Abstract
Obesity increases linearly with age and is associated with impaired vascular endothelial function and increased risk for cardiovascular disease. Mineralocorticoid receptors (MR) contribute to impaired vascular endothelial function in cardiovascular disease; however, their role in uncomplicated human obesity is unknown. Because plasma aldosterone levels are elevated in obesity and adipocytes may be a source of aldosterone, we hypothesized that MR modulate vascular endothelial function in older adults in an adiposity-dependent manner. To test this hypothesis, we administered MR blockade (Eplerenone; 100 mg/day) for 1 month in a balanced, randomized, double-blind, placebo-controlled, crossover study to 22 older adults (10 men, 55–79 years) varying widely in adiposity (body mass index: 20–45 kg/m2) but who were free from overt cardiovascular disease. We evaluated vascular endothelial function (brachial artery flow-mediated dilation [FMD] via ultrasonography) and oxidative stress (plasma F2-isoprostanes and vascular endothelial cell protein expression of nitrotyrosine and NADPH oxidase p47phox) during placebo and MR blockade. In the whole group, oxidative stress (P>0.05) and FMD did not change with MR blockade (6.39±0.67 vs. 6.23±0.73 %, P=0.7, placebo vs. Eplerenone). However, individual improvements in FMD in response to Eplerenone were associated with higher total body fat (body mass index: r=0.45, P=0.02 and DXA-derived % body fat: r=0.50, P=0.009) and abdominal fat (total: r=0.61, P=0.005, visceral: r=0.67, P=0.002 and subcutaneous: r=0.48, P=0.03). In addition, greater improvements in FMD with Eplerenone were related with higher baseline fasting glucose (r=0.53, P=0.01). MR influence vascular endothelial function in an adiposity-dependent manner in healthy older adults.
Keywords: brachial artery, flow-mediated dilation, abdominal visceral and subcutaneous fat
INTRODUCTION
More than one third of adults worldwide is overweight or obese [1] and the prevalence of obesity increases linearly with age [2]. Obesity is associated with increased risk for cardiovascular disease [3], but the underlying mechanisms are not completely understood. Substantial evidence supports an independent role of aldosterone in the development and progression of cardiovascular disease [4–6]. According to the classic view of physiology, aldosterone is secreted by the adrenal gland and is involved in blood pressure regulation by acting on the kidney via activation of epithelial mineralocorticoid receptors (MR) [7]. In the past decade, non-epithelial presence of MR has been demonstrated in cardiac and vascular cells and increasing evidence supports the direct role of MR in modulating vascular function and contributing to cardiovascular disease [8].
Recently, findings from studies in vitro and studies performed in rodents demonstrate that adipose tissue is a secondary source of aldosterone [9] and that adipocyte-derived aldosterone contributes to vascular dysfunction in obesity [10]. In humans, several studies have shown that plasma aldosterone levels are positively related with measures of total and abdominal adiposity including body mass index [11], waist circumference [12], abdominal visceral [13] and subcutaneous adipose tissue [14]. In addition, plasma aldosterone concentrations are elevated in the obese compared with lean human subjects [15, 16]. With weight loss, aldosterone levels are significantly decreased [14, 17–19], highlighting the important role of adipose tissue in the obesity-related increases in aldosterone concentration.
Obesity is also associated with impaired endothelial function [20, 21], an independent predictor of future cardiovascular events, disease progression, and long-term outcome [22, 23]. A key component of endothelial dysfunction is decreased nitric oxide bioavailability resulting from either decreased synthesis or increased degradation due to oxidative stress [24]. Activation of vascular NADPH oxidase, eNOS uncoupling and other factors lead to increased production of reactive oxygen species (ROS), which inactivate nitric oxide, thus leading to impaired vascular smooth muscle relaxation and vasodilation [25].
There is strong evidence supporting that aldosterone activation of MR contributes to oxidative stress and decreased nitric oxide activity. Data from experimental models of cardiovascular disease demonstrated that MR activation increases NADPH oxidase expression and activity leading to increased superoxide production, vascular oxidative stress, decreased nitric oxide bioavailability and impaired vascular endothelial function, while MR blockade reverses these effects [26–29]. Human studies in patients with congestive heart failure found that 1 month of MR blockade improves endothelial function and this improvement is associated with increased nitric oxide bioactivity [30, 31].
Taken together these data support a potential role for MR in obesity-related impairments in endothelial function, but this has not been studied in human obesity. Thus, in the current investigation, we hypothesized that MR modulate vascular endothelial function in an adiposity-dependent manner in healthy older adults. To test this hypothesis we administered the selective MR antagonist Eplerenone (100 mg daily for 1 month) in a balanced randomized, double-blind, placebo-controlled, crossover study in healthy older adults varying widely in total and abdominal adiposity. We measured vascular endothelial function and oxidative stress markers during placebo and MR blockade.
METHODS
Subjects
Twenty-two healthy adults (55–79 years), 10 men and 12 women, of a wide range of adiposity (body mass index: 20.0–44.6 kg/m2; body fat: 25.6–54.1 %) were studied. All subjects were sedentary, non-smokers and were free of overt cardiovascular disease and other clinical disorders (e.g., diabetes, liver and renal disease) as assessed by medical history, physical examination, resting ECG, urinalysis, blood chemistries and hematological evaluation. None of the subjects were taking antihypertensive or vasoactive drugs and subjects who were taking antioxidant supplements completed a 4-week washout prior to study enrolment. All subjects demonstrated normal ECG and blood pressure responses to a graded exercise test on a treadmill. The graded exercise protocol is described below under the aerobic fitness section. Women were all postmenopausal, established by absence of menses for at least 2 years and follicle stimulating hormone >40 IU/L. Postmenopausal women were not on hormone replacement therapy for at least 1 year prior to data collection. The study was carried out in accordance with the Declaration of Helsinki (2008) and was approved by the Institutional Review Boards of the University of Florida, Texas A&M University, and Scott & White Health System. The purpose, nature and risk of all procedures used were explained to the subjects and their written informed consent was obtained prior to participation.
Study design
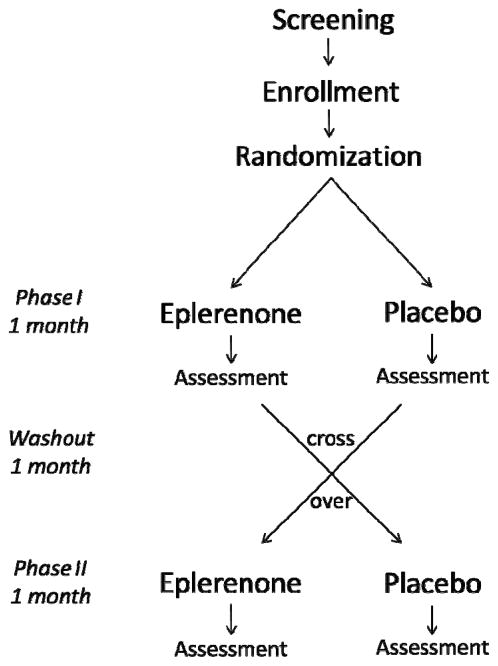
Subjects were assigned to receive an MR antagonist (Eplerenone; 100 mg per day) for 1 month in a balanced randomized, double-blind, placebo-controlled, crossover study with 1-month washout between treatments (Figure 1). Eplerenone was chosen because it has a higher selectivity for mineralocorticoid receptors and fewer side effects than the other mineralocorticoid receptor antagonist that is currently available (i.e., Spironolactone).
Figure 1.
Study design. Subjects were assigned to receive an MR antagonist (Eplerenone; 100 mg per day) or placebo for 1 month in a balanced randomized, double-blind, crossover study with 1-month washout between treatments.
To reduce the risk of hyperkalemia, subjects were not enrolled in the study if their baseline serum potassium was greater than 5.5 mmol/L, serum creatinine was greater than 1.6 mg/dL or creatinine clearance was less than 30 mL/min. Following study enrollment, serum potassium and blood pressure were assessed at baseline, day 3, day 7 and weekly thereafter for each treatment. In response to 1-month treatment with Eplerenone, serum potassium levels did not rise and systolic blood pressure did not decrease excessively requiring subject withdrawal.
General experimental procedures
All measurements were performed in the morning, at the same time each day, in a semi-darkened temperature-controlled room after a 12-hour overnight fast (including abstinence from caffeine and alcohol) and a minimum of 20 minutes of supine rest. Subjects took their morning dose of Eplerenone or placebo exactly one hour prior to data collection.
Vascular endothelial function (flow-mediated dilation; FMD)
Brachial artery FMD was assessed non-invasively following established guidelines [32, 33] by using an ultrasound/Doppler system equipped with a 7.5 MHz vascular transducer (Aplio XV, Toshiba).
Briefly, the subject rested in the supine position with the right arm abducted and fixed in position at heart level by using a Versaform pillow (Sammons Preston Rolyan, Bolingbrook, IL). A pressure cuff connected to a rapid inflator/deflator system (E20 and AG 101, D. E. Hokanson, Bellevue, WA) was placed around the widest part of the subject’s forearm. A duplex ultrasound image of the brachial artery (i.e., 2D image and spectral Doppler waveforms) was obtained ~7 cm proximal to the antecubital fossa. The Doppler angle of insonation for assessing blood velocity was set ≤ 60 degrees. Following image optimization the vascular transducer was clamped (Flexbar, Flexbar Machine Corporation, Islandia, NY) in place to prevent movement during data collection. To ensure the same segment of the brachial artery was imaged in the subsequent ultrasound visit, the distance of the transducer relative to the antecubital crease was recorded, a digital photograph of the arm position was stored, and the ultrasound image was printed.
Reactive hyperemia was induced by inflating the forearm cuff to 250 mmHg for 5 minutes followed by rapid deflation. ECG R-gated duplex ultrasound images of the brachial artery were digitally recorded (Vascular Imager, Medical Imaging Applications, LLC, Coralville, IA) for one minute to establish pre-occlusion baseline and for 2 minutes after cuff deflation to assess peak dilatory response (the maximum brachial artery diameter). End-diastolic diameters were analyzed by using a commercially available edge-detection wall-tracking software package (Brachial Analyzer, Medical Imaging Applications, LLC, Coralville, IA). Individual diameters were averaged (bin: 3 R-gated diameters) before identifying the peak diameter. FMD was expressed as absolute change in mm (maximum diameter − baseline diameter) and as % change ([(maximum − baseline diameter)/baseline diameter] × 100). To quantify the hyperemic response, the first 15 post-occlusion spectral Doppler envelopes and at least 15 baseline spectral Doppler envelopes were recorded on super VHS tape and were analyzed with the Toshiba ultrasound system software to obtain blood velocity. Blood flow (ml/min) was calculated as mean blood velocity × [(baseline diameter)2/4] × μ × 6 × 10−1. Shear stress (dyne/cm2) was calculated as 8 × μ × mean blood velocity/baseline diameter, where μ was blood viscosity, which was assumed to be 0.035 dyne/cm2 [34]. Ultrasound images were analyzed by MH and spectral Doppler was analyzed by ML, both of whom were blind to the treatment (i.e., Eplerenone or placebo) and subject identity.
Vascular endothelial cell collection and protein expression
Endothelial cells were collected from an antecubital vein as previously described [35–38]. Briefly, 2 sterile J-shaped guidewires (Daig, Inc., Minnetonka, MN) were sequentially advanced ~ 10 cm through an 18 gauge intravenous catheter and retracted. Cells were recovered by washing the wires with a dissociation buffer and centrifugation. Cells were fixed with 4% paraformaldehyde (USB corporation, Cleveland, OH), washed thoroughly with PBS, plated on poly-L-lysine coated slides (Sigma Chemical, St. Louis, MO), and stored at −80°C until the immunofluorescence staining was performed.
For immunofluorescence staining, fixed vascular endothelial cells were rehydrated with PBS containing 50 mmol/L glycine and non-specific sites were blocked with 5% donkey serum (Jackson Immunoresearch, West Grove, PA). Slides were incubated with one of the following primary antibodies followed with corresponding secondary antibody with Alexa Fluor 488 (Invitrogen, Carlsbad, CA): nitrotyrosine which is a marker of oxidative stress (Abcam, Inc, Cambridge, MA) and NADPH oxidase p47phox (Millipore, Inc., Billerica, MA) which is one of the major sources of vascular superoxide. Slides were also incubated with a primary antibody for von Willebrand factor (DAKO, Carpinteria, CA) and corresponding secondary antibody with Alexa Fluor 555 (Invitrogen, Carlsbad, CA) to allow identification of endothelial cells. Finally, slides were mounted with Vectashield containing the nuclear stain DAPI (Vector Laboratories, Inc., Burlingame, CA). Because of the large number of slides, staining was performed in several batches, but each subject’s slides from the Eplerenone and placebo visits were included in the same batch to avoid the influence of day-to-day variability in staining. To minimize the potential confound of inter-batch variability in staining, 2 slides of human umbilical venous endothelial cells (HUVEC) were stained in each batch and intensity for each protein of interest was expressed relative to the average HUVEC intensity in that batch.
For analysis, cells were examined with a fluorescence microscope (Eclipse 80i, Nikon Instruments, Inc., Melville, NY) at ×100 magnification using the same exposure time. Images of endothelial cells with intact nuclei were digitally captured by a coolSNAP ES2 camera (Photometrics, Tuscon, AR). Endothelial cells were identified by the presence of von Willebrand factor staining and nuclear integrity was confirmed by DAPI staining. Vascular endothelial cell protein expression was measured with NIS Elements software (version 3.2, Nikon Instruments, Inc., Melville, NY) by quantifying Alexa Fluor 488 intensity while correcting for background fluorescence. Vascular endothelial cell protein expression is reported as intensity per HUVEC intensity.
Blood measures
Standard blood chemistries and hematological evaluation were performed at baseline by a clinical laboratory using conventional assays. Insulin resistance was estimated using the homeostasis model of insulin resistance, [HOMA-IR; HOMA-IR = (fasting insulin μU/ml × fasting glucose mg/dl)/405]. Plasma F2-isoprostanes were measured by the Vanderbilt University Eicosanoid Core Laboratory using gas chromatography-mass spectrometry, as previously described [39].
Height, weight and adiposity measures
Height was measured to the nearest mm using a stadiometer. Body weight was measured to the nearest 0.1 kg with an electronic scale (Tanita, Arlington Heights, IL, USA) while subjects were barefoot and dressed in light clothing. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Total % body fat was assessed with dual-energy x-ray absorptiometry (DPX-IQ, GE/Lunar, Salt Lake City, UT, USA) as described previously [40]. Abdominal total, visceral and subcutaneous fat were measured at the level of L4-L5 using a single slice computed tomography scan and assessed by a commercially available analysis software (Slice-O-Matic v4.3, Tomovision) [41].
Resting blood pressure
Resting blood pressures were recorded over the brachial artery with a semi-automated device (Dinamap, GE, Salt Lake City, UT, USA).
Aerobic fitness
Aerobic fitness was determined using maximal oxygen consumption (VO2max) as previously described [40]. Briefly, online computer-assisted open-circuit spirometry was used during incremental treadmill exercise. After subjects walked for 6 to 10 min at a comfortable speed that corresponded to 70 to 80 % of their age-predicted maximal heart rate to warm-up, the treadmill grade was increased 2.5% every two minutes until volitional exhaustion.
Data Analysis
Statistical analyses were performed using SPSS version 21. Statistical significance for all analyses was set at P< 0.05. Paired t-tests were used to compare FMD, blood and vascular endothelial cell markers of oxidative stress during MR blockade and placebo treatments. Bivariate relations were determined using Pearson product moment correlation coefficients.
RESULTS
Mean values and ranges for baseline subject characteristics are presented in Table 1. Subjects varied widely in total and abdominal adiposity. At baseline, total and abdominal adiposity were negatively associated with FMD (r=−0.37 to 0.49, P<0.05) and positively associated with F2-isoprostanes (r=0.43 to 0.68, P<0.05).
Table 1.
Subject Characteristics
| Mean±SE | Min-Max | |
|---|---|---|
| Sex (male/female) | 10/12 | |
| Age, years | 63.6±1.5 | 55–79 |
| Weight, kg | 89.6±4.3 | 54.8–132.7 |
| Body mass index, kg/m2 | 29.9±1.4 | 20.0–44.6 |
| Body fat, % | 39.7±1.9 | 25.6–54.1 |
| Total abdominal fat, cm2 | 498.7±51.4 | 183.1–1045.0 |
| Abdominal visceral fat, cm2 | 144.3±17.2 | 45.8–329.5 |
| Abdominal subcutaneous fat, cm2 | 354.4±41.1 | 137.3–715.5 |
| VO2max, ml/kg/min | 24.9±1.4 | 14.2–37.7 |
| Total cholesterol, mg/dL | 185±6 | 143–225 |
| LDL cholesterol, mg/dL | 114±6 | 69–162 |
| HDL cholesterol, mg/dL | 47±3 | 30–69 |
| Triglycerides, mg/dL | 120±18 | 58–384 |
| Fasting glucose, mg/dL | 95±2 | 84–109 |
| Fasting insulin, μU/mL | 3.3±0.5 | 2–10 |
| HOMA-IR | 0.8±0.1 | 0.4–2.5 |
| F2-Isoprostanes, pg/mL | 69±9 | 32–198 |
VO2max = Maximal oxygen consumption; LDL = low density lipoprotein; HDL = high density lipoprotein; HOMA-IR= homeostasis assessment model for insulin resistance.
Vascular responses to MR blockade
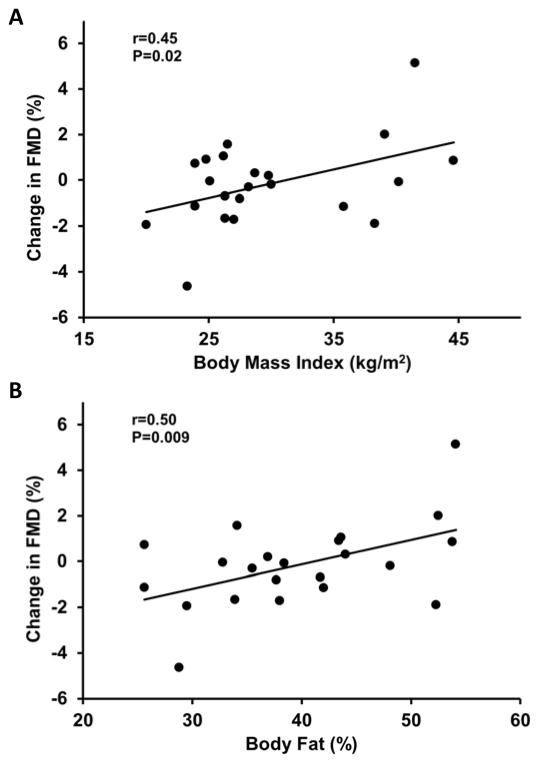
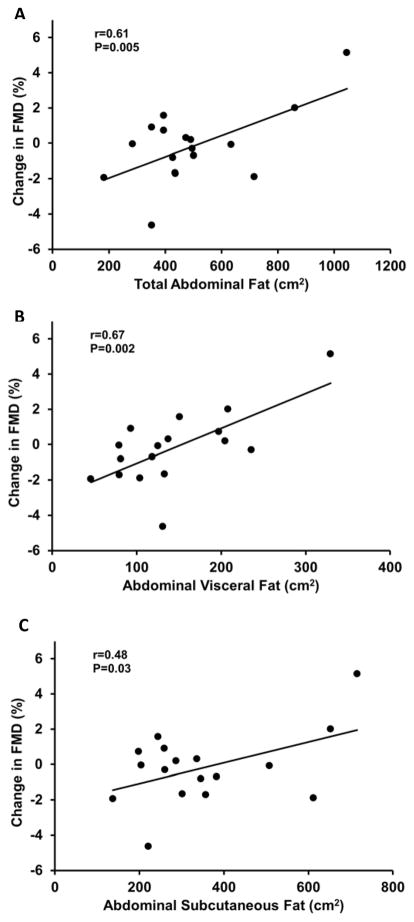
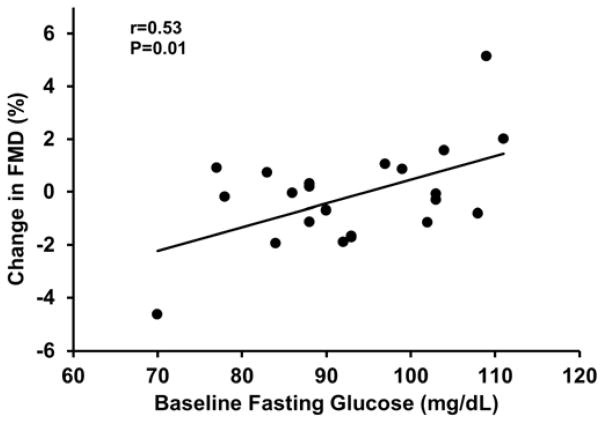
In the whole group, mean brachial artery FMD was not different with MR blockade compared to placebo (P=0.7; Table 2). However, individual responses to MR blockade varied from decreased to increased FMD. Subjects whose FMD improved with MR blockade had ~ 40% higher abdominal visceral fat compared with those whose FMD either decreased or did not change with MR blockade (P=0.03). In agreement with these results, greater improvements in FMD in response to MR blockade were related with greater baseline body mass index, total % body fat and total abdominal, visceral and subcutaneous fat (r=0.45 to 0.67, P≤0.03; Figures 2 and 3). In addition, greater improvements in FMD were associated with higher baseline fasting glucose (r=0.53, P=0.01; Figure 4).
Table 2.
Cardiovascular Responses to Mineralocorticoid Receptor Blockade
| Placebo | MR Blockade | P values | |
|---|---|---|---|
| Heart rate, beats/min | 60±2 | 62±2 | 0.06 |
| Systolic blood pressure, mm Hg | 133±3 | 123±3 | <0.0001 |
| Diastolic blood pressure, mm Hg | 77±2 | 72±1 | 0.07 |
| Baseline diameter, mm | 3.77±0.16 | 3.78±0.15 | 0.9 |
| Baseline SS, dyne/cm2 | 1.75±0.12 | 2.02±0.18 | 0.02 |
| Hyperemic SS, dyne/cm2 | 7.66±0.44 | 7.74±0.46 | 0.8 |
| Change in SS from baseline, % | 348±15 | 314±25 | 0.1 |
| Flow-mediated dilation, mm | 0.23±0.02 | 0.23±0.02 | 0.7 |
| Flow-mediated dilation, % | 6.39±0.67 | 6.23±0.73 | 0.7 |
| Flow-mediated dilation/hyperemic SS | 0.03±0.003 | 0.03±0.003 | 0.5 |
Data are mean±SE. SS: shear stress.
Figure 2.
The relation between body mass index (A) and total % body fat (B) with the change in flow-mediated dilation (FMD) in response to mineralocorticoid receptor blockade.
Figure 3.
The relation between total abdominal fat (A), abdominal visceral fat (B) and abdominal subcutaneous fat (C) with the change in flow-mediated dilation (FMD) in response to mineralocorticoid receptor blockade.
Figure 4.
The relation between baseline fasting glucose and the change in flow-mediated dilation (FMD) in response to mineralocorticoid receptor blockade.
Baseline brachial artery diameter was not different between MR blockade and placebo treatment, whereas, baseline shear stress increased in response to MR blockade (P=0.9 and P=0.02, respectively; Table 2). However, hyperemic shear stress and the change in shear stress from baseline did not differ between MR blockade and placebo, indicating that the post-occlusion stimulus to induce vasodilation was similar (P=0.8 and P=0.1, respectively, Table 2). MR blockade resulted in significant reductions in systolic blood pressure (P<0.0001) and smaller reductions in diastolic blood pressure that did not reach statistical significance (P=0.07; Table 2). However, the change in systolic blood pressure was not related with the change in FMD in response to MR blockade (P>0.05). In addition, accounting for the change in systolic blood pressure in multiple linear regression analysis did not contribute significantly to the model (P>0.05) and did not influence the relation of adiposity with the change in FMD in response to MR blockade.
Plasma oxidative stress and vascular endothelial cell protein expression
MR blockade did not influence plasma F2-isoprostanes (6.5±1.0 vs. 5.9±0.6 pg/mL, P=0.3; placebo vs. MR blockade). Similarly, vascular endothelial cell protein expression of nitrotyrosine (marker of oxidative stress) and NADPH oxidase (vascular source of superoxide) did not significantly change in response to MR blockade (0.79±0.04 vs. 0.73±0.22 intensity/HUVEC intensity, P=0.2, 0.66±0.04 vs. 0.57±0.04 intensity/HUVEC intensity, P=0.1, respectively). There were no correlations between 1) baseline plasma/endothelial cell oxidative stress measures, and baseline adiposity or change in FMD with MR blockade; and 2) change in plasma/endothelial cell oxidative stress markers and change in FMD with MR blockade.
DISCUSSION
We investigated whether MR modulate vascular endothelial function in an adiposity-dependent manner in healthy older adults with widely varying total and abdominal adiposity. Our study demonstrates for the first time that greater improvement in vascular endothelial function with MR blockade is seen in those who have greater total and abdominal adiposity. Another important finding of our study is that greater enhancements in endothelial function in response to MR blockade are associated with higher baseline fasting glucose.
Findings from two recent studies based on animal and in vitro models have shown compelling evidence of aldosterone production in adipocytes and contribution of adipocyte-derived aldosterone to vascular dysfunction in obesity [9, 10]. In humans, several studies have shown elevated plasma aldosterone levels with obesity and some have found that greater BP reduction with MR blockade was associated with higher body mass index [42] and higher waist circumference [43]. Our data extend these findings by demonstrating greater increases in FMD with MR blockade are associated with higher body mass index, total % body fat, total abdominal, visceral and subcutaneous fat.
Aldosterone might be the potential link between adiposity, insulin resistance, and increased risk for cardiovascular disease. A recent review article highlighted data supporting a role for elevated plasma aldosterone levels and MR signaling in the pathophysiology of insulin resistance and vascular dysfunction [44]. Our data demonstrate greater improvements in endothelial function with MR blockade are associated with higher baseline fasting blood glucose. These findings suggest that MR play a larger role in vascular dysfunction in subjects with lower insulin sensitivity.
In our study, systolic blood pressure significantly decreased in response to MR blockade, thus, one might speculate that this could have contributed to the improvements in endothelial function. However, the change in systolic blood pressure was not related with the change in FMD in response to MR blockade. In addition, accounting for the change in systolic blood pressure in multiple linear regression analysis did not significantly contribute to the model and did not influence the relation of adiposity with the change in FMD in response to MR blockade. Taken together these findings argue against the assumption that reductions in blood pressure might have played a significant role in the beneficial effects of Eplerenone on vascular endothelial function.
Our study has several strengths including: 1) novelty of findings; 2) use of balanced randomized, double-blind, placebo-controlled, cross-over design; 3) exclusion of subjects with overt cardiovascular or other clinical disease and medication use, which could confound the independent relation of MR with obesity; 4) quantification of total % body fat using DEXA and total abdominal, visceral and subcutaneous fat using computed tomography; and 5) rigorous procedures to ensure adherence to intervention.
Our study also has some potential limitations. We did not measure baseline plasma aldosterone to determine if it was elevated in our obese subjects. However, several studies have already established a relation between aldosterone levels and obesity. MR have equal affinity for aldosterone and cortisol, however, the presence of the enzyme 11β-hydroxysteroid dehydrogenase (11βHSD) in tissues (including the vascular wall) converts cortisol to corticone making aldosterone the primary MR agonist [45]. Our current data cannot address whether cortisol might have a role in the observed effects of MR blockade in human obesity. In cardiovascular disease, MR appear to contribute to vascular dysfunction by exacerbating ROS production and oxidative stress, but in our study plasma F2-isoprostanes and vascular endothelial cell protein expression of nitrotyrosine and NADPH oxidase p47phox did not change in response to MR blockade. Given our limited oxidative stress measures, we cannot rule out that oxidative stress plays a role in the beneficial effects of MR blockade on endothelial function in human obesity. Our protein expression data of oxidative stress markers focused on vascular endothelial cell samples, which does not reflect whether oxidative stress levels changed in vascular smooth muscle cells. In addition, we measured protein expression of a specific subunit of NADPH oxidase, but it is possible that other isoforms/subunits and/or activation of the enzyme are playing a role, which cannot be addressed using our current methodology. Finally, our subjects were older, thus our results might not be applicable to healthy young adults. Additional research is needed to investigate whether MR blockade also improves vascular endothelial function in an obesity-dependent manner in healthy young adults.
Clinical Significance
Aldosterone contributes to vascular dysfunction in cardiovascular disease. Plasma aldosterone is elevated with total and abdominal adiposity in humans, but its influence on vascular function is unknown. We sought to examine the role of MR in vascular endothelial function in human obesity in a balanced randomized, double-blind, placebo-controlled, crossover study using 1 month MR blockade with Eplerenone. We found that Eplerenone-related improvements in FMD were positively associated with total and abdominal adiposity and baseline fasting glucose in healthy older adults. Aldosterone appears to be an important contributor to vascular endothelial dysfunction in healthy older adults with increased adiposity and fasting blood glucose. These findings have important clinical implications. Therapeutic use of MR blockade to treat hypertension in patients with increased adiposity might confer direct favorable effects on obesity-related vascular alterations and might reduce the risk of developing cardiovascular complications.
CONCLUSIONS
The present findings demonstrate, for the first time, that MR modulate vascular endothelial function in an adiposity-dependent manner in healthy older adults. MR-blockade-related improvements in FMD are positively related with both total and abdominal adiposity. We also demonstrate that changes in vascular endothelial function with MR blockade are related with baseline fasting blood glucose. Our study suggests that MR contribute to the pathophysiology of impaired vascular endothelial function in human obesity.
Acknowledgments
The authors would like to thank Ms. Sharon Greer, R.N., Mr. Creighton Wilson R.N. and the Division of Cardiology and Radiology at the Scott and White Clinic at College Station, Texas, for their contributions. The authors would also like to thank Ms. Molly Cernosek and Ms. Larysa Sautina for technical assistance and the study participants for their time and efforts.
FUNDING
This work was supported by the National Institutes of Health [grant AG 032067] to DDC and American Heart Association [grant 0865117F] to DDC.
Footnotes
AUTHOR CONTRIBUTIONS
M.H.H and D.D.C conceived and designed the study; M.H.H., J.K.Y, M.J.L., T.H.M., M.E. and D.D.C. collected the data; M.H.H., J.K.Y., M.J.L. and H.K.K. analyzed the data; T.H.M. and M.E. provide on-site medical supervision for experiments; M.H.H and D.D.C. performed statistical analysis, prepared figures and drafted manuscript; M.H.H, M.S.S. and D.D.C. interpreted results, edited and revised manuscript; J.K.Y, H.K.K., M.J.L., M.S.S., T.H.M. and M.E. provided feedback for manuscript; all authors approved final version of manuscript.
References
- 1.WHO. Obesity and Overweight Fact Sheet No 311 2013 [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief, 1–8. 2012 [PubMed] [Google Scholar]
- 3.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 4.Rossignol P, Menard J, Fay R, Gustafsson F, Pitt B, Zannad F. Eplerenone survival benefits in heart failure patients post-myocardial infarction are independent from its diuretic and potassium-sparing effects. Insights from an EPHESUS (Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) substudy. J Am Coll Cardiol. 2011;58:1958–1966. doi: 10.1016/j.jacc.2011.04.049. [DOI] [PubMed] [Google Scholar]
- 5.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 6.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 7.Williams GH. Aldosterone biosynthesis, regulation, and classical mechanism of action. Heart Fail Rev. 2005;10:7–13. doi: 10.1007/s10741-005-2343-3. [DOI] [PubMed] [Google Scholar]
- 8.McCurley A, Jaffe IZ. Mineralocorticoid receptors in vascular function and disease. Mol Cell Endocrinol. 2012;350:256–265. doi: 10.1016/j.mce.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen Dinh Cat A, Briones AM, Callera GE, Yogi A, He Y, Montezano AC, Touyz RM. Adipocyte-derived factors regulate vascular smooth muscle cells through mineralocorticoid and glucocorticoid receptors. Hypertension. 2011;58:479–488. doi: 10.1161/HYPERTENSIONAHA.110.168872. [DOI] [PubMed] [Google Scholar]
- 10.Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, Correa JW, Gagnon AM, Gomez-Sanchez CE, Gomez-Sanchez EP, et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012;59:1069–1078. doi: 10.1161/HYPERTENSIONAHA.111.190223. [DOI] [PubMed] [Google Scholar]
- 11.Rossi GP, Belfiore A, Bernini G, Fabris B, Caridi G, Ferri C, Giacchetti G, Letizia C, Maccario M, Mannelli M, et al. Body mass index predicts plasma aldosterone concentrations in overweight-obese primary hypertensive patients. J Clin Endocrinol Metab. 2008;93:2566–2571. doi: 10.1210/jc.2008-0251. [DOI] [PubMed] [Google Scholar]
- 12.Kidambi S, Kotchen JM, Krishnaswami S, Grim CE, Kotchen TA. Aldosterone contributes to blood pressure variance and to likelihood of hypertension in normal-weight and overweight African Americans. Am J Hypertens. 2009;22:1303–1308. doi: 10.1038/ajh.2009.167. [DOI] [PubMed] [Google Scholar]
- 13.Goodfriend TL, Egan BM, Kelley DE. Plasma aldosterone, plasma lipoproteins, obesity and insulin resistance in humans. Prostaglandins Leukot Essent Fatty Acids. 1999;60:401–405. doi: 10.1016/s0952-3278(99)80020-9. [DOI] [PubMed] [Google Scholar]
- 14.Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, Luft FC, Sharma AM. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45:356–362. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- 15.Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, Garg R. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007;92:4472–4475. doi: 10.1210/jc.2007-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andronico G, Cottone S, Mangano MT, Ferraro-Mortellaro R, Baiardi G, Grassi N, Ferrara L, Mule G, Cerasola G. Insulin, renin-aldosterone system and blood pressure in obese people. Int J Obes Relat Metab Disord. 2001;25:239–242. doi: 10.1038/sj.ijo.0801483. [DOI] [PubMed] [Google Scholar]
- 17.Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med. 1981;304:930–933. doi: 10.1056/NEJM198104163041602. [DOI] [PubMed] [Google Scholar]
- 18.Rocchini AP, Katch VL, Grekin R, Moorehead C, Anderson J. Role for aldosterone in blood pressure regulation of obese adolescents. Am J Cardiol. 1986;57:613–618. doi: 10.1016/0002-9149(86)90845-3. [DOI] [PubMed] [Google Scholar]
- 19.Dall’Asta C, Vedani P, Manunta P, Pizzocri P, Marchi M, Paganelli M, Folli F, Pontiroli AE. Effect of weight loss through laparoscopic gastric banding on blood pressure, plasma renin activity and aldosterone levels in morbid obesity. Nutr Metab Cardiovasc Dis. 2009;19:110–114. doi: 10.1016/j.numecd.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arcaro G, Zamboni M, Rossi L, Turcato E, Covi G, Armellini F, Bosello O, Lechi A. Body fat distribution predicts the degree of endothelial dysfunction in uncomplicated obesity. Int J Obes Relat Metab Disord. 1999;23:936–942. doi: 10.1038/sj.ijo.0801022. [DOI] [PubMed] [Google Scholar]
- 22.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: A systematic review with meta-analysis. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 23.Vita JA. Endothelial function. Circulation. 2011;124:e906–912. doi: 10.1161/CIRCULATIONAHA.111.078824. [DOI] [PubMed] [Google Scholar]
- 24.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circulation Research. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 25.Avogaro A, de Kreutzenberg SV. Mechanisms of endothelial dysfunction in obesity. Clin Chim Acta. 2005;360:9–26. doi: 10.1016/j.cccn.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Keidar S, Kaplan M, Pavlotzky E, Coleman R, Hayek T, Hamoud S, Aviram M. Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development: a possible role for angiotensin-converting enzyme and the receptors for angiotensin II and aldosterone. Circulation. 2004;109:2213–2220. doi: 10.1161/01.CIR.0000127949.05756.9D. [DOI] [PubMed] [Google Scholar]
- 27.Rajagopalan S, Duquaine D, King S, Pitt B, Patel P. Mineralocorticoid receptor antagonism in experimental atherosclerosis. Circulation. 2002;105:2212–2216. doi: 10.1161/01.cir.0000015854.60710.10. [DOI] [PubMed] [Google Scholar]
- 28.Thai HM, Do BQ, Tran TD, Gaballa MA, Goldman S. Aldosterone antagonism improves endothelial-dependent vasorelaxation in heart failure via upregulation of endothelial nitric oxide synthase production. J Card Fail. 2006;12:240–245. doi: 10.1016/j.cardfail.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Sartorio CL, Fraccarollo D, Galuppo P, Leutke M, Ertl G, Stefanon I, Bauersachs J. Mineralocorticoid receptor blockade improves vasomotor dysfunction and vascular oxidative stress early after myocardial infarction. Hypertension. 2007;50:919–925. doi: 10.1161/HYPERTENSIONAHA.107.093450. [DOI] [PubMed] [Google Scholar]
- 30.Farquharson CA, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation. 2000;101:594–597. doi: 10.1161/01.cir.101.6.594. [DOI] [PubMed] [Google Scholar]
- 31.Abiose AK, Mansoor GA, Barry M, Soucier R, Nair CK, Hager D. Effect of spironolactone on endothelial function in patients with congestive heart failure on conventional medical therapy. Am J Cardiol. 2004;93:1564–1566. doi: 10.1016/j.amjcard.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force.[erratum appears in J Am Coll Cardiol 2002 Mar 20;39(6):1082] Journal of the American College of Cardiology. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 33.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 35.Colombo PC, Ashton AW, Celaj S, Talreja A, Banchs JE, Dubois NB, Marinaccio M, Malla S, Lachmann J, Ware JA, et al. Biopsy coupled to quantitative immunofluorescence: a new method to study the human vascular endothelium. J Appl Physiol. 2002;92:1331–1338. doi: 10.1152/japplphysiol.00680.2001. [DOI] [PubMed] [Google Scholar]
- 36.Silver A, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H Oxidase-p47phox expression and evidence of endothelial oxidative stress. Circulation. 2007;115 doi: 10.1161/CIRCULATIONAHA.106.657486. [DOI] [PubMed] [Google Scholar]
- 37.Silver AE, Christou DD, Donato AJ, Beske SD, Moreau KL, Magerko KA, Seals DR. Protein expression in vascular endothelial cells obtained from human peripheral arteries and veins. J Vasc Res. 2010;47:1–8. doi: 10.1159/000231715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2:221–226. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- 40.Christou DD, Gentile CL, DeSouza CA, Seals DR, Gates PE. Fatness is a better predictor of cardiovascular disease risk factor profile than aerobic fitness in healthy men. Circulation. 2005;111:1904–1914. doi: 10.1161/01.CIR.0000161818.28974.1A. [DOI] [PubMed] [Google Scholar]
- 41.Christou DD, Pierce GL, Walker AE, Hwang MH, Yoo JK, Luttrell M, Meade TH, English M, Seals DR. Vascular smooth muscle responsiveness to nitric oxide is reduced in healthy adults with increased adiposity. Am J Physiol Heart Circ Physiol. 2012;303:H743–750. doi: 10.1152/ajpheart.00394.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khosla N, Kalaitzidis R, Bakris GL. Predictors of hyperkalemia risk following hypertension control with aldosterone blockade. Am J Nephrol. 2009;30:418–424. doi: 10.1159/000237742. [DOI] [PubMed] [Google Scholar]
- 43.de Souza F, Muxfeldt E, Fiszman R, Salles G. Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension. 2010;55:147–152. doi: 10.1161/HYPERTENSIONAHA.109.140988. [DOI] [PubMed] [Google Scholar]
- 44.Bender SB, McGraw AP, Jaffe IZ, Sowers JR. Mineralocorticoid receptor-mediated vascular insulin resistance: an early contributor to diabetes-related vascular disease? Diabetes. 2013;62:313–319. doi: 10.2337/db12-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Funder JW. Aldosterone and Mineralocorticoid Receptors in the Cardiovascular System. Progress in Cardiovascular Diseases. 2010;52:393–400. doi: 10.1016/j.pcad.2009.12.003. [DOI] [PubMed] [Google Scholar]