Abstract
The malaria parasite harbors a relict plastid called the apicoplast and its discovery opened a new avenue for drug discovery and development due to its unusual, nonmammalian metabolism. The apicoplast is essential during the asexual intraerythrocytic and hepatic stages of the parasite, and there is strong evidence supporting its essential metabolic role during the mosquito stages of the parasite. Supply of the isoprenoid building blocks isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) is the essential metabolic function of the apicoplast during the asexual intraerythrocytic stages. However, the metabolic role of the apicoplast during gametocyte development, the malaria stages transmitted to the mosquito, remains unknown. In this study, we showed that production of IPP for isoprenoid biosynthesis is the essential metabolic function of the apicoplast during gametocytogenesis, by obtaining normal gametocytes lacking the apicoplast when supplemented with IPP. When IPP supplementation was removed early in gametocytogenesis, developmental defects were observed, supporting the essential role of isoprenoids for normal gametocytogenesis. Furthermore, mosquitoes infected with gametocytes lacking the apicoplast developed fewer and smaller oocysts that failed to produce sporozoites. This finding further supports the essential role of the apicoplast in establishing a successful infection in the mosquito vector. Our study supports isoprenoid biosynthesis as a valid drug target for development of malaria transmission-blocking inhibitors.
INTRODUCTION
Human malaria is a devastating disease caused by five species of Plasmodium. Around 250 million cases of malaria occur every year, with Plasmodium falciparum and Plasmodium vivax accounting for 95% of all infections (1). P. falciparum, the deadliest species, is resistant to most current antimalarials, and emerging resistance to artemisinin, the current first-line treatment, has already been observed (2). Thus, in the absence of an effective vaccine, drugs with novel mechanisms of action are urgently needed. Traditionally, antimalarial drug development has focused on the asexual intraerythrocytic stages of the parasite's life cycle because these stages are responsible for the pathology associated with malaria. However, a renewed priority for malaria control and elimination has stimulated researchers to employ multistage approaches to discover new drug candidates that will not only relieve malaria symptoms but also stop its transmission (3).
All Plasmodium species have a complex life cycle that occurs between the human host and the Anopheles mosquito vector. Mature female and male gametocytes, the malarial stages transmitted to the mosquito, are asymptomatic, nonreplicating forms that can persist for weeks in circulating blood. In contrast to the case with other species of Plasmodium, P. falciparum gametocytes develop through five morphologically distinct stages (I to V), taking 10 to 12 days to fully mature into stage V gametocytes (4–6). Additionally, only mature stage V gametocytes are found in the peripheral blood circulation of an infected patient. Once gametocytes are released into the circulatory blood system, they require an additional 2 to 3 days to become infective to a mosquito (7–10). Immature or early stage gametocytes (I to III) are often sensitive to antimalarials that kill asexual intraerythrocytic stages, while mature gametocytes are insensitive. Consequently, a person may have their clinical infection cleared but still transmit the parasite to Anopheles mosquitoes for up to 3 weeks (7, 9, 11). Once the parasite enters the mosquito midgut, gametogenesis begins, in which both male (microgamete) and female (macrogamete) forms egress from host red blood cells. Gametogenesis occurs within 12 min post-blood meal in the mosquito. A motile microgamete will fertilize a macrogamete to form a zygote that eventually develops into a motile form called the ookinete. The ookinete traverses the midgut epithelium to reach the basal lamina, where it develops into an oocyst. The oocyst generates and releases sporozoites that invade the salivary glands of the mosquito and are injected into another human host upon the mosquito's next blood meal (12).
The malaria parasite harbors a relict plastid called the apicoplast, and its discovery opened new avenues for drug discovery and development due to its unusual, nonmammalian metabolism (13, 14). The apicoplast supports three metabolic functions: type II fatty acid biosynthesis, de novo heme biosynthesis, and isoprenoid biosynthesis. Type II fatty acid and de novo heme biosynthesis are not essential during the asexual and gametocyte intraerythrocytic stages when parasites scavenge lipids and heme from the human host (15–17); however, both of these biosynthetic pathways are essential for development of liver and mosquito stages of the parasite (15–20). In contrast, biosynthesis of the isoprenoid precursors isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) is the essential metabolic function of the apicoplast in the asexual intraerythrocytic stages (21). To investigate if supply of isoprenoid precursors is also the essential metabolic role of the apicoplast during gametocytogenesis, we generated asexual intraerythrocytic stage parasites lacking the apicoplast by treating them with doxycycline (DOX) as described previously (21) and inducing gametocytogenesis in the presence of IPP. Using this approach, morphologically normal stage V gametocytes lacking the apicoplast (API-minus) were successfully generated. However, if IPP supplementation was stopped earlier in the development of API-minus gametocytes, developmental defects were observed, supporting the hypothesis that the supply of isoprenoid precursors is the essential metabolic role of the apicoplast during gametocytogenesis. In addition, Anopheles gambiae mosquitoes infected with API-minus gametocytes developed fewer and smaller oocysts that failed to produce sporozoites, supporting the premise that the apicoplast is essential to establishing a successful infection in the mosquito vector.
MATERIALS AND METHODS
Parasite cultures.
P. falciparum strain NF54 (MRA-1000), deposited by M. Dowler, Walter Reed Army Institute of Research, was obtained through the MR4 Malaria Reagent Repository (ATCC, Manassas, VA) as part of the BEI Resources Repository, NIAID, NIH. NF54 is a chloroquine-sensitive strain capable of producing gametocytes. As with all gametocyte-producing strains, NF54 loses its ability to produce gametocytes after continuous in vitro culturing. Therefore, a new aliquot of NF54 was used every 1 to 2 months to retain high production of gametocytes. P. falciparum NF54 parasites were cultured in O-positive human erythrocytes (Interstate Blood Bank Inc., Memphis, TN) at 5% hematocrit in RPMI 1640 medium supplemented with 5 g/liter of Albumax I (GIBCO Life Technologies), 2 g/liter of glucose (Sigma-Aldrich), 2.3 g/liter of sodium bicarbonate (Sigma-Aldrich), 370 μM hypoxanthine (Sigma-Aldrich), 25 mM HEPES (Gibco Life Technologies), and 20 mg/liter of gentamicin (GIBCO Life Technologies). Parasites were kept at 37°C under reduced-oxygen conditions (5.06% CO2, 4.99% O2, and 89.95% N2).
Generation of API-minus gametocytes.
P. falciparum NF54 strain cultures less than 2 months old were maintained under normal culturing conditions described above (5% hematocrit, parasitemia < 10%). API-minus asexual stages were generated using DOX, an inhibitor of apicoplast translation that causes irreversible loss of the organelle, and IPP supplementation as a chemical rescue (21). Before starting DOX treatment, the culture was passed through a magnetic (magnetic-activated cell sorting [MACS]) column to remove any existing gametocytes, trophozoites, and schizonts. The ring stages were saved and adjusted to 5% hematocrit, and then 1 ml was recovered for quantitative PCR (qPCR) analysis. The remaining culture was split into two 25-cm2 (5-ml) flasks each containing 5% hematocrit and 2% parasitemia. One of the 5-ml cultures was treated with 1 μM DOX in the presence of 200 μM IPP for 8 days to remove the apicoplast, as described previously (21). Control and DOX-treated cultures were smeared and subcultured as needed to maintain around 10% parasitemia. After 8 days of DOX treatment, control and API-minus asexual cultures were passed again through a MACS column to remove any gametocytes and mature asexual stages. NF54 ring stages (>99% purity as assessed by Giemsa-stained smears) were recovered (day 0). One milliliter of control and API-minus cultures each was saved for qPCR analysis to confirm loss of the apicoplast genome. The remaining parasites were subcultured at 5% hematocrit and 1% parasitemia for gametocytogenesis using the “crash” method (nutrient stress) as described previously (22). It is worth mentioning that the crash method used generates an asynchronous gametocyte culture even though highly synchronic ring-stage cultures were used to set the subcultures for gametocytogenesis. Cultures for gametocytogenesis from DOX-treated asexual parasites without IPP supplementation were also included as a control to confirm loss of the apicoplast as described previously (21). We did not observe any parasite (asexual or sexual stages) after 13 days of continuous culture, confirming that DOX-treated asexual parasites were API-minus at the time that the experiment was set. DOX treatment was stopped at this point because parasites no longer possessed an apicoplast. Control and API-minus parasites were supplemented with 200 μM IPP only, as indicated in Fig. 1A. Control parasites were also supplemented with 200 μM IPP for different amounts of time (days) to assess if IPP alone has any adverse effects on gametocytogenesis. From days 4 to 13, medium changes were performed daily. To remove the IPP present in the media on the day indicated in Fig. 1A, each well was resuspended, cells were pelleted by centrifugation at 1,000 × g for 5 min, and IPP-containing medium was discarded and replaced with fresh medium without IPP. Cultures were smeared before stopping IPP supplementation to assess gametocyte stage distribution. On day 13 (Fig. 1A), 1 ml of culture was recovered from all conditions for qPCR analysis to confirm the absence or presence of the apicoplast genome. All cultures were smeared and stained with Giemsa for stage distribution and morphological assessment of API-minus gametocytes and controls. The remainder of each culture was processed for fluorescence microscopy as described below. Thirty microscopic fields from each culture were observed for morphological assessment. Classification of gametocyte stages II to V was based on the description by Carter and Miller (23). Stage distribution was calculated as a percentage of the total number of parasites, and the percentage gametocytemia was determined by counting gametocytes in 1,000 red blood cells. Morphological and stage distribution assessments were independently performed by two microscopists that were blinded. Experiments comparing controls and API-minus gametocyte development were carried out five times with IPP supplementation for 13 days, three times with IPP supplementation for 5 days, and twice with IPP supplementation for 8 and 11 days each.
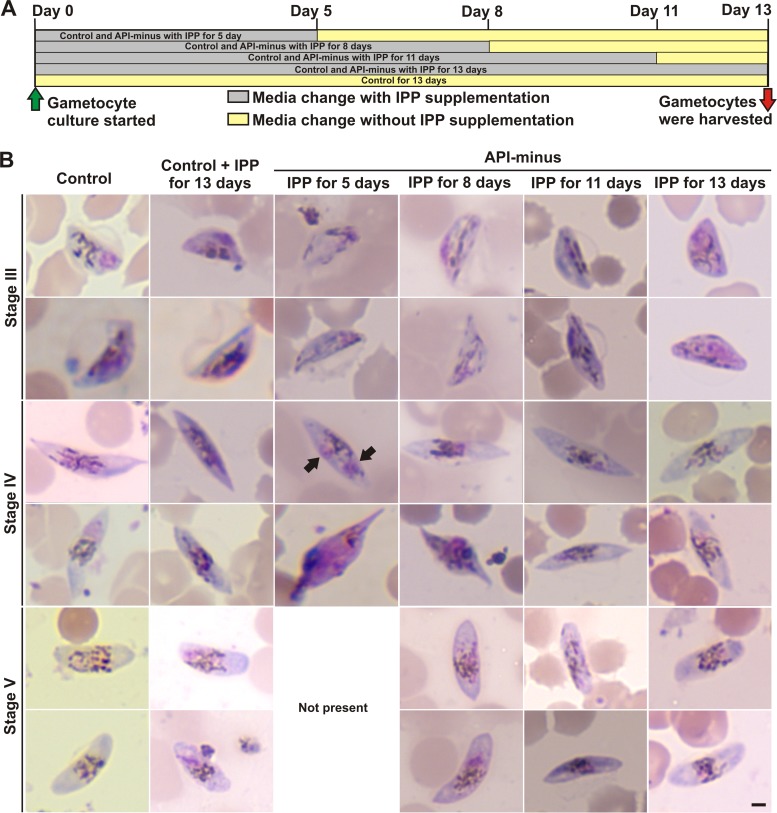
FIG 1.
P. falciparum parasites lacking apicoplasts undergo gametocytogenesis in the presence of IPP. (A) Scheme showing the experimental design where control and API-minus rings were subcultured for gametocytogenesis on day 0. Control and API-minus parasites were supplemented with IPP for the indicated times, and then medium without IPP supplementation was used until gametocytes were harvested for analysis. All conditions were harvested at day 13. (B) Representative Giemsa-stained smears of P. falciparum gametocytes at day 13 from controls with and without IPP supplementation and from API-minus gametocytes for which IPP supplementation was stopped as indicated in panel A. Stage V was not present in API-minus gametocytes for which IPP supplementation was stopped after 5 days of culture for gametocytogenesis. Nuclei are observed in purple and hemozoin crystals as dark dashes. Black arrows indicate abnormal nuclei. Scale bar indicates 2 μm.
Fluorescence microscopy.
Potential changes in mitochondrial membrane potential as an indicator of parasite viability were tracked in API-minus gametocytes for which IPP was removed at different times as indicated in Fig. 1A using MitoTracker; DNA (Hoechst) localization was used for reference. The control and gametocytes lacking the apicoplast supplemented with IPP for 5, 8, 11, or 13 days were generated as described above. On day 13, gametocytes were recovered and washed one time with phosphate-buffered saline solution (PBS), followed by the addition of 100 nM MitoTracker Red CM-H2XRos (Life Technologies) in complete medium for 15 min at 37°C and protected from light. Each sample was washed again one time with PBS and resuspended in 100 μl of complete medium. Hoechst 33342 at 0.25 μg/ml was added to each sample right before imaging. A Zeiss Axioimager M1 microscope with a 100×/1.4 numerical aperture (NA) oil immersion objective and an Axiocam MRm digital camera was used to obtain fluorescent images as reported previously (24). Adobe Photoshop CS2 was used to prepare final figures.
alamarBlue assay for gametocyte viability assessment.
To verify that API-minus P. falciparum gametocytes were viable at the time of collection for morphology studies, control and API-minus gametocytes supplemented with IPP for 5 or 13 days were generated as described above. Recovery and concentration was achieved on day 12 using a NycoPrep 1.077 cushion (25), and the number of gametocytes was calculated using a Neubauer chamber. About 30,000 gametocytes per well were added to black flat-bottom half-area 96-well plates in a 100-μl final volume and alamarBlue was added at 10% of the well volume (26). The plate was incubated in a humidified chamber at 37°C under reduced-oxygen conditions (5.06% CO2, 4.99% O2, and 89.95% N2) for 24 h and read in a microplate reader (Synergy H1 BioTek) at 585 nm after excitation at 540 nm. Values are reported as percent mitochondrial activity compared to control values and represent the averages and standard deviation from biological triplicates.
Generation of control and API-minus gametocytes for metabolite analysis.
API-minus asexual stages were generated as described in the section above. API-minus and control asexual parasites were synchronized by sorbitol (Sigma-Aldrich) treatment and passed through a MACS column before induction of gametocytogenesis in subcultures by nutrient stress (22). On day 9 after setting cultures for gametocytogenesis, parasites were treated with 5% sorbitol for 10 min at 37°C to remove asexual stages. Sorbitol treatment was repeated for 4 consecutive days, which effectively removed >99% of asexual parasites (assessed by Giemsa-stained smears). Gametocytes were recovered on day 13 using a NycoPrep 1.077 cushion (Axis-Shield) (25), and the number of gametocytes was calculated using a Neubauer chamber. Cell pellets were washed twice with PBS (pH 7.2), lyophilized, and stored at −80°C until extraction.
qRT-PCR.
Each gametocyte pellet was resuspended in 180 μl of PBS before DNA extraction. DNA was purified using a QiaAMP blood kit (Qiagen) by following the manufacturer's protocol and quantified. To perform relative quantification of the apicoplast and the mitochondrial and nuclear genomes in API-minus gametocytes, and their controls, we used three target genes (tufa, cytb, and CHT1) as described previously (21). Their primer sequences were as follows: tufA (apicoplast, PFC10_API0028), 5′-GATATTGATTCAGCTCCAGAAGAAA-3′ (forward) and 5′-ATATCCATTTGTGTGGCTCCTATAA-3′ (reverse); cytb3 (mitochondria, mal_mito_3), 5′-AGATACATGCACGCAACAGG-3′ (forward) and 5′-TCATTTGACCCCATGGTAAGA-3′ (reverse); and CHT1 (nuclear, PF3D7_1252200), 5′-TGTTTCCTTCAACCCCTTTT-3′ (forward) and 5′-TAATCCAAACCCGTCTGCTC-3′ (reverse). In addition, an endogenous control gene (tRNA synthase) was used as an active reference, which allowed normalizing quantification of DNA target genes for differences in the amount of DNA added to each reaction. Primer sequences of tRNA synthase (PF3D7_0717700) were 5′-AAGTAGCAGGTCATCGTGGTT-3′ (forward) and 5′-GTTCGGCACATTCTTCCATAA-3′ (reverse). Reaction mixtures contained 10 to 100 ng of template DNA, a 0.1 μM concentration of each primer, 1× GoTaq qPCR master mix, and 0.2 μl carboxy-X-rhodamine (CXR) reference dye in a final volume of 20 μl. Quantitative real-time PCRs (qRT-PCRs) were performed on an Applied Biosystems 7300 real-time PCR system. Relative quantification of target genes was determined using the comparative method (threshold cycle [2−ΔΔCT]).
Methylerythritol phosphate (MEP) intermediate analysis by IP-RP-UPLC-MS/MS analysis.
Metabolite extraction was performed as described previously for asexual stages (27) from lyophilized cell pellets. The same number of gametocytes was analyzed for all conditions (2 × 106). Briefly, isopentenyl S-thiolodiphosphate (ISPP) was used as the internal standard at a final concentration of 100 μM. ISPP was diluted in water, and 1 ml was added to each sample and sonicated for 10 min in a water bath sonicator, followed by incubation at 60°C for 1.5 h. Samples were chilled on ice and centrifuged for 10 min at 9,400 × g at room temperature. Supernatants were filtered through a 10-kDa Centricom filter (Millipore), and the flowthroughs were dried in a SpeedVac with heat. Samples were resuspended in 110 μl of water and centrifuged for 10 min at 9,400 × g at 4°C. Samples were transferred to a total recovery vial (Waters), and two injections of 40 μl of each sample underwent ion pair reversed-phase ultraperformance liquid chromatography in tandem with mass spectrometry (IP-RP-UPLC-MS/MS) analysis.
A previously described method involving IP-RP-UPLC-MS/MS (28) was used for analysis of the intermediates 1-deoxy-d-xylulose 5-phosphate (DOXP), MEP, 4-diphosphocytidyl-2-C-methyl-d-erythritol (CDP-ME), 4-diphosphocytidyl-2-C-methyl-d-erythritol 2-phosphate (CDP-MEP), 2-C-methyl-d-erythritol 2,4-cyclodiphosphate (MEcPP), (E)-4-hydroxy-3-methyl-but-2-enyl diphosphate (HMBPP), and IPP. Briefly, separations and analyses were performed using a Waters ACQUITY H-class UPLC (Waters, USA) liquid chromatography system in tandem with a XEVO TQ-MS mass spectrometer (Waters, USA) equipped with an electrospray ionization (ESI) source. The LC system was equipped with a quaternary pump, and the column temperature was maintained at 40°C. A Waters ACQUITY UPLC HSS T3 column (1.8 μm; 2.1 mm by 100 mm) and an ACQUITY column in-line filter were used. Standards and samples were separated using a gradient mobile phase consisting of 1.25 mM dibutylamine acetate (DBAA), 10 mM ammonium formate in water, and 1% formic acid to adjust the pH to 5.2 (A) and 1.25 mM DBAA in acetonitrile (B). The flow rate was set at 0.3 ml/min, and the gradient conditions were 0 to 2 min (100% A), 2 to 7 min (100% A to 60% A), 7 to 9 min (60% A to 50% A), 9 to 11 min (50% A to 100% A), and 11 to 15 min (100% A). The autosampler was set at 10°C. The following conditions for the mass spectrometer were used for the MEP intermediate analysis. Capillary voltage was set at 3.17 kV for the negative-ion mode. The source and desolvation gas temperatures of the mass spectrometer were set at 150°C and 450°C, respectively. The desolvation gas (nitrogen) was set at 700 liters/h. Relative quantitative determination was performed in ESI negative-ion mode using multiple-reaction monitoring (MRM) mode. The ion transitions, cone voltage (CV), and collision energy (CE) used for ESI-MS/MS analysis were determined using MassLynx V4.1 Intellistart software and are as follows: for DOXP, ion transition of 212.93 > 78.75, CV of 20 V, and CE of 24 eV; for MEP, ion transition of 214.94 > 96.77, CV of 96 V, and CE of 20 eV; for MEcPP, ion transition of 276.96 > 78.74, CV of 38 V, and CE of 24 eV; for CDP-ME, ion transition of 520.05 > 321.99, CV of 38 V, and CE of 22 eV; for CDP-MEP, ion transition of 600.14 > 78.80, CV of 34 V, and CE of 62 eV; for HMBPP, ion transition of 260.90 > 78.75, CV of 92 V, and CE of 28 eV; for IPP, ion transition of 245.1 > 78.8, CV of 24 V, and CE of 16 eV; and for ISPP, ion transition of 260.95 > 158.76, CV of 22 V, and CE of 14 eV. Data were acquired using MassLynx software, v. 4.1, and processed using TargetLynx Application Manager (Waters). Relative quantification of the level of each metabolite was performed by determining the analyte-to-internal standard ratio (response), calculated by dividing the area of the analyte peak by the area of the internal standard (ISPP) peak. The limit of detection was defined as three times the signal-to-noise ratio (29). The response of each detected metabolite is expressed as the mean and standard deviation from two biological replicates.
Mosquito rearing and P. falciparum infections.
API-minus asexual rings were generated as described above and adapted to the protocol of the Johns Hopkins parasitology core facility for gametocyte production where Albumax I was replaced by human serum (22). Expression levels of apicoplast, mitochondrial, and nuclear genomes in API-minus gametocytes and their controls were confirmed by qRT-PCR as described above. The colony of A. gambiae (Keele strain) was obtained from the Johns Hopkins insectary core facility. Mosquitoes were maintained on 10% sucrose at 27°C and 80% relative humidity with a 14-h/10-h light/dark cycle as reported previously (30). P. falciparum infections were performed by diluting stage V control and API-minus gametocytes to ∼0.3% gametocytemia and fed to A. gambiae mosquitoes using an artificial membrane feeder for 1 h in the absence of IPP. Fed mosquitoes were sorted immediately. Oocyst counts were performed by midgut dissection in 1× PBS at 6, 12, and 17 days postfeeding, staining with 0.2% mercurochrome, and visualization with a compound microscope as described previously (30). Pictures of oocysts were processed using Adobe Photoshop CS2. Sporozoite counts were performed by salivary gland dissection at day 17 postfeeding based on the work of Vega-Rodriguez et al. (31), with the following modifications. Salivary glands from 5 mosquitoes were pooled into 1.5-ml tubes containing 100 μl of PBS. Each tube was homogenized and spun down at 7,000 rpm for 5 min. After the removal of 80 μl of PBS, the remaining ∼20 μl was loaded onto a hemocytometer for counting by light microscopy. Graphs were generated using GraphPad Prism 6.
RESULTS
P. falciparum parasites lacking apicoplasts undergo gametocytogenesis in the presence of IPP. (i) Gametocyte morphology.
Recently, it was demonstrated that type II fatty acid and de novo heme biosynthesis are not essential during gametocyte stages (15–17), suggesting that supply of isoprenoid precursors may remain as the essential metabolic function of the apicoplast during gametocytogenesis. To test this hypothesis, API-minus ring-stage parasites were generated and purified as described in Materials and Methods, and subcultures were set for induction of gametocytogenesis (day 0) (Fig. 1A) using the crash method (22). Control parasites were processed in parallel with and without IPP supplementation to investigate the potential effect of an excess of IPP on the development of gametocytes. IPP supplementation was stopped at different times (days), as indicated in Fig. 1A in both control and API-minus parasites, and replaced by regular medium until day 13, when all conditions were recovered and processed for microscopic evaluation and qPCR analysis.
Gametocytes develop through five stages (I to V); only stages II to V are morphologically recognizable in Giemsa-stained smears, while stage I is not easily distinguished from trophozoites. During gametocytogenesis, the parasites undergo morphological transformations without cell replication (see Fig. S1 in the supplemental material) (4). Briefly, at stage II, gametocytes start to adopt a spindle shape and hemozoin crystals (dark dashes) are more disperse throughout the cell, rather than being localized in one area as in the asexual stages (see Fig. S1). Stage II has only one nucleus, where as an asexual parasite of the same size would have four nuclei. As stage II progresses, one side starts to flatten to adopt a small capital “D” shape, which grows. Stage III is characterized by the typical “hat” shape, and hemozoin crystals are distributed throughout the cell, adopting different patterns. As the gametocytes progress to stage IV, further elongation occurs, with the gametocytes again adopting a spindle shape with pointed ends. Hemozoin crystals start to concentrate in the center of the cell near the nucleus (purple). As the gametocytes progress to stage V, the ends become more rounded and gametocytes adopt a “sausage” or crescent shape. Morphological evaluations from five independent experiments revealed that asexual parasites lacking the apicoplast were able to generate normal gametocytes when maintained in IPP-supplemented medium for 13 days compared to controls (Fig. 1B, control, control + IPP for 13 days, and API-minus + IPP for 13 days; see also Fig. S1). Morphological examinations were performed blinded by two microscopists who were unable to distinguish controls from API-minus gametocytes. An excess of IPP in control gametocytes did not alter their morphology (Fig. 1B). Similar results were obtained when IPP supplementation was maintained for only 11 days and then replaced by regular medium until day 13 when parasites were recovered for analysis (Fig. 1B, API-minus + IPP for 11 days; see also Fig. S1).
On the other hand, API-minus parasites did not develop normally when IPP supplementation was removed early during gametocytogenesis. Giemsa-stained smears of API-minus gametocytes for which IPP supplementation was stopped after 5 days parasites were set for gametocytogenesis and then maintained in medium without IPP supplementation until day 13 (Fig. 1A) showed that 100% of the observed gametocytes, from three independent experiments, presented morphological abnormalities (Fig. 1B, API-minus + IPP for 5 days; see also Fig. S1). Interestingly, we were able to identify the characteristic shape of each stage (II to IV). In general, API-minus gametocytes presented a “ghost” or hollow appearance, especially in stages II and III, with fewer hemozoin crystals than controls. In some stage IV gametocytes, an abnormal nucleus was observed as two separate structures stained in purple (Fig. 1B, black arrows; see also Fig. S1). API-minus gametocytes at stage V were not present under this experimental condition (IPP for 5 days). When IPP supplementation was stopped after 8 days and then gametocytes were maintained in medium without IPP supplementation until day 13, 30% ± 10% of the observed API-minus gametocytes presented the abnormalities described above compared to controls (Fig. 1B, API-minus + IPP for 8 days; see also Fig. S1).
We confirmed by qPCR analysis the absence of the apicoplast genome in ring-stage parasites before setting the subcultures for gametocytogenesis and in API-minus gametocytes at the end of the each experiment (Fig. 2). Our results showed that P. falciparum parasites lacking apicoplasts undergo normal gametocytogenesis in the presence of IPP, suggesting that biosynthesis of isoprenoid precursors remains the essential metabolic function of the apicoplast during in vitro gametocytogenesis.
FIG 2.
Relative quantification of nuclear, mitochondrial, and apicoplast genes by qPCR, showing that API-minus parasites have lost their apicoplast genome while maintaining their mitochondrial and nuclear genomes.
(ii) Gametocyte stage distribution.
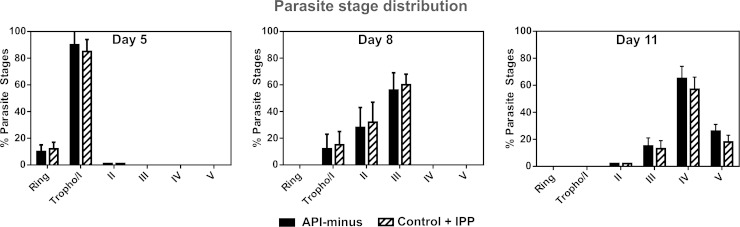
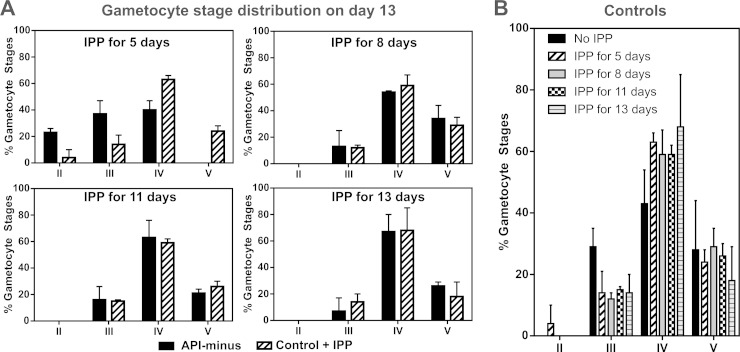
We assessed the gametocyte stage distribution for all conditions before IPP supplementation was stopped at the times indicated in Fig. 1A. Because it is difficult to distinguish stage I gametocytes from early trophozoite stages using Giemsa-stained smears, trophozoite-like parasites were counted and are referred to here as tropho/I. As shown in Fig. 3, control and API-minus cultures did not show differences in the gametocyte stage distribution at the time that IPP supplementation was removed. On day 5, both control and API-minus cultures contained mainly tropho/I stage parasites. On day 8, both cultures were mainly at stages II and III. On day 11, control and API-minus gametocytes were a mix of stages III to V, with both being predominantly stage IV. The observed stage distribution was expected because sexually committed merozoites take 10 to 12 days to develop to stage V (22). Therefore, in the presence of IPP, API-minus parasites developed at a rate similar to that of the control. At days 5, 8, and 11, IPP-containing medium was replaced with medium without IPP supplementation as indicated in Fig. 1A and the cells continued to receive fresh daily medium changes without IPP until recovery on day 13, when stage distribution was determined again by Giemsa-stained smears (Fig. 4A). Both control and API-minus gametocyte distributions were similar (stages III to V) for subcultures where IPP was removed on day 8 or later. However, we observed that when IPP was removed from gametocytes early in development (day 5 after setting subcultures for gametocytogenesis), API-minus parasites exhibited delayed development (Fig. 4A) in addition to the morphological abnormalities described above. API-minus parasites that received IPP for only 5 days were in stages II to IV when observed at day 13, while control gametocytes were at stages III to V (Fig. 4A). Therefore, our results suggest that biosynthesis of isoprenoid precursors is essential for gametocyte development, especially during early stages (I to II), and that requirements may decrease as the gametocytes mature from stages III to V.
FIG 3.
Parasite stage distribution of control and API-minus cultures on the day that IPP supplementation was stopped. Cultures were smeared before medium was replaced without IPP supplementation and stage distribution was assessed by Giemsa-stained smears and light microscopy based on the Carter description. Values represent means ± SD from three independent experiments for day 5 and two independent experiments for days 8 and 11.
FIG 4.
(A) Gametocyte stage distribution of control and API-minus cultures on day 13 was assessed by Giemsa-stained smears and light microscopy based on the Carter description. Values represent means ± SD from three independent experiments for IPP for 5 days, two independent experiments for IPP for 8 and 11 days, and five independent experiments for IPP for 13 days. (B) Control cultures supplemented with 200 μM IPP for different days during gametocytogenesis did not show statistically significant differences (one-way analysis of variance [ANOVA], P = 0.9296) on gametocyte stage distribution compared to controls without IPP supplementation. Gametocyte stage distribution was assessed on day 13 after setting subcultures for gametocytogenesis. Error bars represent the standard deviations of the means from independent experiments, as indicated in panel A, and from five independent experiments for control gametocytes without IPP supplementation.
An excess of IPP in control gametocytes did not change the gametocyte stage distribution (Fig. 4B). In addition, all conditions reached similar gametocytemia by day 13 compared to their respective control within the same experiment (± 1%), taking into consideration that initial ring-stage cultures came from different preparations (control and API-minus). Gametocytemia between experiments performed at different times varied around ±2%.
(iii) Gametocyte viability.
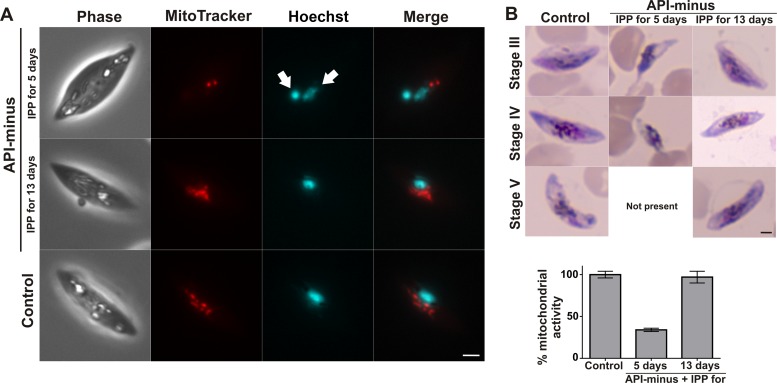
To assess whether API-minus gametocytes for which IPP supplementation was stopped at different times were viable at the time of collection on day 13, parasites were incubated with MitoTracker to examine the mitochondrial membrane potential. Isoprenoid precursors are necessary to assemble the isoprenic side chain of ubiquinones (32, 33) and are vital for the integrity of mitochondrial electron transport (34). Therefore, we hypothesized that API-minus parasites may also present a reduced mitochondrial electron transport when IPP is removed earlier in development. Control and API-minus gametocytes that received IPP until day 13 exhibited a robust mitochondrial membrane potential, as evidenced by strong MitoTracker labeling (Fig. 5A; see also Fig. S2 in the supplemental material). Gametocytes for which IPP was supplied for 5 days and then replaced with medium without IPP supplementation showed reduced mitochondrial membrane potential (Fig. 5A; see also Fig. S2). Interestingly, Hoechst labeling of the DNA also revealed the presence of an abnormal nucleus (Fig. 5A, white arrows). Gametocytes for which IPP was supplied for 8 or 11 days and then replaced by regular medium showed a normal mitochondrial membrane potential compared to the control (see Fig. S2).
FIG 5.
Gametocyte viability. (A) Representative wide-field epifluorescence microscopy at day 13 for nucleus (Hoechst) and mitochondria (MitoTracker) comparing control gametocytes and API-minus gametocytes for which IPP was supplemented for 5 or 13 days. Hemozoin crystals can be identified as white spots in the phase. White arrows indicate abnormal nuclei. Scale bar indicates 2 μm. (B) (Top) Representative Giemsa-stained smears of P. falciparum gametocytes at day 13 from controls and API-minus gametocytes for which IPP was supplemented for 5 or 13 days after fluorescence determination by alamarBlue. Scale bar indicates 2 μm. (Bottom) alamarBlue assay demonstrating mitochondrial activity of API-minus gametocytes for which IPP was supplemented for 5 or 13 days. Values represent means ± SD from biological triplicates.
To confirm that API-minus gametocytes for which IPP supplementation was stopped at day 5 have reduced mitochondrial activity as revealed by MitoTracker labeling, control and API-minus gametocyte (IPP for 5 or 13 days) cultures were set up as described in Materials and Methods and were incubated with alamarBlue, an oxidoreduction indicator (26). alamarBlue is a reporter of metabolic activity, and the active ingredient (resazurin) is thought to be reduced by mitochondrial enzymes (26, 35, 36). Both MitoTracker and alamarBlue have been successfully developed for gametocytocidal assays (24, 26, 37, 38). After fluorescence was read, all wells were smeared and stained with Giemsa for morphological evaluation (Fig. 5B). API-minus gametocytes that received IPP supplementation for only 5 days and then medium without IPP supplementation until recovery on day 13 showed a 65% reduction of mitochondrial activity and morphological abnormalities compared to the control, while API-minus gametocytes that were under continuous supply of IPP showed mitochondrial activity and morphology similar to those of the control. Our results indicate that withdrawal of IPP supplementation from API-minus gametocytes early during gametocytogenesis causes not only a delay in development but also a reduction in viability.
MEP pathway intermediates are present in late stage gametocytes.
IPP and DMAPP are used to synthesize all isoprenoid derivatives and are obtained through the apicoplast-localized methylerythritol phosphate (MEP) pathway, which is absent in humans (27, 39). The results described above suggest a lower requirement for isoprenoid precursors in late-stage gametocytes and raised the question of whether intermediates of the MEP pathway are present in late-stage gametocytes. To our knowledge, the presence of MEP pathway intermediates has not been investigated in gametocytes. In addition, API-minus gametocytes were also analyzed to assess the levels of MEP pathway intermediates. To assess the presence of MEP intermediates, 2 × 106 gametocytes in stages IV and V from control and API-minus cultures were purified as described in Materials and Methods and MEP pathway intermediates were analyzed by IP-RP-UPLC-MS/MS (Table 1). We detected most of the MEP pathway intermediates in late-stage control gametocytes. The MEP pathway intermediates were below the limit of detection in API-minus gametocytes, and only IPP from exogenous supplementation was detected.
TABLE 1.
Relative metabolite levels of MEP pathway intermediates detected by IP-RP-UPLC-MS/MS in control and API-minus late-stage (IV and V) gametocytes
| MEP intermediate | Ratioa in: |
|
|---|---|---|
| Control gametocytesb | API-minus gametocytesc | |
| DOXP | 0.10 ± 0.07 | ND |
| MEP | 0.75 ± 0.21 | ND |
| CDP-ME | ND | ND |
| CDP-MEP | 2.00 ± 1.41 | ND |
| MEcPP | 0.65 ± 0.14 | ND |
| HMBPP | 0.13 ± 0.04 | ND |
| IPP | 2.78 ± 0.18 | 2,733 ± 387 |
The analyte-to-internal standard ratio (response) was calculated for each metabolite by dividing the area of the analyte peak by the area of the internal standard (ISPP) peak. Values are means ± SD from two biological replicates. ND, not detected.
Control gametocytes without IPP supplementation.
API-minus gametocytes with IPP supplementation until were recovered for analysis (13 days after gametocytogenesis was induced).
The apicoplast is essential for oocyst and sporozoite development in mosquitoes.
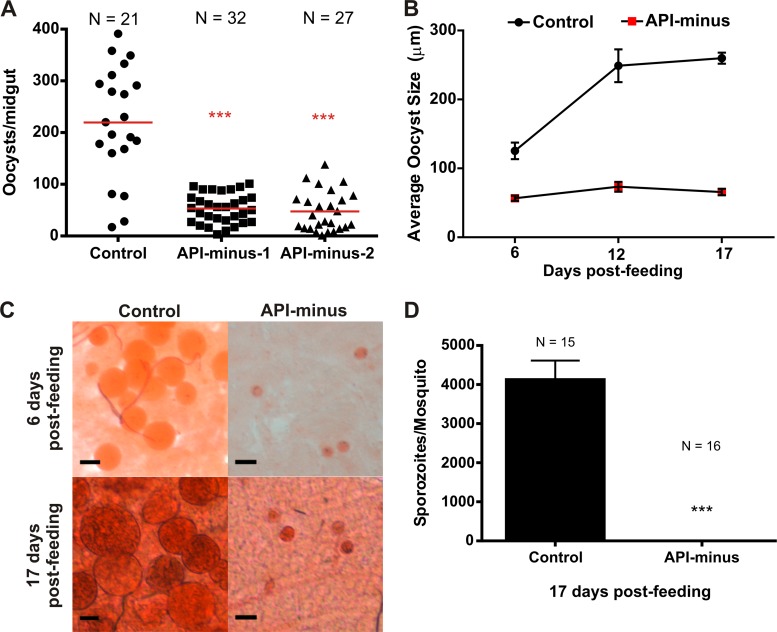
Our results described above support the hypothesis that isoprenoids are essential for gametocytogenesis. Therefore, inhibitors directly targeting their biosynthesis or indirectly targeting their biosynthesis by affecting apicoplast replication and maintenance may serve as transmission-blocking agents. In addition, there is strong evidence supporting the essential metabolic role of the apicoplast during the mosquito stages (15, 16, 40, 41). Therefore, we hypothesized that gametocytes lacking the apicoplast will fail to develop oocysts and sporozoites. To assess this hypothesis, API-minus gametocytes were generated and fed to A. gambiae mosquitoes in the absence of IPP. Unlike in our previous experiments, API-minus gametocytes used to feed mosquitoes were generated using human serum instead of Albumax I (a purified serum substitute); however, no differences were observed in gametocyte development (data not shown). At 6 days postfeeding, midguts from a subgroup of mosquitoes from control and API-minus gametocytes were dissected. Mosquitoes infected with gametocytes lacking the apicoplast showed a marked reduction in the number of oocysts formed (Fig. 6A) and their sizes were smaller than for the control (Fig. 6B and C). To address whether the oocysts formed from API-minus gametocytes delayed growth or were arrested early in development, midguts from another subgroup of mosquitoes fed with control or API-minus gametocytes were dissected after 12 days postfeeding and oocysts were measured. After 17 days postfeeding, all the remaining mosquitoes were collected for oocyst measurement and sporozoite counting. As shown in Fig. 6B and C, the size of oocysts from API-minus gametocytes at 12 or 17 days postfeeding was significantly smaller than for the control (P < 0.0001), and oocysts remained at a size comparable to that of API-minus oocysts at 6 days postfeeding. Importantly, no sporozoites were detected in the salivary glands of mosquitoes infected with API-minus gametocytes (Fig. 6D). Our results show that the apicoplast is essential for oocyst and sporozoite development. Although further studies are required to confirm if isoprenoid biosynthesis is essential in the mosquito stages, our results suggest that targeting isoprenoid biosynthesis during gametocytogenesis may block parasite transmission to the mosquito vector.
FIG 6.
Oocyst and sporozoite formation in control and API-minus P. falciparum-infected An. gambiae mosquitoes. (A) Quantification of oocysts in control and two groups of API-minus P. falciparum-infected mosquitoes after 6 days postfeeding. P values (***) of API-minus mosquitoes with respect to controls are <0.0001. “N” represents the number of mosquitoes dissected from each group. Horizontal bars represent the average of oocyst number per midgut of infections from two independent gametocyte cultures. (B) Average of oocyst size from control and API-minus P. falciparum-infected mosquitoes over time. Error bars represent the standard deviations of the means. (C) Mercurochrome staining of oocysts in the midgut preparations after 6 and 17 days postinfection. Scale bar indicates 100 μm. (D) Quantification of salivary gland sporozoites from control and API-minus infected mosquitoes. The P value (***) for API-minus mosquitoes with respect to the control is <0.0001. “N” represents the total number of mosquitoes dissected from each group. Error bars represent the standard deviations of the means.
DISCUSSION
Since the apicoplast was discovered (13), a series of genetic and pharmacological studies has demonstrated that this organelle is essential for malaria parasite growth (16, 18, 19, 21, 40–42). Recently, it was demonstrated that type II fatty acid and de novo heme biosynthesis are not essential for gametocyte development (15–17), suggesting that supply of isoprenoid precursors may remain the essential metabolic function of the apicoplast during gametocytogenesis. Our findings presented here support the idea that, similar to the case with asexual intraerythrocytic stages, the supply of isoprenoid precursors is the essential metabolic role of the apicoplast during gametocytogenesis. We were able to generate API-minus gametocytes that were indistinguishable from control gametocytes under continuous supply of IPP. Although detailed biochemical and structural characterizations of these API-minus gametocytes were not conducted in this study, two microscopists who were blinded observed seven independent preparations (five for API-minus gametocytes with IPP for 13 days and two for API-minus gametocytes with IPP for 11 days) and were unable to distinguish controls from API-minus gametocytes. It is important to acknowledge that the mechanism by which IPP is incorporated into the parasite both in asexual and gametocyte stages remains unknown.
In contrast to the asexual stages, P. falciparum gametocytes progress through five morphologically distinct developmental stages (I to V), ultimately reaching full maturity (stage V) after 10 to 12 days (4–6). Therefore, it is valid to speculate that metabolic requirements may change both throughout gametocytogenesis and in comparison to asexual stages (43). When IPP supplementation was stopped at day 5 in API-minus parasites when parasites were at stage tropho/I (Fig. 3), by the time of analysis on day 13, gametocytes presented morphological defects compared to controls. These gametocytes were also delayed in development, as assessed by stage distribution, and failed to mature to stage V, in addition to showing reduced viability as indicated by reduction of their mitochondrial metabolic activity (Fig. 4A and 5). When IPP supplementation was stopped in API-minus cultures on day 8 after subcultures were set, gametocytes were approximately 35% in the tropho/I-II stage and 65% in stage III (Fig. 3). When they were recovered for analysis on day 13, 30% ± 10% of the gametocytes presented morphological defects, which may represent those gametocytes that were in the tropho/I-II stage when IPP supplementation was stopped. However, the overall stage development was not affected (Fig. 1B and 4A; see also Fig. S1 in the supplemental material). The morphological defects were not present when IPP supplementation was maintained until stage III-IV of gametocytogenesis (IPP removed on day 11) (Fig. 1B and 3; see also Fig. S1). Therefore, these results suggest that the necessary isoprenoid products for normal gametocytogenesis are mostly synthesized during early stages and that the demand for isoprenoid precursors may be reduced as the gametocyte matures. In addition, our results support the hypothesis that the apicoplast remains metabolically active throughout gametocytogenesis despite the fact that the organelle does not elongate and divide during sexual intraerythrocytic stages (44). Interestingly, the MEP pathway-specific inhibitor fosmidomycin (FOS) does not affect gametocytes at any stage (24, 38, 45). FOS is a specific inhibitor of the second enzyme in the MEP pathway, 1-deoxy-d-xylulose 5-phosphate reductoisomerase, which is essential for P. falciparum survival (27, 39, 46). We previously identified a new antiapicoplast inhibitor from the Malaria Box collection, MMV008138, and suggested that the molecular target may be present within the MEP pathway (24). Recently, the enzyme 2-C-methyl-d-erythritol 4-phosphate cytidylyltransferase (IspD), the third enzyme of the MEP pathway, has been identified as the molecular target of MMV008138 (34). Unfortunately, similar to FOS, MMV008138 does not kill gametocytes at any stage (24, 37, 38, 47, 48). Our results showed that MEP intermediates are present in gametocytes (Table 1) and that isoprenoids are essential for gametocytogenesis, suggesting that the loss of FOS and MMV008138 activity in gametocytes, especially during the early stages (38, 48), may be due to lack of transport mechanisms for these inhibitors or unknown metabolic regulation factors.
The apicoplast is the sole source of isoprenoid precursors through the MEP pathway during the intraerythrocytic stages, as the human erythrocytes do not have an active mevalonate pathway to synthesize IPP (27, 49–51). In addition, it is unlikely that IPP can be scavenged from human plasma at the required concentration (200 μM) because it has not been detected in the human serum metabolome (52). However, gametocyte developing stages (I to IV) are usually absent from the peripheral circulation and are sequestered mainly in the extravascular space of the bone marrow of the human host (53). A recent study supports the idea that gametocytes can develop in erythroid precursors in the bone marrow (53), which may offer a different metabolic environment for the parasite to scavenge isoprenoid precursors, but this remains to be investigated further.
Another interesting aspect of the apicoplast is that it contains its own 35-kb prokaryotic-like genome but is not self-sufficient, requiring many nuclear genes for proteins that are then targeted to the organelle. It is predicted that approximately 540 gene products in the P. falciparum nuclear genome are targeted to the apicoplast, representing about 10% of the total predicted parasite genes and including all of the MEP pathway enzymes (54). It was previously shown that asexual API-minus parasites still continue to transcribe and translate apicoplast-targeted gene products (21). Our IP-RP-UPLC-MS/MS analysis showed that MEP pathway intermediates are not detected in API-minus gametocytes, supporting the hypothesis that even if the MEP pathway genes are translated, the enzymes may not be able to sustain metabolic activity outside the apicoplast.
Isoprenoids represent the largest class of small molecules on earth, with more than 40,000 compounds described. Isoprenoids are essential for life and are involved in a wide variety of biological functions, spanning from compartmentalization and stability to signaling and cell cycle control (55). Once IPP and DMAPP are synthesized and transported out of the apicoplast, a myriad of isoprenoid products with different cellular localization and functions are synthesized in the malaria parasite. Many isoprenoid products have been identified as being present in the intraerythrocytic asexual stages of P. falciparum, such as ubiquinones and menaquinone (32, 33, 56), carotenoids and vitamin E (57–59), dolichols (50, 60, 61), and prenylated proteins (60, 62–65). The presence and function of these isoprenoids in gametocytes remain to be confirmed and are under investigation. Nevertheless, the morphological abnormalities, delayed development, and reduced viability observed in API-minus gametocytes for which IPP supplementation was stopped early in gametocytogenesis is likely to be a result of a pleiotropic effect on multiple cellular processes.
The apicoplast is essential for parasite growth and development and is, therefore, a potential target for drug development. Asexual stages of the parasite treated with known antibiotics targeting apicoplast functions, such as DNA replication, transcription, and translation, display a “delayed-death” phenotype (66). These parasites initially present normal morphology and continue to grow and release daughter cells (merozoites) that are capable of invading new host cells. However, this progeny will not complete its intraerythrocytic asexual cycle because the apicoplast will fail to replicate and segregate (40, 66, 67). These apicoplast-targeting drugs do not affect gametocyte stages (24, 38, 45), which is not unexpected because the apicoplast does not elongate and divide during gametocytogenesis (44). However, apicoplast-targeting antibiotics may prevent sexual development in the mosquito stages due to inhibition of apicoplast functions that may not be apparent during gametocytogenesis. This appears to be the case for azithromycin (AZM), an antibacterial macrolide that inhibits protein synthesis and is well-tolerated in patients, including children and pregnant women (40, 68–71). The effect of AZM on mosquito stages was studied in the rodent malaria parasite P. berghei (40), a related species often used to model malaria transmission. AZM had no effect on P. berghei male gametocyte exflagellation, but a dose of AZM of 400 mg/kg inhibited oocyst and sporozoite development in the mosquito vector, suggesting an essential role of the apicoplast during the mosquito stages (40). The efficacy of AZM in mosquito stages has been called into question by another study by Delves and colleagues, which showed that stage V gametocytes pretreated with AZM and DOX 24 h before feeding to mosquitoes resulted in similar numbers of oocysts compared to the control (11); however, generation of sporozoites was not evaluated. Our results from feeding mosquitoes with API-minus gametocytes confirmed that parasites lacking the apicoplast develop fewer and smaller oocysts that fail to produce sporozoites. However, the lack of isoprenoid biosynthesis is not the sole reason for failing sporozoite production. Previous studies of the role of two other apicoplast pathways, type II fatty acid (16) and de novo heme biosynthesis (15, 17), during development of the malaria parasite in the mosquito revealed that these pathways are essential in mosquito stages, providing further evidence to support the essential metabolic role of the apicoplast.
Heme biosynthesis in the malaria parasite is unique since it encompasses several enzymes localized in three cellular compartments (mitochondria, apicoplast, and cytosol) (72, 73). Recently, it was demonstrated that disruption of the gene for ferrochelatase, the last enzyme of the de novo heme biosynthesis that localizes in the mitochondrion, caused no defects in asexual intraerythrocytic stages and gametocyte generation or maturation, suggesting that a heme salvage mechanism is likely to occur in blood stages. However, this resulted in a >70% reduction in male gamete formation and completely prevented oocyst formation in Anopheles stephensi mosquitoes (17). We observed formation of oocysts, although they were fewer and smaller than for the control, which could suggest that API-minus gametocytes have their gamete formation compromised; further studies will be required to confirm this hypothesis.
To our knowledge, this is the first demonstration that isoprenoid biosynthesis is vital for gametocytogenesis. Our results support the notion that the essential metabolic role of the apicoplast during P. falciparum gametocytogenesis is to provide precursors for isoprenoid biosynthesis, as previously suggested from genetic approaches. Discovering the myriad of isoprenoid products and their biological functions in gametocyte stages will unveil novel drug targets to develop malaria transmission-blocking inhibitors.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI108819 to M.B.C. J.D.W. and P.M.K. were recipients of a graduate scholarship from the National Science Foundation S-STEM project under award number DUE-0850198.
We thank the Johns Hopkins Malaria Research Institute parasite and insectary core facilities for assistance with our experiments. We thank researchers at Keele University for the original supply of the A. gambiae mosquitoes. We also thank Ryan C. Smith for valuable discussions on the mosquito results. We thank Janet Webster for comments and corrections.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00198-14.
REFERENCES
- 1.World Health Organization 2010. World malaria report (WHO/HTM/GMP/2010.1). World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Dondorp AM, Yeung S, White L, Nguon C, Day NP, Socheat D, von Seidlein L. 2010. Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol 8:272–280. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- 3.Flannery EL, Chatterjee AK, Winzeler EA. 2013. Antimalarial drug discovery—approaches and progress towards new medicines. Nat Rev Microbiol 11:849–862. doi: 10.1038/nrmicro3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker DA. 2010. Malaria gametocytogenesis. Mol Biochem Parasitol 172:57–65. doi: 10.1016/j.molbiopara.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bousema T, Drakeley C. 2011. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev 24:377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lobo CA, Kumar N. 1998. Sexual differentiation and development in the malaria parasite. Parasitology Today 14:146–150. doi: 10.1016/S0169-4758(97)01210-6. [DOI] [PubMed] [Google Scholar]
- 7.Delves MJ. 2012. Plasmodium cell biology should inform strategies used in the development of antimalarial transmission-blocking drugs. Future Med Chem 4:2251–2263. doi: 10.4155/fmc.12.182. [DOI] [PubMed] [Google Scholar]
- 8.Lensen A, Bril A, van de Vegte M, van Gemert GJ, Eling W, Sauerwein R. 1999. Plasmodium falciparum: infectivity of cultured, synchronized gametocytes to mosquitoes. Exp Parasitol 91:101–103. doi: 10.1006/expr.1998.4354. [DOI] [PubMed] [Google Scholar]
- 9.Sinden RE, Smalley ME. 1979. Gametocytogenesis of Plasmodium falciparum in vitro: the cell-cycle. Parasitology 79:277–296. doi: 10.1017/S003118200005335X. [DOI] [PubMed] [Google Scholar]
- 10.Talman AM, Domarle O, McKenzie FE, Ariey F, Robert V. 2004. Gametocytogenesis: the puberty of Plasmodium falciparum. Malar J 3:24. doi: 10.1186/1475-2875-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delves M, Plouffe D, Scheurer C, Meister S, Wittlin S, Winzeler EA, Sinden RE, Leroy D. 2012. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLoS Med 9:e1001169. doi: 10.1371/journal.pmed.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith RC, Vega-Rodriguez J, Jacobs-Lorena M. 2014. The Plasmodium bottleneck: malaria parasite losses in the mosquito vector. Mem Inst Oswaldo Cruz 109:644–661. doi: 10.1590/0074-0276130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McFadden GI, Reith ME, Munholland J, Lang-Unnasch N. 1996. Plastid in human parasites. Nature 381:482. doi: 10.1038/381482a0. [DOI] [PubMed] [Google Scholar]
- 14.McFadden GI, Roos DS. 1999. Apicomplexan plastids as drug targets. Trends Microbiol 7:328–333. doi: 10.1016/S0966-842X(99)01547-4. [DOI] [PubMed] [Google Scholar]
- 15.Nagaraj VA, Sundaram B, Varadarajan NM, Subramani PA, Kalappa DM, Ghosh SK, Padmanaban G. 2013. Malaria parasite-synthesized heme is essential in the mosquito and liver stages and complements host heme in the blood stages of infection. PLoS Pathog 9:e1003522. doi: 10.1371/journal.ppat.1003522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Schaijk BC, Kumar TR, Vos MW, Richman A, van Gemert GJ, Li T, Eappen AG, Williamson KC, Morahan BJ, Fishbaugher M, Kennedy M, Camargo N, Khan SM, Janse CJ, Sim KL, Hoffman SL, Kappe SH, Sauerwein RW, Fidock DA, Vaughan AM. 2014. Type II fatty acid biosynthesis is essential for Plasmodium falciparum sporozoite development in the midgut of Anopheles mosquitoes. Eukaryot Cell 13:550–559. doi: 10.1128/EC.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ke H, Sigala PA, Miura K, Morrisey JM, Mather MW, Crowley JR, Henderson JP, Goldberg DE, Long CA, Vaidya AB. 28 October 2014. The heme biosynthesis pathway is essential for Plasmodium falciparum development in mosquito stage but not in blood stages. J Biol Chem doi: 10.1074/jbc.M114.615831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaughan AM, O'Neill MT, Tarun AS, Camargo N, Phuong TM, Aly AS, Cowman AF, Kappe SH. 2009. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol 11:506–520. doi: 10.1111/j.1462-5822.2008.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu M, Kumar TR, Nkrumah LJ, Coppi A, Retzlaff S, Li CD, Kelly BJ, Moura PA, Lakshmanan V, Freundlich JS, Valderramos JC, Vilcheze C, Siedner M, Tsai JH, Falkard B, Sidhu AB, Purcell LA, Gratraud P, Kremer L, Waters AP, Schiehser G, Jacobus DP, Janse CJ, Ager A, Jacobs WR Jr, Sacchettini JC, Heussler V, Sinnis P, Fidock DA. 2008. The fatty acid biosynthesis enzyme FabI plays a key role in the development of liver-stage malarial parasites. Cell Host Microbe 4:567–578. doi: 10.1016/j.chom.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Botte CY, Yamaryo-Botte Y, Rupasinghe TW, Mullin KA, MacRae JI, Spurck TP, Kalanon M, Shears MJ, Coppel RL, Crellin PK, Marechal E, McConville MJ, McFadden GI. 2013. Atypical lipid composition in the purified relict plastid (apicoplast) of malaria parasites. Proc Natl Acad Sci U S A 110:7506–7511. doi: 10.1073/pnas.1301251110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeh E, DeRisi JL. 2011. Chemical rescue of malaria parasites lacking an apicoplast defines organelle function in blood-stage Plasmodium falciparum. PLoS Biol. 9:e1001138. doi: 10.1371/journal.pbio.1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saliba KS, Jacobs-Lorena M. 2013. Production of Plasmodium falciparum gametocytes in vitro. Methods Mol Biol 923:17–25. doi: 10.1007/978-1-62703-026-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter R, Miller LH. 1979. Evidence for environmental modulation of gametocytogenesis in Plasmodium falciparum in continuous culture. Bull World Health Organ 57(Suppl 1):S37–S52. [PMC free article] [PubMed] [Google Scholar]
- 24.Bowman JD, Merino EF, Brooks CF, Striepen B, Carlier PR, Cassera MB. 2014. Antiapicoplast and gametocytocidal screening to identify the mechanisms of action of compounds within the Malaria Box. Antimicrob Agents Chemother 58:811–819. doi: 10.1128/AAC.01500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lelièvre J, Almela MJ, Lozano S, Miguel C, Franco V, Leroy D, Herreros E. 2012. Activity of clinically relevant antimalarial drugs on Plasmodium falciparum mature gametocytes in an ATP bioluminescence “transmission blocking” assay. PLoS One 7:e35019. doi: 10.1371/journal.pone.0035019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka TQ, Williamson KC. 2011. A malaria gametocytocidal assay using oxidoreduction indicator, alamarBlue. Mol Biochem Parasitol 177:160–163. doi: 10.1016/j.molbiopara.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassera MB, Gozzo FC, D'Alexandri FL, Merino EF, del Portillo HA, Peres VJ, Almeida IC, Eberlin MN, Wunderlich G, Wiesner J, Jomaa H, Kimura EA, Katzin AM. 2004. The methylerythritol phosphate pathway is functionally active in all intraerythrocytic stages of Plasmodium falciparum. J Biol Chem 279:51749–51759. doi: 10.1074/jbc.M408360200. [DOI] [PubMed] [Google Scholar]
- 28.Laourdakis CD, Merino EF, Neilson AP, Cassera MB. 2014. Comprehensive quantitative analysis of purines and pyrimidines in the human malaria parasite using ion-pairing ultra-performance liquid chromatography–mass spectrometry. J Chromatogr B 967:127–133. doi: 10.1016/j.jchromb.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klawitter J, Schmitz V, Leibfritz D, Christians U. 2007. Development and validation of an assay for the quantification of 11 nucleotides using LC/LC-electrospray ionization-MS. Anal Biochem 365:230–239. doi: 10.1016/j.ab.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Smith RC, Eappen AG, Radtke AJ, Jacobs-Lorena M. 2012. Regulation of anti-Plasmodium immunity by a LITAF-like transcription factor in the malaria vector Anopheles gambiae. PLoS Pathog 8:e1002965. doi: 10.1371/journal.ppat.1002965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vega-Rodriguez J, Franke-Fayard B, Dinglasan RR, Janse CJ, Pastrana-Mena R, Waters AP, Coppens I, Rodriguez-Orengo JF, Srinivasan P, Jacobs-Lorena M, Serrano AE. 2009. The glutathione biosynthetic pathway of Plasmodium is essential for mosquito transmission. PLoS Pathog 5:e1000302. doi: 10.1371/journal.ppat.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Macedo CS, Uhrig ML, Kimura EA, Katzin AM. 2002. Characterization of the isoprenoid chain of coenzyme Q in Plasmodium falciparum. FEMS Microbiol Lett 207:13–20. doi: 10.1016/S0378-1097(01)00547-X. [DOI] [PubMed] [Google Scholar]
- 33.Tonhosolo R, D'Alexandri FL, Genta FA, Wunderlich G, Gozzo FC, Eberlin MN, Peres VJ, Kimura EA, Katzin AM. 2005. Identification, molecular cloning and functional characterization of an octaprenyl pyrophosphate synthase in intra-erythrocytic stages of Plasmodium falciparum. Biochem J 392:117–126. doi: 10.1042/BJ20050441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Meer JY, Hirsch AK. 2012. The isoprenoid-precursor dependence of Plasmodium spp. Nat Prod Rep 29:721–728. doi: 10.1039/c2np20013a. [DOI] [PubMed] [Google Scholar]
- 35.De Fries R, Mitsuhashi M. 1995. Quantification of mitogen induced human lymphocyte proliferation: comparison of alamarbluetm assay to 3h-thymidine incorporation assay. J Clin Lab Anal 9:89–95. doi: 10.1002/jcla.1860090203. [DOI] [PubMed] [Google Scholar]
- 36.O'Brien J, Wilson I, Orton T, Pognan F. 2000. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 37.Sun W, Tanaka TQ, Magle CT, Huang W, Southall N, Huang R, Dehdashti SJ, McKew JC, Williamson KC, Zheng W. 2014. Chemical signatures and new drug targets for gametocytocidal drug development. Sci Rep 4:3743. doi: 10.1038/srep03743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffy S, Avery VM. 2013. Identification of inhibitors of Plasmodium falciparum gametocyte development. Malar J 12:408. doi: 10.1186/1475-2875-12-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Turbachova I, Eberl M, Zeidler J, Lichtenthaler HK, Soldati D, Beck E. 1999. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 285:1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu S, Osada Y, Kanazawa T, Tanaka Y, Arai M. 2010. Suppressive effect of azithromycin on Plasmodium berghei mosquito stage development and apicoplast replication. Malar J 9:73–80. doi: 10.1186/1475-2875-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sullivan M, Li J, Kumar S, Rogers MJ, McCutchan TF. 2000. Effects of interruption of apicoplast function on malaria infection, development, and transmission. Mol Biochem Parasitol 109:17–23. doi: 10.1016/S0166-6851(00)00226-7. [DOI] [PubMed] [Google Scholar]
- 42.Dahl EL, Shock JL, Shenai BR, Gut J, DeRisi JL, Rosenthal PJ. 2006. Tetracyclines specifically target the apicoplast of the malaria parasite Plasmodium falciparum. Antimicrob Agents Chemother 50:3124–3131. doi: 10.1128/AAC.00394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacRae J, Dixon M, Dearnley M, Chua H, Chambers J, Kenny S, Bottova I, Tilley L, McConville M. 2013. Mitochondrial metabolism of sexual and asexual blood stages of the malaria parasite Plasmodium falciparum. BMC Biol 11:67. doi: 10.1186/1741-7007-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okamoto N, Spurck TP, Goodman CD, McFadden GI. 2009. Apicoplast and mitochondrion in gametocytogenesis of Plasmodium falciparum. Eukaryot Cell 8:128–132. doi: 10.1128/EC.00267-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peatey CL, Leroy D, Gardiner DL, Trenholme KR. 2012. Anti-malarial drugs: how effective are they against Plasmodium falciparum gametocytes? Malar J 11:34. doi: 10.1186/1475-2875-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Odom AR, Van Voorhis WC. 2010. Functional genetic analysis of the Plasmodium falciparum deoxyxylulose 5-phosphate reductoisomerase gene. Mol Biochem Parasitol 170:108–111. doi: 10.1016/j.molbiopara.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lucantoni L, Duffy S, Adjalley SH, Fidock DA, Avery VM. 2013. Identification of MMV malaria box inhibitors of Plasmodium falciparum early-stage gametocytes using a luciferase-based high-throughput assay. Antimicrob Agents Chemother 57:6050–6062. doi: 10.1128/AAC.00870-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanders NG, Sullivan DJ, Mlambo G, Dimopoulos G, Tripathi AK. 2014. Gametocytocidal screen identifies novel chemical classes with Plasmodium falciparum transmission blocking activity. PLoS One 9:e105817. doi: 10.1371/journal.pone.0105817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cassera MB, Merino EF, Peres VJ, Kimura EA, Wunderlich G, Katzin AM. 2007. Effect of fosmidomycin on metabolic and transcript profiles of the methylerythritol phosphate pathway in Plasmodium falciparum. Mem Inst Oswaldo Cruz 102:377–383. doi: 10.1590/S0074-02762007000300019. [DOI] [PubMed] [Google Scholar]
- 50.Couto AS, Kimura EA, Peres VJ, Uhrig ML, Katzin AM. 1999. Active isoprenoid pathway in the intra-erythrocytic stages of Plasmodium falciparum: presence of dolichols of 11 and 12 isoprene units. Biochem J 341(Part 3):629–637. [PMC free article] [PubMed] [Google Scholar]
- 51.Mbaya B, Rigomier D, Edorh GG, Karst F, Schrevel J. 1990. Isoprenoid metabolism in Plasmodium falciparum during the intraerythrocytic phase of malaria. Biochem Biophys Res Commun 173:849–854. doi: 10.1016/S0006-291X(05)80864-2. [DOI] [PubMed] [Google Scholar]
- 52.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS. 2011. The human serum metabolome. PLoS One 6:e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joice R, Nilsson SK, Montgomery J, Dankwa S, Egan E, Morahan B, Seydel KB, Bertuccini L, Alano P, Williamson KC, Duraisingh MT, Taylor TE, Milner DA, Marti M. 2014. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci Transl Med; 6:244re5. doi: 10.1126/scitranslmed.3008882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ralph SA, Van Dooren GG, Waller RF, Crawford MJ, Fraunholz MJ, Foth BJ, Tonkin CJ, Roos DS, McFadden GI. 2004. Tropical infectious diseases: metabolic maps and functions of the Plasmodium falciparum apicoplast. Nat Rev Microbiol 2:203–216. doi: 10.1038/nrmicro843. [DOI] [PubMed] [Google Scholar]
- 55.Holstein SA, Hohl RJ. 2004. Isoprenoids: remarkable diversity of form and function. Lipids 39:293–309. doi: 10.1007/s11745-004-1233-3. [DOI] [PubMed] [Google Scholar]
- 56.Tonhosolo R, Gabriel HB, Matsumura MY, Cabral FJ, Yamamoto MM, D'Alexandri FL, Sussmann RA, Belmonte R, Peres VJ, Crick DC, Wunderlich G, Kimura EA, Katzin AM. 2010. Intraerythrocytic stages of Plasmodium falciparum biosynthesize menaquinone. FEBS Lett 584:4761–4768. doi: 10.1016/j.febslet.2010.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tonhosolo R, D'Alexandri FL, de Rosso VV, Gazarini ML, Matsumura MY, Peres VJ, Merino EF, Carlton JM, Wunderlich G, Mercadante AZ, Kimura EA, Katzin AM. 2009. Carotenoid biosynthesis in intraerythrocytic stages of Plasmodium falciparum. J Biol Chem 284:9974–9985. doi: 10.1074/jbc.M807464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.D'Alexandri FL, Tonhosolo R, Kimura EA, Katzin AM. 2009. Mass spectrometry analysis of polyisoprenoids alcohols and carotenoids via ESI(Li(+))-MS/MS. Methods Mol Biol 580:109–128. doi: 10.1007/978-1-60761-325-1_6. [DOI] [PubMed] [Google Scholar]
- 59.Sussmann RA, Angeli CB, Peres VJ, Kimura EA, Katzin AM. 2011. Intraerythrocytic stages of Plasmodium falciparum biosynthesize vitamin E. FEBS Lett 585:3985–3991. doi: 10.1016/j.febslet.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 60.D'Alexandri FL, Kimura EA, Peres VJ, Katzin AM. 2006. Protein dolichylation in Plasmodium falciparum. FEBS Lett 580:6343–6348. doi: 10.1016/j.febslet.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 61.Shams-Eldin H, de Macedo CS, Niehus S, Dorn C, Kimmel J, Azzouz N, Schwarz RT. 2008. Plasmodium falciparum dolichol phosphate mannose synthase represents a novel clade. Biochem Biophys Res Commun 370:388–393. doi: 10.1016/j.bbrc.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 62.Chakrabarti D, Da Silva T, Barger J, Paquette S, Patel H, Patterson S, Allen CM. 2002. Protein farnesyltransferase and protein prenylation in Plasmodium falciparum. J Biol Chem 277:42066–42073. doi: 10.1074/jbc.M202860200. [DOI] [PubMed] [Google Scholar]
- 63.Jordão FM, Saito AY, Miguel DC, de Jesus Peres V, Kimura EA, Katzin AM. 2011. In vitro and in vivo antiplasmodial activities of risedronate and its interference with protein prenylation in Plasmodium falciparum. Antimicrob Agents Chemother 55:2026–2031. doi: 10.1128/AAC.01820-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wiesner J, Kettler K, Sakowski J, Ortmann R, Katzin AM, Kimura EA, Silber K, Klebe G, Jomaa H, Schlitzer M. 2004. Farnesyltransferase inhibitors inhibit the growth of malaria parasites in vitro and in vivo. Angew Chem Int Ed Engl 43:251–254. doi: 10.1002/anie.200351169. [DOI] [PubMed] [Google Scholar]
- 65.Howe R, Kelly M, Jimah J, Hodge D, Odom AR. 2013. Isoprenoid biosynthesis inhibition disrupts Rab5 localization and food vacuolar integrity in Plasmodium falciparum. Eukaryot Cell 12:215–223. doi: 10.1128/EC.00073-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dahl EL, Rosenthal PJ. 2007. Multiple antibiotics exert delayed effects against the Plasmodium falciparum apicoplast. Antimicrob Agents Chemother 51:3485–3490. doi: 10.1128/AAC.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noedl H, Krudsood S, Leowattana W, Tangpukdee N, Thanachartwet W, Looareesuwan S, Miller RS, Fukuda M, Jongsakul K, Yingyuen K, Sriwichai S, Ohrt C, Knirsch C. 2007. In vitro antimalarial activity of azithromycin, artesunate, and quinine in combination and correlation with clinical outcome. Antimicrob Agents Chemother 51:651–656. doi: 10.1128/AAC.01023-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sidhu ABS, Sun Q, Nkrumah LJ, Dunne MW, Sacchettini JC, Fidock DA. 2007. In vitro efficacy, resistance selection, and structural modeling studies implicate the malarial parasite apicoplast as the target of azithromycin. J Biol Chem 282:2494–2504. doi: 10.1074/jbc.M608615200. [DOI] [PubMed] [Google Scholar]
- 69.Carter R, Mendis KN, Miller LH, Molineaux L, Saul A. 2000. Malaria transmission-blocking vaccines—how can their development be supported? Nat Med 6:241–244. doi: 10.1038/73062. [DOI] [PubMed] [Google Scholar]
- 70.Sarkar M, CCW, Koren G, Einarson ARN. 2006. Pregnancy outcome following gestational exposure to azithromycin. BMC Pregnancy Childbirth 6:18. doi: 10.1186/1471-2393-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Treadway G, Pontani D. 1996. Paediatric safety of azithromycin: worldwide experience. J Antimicrob Chemother 37:143–149. doi: 10.1093/jac/37.suppl_C.143. [DOI] [PubMed] [Google Scholar]
- 72.Koreny L, Obornik M, Lukes J. 2013. Make it, take it, or leave it: heme metabolism of parasites. PLoS Pathog 9:e1003088. doi: 10.1371/journal.ppat.1003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sheiner L, Vaidya AB, McFadden GI. 2013. The metabolic roles of the endosymbiotic organelles of Toxoplasma and Plasmodium spp. Curr Opin Microbiol 16:452–458. doi: 10.1016/j.mib.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.