Abstract
Angiotensin converting enzyme (ACE) plays an important role in blood pressure control. ACE also has effects on renal function, reproduction, hematopoiesis and several aspects of the immune response. ACE 10/10 mice over express ACE in monocytic cells; macrophages from ACE 10/10 mice demonstrate increased polarization towards a proinflammatory phenotype. As a result, ACE 10/10 mice have a highly effective immune response following challenge with either melanoma, bacterial infection or Alzheimer’s disease. The ACE 10/10 mice suggest that enhanced monocytic function greatly contributes to the ability of the immune response to defend against a wide variety of antigenic and non-antigenic challenges.
Keywords: ACE 10/10, Alzheimer’s disease, macrophages, monocytes, peptidase
Introduction
The renin angiotensin system is a series of enzymes and substrates that plays a central role in blood pressure control (Corvol et al., 2004). Angiotensinogen, a protein produced by the liver, is cleaved by renin to release the amino terminal 10 amino acid, a peptide called angiotensin I. This is further cleaved or ‘converted’ to the 8 amino acid peptide angiotensin II by angiotensin converting enzyme (ACE). Angiotensin II binds to cell surface receptors found in many tissues, initiating a coordinated series of physiologic changes that reduces renal sodium excretion and increases peripheral vascular smooth muscle constriction. The net result is blood pressure elevation. Renin, an aspartyl protease, is the major enzyme regulating angiotensin II production and ultimately the role of the RAS in blood pressure control. ACE, the second enzyme in this system, is very different from renin. It is a zinc-dependent dicarboxypeptidase that cleaves many different peptides in addition to angiotensin I.
While the RAS was initially studied for its role in blood pressure control, a mountain of evidence now suggests that the role of the RAS is far more complex than originally envisioned (Bernstein et al., 2013). This is because angiotensin II has many effects beyond those directly related to blood pressure. Further, ACE, a relatively non-specific peptidase, can cleave a variety of peptide substrates with diverse physiologic effects. This review will focus on the several roles of ACE and emphasizes the newly recognized role of this peptidase in the immune response. Most of these new observations benefit from mouse models that contain mutations in the ACE gene. Several groups have studied ACE null mice. In addition, we have used gene targeting approaches to substitute tissue specific promoters in place of the natural somatic ACE promoter. This results in ACE expression by those mouse tissues recognizing the ‘new’ ACE promoter.
ACE null mice
A detailed analysis of a mouse null for all ACE expression clearly defined some of the central roles of this peptidase (Krege et al., 1995; Esther et al., 1996). Null animals (knockout out, KO) have a blood pressure far lower than wild type (WT) mice. Further, male ACE KO mice also reproduce very poorly due to their lack of a testis specific isozyme of ACE, often termed testis ACE. An ACE null mouse produces large amounts of a dilute urine; in the absence of a functional RAS, renal dysfunction is caused by both the lack of angiotensin II and renal structural defects secondary to the very low intrauterine blood pressure present in the fetus. Finally, ACE null mice have defects in hematopoiesis. Such animals are anemic due to the lack of angiotensin II production (Cole et al., 2000). ACE null mice also have developmental defects of myelopoiesis and in the functional behavior of macrophages (Lin et al., 2011).
Recent studies have identified a very unexpected role for ACE, namely as an enzyme that participates in shaping the peptide repertoire displayed on the surface of cells as part of the MHC class I complex (Shen et al., 2011). With the exception of red blood cells, all cells express MHC class I molecules. These molecules identify tissues as ‘self’; tissues bearing syngeneic MHC class I molecules are ignored by the immune system. MHC class I molecules are composed of two protein chains which are assembled in the endoplasmic reticulum (ER). There, MHC class I molecules bind peptides derived from the degradation of cellular proteins. In the presence of viral infection, or following organ transplantation, the immune system detects the cell surface display of MHC class I bound peptides that are not ‘self’. Cells displaying such peptides are then destroyed.
While several different approaches were used to explore the role of ACE in shaping the MHC class I peptide repertoire, perhaps the critical experiments were cross immunization studies in which macrophages or splenocytes were transferred from a donor mouse wild-type for ACE into a recipient KO mouse lacking ACE (Shen et al., 2011). Equivalent experiments were also performed with an ACE KO donor and a WT ACE recipient, as well as control experiments in which WT was transferred into WT and KO into KO. All other aspects of the immune makeup of these mice was carefully controlled, including the MHC class I alleles and the sex of the animals. Normally, the transfer of macrophages from one mouse (for example C57BL/6) to a mouse with an identical genetic background does not result in activation of the immune response. However, when tissues from either an animal WT for ACE (or null for ACE) was transferred into an animal null for ACE (or WT for ACE), the result was immune activation in the recipient, as detected by CD8+ T cell cytokine expression. While it was previously recognized that peptides for MHC class I display were trimmed in the endoplasmic reticulum by aminopeptidases, this was the first solid evidence that a carboxypeptidase, specifically ACE, played a role in C-terminal cleavage and the shaping of the MHC class I peptide repertoire. Additional data indicates that infection induces ACE upregulated by macrophages and dendritic cells (Shen et al., 2011). It should be noted that there is no evidence of autoimmunity in humans taking ACE inhibitors.
ACE over expression in myelomonocytic cells
In addition to affecting MHC peptide repertoire, ACE has been shown to have a number of other effects on the immune response. Perhaps most dramatic is a mouse model, called ACE 10/10, in which the natural ACE gene was modified such that ACE expression is regulated by the c-fms promoter (Shen et al., 2007). The result is that these animals markedly over express ACE in myelomonocytic cells, particularly monocytes and macrophages. Further, since endothelial cells do not normally transcribe the c-fms protein (the receptor for macrophage colony-stimulating factor), they do not express ACE in the ACE 10/10 mouse. Nonetheless, these mice have normal blood pressure and normal basal renal function.
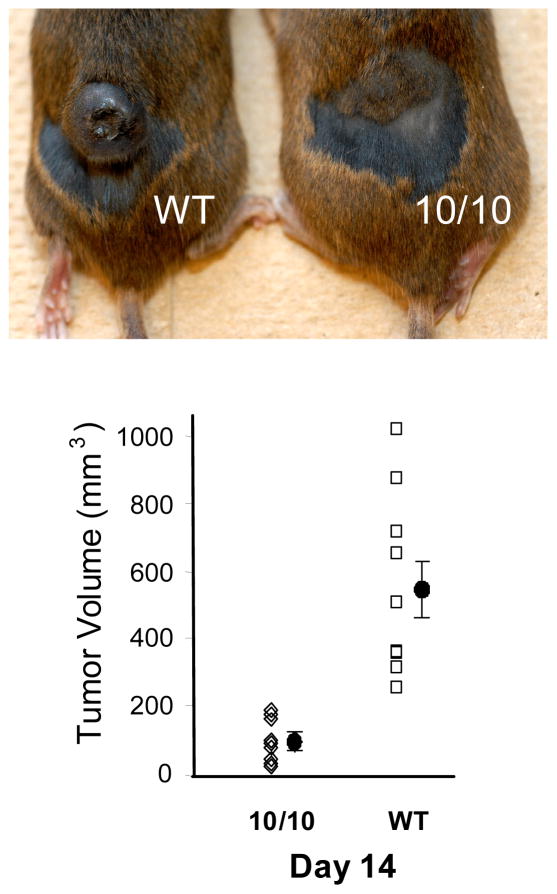
The first experiment to reveal a unique immune response in ACE 10/10 mice was when these animals were challenged with the B10 melanoma cell line. Typically, two weeks following an intra-dermal injection of tumor cells, mice present with local melanotic nodules. Remarkably, ACE 10/10 mice developed very much smaller tumors than control mice, irrespective of genetic background (Figure 1) (Shen et al., 2007). Further, the number of inflammatory cells observed adhered to endothelial cells and within tumor tissue was much higher in the ACE 10/10 mice. Additional experiments defined several aspects of the immune response that were unique in these animals. As compared to WT mice, macrophages from ACE 10/10 mice make more of the pro-inflammatory cytokines IL-12 and TNF-α; ACE 10/10 cells make less of the anti-inflammatory cytokine IL-10. Also, ACE 10/10 macrophages express increased amounts of iNOS and NO. These and other phenotypic characteristics of the monocytic immune response are often referred to as an M1 pattern of macrophage differentiation (Mantovani et al., 2004). These cells are pro-inflammatory and associated with a vigorous response to immune challenge by tumor or bacterial infection. Indeed, when ACE 10/10 mice were challenged with the bacteria L. monocytogenes or methicillin resistant S. aureus (MRSA), infection was cleared more rapidly in ACE 10/10 mice as compared to WT animals (Okwan-Duodu et al., 2010).
Figure 1.
Reduced tumor size in ACE 10/10 mice. ACE 10/10 and WT mice received an intradermal injection of 1 × 106 B16-F10 myeloma cells. After 14 days, the mice were sacrificed and tumor size was measured. The top panel is a typical result where ACE 10/10 mice have much smaller tumors than WT mice. The bottom panel shows individual data points as well as the group means and the standard error of the mean.
Macrophages have diverse physiologic roles, varying from processing and presenting MHC antigens, to the phagocytosis and killing of bacteria, and even the downregulation and termination of immune reactions. A variety of studies in the ACE 10/10 model indicate that under basal conditions there was no intrinsic activation of the immune response (Okwan-Duodu et al., 2010). Only following challenge with a tumor, bacteria or virus did there appear to be an enhanced immune response. Further, the termination of the immune response appeared relatively normal. Immunologists have given the designation M1 and M2 to extremes of macrophage behavior (Gordon and Taylor, 2005). The M1 cell is an aggressive cell that responds to bacteria or tumor with the goal of destroying the immune challenge. M2 macrophages are thought to suppress and help terminate the immune response leading to resolution of an inflammatory challenge. M2 like cells have been implicated as possibly contributing to tumor evasion of the immune response. In the case of the ACE 10/10 mice, it appeared as if the monocytes and macrophages were M1-like. Put more simply, it seemed as if, in this animal model, monocytes and macrophages were more efficient at eliminating pathogens and cell debris than equivalent cells from a WT mouse.
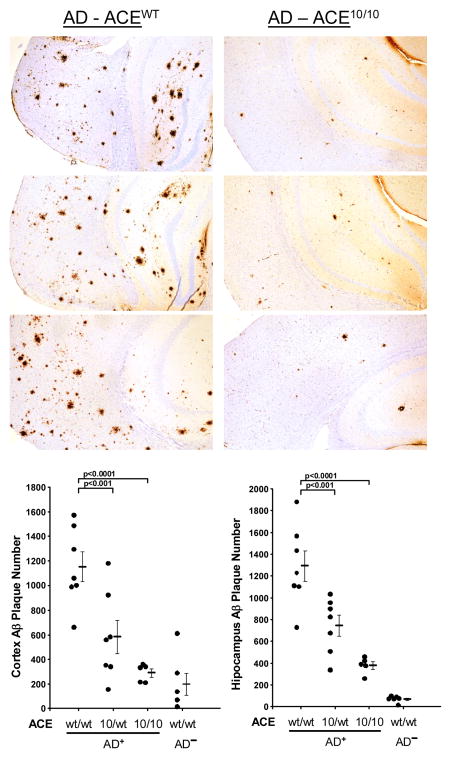
One disease in which precipitated proteins are thought to be pathogenic is Alzheimer’s disease (AD). Several studies have implicated peptide breakdown products of amyloid precursor protein as being pathogenic (Selkoe, 2008). These amyloid β peptides are commonly 38–43 amino acids in length with the peptide Aβ1-42 thought to be most neurotoxic. Brain plaques typically associated with AD are insoluble precipitates of Aβ peptides. To investigate the ability of ACE 10/10 to affect the progression of AD, a mouse strain genetically prone to Alzheimer’s-like pathology (cerebral and hippocampal amyloid plaques and cognitive defects associated with aging) were crossed with ACE 10/10 mice (Bernstein et al., 2014). An extensive investigation of AD+ ACE 10/10 versus AD+ ACE WT/WT demonstrated significantly less Alzheimer’s pathology in the AD+ ACE 10/10 mice (Figure 2). For example, the Alzheimer’s associated plaque burden was reduced by as much as 79% at 7 months and 48% at 13 months in AD+ ACE 10/10 versus AD+ ACE WT/WT mice. However, most remarkable was the cognitive assessment of these mice as measured by a Barnes maze, which tests the ability of the animals to learn and remember the location of an escape box using spatial clues. Two cohorts of AD mice, aged 11 and 12 months, were evaluated by two separate groups of evaluators, blinded to the genotypes of the mice. This study showed that AD+ ACE 10/10 mice demonstrated cognitive learning ability essentially indistinguishable from similarly aged non-AD (normal) mice. In contrast, the AD+ ACE WT/WT mice demonstrated the expected cognitive defects due to the progression of the Alzheimer’s-like disease. Thus, in this study we took advantage of two features of the ACE 10/10 model: over expression of ACE by monocytes and macrophages induces an enhanced immune response that appears to facilitate clearance of Aβ peptides, and the positioning of super physiologic amounts of ACE on the surface of monocytes and macrophages allows this enzyme to directly interact with and potentially cleave Aβ peptides into smaller peptide fragments.
Figure 2.
Reduced Alzheimer’s disease-like pathology in ACE 10/10 mice. (Top) All mice were genetically prone to develop Alzheimer’s-like disease; the mice were either ACE WT (left panels) or ACE 10/10 (right panels) for ACE expression. All mice were sacrificed at 255 days Paraffin embedded brain sections were immunohistochemical stained for human amyloid β using the monoclonal antibody 6E10. (Bottom) Quantitative analysis indicates that there are far fewer amyloid plaques in the brains of ACE 10/10 mice than ACE WT mice.
Perspective
ACE has been shown to be associated with the epithelioid histiocytes in sarcoid and perhaps other granulomatous processes (Kurata et al., 2005). Thus, it appears very likely that under natural conditions, the up-regulation of ACE expression is a part of the natural monocytic response to chronic immune challenge. In the ACE 10/10 mouse, monocytes and macrophages over express ACE by at least 16-fold (Shen et al., 2007). Such high level expression treats ACE almost as a drug, and undoubtedly reveals effects that are beyond those observed with the natural levels of ACE expression. Our discovery that ACE over expression in monocytes and macrophages markedly enhances the immune response was fortuitous. However, further research will be necessary to determine if the ACE 10/10 effect is unique or whether it is an exaggeration of the normal process of ACE over expression during macrophage activation. Whether normal or not, our studies have revealed a highly unexpected finding and have led to the development of a novel concept. The new finding is that, at least in mice, ACE over expression is associated with a substantially enhanced immune response observed with models of cancer, bacterial infection, viral challenge, and now Alzheimer’s disease. Perhaps even more important is the concept that the empowerment of macrophage function has profound consequences. For example, in atherosclerosis, it is commonly postulated that the development of foamy macrophages, macrophages incapable of dealing with the stress of oxidized lipid, directly contribute to the development of atherosclerotic plaque (Yu et al., 2013; Maiolino et al., 2013). In such a case, the failure of macrophages to dispose of the lipid and other cell debris is directly contributory to the major disease affecting Western societies. Understanding how to enhance macrophage function and perhaps endowing macrophages with increased capacity to clear toxic products, may provide a new approach to chronic diseases. Indeed, our findings with the ACE 10/10 mice suggest that enhanced monocytic function greatly contributes to the ability of the immune response to defend against a wide variety of both antigenic and non-antigenic challenges.
Acknowledgments
The authors acknowledge the tireless support of Mr. Brian Taylor. This study was supported by the National Institute of Health grants R01 HL110353 and R00 HL088000; Beginning Grant-in-Aid 13BGIA14680069 and Scientist Development Grant 11SDG6770006 from the American Heart Association; the Coins for Alzheimer’s Research Trust (C.A.R.T) Fund; the BrightFocus Foundation (former AHAF), the Maurice Marciano Family Foundation, and by the National Center for Advancing Translational Sciences through CTSI Grant UL1TR000124.
Footnotes
The authors dedicate the manuscript to the memory of Natalie Radom Bernstein who died April 9, 2013, and Salomon Moni Hamaoui who died March 6, 1994, both of Alzheimer’s disease.
References
- Bernstein KE, Koronyo Y, Salumbides BC, Sheyn J, Pelissier L, Lopes DHJ, Shah KH, Bernstein EA, Fuchs D-T, Yu JJ, et al. Angiotensin-converting enzyme overexpression in myelomonocytes prevents Alzheimer’s-like cognitive decline. J Clin Invest. 2014 doi: 10.1172/JCI66541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein KE, Ong FS, Blackwell WL, Shah KH, Giani JF, Gonzalez-Villalobos RA, Shen XZ, Fuchs S. A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme. Pharmacol Rev. 2013;65:1–46. doi: 10.1124/pr.112.006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J, Ertoy D, Lin H, Sutliff RL, Ezan E, Guyene TT, Capecchi M, Corvol P, Bernstein KE. Lack of angiotensin II-facilitated erythropoiesis causes anemia in angiotensin-converting enzyme-deficient mice. J Clin Invest. 2000;106:1391–1398. doi: 10.1172/JCI10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvol P, Eyries M, Soubrier F. Peptidyl-dipeptidase A/Angiotensin I-converting enzyme. In: Barret A, Rawlings N, Woessner J, editors. Handbook of Proteolytic Enzymes. New York: Elsevier Academic Press; 2004. pp. 332–349. [Google Scholar]
- Esther CR, Jr, Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest. 1996;74:953–965. [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Krege JH, John SW, Langenbach LL, Hodgin JB, Hagaman JR, Bachman ES, Jennette JC, O’Brien DA, Smithies O. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature. 1995;375:146–148. doi: 10.1038/375146a0. [DOI] [PubMed] [Google Scholar]
- Kurata A, Terado Y, Schulz A, Fujioka Y, Franke FE. Inflammatory cells in the formation of tumor-related sarcoid reactions. Hum Pathol. 2005;36:546–554. doi: 10.1016/j.humpath.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Lin C, Datta V, Okwan-Duodu D, Chen X, Fuchs S, Alsabeh R, Billet S, Bernstein KE, Shen XZ. Angiotensin-converting enzyme is required for normal myelopoiesis. FASEB J. 2011;25:1145–1155. doi: 10.1096/fj.10-169433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiolino G, Rossitto G, Caielli P, Bisogni V, Rossi GP, Calò LA. The Role of Oxidized Low-Density Lipoproteins in Atherosclerosis: The Myths and the Facts. Mediators Inflamm. 2013;2013:714653. doi: 10.1155/2013/714653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Okwan-Duodu D, Datta V, Shen XZ, Goodridge HS, Bernstein EA, Fuchs S, Liu GY, Bernstein KE. Angiotensin-converting enzyme overexpression in mouse myelomonocytic cells augments resistance to Listeria and methicillin-resistant Staphylococcus aureus. J Biol Chem. 2010;285:39051–39060. doi: 10.1074/jbc.M110.163782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XZ, Billet S, Lin C, Okwan-Duodu D, Chen X, Lukacher AE, Bernstein KE. The carboxypeptidase ACE shapes the MHC class I peptide repertoire. Nat Immunol. 2011;12:1078–1085. doi: 10.1038/ni.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XZ, Li P, Weiss D, Fuchs S, Xiao HD, Adams JW, Williams IR, Capecchi MR, Taylor WR, Bernstein KE. Mice with enhanced macrophage angiotensin converting enzyme are resistant to melanoma. Am J Pathol. 2007;170:2122–2134. doi: 10.2353/ajpath.2007.061205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XH, Fu YC, Zhang DW, Yin K, Tang CK. Foam cells in atherosclerosis. Clin Chim Acta. 2013;424:245–252. doi: 10.1016/j.cca.2013.06.006. [DOI] [PubMed] [Google Scholar]