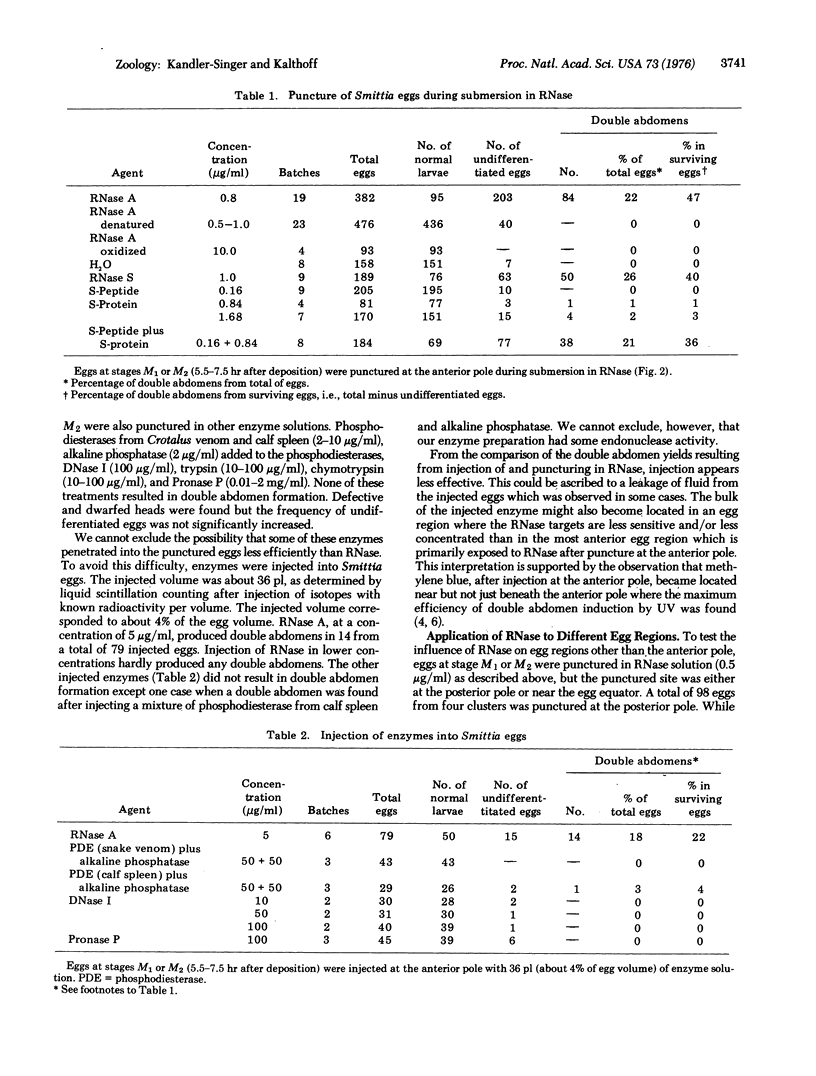
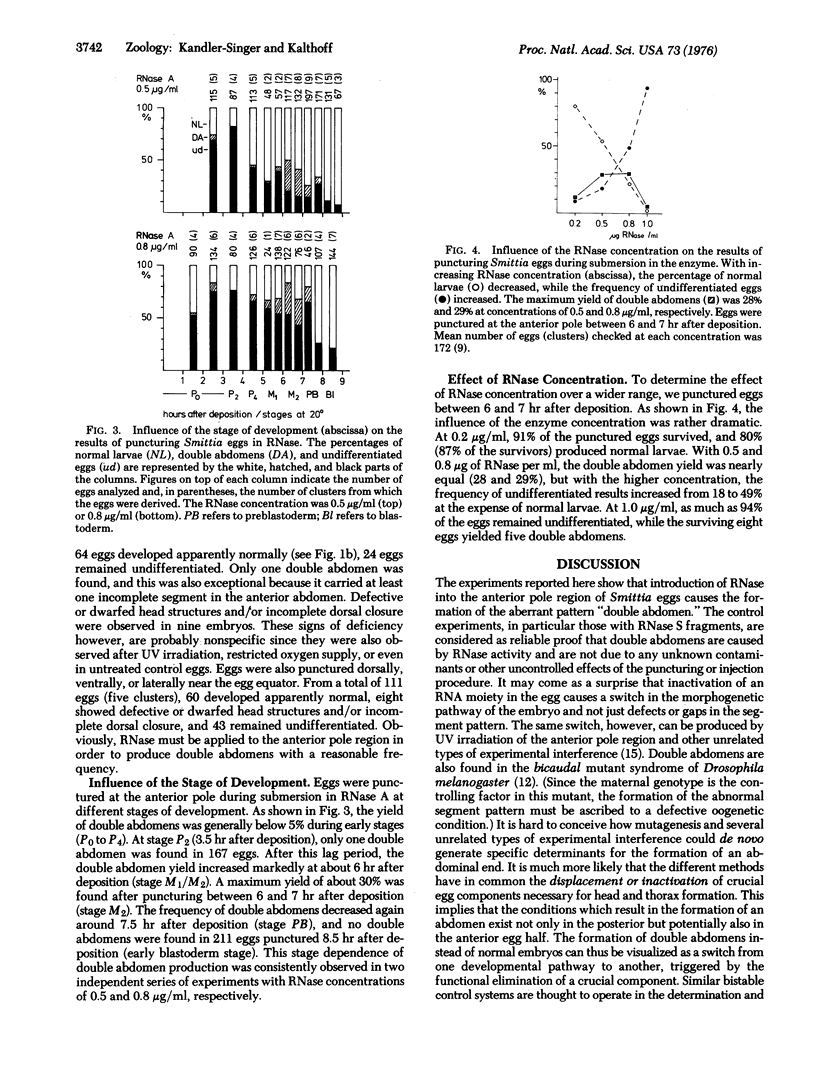
Abstract
In chironomid midges, the development of head and thorax in the embryo requires the function of cytoplasmic determinants localized near the anterior pole of the egg. Experimental inactivation of these determinants causes a dramatic switch in the developmental program of the embryo. Instead of the normal segment pattern, the aberrant pattern "double abdomen" is formed. Head, thorax, and anterior abdominal segments are then replaced by an additional set of posterior abdominal segments joined in mirror image symmetry to the original abdomen. Such double abdomens have been produced, with a maximum yield of 29%, by application of RNase (ribonuclease I, ribonucleate 3'-pyrimidino-oligonucleotidohydrolase, EC 3.1.4.22) to the anterior pole region of the egg. This was achieved by microinjection or puncturing the eggs during submersion in RNase. Control experiments with inactive RNase S fragments reliably proved that double abdomen formation resulted from RNase activity. Neither application of other enzymes to the anterior pole region nor application of RNase to other egg regions produced double abdomens in significant yields. The effects of RNase concentration and stage of development were determined. The data from these and earlier experiments are compatible with the idea that stored cytoplasmic RNP particles act as anterior determinants. Similarities to genetically caused switches in developmental pathways (homeotic mutations) are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kauffman S. A. Control circuits for determination and transdetermination. Science. 1973 Jul 27;181(4097):310–318. doi: 10.1126/science.181.4097.310. [DOI] [PubMed] [Google Scholar]
- Ouweneel W. J. Developmental genetics of homoeosis. Adv Genet. 1976;18:179–248. doi: 10.1016/s0065-2660(08)60439-3. [DOI] [PubMed] [Google Scholar]
- RICHARDS F. M., VITHAYATHIL P. J. The preparation of subtilisn-modified ribonuclease and the separation of the peptide and protein components. J Biol Chem. 1959 Jun;234(6):1459–1465. [PubMed] [Google Scholar]
- Schmidt O., Zissler D., Sander K., Kalthoff K. Switch in pattern formation after puncturing the anterior pole of Smittia eggs (Chironomidae, Diptera). Dev Biol. 1975 Sep;46(1):216–221. doi: 10.1016/0012-1606(75)90099-8. [DOI] [PubMed] [Google Scholar]
- Spirin A. S. The second Sir Hans Krebs Lecture. Informosomes. Eur J Biochem. 1969 Aug;10(1):20–35. doi: 10.1111/j.1432-1033.1969.tb00651.x. [DOI] [PubMed] [Google Scholar]
- YAJIMA H. Studies on embryonic determination of the harlequin-fly, Chironomus dorsalis. I. Effects of centrifugation and of its combination with constriction and puncturing. J Embryol Exp Morphol. 1960 Jun;8:198–215. [PubMed] [Google Scholar]
- Yajima H. Study of the development of the internal organs of the double malformations of Chironomus dorsalis by fixed and sectioned materials. J Embryol Exp Morphol. 1970 Sep;24(2):287–303. [PubMed] [Google Scholar]




