data given as log10 values); n.d., not done.


