Abstract
Background
Drug resistance mutations archived in resting memory CD4+ cells may persist despite suppression of HIV RNA to <50 copies/ml. We sequenced pol gene from proviral DNA among viremic and suppressed patients to identify drug resistance mutations.
Methods
The Peninsula AIDS Research Cohort study enrolled and followed over 2 years 120 HIV infected patients from San Mateo and San Francisco Counties. HIV-1 pol genotyping by bulk sequencing was performed on 38 DNA and RNA from viremic patients and DNA only among 82 suppressed patients at baseline. Antiretroviral susceptibility was predicted by HIVDB.stanford.edu.
Results
Among 120 subjects, 81% were on antiretroviral therapy and had been treated for a median time of 7 years. Thirty-two viremic patients showed concordant RNA and DNA genotypes (84%); the discordant profiles were mainly observed in patients with low-level viremia. Among suppressed patients, 21 had drug resistance mutations in proviral DNA (26%) with potential resistance to one, two or three ARV classes in 16, 4 and 1 samples respectively.
Conclusions
The high level of genotype concordance between DNA and RNA in viremic patients suggested that DNA genotyping might be used to assess drug resistance in resource-limited settings, and further investigation of extracted DNA from dried blood spots is needed. Drug resistance mutations in proviral DNA in 26% of subjects with less than 50 copies/ml pose a risk for the transmission of drug resistant virus with virologic failure, treatment interruption or decreased adherence.
Introduction
Antiretroviral therapy (ART) has changed the dynamic of the HIV epidemic through suppression of HIV-1 RNA to undetectable plasma levels (<50 copies/ml). However, latent HIV infection of CD4+ T cells, established early after infection, necessitates lifelong ART [1–3]. Treatment failure can lead to the selection of drug resistance mutations (DRM) in the viral genome, which may be archived in the cellular reservoir. Even when viral RNA (vRNA) is suppressed, DRM may be detected in proviral DNA by sequencing extracted DNA from buffy coat, whole blood or peripheral blood mononuclear cells (PBMC) [4–7]. DRM archived in proviral DNA may be expressed upon virologic failure, transmit drug resistant virus and impact management of long-term ART [8–11]. Although it is the current standard for assessing drug susceptibility, the plasma genotype may underestimate drug resistance if treatment has been interrupted or adherence compromised with the overgrowth of susceptible virus in the absence of drug pressure [12–14].
Genotyping of the proviral reservoir may inform treatment decisions, identify occult DRM and uncover community phylogenetic relationships. Retrieving HIV resistance data from PBMC may be less expensive, but the clinical significance of DRM in proviral DNA remains controversial [9,15–17]. Moreover, consensus genotype from plasma RNA or proviral DNA does not detect DRM as minority variants <20% [18–20].
Integrated HIV proviral DNA in T central memory cells can be transcriptionally reactivated leading to virological failure [1,2,21]. Some studies show that HIV proviral populations may evolve despite apparent suppression of viremia [15], while others find minimal changes in the composition of the proviral population during suppressive treatment [22,23]. The proviral reservoir is most likely the source of the persistent expression of virus at <50 copies/ml among patients on suppressive ART, despite the addition of potent integrase inhibitors [24–26]. Whether these low levels of HIV RNA result from the continued replication in reservoirs, tissues or cells unaffected by ART, or are derived from activation of cells in the latent reservoir is controversial, and both mechanisms may be occurring.
The resistance mutations detected by genotyping proviral DNA after virologic failure have been found to closely mirror those obtained from concomitant vRNA [4,27–29] unless there has been a treatment interruption or poor adherence [30]. Significantly fewer mutations have been found in proviral DNA compared to historic vRNA genotypes showing that some DRM are not archived or are not detectable in the latent reservoir [30–32]. Conversely, the archived provirus mutations in T cells may not be detected in plasma RNA in the absence of drug pressure [33,34].
Here, to assess the prevalence and the pertinence of DRM archived in proviral DNA, 120 subjects, 82 suppressed to <50 copies/ml and 38 with 68-171,000 copies/ml of plasma RNA, were sequenced from one or both compartments. We identified virologic failures among suppressed patients by evaluating the longitudinal relationship between archival DNA and RNA drug resistance patterns. PBMC genotyping may identify transmission linkages and add information to optimize ART among suppressed patients.
Material and Methods
Study population
One hundred and twenty HIV infected individuals were recruited from the Peninsula AIDS Research Consortium clinic at the San Mateo County AIDS Program and the San Francisco Public Health Department clinic, and enrolled in a longitudinal study of drug resistance. Participants were recruited from public health clinics during attendance for routine HIV care.
Ethics statements
Written informed consent was obtained for each participant and the study was approved by the institutional review board “Administrative Panels for the Protection of Human Subjects” from Stanford University for the protocol 7861 “Antiretroviral Drug Resistance in Blood, Oral, and Genital HIV-1: Implications for Pathogenesis and Transmission of Drug Resistance and Secondary Prevention in Community Based Treatment Programs”.
Sample collection
Study visits occurred every 6 months over a total of 2 years. Blood specimens were obtained at each visit and transported to the laboratory within 6 hours of collection. Plasma was removed after centrifugation and PBMC were separated from whole blood by density gradient centrifugation and stored at -70°C in 90% human serum, 10% Dimethyl Sulfoxyde (DMSO). Viral load (VL) and CD4 measures were performed by the clinical laboratories at San Mateo County hospital using Roche Cobas Amplicor Monitor v1.5 (Roche Molecular Diagnostics, Inc., Branchburg, NJ, USA) and BD FACSCalibur (Becton Dickinson, Franklin Lakes, NJ, USA), respectively.
DNA and RNA extractions
Virus was concentrated from 1 ml of plasma by centrifugation for 20 min at 20,000g to pellet the virus, 800μl of supernatant was discarded and RNA extracted from the 200μl residua with QIAamp viral RNA Mini kit (QIAGEN Inc., Hilden, Germany) as per the manufacturer’s protocol. vRNA was used as template for reverse transcription using SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) and random hexamer primers, using cycling parameters of 10min at 25°C, 30min at 45°C and 15min at 70°C, and the RNA template degraded with RNAse H for 20 min at 37°C. DNA was extracted from pelleted PBMC using QIAamp DNA Blood Mini kit (QIAGEN Inc.).
Genotyping
At baseline, vRNA and proviral DNA, were sequenced from viremic patients (n = 38) while suppressed patients (n = 82) provided only a proviral DNA genotype. Only the vRNA was sequenced from patients who virologically failed during follow-up (n = 11).
A 1,300-bp fragment encompassing the protease (PR) and the first 300 amino acids of the reverse transcriptase (RT) gene was generated from DNA and cDNA for PCR amplification, followed by a nested PCR with 1st round PCR primers; MAW26 (5´-TTGGAAATGTGGAAAGGAAGGAC-3´) and RT21 (5´-CTGTATTTCTGCTAT TAAGTCTTTTGATGGG-3´) and 2nd round with PRO1 (5´-CAGAGCCAACAGCCC CACCA-3´) and RT20 (5´-CTGCCAGTTCTAGCTCTG CTTC-3´), with Platinum Taq DNA Polymerase (Invitrogen), 50pmoles of each primer, 2mM of Magnesium Chloride and 0.5mM of dNTPs. Forty cycles for the 1st and 35 for the 2nd round of 94°C for 15s, 55°C for 20s and 72°C for 2 min, then 10min at 72°C produced a nested PCR product, purified using QIAquick PCR Purification kit (QIAGEN Inc.), as per manufacturer’s protocol. After quantification, 30ng were sequenced using the Big Dye Terminator kit (Life Technologies, Foster City, CA, USA) and a set of 4 bidirectional primers by capillary electrophoresis on a 3130xl Genetic Analyzer (Life Technologies).
Sequence analysis
Sequences were assembled using Geneious R6 genetic analyzer (Biomatters, Auckland, NZ) [35]. DRM were identified using the Stanford HIV Drug Resistance Database [36]. A Neighbor Joining phylogenetic tree was inferred with Phylip package [37] with 2-Kimura parameters and support values for the branching pattern were calculated from 1000 bootstrap replicates, visualized using FigTree [38] and analyzed for potential transmission clusters and linked infections. The frequency of apolipoprotein B mRNA-editing, enzyme-catalytic, polypeptide-like 3G/F (APOBEC3G/F) G to A mutations have been determined with the Calibrated Population Resistance tool from the HIV Stanford Database for Quality Assessment [39]. More than 3 characteristic mutations in the RT and 2 in the PR genes associated with APOBEC3G/F activity were considered as evidence for hypermutation. HIV-1 PR and RT sequences recovered from PBMC and viral RNA have been submitted to GenBank with the accession numbers KJ69464 to KJ769641.
Results
Baseline characteristics
One hundred and twenty subjects were enrolled: their demographic, immunologic and virologic baseline characteristics are presented in Table 1. The median age of the study population was 44 years (ranging from 20 to 64), 82% were male, 38% White, 28% African-American, 25% Hispanic/Latino and 9% Asian/Pacific Island/Native American (reflecting the ethnic and gender distributions of HIV infection in the San Francisco Bay Area). Among all study participants, 97 (81%) had been on ART for a median of 7 years. Of these patients, 41% remained on a non-nucleoside reverse transcriptase (RT) inhibitor (NNRTI) and 59% on a Protease inhibitor (PI) based regimen.
Table 1. Demographic, immunologic and virologic characteristics of the study population n = 120.
| Characteristic | N = 120 | |
|---|---|---|
| Gender | Female | 21 (17.5%) |
| Male | 98 (81.7%) | |
| Transgender | 1 (0.8%) | |
| Ethnicity | White | 46 (8.37%) |
| African American | 33 (27.5%) | |
| Hispanic / Latino | 30 (25%) | |
| Other (Asian, Natives) | 11 (9.2%) | |
| Age | Median (range) | 44 (20–64) |
| On HAART | Yes | 97 (81%) |
| CD4 count (cells/mm3) | <200 | 22 (18%) |
| >200 | 98 (82%) | |
| Viral Load (copies/ml) | <50 | 82 (68.3%) |
| >50 | 38 (31.7%) | |
| Patients on HAART | Viral Load < 50 | 79 (81.5%) |
| Viral Load >50 | 18 (18.5%) | |
| CD4 count < 200 | 17 (17.5%) | |
| CD4 count > 200 | 80 (82.5%) | |
| Patients not currently on HAART | Viral Load < 50 | 3 (13%) |
| Viral Load >50 | 20 (87%) | |
| CD4 count < 200 | 5 (12%) | |
| CD4 count > 200 | 18 (78%) |
At study entry 82 subjects (68%) were suppressed to <50 copies/ml and 38 (32%) had >50 copies/ml of plasma HIV-1 RNA with a median of 5,865 copies/ml. The median CD4+ cells count was 422/mm3 among suppressed patients and 305/mm3 among viremic patients. Cumulative ART history included exposure to TDF (85%), AZT and/or d4T (57%) and 57% and 66% had received an NNRTI- or PI- based regimen respectively.
Baseline resistance profiles in viremic patients
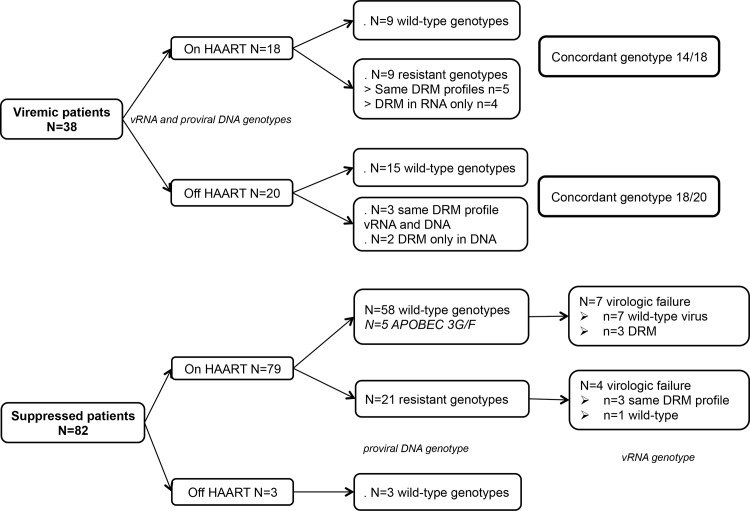
Overall, resistance profiles in both vRNA and proviral DNA were concordant in 32/38 viremic patients at baseline (84%). Twenty-four patients had wild-type genotype in both vRNA and DNA and 14 had DRM (see Table 2); 12 had resistance mutations in vRNA and DNA with identical DRM in 8/12 (some present in vRNA were absent in proviral DNA), and 2 had DRM only in DNA (see Fig. 1 and Table 2).
Table 2. Drug resistance mutations profiles of 14 viremic patients at baseline with drug resistance with their viral load and treatment status at entry.
| PID | ART at baseline | VL at baseline | Proviral DNA | Viral RNA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TAMs | Non TAMS | NNRTI | PI | TAMs | Non TAMs | NNRTI | PI | |||
| 10064 | yes | 67 | K103N | M184MV | K103N | |||||
| 10020 | 68 | T215FIST, K70KR, D67DN, K219KQ | T215F, K70R, D67N, K219Q, M41L, T69ADNT | M184V | ||||||
| 20012 | 149 | T215Y, M41L | M184MV | D30N, M46L | T215Y, M41L, L210W | M184V | D30N, M46L | |||
| 10041 | 788 | T215ILPT | I54V, V82IT | T215IL | I54V, V82IT | |||||
| 50007 | 969 | D67DG, K219KN | M184MV | K103KN, V106AV | D67G, K219N | M184V, L74IL | K103N, V106A | |||
| 10014 | 3,320 | T215F | M184MV | T215F | M184V | |||||
| 10056 | 7,190 | T215NSTY | M184V | K103N | T215Y | M184V | K103N | |||
| 60009 | 41,700 | K103N, Y181C | K103KN, Y181YC | |||||||
| 10047 | 49,100 | V179D | V179D | |||||||
| 60001 | no | 156 | M184V | V82AITV | ||||||
| 10046 | 2,770 | M184MV | ||||||||
| 30018 | 4,114 | K103R | K103R | |||||||
| 30014 | 12,548 | K103N | K103N | |||||||
| 30023 | 29,268 | K103N | K103N | |||||||
The other 24 viremic patients did not have DRM in either RNA or DNA.
Discordant DRM between proviral DNA and viral RNA are shown in blue.
Fig 1. Flow chart for the 120 patients enrolled in the study.
Among viremic patients, 18/38 (47%) had been on treatment at baseline for a median of 4 years (ranging from 1 to 13 years). Half were wild-type and the other half harbored resistance in vRNA and proviral DNA, and 4 of them displayed DRM in vRNA that were not present in proviral DNA (see Table 2 – upper section).
Twenty viremic patients were not treated or had interrupted treatment prior to enrollment, 15 of whom had wild-type genotype in both vRNA and proviral DNA. Three of the twenty untreated patients had DRM in both vRNA and DNA, and 2 had DRM only in proviral DNA (see Table 2 – lower section).
Discordant resistance profiles in vRNA and DNA were seen in 6 patients with low viral load (mean of 697 copies/ml) in contrast with the concordant genotypes among 32 with higher virus load (mean 31,252 copies/ml). Considering only viremic patients with VL >1,000 copies/ml, concordance between vRNA and proviral DNA was 97%. Finally, among 12 patients known to have been previously exposed to NNRTI, 4 had NNRTI resistance mutations in proviral DNA while among 22 exposed to PI, only 3 had PI resistance mutations.
Baseline resistance profiles in suppressed patients
Most of the 82 suppressed subjects were on treatment, 3 were reported to be off of treatment and harbored wild-type genotypes in proviral DNA (see Fig. 1). Twenty-one harbored archived DRM in proviral DNA (26%); 11 to NRTI, 5 to NNRTI only, 3 to NNRTI and NRTI, 1 to PI and NRTI, and 1 had DRM to all the 3 classes. Three of the subjects had NNRTI resistance mutations in proviral DNA although previous NNRTI exposure had not been recorded. Among the 12 subjects exposed to NNRTI, 6 (50%) had NNRTI resistance mutations, while among 17 patients who have been exposed to a PI only 2 (12%) harbored PI resistance mutations. Among the 61 without detectable DRM in proviral DNA, 5 (8%) had ≥3 APOBEC3G/F signature mutations in pol gene with modestly higher CD4 cell counts (mean value 435 vs 578 CD4 cells/mm3).
Emergence of viremia and virologic failure
Of the 82 study subjects with <50 copies/ml at study entry, 11/82 (13%) subsequently had a VL >50 copies/ml at a mean of 39 weeks. Seven of the 11 had virologic failure with >1,000 copies/ml (mean VL = 28,331 copies/ml), among them 3 had DRM in proviral DNA at baseline (upper section of Table 3); 1 had an NRTI mutation (PID 10069), 1 had NNRTI mutations (PID 10082) and 1 had both NRTI and NNRTI mutations (PID 10033). Two of them had the same DRM at virological failure and one had wild-type genotype. Two patients among 4 with wild-type genotype at baseline developed new DRM consistent with NNRTI based regimen (PIDs 10017 and 50002, middle section of Table 3). Among the 4 with low level VF (mean VL = 160 copies/ml) (lower section of Table 3); one patient had NRTI mutations at baseline and virologic failure (PID 10059), and another one wild-type at baseline had a K70KR mixture arose at VF (PID 10018). The 2 other patients (PIDs 10010 and 20014) had a wild-type genotype at both baseline and virological failure.
Table 3. Drug resistance mutations profiles among 11 patients who were suppressed at baseline (BL) and failed ART during the study follow-up.
| PID | BL DRM in DNA | Week of virologic failure—VL | vRNA TAMs | vRNA Non TAMs | vRNA NNRTI | ART regimen |
|---|---|---|---|---|---|---|
| 10033 | T69ADNT, M184MV, G190AG | 48–4,710 | AZT 3TC TDF NVP | |||
| 72–1,670 | ||||||
| 10069 | T215D | 24–1,680 | T215D | ABC AZT 3TC NVP | ||
| 10082 | K103KN, K238KT | 48–2,130 | K103KN, K238KT | TDF FTC AZT ATV | ||
| 10017 | 24–74,200 | TDF FTC EFV | ||||
| 48–7,610 | K65KR / M184MV | K103N, Y188FLHY, V108IV, M230ML | ||||
| 10075 | 48–32,000 | TDF FTC ATV | ||||
| 10090 | 48–73,300 | TDF FTC ATV | ||||
| 50002 | 24–10,300 | Y188CY | TDF FTC EFV | |||
| 10010 | 24–59 | TDF FTC NVP | ||||
| 96–69 | ||||||
| 20014 | 48–157 | NA | ||||
| 10018 | 48–119 | K70KR | TDF ABC LPV | |||
| 10059 | M41L, T215S | 48–303 | M41L, T215S | TDF FTC LPV | ||
| 72–276 | M41L, T215S |
Phylogenetic analysis
A phylogenetic tree shown as Fig. 2 shows 2 pairs of sequences that cluster with high bootstrap values. The first pair includes 2 men in their late 30s, one heterosexual and one a former intravenous drug user (IDU). The second pair of men in their late 30s, included a former IDU who had sex with men and a heterosexual. These risk group characteristics suggest that direct transmission was unlikely to occur, but the clustering suggests a common source of infection.
Fig 2. Neighbor Joining tree with 2-Kimura parameters and 1,000 replicates.
Ethnicities are represent with different color codes: blue = White, green = Hispanic, red = African American and black = Asian and others. Bootstrap values for transmission clusters are showed. Drug resistance mutations are also represented ● NRTI ■ NNRTI and ▲ PI.
Another cluster distinguished from the tree includes 18 subjects. Their median age is 50 years, and 16 are African-American, 15 are former IDU, 10 are female and 10 currently live in East Palo Alto. Despite a low bootstrap value (430), this group shares common characteristics, which highlight the dynamic of HIV epidemic among drug users and their sexual partners in the 1990s [40].
Discussion
In summary, our study showed that proviral DNA and vRNA drug resistance profiles of viremic subjects are concordant (84%, CI95%: 69–92%) and are consistent with a median of 87% describe in the literature [4,13,41–45]. Discordance was most common among those with low virus loads (50–1,000 copies/ml). Among those with >1,000 copies/ml, there was 97% concordance between mutations detected in viral RNA and PBMC DNA.
RNA genotyping is the gold-standard template for HIV drug resistance testing and requires RNA extraction, reverse-transcription followed by PCR and sequencing reactions. vRNA is labile and genotyping protocols mandate separation and storage of plasma at -70°C within 6 hours. In addition, reverse transcription is the most critical and costly step in RNA genotyping [46]. While this study used cryopreserved PBMC, which can be labor intensive to prepare and requires cold storage as well, DNA may be extracted from whole blood or dried blood spots, and can be transported at room temperature for several days. These results suggest that proviral DNA sequencing may be a less resource intensive means to assess drug resistance in resource-limited settings among patients failing ART. Further studies should compare genotyping of vRNA and proviral DNA extracted from dried blood spots to approximate to “real-life” conditions in RLS. Cheaper, easier and reliable proviral DNA genotyping may offer communities and clinics, distant from laboratory facilities, improved clinical management of HIV patients in decentralized treatment programs.
DRM, predominantly NRTI and NNRTI resistance mutations, were found in PBMC proviral DNA among 26% of subjects with suppressed plasma RNA levels who enrolled in a community treatment program in California. Those with PBMC DRM had failed previous ART and were subsequently suppressed on either a boosted PI or NNRTI based regimen. Stable reservoirs of NRTI and NNRTI DRM (particularly M184V and K103N) in PBMC are common at virologic failure of a first-line NNRTI based regimen [23,34,47–51]. Archived DRM in proviral DNA despite suppression of plasma RNA for years, raises concern about the potential for transmitted drug resistance (TDR). Among the viremic subjects in this study who denied previous NNRTI exposure, 2/16 (12.5%) had DRM in vRNA and proviral DNA, which is consistent with levels of TDR associated with acute infection and newly diagnosed patients in San Francisco [52]. The NNRTI and NRTI mutations observed included K103N, G190A and K238T and K65R, T215Y/F and M184V associated with rapid virologic failure on NNRTI first-line regimens as well as transmission [53–56]. The DRM observed in proviral DNA of suppressed subjects, may be expressed in vRNA in the genital track, and thus potentially may be transmitted by genital fluids [57].
The significance of low level viremia (between 50 and 1,000 copies/ml) remains controversial [58–61]. These values require confirmation, and few authorities would recommend drug switching with VL measures based on a single value between 50 and 1,000 copies/ml [62,63]. Among samples with <1,000 copies/ml, genotyping of PBMC and plasma demonstrated DRM in 5 of 9 from both components, and those with DRM in the plasma had similar DRM in PBMC DNA. Some discordance in specific mutations (present in one compartment or the other) was observed, but differences between plasma and PBMC did not identify new class resistance nor were they sufficient to alter treatment considerations. The sensitivity of detection of individual DRM in PBMC as compared to plasma was 84% (32/38). There were 2 instances at low virus load (156 and 2770 copies/ml) where DRM found in PBMC (M184V, V82F) were not expressed in plasma. This may be due to the re-emergence of wild-type virus with waning drug pressure and early virologic failure. Also, 4 samples displayed DRM in vRNA that were absent from proviral DNA that may be explained by the emergence of DRM not yet archived in proviral DNA or present but at levels undetectable by bulk sequencing <20%. Further studies may be required to examine minor viral quasispecies in proviral DNA with Next Generation Sequencing technologies.
In assigning clinical significance to DRM in PBMC, the study is limited by the potency of modern suppressive regimens with relatively small numbers with virologic failure over a mean follow-up of 46 weeks. However the frequency of DRM at entry among suppressed individuals who developed virologic failure 4/11 (36%) was similar to DRM found among those who remained suppressed, 17/68 (25%), suggesting that DRM in PBMC are not themselves a risk for virologic failure, and were only expressed in a fraction of those with later virologic failure.
APOBEC3 cytidine deaminases restrict endogenous and exogenous retroviruses by inducing G to A hypermutations in GG or GA dinucleotides context. HIV-1 counteracts this antiviral activity through Vif. Kieffer et al showed that hypermutated sequences comprise >9% of archived species in resting CD4+ cells [64]. In our study 5/81 proviral sequences were hypermutated (6%), consistent with recent studies [65,66], where E138K and M184I were the most common APOBEC3G/F associated mutations. These were clearly defined as codons that were hypermutated rather than mutations selected by 2nd generation NNRTI and 3TC/FTC respectively and can be attributed to APOBEC3G/F editing [67,68].
Fourati et al demonstrated that the HIV-1 genome is often defective in PBMC after a long-term HAART [69]. In our study, subjects with APOBEC3G/F signature mutations had higher median CD4 counts than those without (578 vs 435 cells/mm3) reflecting a robust immune response. However, archival DRM, even in hypermutated sequences, can shape the phenotype of the circulating viral population through recombination with replication competent virus, leading to resistant strains [67]. Defective HIV-1 quasispecies in the form of multiple drug-resistant proviral DNA within cells can be rescued by superinfection with different subtype variants of HIV-1, HIV-2 and SIV [70].
Sequencing proviral DNA provided additional information about DRM, which may impact treatment decisions, particularly in the setting of low level viremia or suppression. Moreover, new information about phylogenetic relationships and the prevalence of DRM, albeit as archival mutations among suppressed individuals may identify historic transmission dynamics in a community. Here we found linkage among African American men and women in East Palo Alto, consistent with a reported peak in the IDU epidemic more than 20 years ago in this community [71]. Although the ultimate duration and clinical significance of proviral resistance is not fully defined, archival mutations often are expressed upon virologic failure, potentially contributing to TDR, and archived mutations may limit future treatment options. Further studies of proviral DNA sequences and sequence analysis in monitoring ART are warranted.
Acknowledgments
We want to acknowledge support for the PARC Peninsula AIDS Research Collaboration from the California HIV Research Program and thank all the study participants.
Data Availability
HIV-1 PR and RT sequences recovered from PBMC and viral RNA have been submitted to GenBank with the accession numbers KJ69464 to KJ769641.
Funding Statement
This work was funded by California HIV Research Program CH05-SMCH-612. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chun TW, Engel D, Mizell SB, Ehler LA, Fauci AS (1998) Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J Exp Med Jul 6;188(1):83–91. Erratum in: J Exp Med 1998 Aug 3;188(3):following 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, et al. (1997) Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science Nov 14;278(5341):1295–300. [DOI] [PubMed] [Google Scholar]
- 3. Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, et al. (2003) Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med Jun;9(6):727–8. [DOI] [PubMed] [Google Scholar]
- 4. Chew CB, Potter SJ, Wang B, Wang YM, Shaw CO, et al. (2005) Assessment of drug resistance mutations in plasma and peripheral blood mononuclear cells at different plasma viral loads in patients receiving HAART. J Clin Virol Jul;33(3):206–16. [DOI] [PubMed] [Google Scholar]
- 5. Coovadia A, Hunt G, Abrams EJ, Sherman G, Meyers T, et al. (2009) Persistent minority K103N mutations among women exposed to single-dose nevirapine and virologic response to nonnucleoside reverse-transcriptase inhibitor-based therapy. Clin Infect Dis Feb 15;48(4):462–72. 10.1086/596486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. MacLeod IJ, Rowley CF, Thior I, Wester C, Makhema J, et al. (2010) Minor resistant variants in nevirapine-exposed infants may predict virologic failure on nevirapine-containing ART. J Clin Virol Jul;48(3):162–7. 10.1016/j.jcv.2010.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neogi U, Shet A, Sahoo PN, Bontell I, Ekstrand ML, et al. (2013) Human APOBEC3G-mediated hypermutation is associated with antiretroviral therapy failure in HIV-1 subtype C-infected individuals. J Int AIDS Soc Feb 25;16:18472 10.7448/IAS.16.1.18472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bi X, Gatanaga H, Ida S, Tsuchiya K, Matsuoka-Aizawa S, et al. (2003) Emergence of protease inhibitor resistance-associated mutations in plasma HIV-1 precedes that in proviruses of peripheral blood mononuclear cells by more than a year. J Acquir Immune Defic Syndr Sep 1;34(1):1–6. [DOI] [PubMed] [Google Scholar]
- 9. Lambotte O, Chaix ML, Gubler B, Nasreddine N, Wallon C, et al. (2004) The lymphocyte HIV reservoir in patients on long-term HAART is a memory of virus evolution. AIDS May 21;18(8):1147–58. [DOI] [PubMed] [Google Scholar]
- 10. Verhofstede C, Wanzeele FV, Van Der Gucht B, De Cabooter N, Plum J (1999) Interruption of reverse transcriptase inhibitors or a switch from reverse transcriptase to protease inhibitors resulted in a fast reappearance of virus strains with a reverse transcriptase inhibitor-sensitive genotype. AIDS Dec 24;13(18):2541–6. [DOI] [PubMed] [Google Scholar]
- 11. Yerly S, Kaiser L, Race E, Bru JP, Clavel F, et al. (1999) Transmission of antiretroviral-drug-resistant HIV-1 variants. Lancet Aug 28;354(9180):729–33. [DOI] [PubMed] [Google Scholar]
- 12. Deeks SG, Wrin T, Liegler T, Hoh R, Hayden M, et al. (2001) Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N Engl J Med Feb 15;344(7):472–80. [DOI] [PubMed] [Google Scholar]
- 13. Devereux HL, Loveday C, Youle M, Sabin CA, Burke A, et al. (2000) Substantial correlation between HIV type 1 drug-associated resistance mutations in plasma and peripheral blood mononuclear cells in treatment-experienced patients. AIDS Res Hum Retroviruses Jul 20;16(11):1025–30. [DOI] [PubMed] [Google Scholar]
- 14. Verhofstede C, Noë A, Demecheleer E, De Cabooter N, Van Wanzeele F, et al. (2004) Drug-resistant variants that evolve during nonsuppressive therapy persist in HIV-1-infected peripheral blood mononuclear cells after long-term highly active antiretroviral therapy. J Acquir Immune Defic Syndr Apr 15;35(5):473–83. [DOI] [PubMed] [Google Scholar]
- 15. Falasca F, Montagna C, Maida P, Bucci M, Fantauzzi A, et al. (2013) Analysis of intracellular human immunodeficiency virus (HIV)-1 drug resistance mutations in multi-failed HIV-1-infected patients treated with a salvage regimen: 72-week follow-up. Clin Microbiol Infect Jul;19(7):E318–21. 10.1111/1469-0691.12175 [DOI] [PubMed] [Google Scholar]
- 16. Noë A, Plum J, Verhofstede C (2005) The latent HIV-1 reservoir in patients undergoing HAART: an archive of pre-HAART drug resistance. J Antimicrob Chemother Apr;55(4):410–2. [DOI] [PubMed] [Google Scholar]
- 17. Turriziani O, Andreoni M, Antonelli G (2010) Resistant viral variants in cellular reservoirs of human immunodeficiency virus infection. Clin Microbiol Infect Oct;16(10):1518–24. 10.1111/j.1469-0691.2010.03329.x [DOI] [PubMed] [Google Scholar]
- 18. Mohamed S, Ravet S, Camus C, Khiri H, Olive D, et al. (2014) Clinical and analytical relevance of NNRTIs minority mutations on viral failure in HIV-1 infected patients. J Med Virol Mar;86(3):394–403. 10.1002/jmv.23853 [DOI] [PubMed] [Google Scholar]
- 19. Rowley CF, Boutwell CL, Lee EJ, MacLeod IJ, Ribaudo HJ, et al. (2010) Ultrasensitive detection of minor drug-resistant variants for HIV after nevirapine exposure using allele-specific PCR: clinical significance. AIDS Res Hum Retroviruses Mar;26(3):293–300. 10.1089/aid.2009.0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stekler JD, Ellis GM, Carlsson J, Eilers B, Holte S, et al. (2011) Prevalence and impact of minority variant drug resistance mutations in primary HIV-1 infection. PLoS One 6(12):e28952 10.1371/journal.pone.0028952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, et al. (1997) Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A Nov 25;94(24):13193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nottet HS, van Dijk SJ, Fanoy EB, Goedegebuure IW, de Jong D, et al. (2009) HIV-1 can persist in aged memory CD4+ T lymphocytes with minimal signs of evolution after 8.3 years of effective highly active antiretroviral therapy. J Acquir Immune Defic Syndr Apr 1;50(4):345–53. 10.1097/QAI.0b013e318197eb04 [DOI] [PubMed] [Google Scholar]
- 23. Palmisano L, Giuliano M, Galluzzo CM, Amici R, Andreotti M, et al. (2009) The mutational archive in proviral DNA does not change during 24 months of continuous or intermittent highly active antiretroviral therapy. HIV Med Sep;10(8):477–81. 10.1111/j.1468-1293.2009.00715.x [DOI] [PubMed] [Google Scholar]
- 24. Delaugerre C, Charreau I, Braun J, Néré ML, de Castro N, et al. ANRS 138 study group (2010) Time course of total HIV-1 DNA and 2-long-terminal repeat circles in patients with controlled plasma viremia switching to a raltegravir-containing regimen. AIDS Sep 24;24(15):2391–5. 10.1097/QAD.0b013e32833d214c [DOI] [PubMed] [Google Scholar]
- 25. Gandhi RT, Zheng L, Bosch RJ, Chan ES, Margolis DM, et al. AIDS Clinical Trials Group A5244 team (2010) The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med Aug 10;7(8). 10.1371/journal.pmed.1000334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McMahon D, Jones J, Wiegand A, Gange SJ, Kearney M, et al. (2010) Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clin Infect Dis Mar 15;50(6):912–9. 10.1086/650749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Banks L, Gholamin S, White E, Zijenah L, Katzenstein DA (2012) Comparing Peripheral Blood Mononuclear Cell DNA and Circulating Plasma viral RNA pol Genotypes of Subtype C HIV-1. J AIDS Clin Res Feb;3(2):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Geretti AM, Conibear T, Hill A, Johnson JA, Tambuyzer L, et al. SENSE Study Group (2014) Sensitive testing of plasma HIV-1 RNA and Sanger sequencing of cellular HIV-1 DNA for the detection of drug resistance prior to starting first-line antiretroviral therapy with etravirine or efavirenz. J Antimicrob Chemother Apr;69(4):1090–7. 10.1093/jac/dkt474 [DOI] [PubMed] [Google Scholar]
- 29. Diallo K, Murillo WE, de Rivera IL, Albert J, Zhou Z, et al. (2012) Comparison of HIV-1 resistance profiles in plasma RNA versus PBMC DNA in heavily treated patients in Honduras, a resource-limited country. Int J Mol Epidemiol Genet 3(1):56–65. [PMC free article] [PubMed] [Google Scholar]
- 30. Imaz A, Olmo M, Peñaranda M, Gutiérrez F, Romeu J, et al. STOPAR study team (2013) Short-term and long-term clinical and immunological consequences of stopping antiretroviral therapy in HIV-infected patients with preserved immune function. Antivir Ther 18(1):125–30. 10.3851/IMP2249 [DOI] [PubMed] [Google Scholar]
- 31. Delaugerre C, Braun J, Charreau I, Delarue S, Nere ML, et al. ANRS 138-EASIER study group (2012) Comparison of resistance mutation patterns in historical plasma HIV RNA genotypes with those in current proviral HIV DNA genotypes among extensively treated patients with suppressed replication. HIV Med Oct;13(9):517–25. 10.1111/j.1468-1293.2012.01002.x [DOI] [PubMed] [Google Scholar]
- 32. Wirden M, Soulie C, Valantin MA, Fourati S, Simon A, et al. (2011) Historical HIV-RNA resistance test results are more informative than proviral DNA genotyping in cases of suppressed or residual viraemia. J Antimicrob Chemother Apr;66(4):709–12. 10.1093/jac/dkq544 [DOI] [PubMed] [Google Scholar]
- 33. Bon I, Gibellini D, Borderi M, Alessandrini F, Vitone F, et al. (2007) Genotypic resistance in plasma and peripheral blood lymphocytes in a group of naive HIV-1 patients. J Clin Virol Apr;38(4):313–20. [DOI] [PubMed] [Google Scholar]
- 34. Kabamba-Mukadi B, Duquenne A, Henrivaux P, Musuamba F, Ruelle J, et al. (2010) HIV-1 proviral resistance mutations: usefulness in clinical practice. HIV Med Sep;11(8):483–92. 10.1111/j.1468-1293.2009.00814.x [DOI] [PubMed] [Google Scholar]
- 35.Geneious. http://www.geneious.com/.
- 36.Stanford University HIV Drug Resistance Database. http://hivdb.stanford.edu.
- 37. Felsenstein J (2008) PHYLIP Phylogeny Inference Package version 3.6. Department of Genome Sciences University of Washington, Seattle: 10.14219/jada.archive.2008.0268 [DOI] [Google Scholar]
- 38.Vlad IM, Srinivasan BV, Raykar VC, Duraiswami R, Davis LS (2008) Automatic online tuning for fast Gaussian summation. Advances in Neural Information Processing Systems.
- 39.Database SUHDR Calibrated Population Resistance tool. http://cpr.stanford.edu/cpr.cgi.
- 40. Bowser BP, Worl CO, Fulliove RE, Fullilove MT (2014) Post-Script to the Crack Epidemic and Its Link to HIV. Journal of Equity in Health Vol. 3, No 1, 45–54. [Google Scholar]
- 41. Bon I, Alessandrini F, Borderi M, Gorini R, Re MC (2007) Analysis of HIV-1 drug-resistant variants in plasma and peripheral blood mononuclear cells from untreated individuals: implications for clinical management. New Microbiol Jul;30(3):313–7. [PubMed] [Google Scholar]
- 42. Günthard HF, Wong JK, Ignacio CC, Guatelli JC, Riggs NL, et al. (1998) Human immunodeficiency virus replication and genotypic resistance in blood and lymph nodes after a year of potent antiretroviral therapy. J Virol Mar;72(3):2422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Monno L, Punzi G, Scarabaggio T, Saracino A, Brindicci G, et al. (2003) Mutational patterns of paired blood and rectal biopsies in HIV-infected patients on HAART. J Med Virol May;70(1):1–9. [DOI] [PubMed] [Google Scholar]
- 44. Murillo W, de Rivera IL, Parham L, Jovel E, Palou E, et al. (2010) Prevalence of drug resistance and importance of viral load measurements in Honduran HIV-infected patients failing antiretroviral treatment. HIV Med Feb;11(2):95–103. 10.1111/j.1468-1293.2009.00747.x [DOI] [PubMed] [Google Scholar]
- 45. Soto-Ramirez LE, Rodriguez-Diaz R, Durán AS, Losso MH, Salomón H, et al. NISDI Perinatal Study Group (2008) Antiretroviral resistance among HIV type 1-infected women first exposed to antiretrovirals during pregnancy: plasma versus PBMCs. AIDS Res Hum Retroviruses Jun;24(6):797–804. 10.1089/aid.2007.0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prakash R (2009) DNA structure is more stable than RNA structure. http://www.brighthub.com/science/genetics/articles/16332.aspx.
- 47. Hassan AS, Nabwera HM, Mwaringa SM, Obonyo CA, Sanders EJ, et al. (2014) HIV-1 virologic failure and acquired drug resistance among first-line antiretroviral experienced adults at a rural HIV clinic in coastal Kenya: a cross-sectional study. AIDS Res Ther Jan 23;11(1):9 10.1186/1742-6405-11-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pillay S, Bland RM, Lessells RJ, Manasa J, de Oliveira T, et al. (2014) Drug resistance in children at virological failure in a rural KwaZulu-Natal, South Africa, cohort. AIDS Res Ther Jan 20;11(1):3 10.1186/1742-6405-11-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Trotter AB, Hong SY, Srikantiah P, Abeyewickreme I, Bertagnolio S, et al. (2013) Systematic review of HIV drug resistance in Southeast Asia. AIDS Rev Jul-Sep;15(3):162–70. [PMC free article] [PubMed] [Google Scholar]
- 50. Delaugerre C, Chaix ML, Blanche S, Warszawski J, Cornet D, et al. ANRS French Perinatal Cohort (2009) Perinatal acquisition of drug-resistant HIV-1 infection: mechanisms and long-term outcome. Retrovirology Sep 19;6:85 10.1186/1742-4690-6-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Josefsson L, von Stockenstrom S, Faria NR, Sinclair E, Bacchetti P, et al. (2013) The HIV-1 reservoir in eight patients on long-term suppressive antiretroviral therapy is stable with few genetic changes over time. Proc Natl Acad Sci U S A Dec 17;110(51):E4987–96. 10.1073/pnas.1308313110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jain V, Liegler T, Vittinghoff E, Hartogensis W, Bacchetti P, et al. (2010) Transmitted drug resistance in persons with acute/early HIV-1 in San Francisco, 2002–2009. PLoS One Dec 10;5(12):e15510 10.1371/journal.pone.0015510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cambiano V, Bertagnolio S, Jordan MR, Lundgren JD, Phillips A (2013) Transmission of drug resistant HIV and its potential impact on mortality and treatment outcomes in resource-limited settings. J Infect Dis Jun 15;207 Suppl 2:S57–62. 10.1093/infdis/jit111 [DOI] [PubMed] [Google Scholar]
- 54. Imade GE, Sagay AS, Chaplin B, Chebu P, Musa J, et al. (2014) Short Communication: Transmitted HIV Drug Resistance in Antiretroviral-Naive Pregnant Women in North Central Nigeria. AIDS Res Hum Retroviruses Feb;30(2):127–33. 10.1089/AID.2013.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kleyn TJ, Liedtke MD, Harrison DL, Lockhart SM, Salvaggio MR, et al. (2014) Incidence of Transmitted Antiretroviral Drug Resistance in Treatment-Naive HIV-1-Infected Persons in a Large South Central United States Clinic. Ann Pharmacother Jan 28. [DOI] [PubMed]
- 56. Lai CC, Hung CC, Chen MY, Sun HY, Lu CL, et al. (2012) Trends of transmitted drug resistance of HIV-1 and its impact on treatment response to first-line antiretroviral therapy in Taiwan. J Antimicrob Chemother May;67(5):1254–60. 10.1093/jac/dkr601 [DOI] [PubMed] [Google Scholar]
- 57. Kantor R, Bettendorf D, Bosch RJ, Mann M, Katzenstein D, et al. ACTG A5077 Study Team (2014) HIV-1 RNA Levels and Antiretroviral Drug Resistance in Blood and Non-Blood Compartments from HIV-1–Infected Men and Women enrolled in AIDS Clinical Trials Group Study A5077. PLoS One Apr 3;9(4):e93537 10.1371/journal.pone.0093537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Greub G, Cozzi-Lepri A, Ledergerber B, Staszewski S, Perrin L, et al. Frankfurt HIV Clinic Cohort and the Swiss HIV Cohort Study (2002) Intermittent and sustained low-level HIV viral rebound in patients receiving potent antiretroviral therapy. AIDS Sep 27;16(14):1967–9. [DOI] [PubMed] [Google Scholar]
- 59. Macias J, Palomares JC, Mira JA, Torres MJ, García-García JA, et al. (2005) Transient rebounds of HIV plasma viremia are associated with the emergence of drug resistance mutations in patients on highly active antiretroviral therapy. J Infect Oct;51(3):195–200. [DOI] [PubMed] [Google Scholar]
- 60. Martinez V, Marcelin AG, Morini JP, Deleuze J, Krivine A, et al. (2005) HIV-1 intermittent viraemia in patients treated by non-nucleoside reverse transcriptase inhibitor-based regimen. AIDS Jul 1;19(10):1065–9. [DOI] [PubMed] [Google Scholar]
- 61. Tobin NH, Learn GH, Holte SE, Wang Y, Melvin AJ, et al. (2005) Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: expression of archival virus and replication of virus. J Virol Aug;79(15):9625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Africa NDoHS (2010) Clinical guidelines for the management of HIV & AIDS in adults and adolescents. http://www.who.int/hiv/pub/guidelines/south_africa_art.pdf?ua=1.
- 63.Organization WH (March 2014 Supplement) To the 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventinh HIV infection. http://apps.who.int/iris/bitstream/10665/104264/1/9789241506830_eng.pdf?ua=1.
- 64. Kieffer TL, Kwon P, Nettles RE, Han Y, Ray SC, et al. (2005) G—>A hypermutation in protease and reverse transcriptase regions of human immunodeficiency virus type 1 residing in resting CD4+ T cells in vivo. J Virol Feb;79(3):1975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fourati S, Malet I, Lambert S, Soulie C, Wirden M, et al. (2012) E138K and M184I mutations in HIV-1 reverse transcriptase coemerge as a result of APOBEC3 editing in the absence of drug exposure. AIDS Aug 24;26(13):1619–24. 10.1097/QAD.0b013e3283560703 [DOI] [PubMed] [Google Scholar]
- 66. Pace C, Keller J, Nolan D, James I, Gaudieri S, et al. (2006) Population level analysis of human immunodeficiency virus type 1 hypermutation and its relationship with APOBEC3G and vif genetic variation. J Virol Sep;80(18):9259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim EY, Bhattacharya T, Kunstman K, Swantek P, Koning FA, et al. (2010) Human APOBEC3G-mediated editing can promote HIV-1 sequence diversification and accelerate adaptation to selective pressure. J Virol Oct;84(19):10402–5. 10.1128/JVI.01223-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mulder LC, Harari A, Simon V (2008) Cytidine deamination induced HIV-1 drug resistance. Proc Natl Acad Sci U S A Apr 8;105(14):5501–6. 10.1073/pnas.0710190105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fourati S, Lambert-Niclot S, Soulie C, Malet I, Valantin MA, et al. (2012) HIV-1 genome is often defective in PBMCs and rectal tissues after long-term HAART as a result of APOBEC3 editing and correlates with the size of reservoirs. J Antimicrob Chemother Oct;67(10):2323–6. 10.1093/jac/dks219 [DOI] [PubMed] [Google Scholar]
- 70. Quan Y, Xu H, Wainberg MA (2014) Defective HIV-1 quasispecies in the form of multiply drug-resistant proviral DNA within cells can be rescued by superinfection with different subtype variants of HIV-1 and by HIV-2 and SIV. J Antimicrob Chemother Jan;69(1):21–7. 10.1093/jac/dkt326 [DOI] [PubMed] [Google Scholar]
- 71. Watters JK (1994) Trends in risk behavior and HIV seroprevalence in heterosexual injection drug users in San Francisco, 1986–1992. J Acquir Immune Defic Syndr Dec;7(12):1276–81. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
HIV-1 PR and RT sequences recovered from PBMC and viral RNA have been submitted to GenBank with the accession numbers KJ69464 to KJ769641.