Abstract
The challenges of healing have led investigators to question existing paradigms in the hopes of uncovering overlooked solutions. Such is the case in a recent study showing that introduction of a cartilage construct into a mouse tibial defect induces remarkable healing owing to the transformation of donor chondrocytes into new bone.
Despite major advances in our ability to treat bone fractures, no definitive procedure exists to heal critical-size (>3cm) defects, and long-term outcomes of current procedures suffer high rates of failure and complications.1 These problems are further evidenced by the dissatisfaction reported following limb-salvage surgery, as the results of such procedures, in terms of the patient’s quality of life, are no better than amputation.2 Although the gold standard of care for bone defects is autograft of live tissue (i.e. transport of the patient’s fibula to a segmental defect in their radius), this approach is not possible for most critical-size defect situations that would require too much host bone to transport. Thus, devitalized bone allografts are commonly used in combination with pharmacological, biological and cellular adjuvants to improve healing. Conventional thinking is that these cellular adjuvants should be osteoblastic, to promote primary bone healing via intramembranous bone formation with rigid fixation. Contrary to this established paradigm, Bahney et al. hypothesized that the introduction of a cartilage construct into a segmental defect efficiently heals the bone via endochondral ossification (Figure 1), and they have gone on to demonstrate the feasibility of this technique in a mouse model of tibial fracture.3
Figure 1. Cartilage grafts for segmental defect healing.
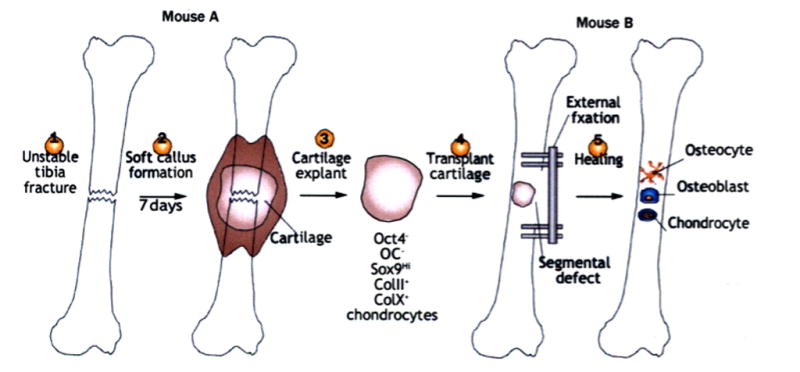
The generation of an unstable fracture (1) in mouse A produces a lot of cartilage in the soft callus tissue by day 7 (2). The explanted fracture callus/cartilage is made into a graft (3), and transplanted (4) into a tibial segmental defect in mouse B that heals with external fixation. During this healing process (5), the grafted chondrocytes de-differentiate into Oct4+ progenitor cells, and then differentiate into hypertrophic chondrocytes, osteoblasts and osteocytes in the regenerate tissue. Abbreviations: col II, type II collagen; col X, type 10 collagen; OC, osteocalcin; Oct4, octamer-binding protein 4.
The similarities between endochondral ossification in embryonic bone development and during fracture healing are well known. Investigators have also shown that endochondral ossification follows ectopic transplantation of cartilage-like tissue derived from mesenchymal stem cells (MSCs).4 Moreover, “bone organs”, with mature vasculature and functional haematopoietic compartments, can be generated from ectopic transplantation of engineered hypertrophic cartilage.5 The study by Bahney et al. expands on this previous work by use of a translational model of bone regeneration.3 They harvested endochondral cartilage from callus tissue generated at the site of an unstable tibia fracture in mice. This cartilage construct was then transplanted as a graft into a critical-size tibia defect. The authors showed that the bone regenerate healed the defect with similar radiographic, biomechanical, and histologic properties to those observed with the live isograft control. More surprisingly, when they repeated these experiments using cartilage graft from genetically labelled mouse strains (LacZ and GFP) in order to assess cell fate in their model, they found that the majority of the regenerated bone was composed of donor cells, which had apparently transformed (dedifferentiated and then differentiated) into osteoblasts and osteocytes.
Another interesting finding in this study was the persistence of donor-derived hypertrophic chondrocytes at the fracture site.3 Apoptosis of terminal hypertrophic chondrocytes is widely believed to be required for bone formation during embryonic development, and impairment of this process gives rise to rickets.6 Thus, the observation that hypertrophic chondrocytes showed no sign of apoptosis suggests some remarkable quality of this fracture-callus-derived cartilage construct. As the authors point out, this observation warrants further study to formally elucidate the mechanism by which the donor cells undergo a morphological change from chondrocytes to osteocytes.
Bahney et al. also performed in vitro studies to better understand the role of angiogenesis and vascular endothelial cell effects on the morphological changes of cartilage explants, which is another revolving concept of fracture healing. Although it is well known that vascularization of the fracture callus is critical for its mineralization and remodeling into lamellar bone, recent studies have shown that the formation of fibrous tissue during allograft healing is associated with large-vessel (>100μm) arteriogenesis, which promotes fracture non-union.7 Additionally, treatment with teriparatide, which increases cartilage formation at the host–graft interface, substantially inhibits arteriogenesis. Thus, another critical area for future study is the importance of the hypoxic environment, generated by cartilage in the early phase of bone healing, to inhibition of the chronic inflammation and fibrosis that usually causes the bone non-union that follows massive allografting.
Some limitations to the study also warrant discussion. The first is the challenge of translating results from mice, which have extraordinary bone-healing potential, to humans. One of the most serious complications following reconstructive surgery for a massive bone defect is re-fracture.1 Thus, beyond the obvious issues of scale and long-term outcome, the novel approach developed by Bahney et al. seems to rely on the persistence of cartilage at the fracture site, which could be highly susceptible to fracture, and potentially to hypertrophic non-union in humans.
Another question is whether something intrinsic to cartilage produced in fracture callus exists that engenders it with unique bone-healing properties that cannot be attained by differentiated MSCs or other chondrocytes. As Bahney et al. described, human MSCs embedded in a hydrogel scaffold produced cartilage-like matrix with robust expression of type II and type X collagen and MMP-13 only in vitro, and the bone-healing potential of this construct is unknown. Even though human articular cartilage and chondrocyte allograft transplantation products are currently used for cartilage repair, the results of this study do not support their use for bone repair. Thus, even if human fracture-callus-derived cartilage proves to be highly osteogenic for this purpose, how it could be obtained to treat patients remains unknown.
Another challenge for this novel cartilage-construct approach to bone healing is the need to demonstrate its superiority over more practical cell sources; MSCs, for example, have been widely used experimentally and have great potential for cell-based therapies for a diverse range of diseases.8 Likewise, induced pluripotent stem cells (iPS) also hold promise for cell-based therapies.9 In a 2011 study, genetic stem cell technology was used to directly induce cartilaginous tissue from dermal fibroblasts by use of two cell-reprogramming factors (c-Myc and Klf4) and a chondrogenic factor (SOX9), without generating iPS.10 On the basis of results obtained with their cartilage graft model, Bahney et al.3 concluded that the chondrocytes in the graft construct, which do not express Oct4, re-expressed this pluripotent transcription factor during their transformation into bone cells. This is an interesting observation because other studies that utilized iPS cells as osteoprogenitors showed that Oct4 was not expressed during cellular transformation,9, 10 suggesting that the cartilage grafts differentiate through a different pathway.
In conclusion, the paper by Bahney et al.3 highlights many unknowns in the field of bone healing for large segmental defects, and underscores the need to challenge existing paradigms and clinical practices. The demonstration, for the first time, that cartilage grafts efficiently heal critical-size bone defects in a mouse model by mimicking embryonic endochondral ossification could be an important milestone. Nonetheless, future studies are needed to translate these finding and elucidate the mechanism by which the donor cartilage differentiates into bone, which could be the next breakthrough in this field.
Acknowledgments
The authors are supported by NIH grants P30 AR061307; P50 AR054041; and R01 DE019902.
Biographies
Dr. Kohei Nishitani gained his MD in 2002 and his PhD in 2010 from Kyoto University, Kyoto, Japan. Currently, Dr. Nishitani is a Postdoctoral Associate of Center for Musculoskeletal Research, University of Rochester, Rochester, NY, USA. He is a board-certified member of the Japanese Orthopaedic Association. His research is focused on bone and cartilage biology, and translational therapies for massive bone defects.
Dr Edward Schwarz gained his PhD in 1993 from the Albert Einstein College of Medicine, Yeshiva University, NY, USA. Currently, Dr Schwarz is the Burton Professor of Orthopaedics and Director of the Center for Musculoskeletal Research at the University of Rochester, Rochester, NY, USA. He has published over 230 papers and is an expert in the field of Osteoimmunology. Since 2000, Dr. Schwarz has focused on drug, gene and stem cell therapies to revitalize structural allografts.
Footnotes
Competing interests:
The authors declare no competing interests.
References
- 1.Wheeler DL, Enneking WF. Allograft bone decreases in strength in vivo over time. Clin Orthop Relat Res. 2005;435:36–42. doi: 10.1097/01.blo.0000165850.58583.50. [DOI] [PubMed] [Google Scholar]
- 2.Bosse MJ, et al. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. N Engl J Med. 2002;347:1924–1931. doi: 10.1056/NEJMoa012604. [DOI] [PubMed] [Google Scholar]
- 3.Bahney CS, et al. Stem cell derived endochondral cartilage stimulates bone healing by tissue transformation. J Bone Miner Res. doi: 10.1002/jbmr.2148. http://dx.doi.org/10.1002/jbmr.2148. [DOI] [PMC free article] [PubMed]
- 4.Pelttari K, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 5.Scotti C, et al. Engineering of a functional bone organ through endochondral ossification. Proc Natl Acad Sci USA. 2013;110:3997–4002. doi: 10.1073/pnas.1220108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabbagh Y, Carpenter TO, Demay MB. Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc Natl Acad Sci USA. 2005;102:9637–9642. doi: 10.1073/pnas.0502249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhillon RS, et al. PTH-enhanced structural allograft healing is associated with decreased angiopoietin-2-mediated arteriogenesis, mast cell accumulation, and fibrosis. J Bone Miner Res. 2012;28:586–597. doi: 10.1002/jbmr.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianco P, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lian Q, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113–1123. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 10.Hiramatsu K, et al. Generation of hyaline cartilaginous tissue from mouse adult dermal fibroblast culture by defined factors. J Clin Invest. 2011;121:640–657. doi: 10.1172/JCI44605. [DOI] [PMC free article] [PubMed] [Google Scholar]


