Abstract
Heterotrimeric G-proteins are versatile regulators involved in diverse cellular processes in eukaryotes. In plants, the function of G-proteins is primarily associated with ABA signaling. However, the downstream effectors and the molecular mechanisms in the ABA pathway remain largely unknown. In this study, an AGB1 mutant (agb1-2) was found to show enhanced drought tolerance, indicating that AGB1 might negatively regulate drought tolerance in Arabidopsis. Data showed that AGB1 interacted with protein kinase AtMPK6 that was previously shown to phosphorylate AtVIP1, a transcription factor responding to ABA signaling. Our study found that transcript levels of three ABA responsive genes, AtMPK6, AtVIP1 and AtMYB44 (downstream gene of AtVIP1), were significantly up-regulated in agb1-2 lines after ABA or drought treatments. Other ABA-responsive and drought-inducible genes, such as RD29A (downstream gene of AtMYB44), were also up-regulated in agb1-2 lines. Furthermore, overexpression of AtVIP1 resulted in hypersensitivity to ABA at seed germination and seedling stages, and significantly enhanced drought tolerance in transgenic plants. These results suggest that AGB1 was involved in the ABA signaling pathway and drought tolerance in Arabidopsis through down-regulating the AtMPK6, AtVIP1 and AtMYB44 cascade.
Introduction
Heterotrimeric GTP-binding proteins (G-proteins) are evolutionarily conserved plasma membrane-bound proteins that regulate a number of fundamental processes in eukaryotic organisms. G-proteins consist of three subunits, Gα, Gβ, and Gγ. In contrast to humans, which have 23 Gα, 5 Gβ and 14 Gγ genes [1], Arabidopsis has only one Gα (GPA1) [2], one Gβ (AGB1) [3] and three Gγ (AGG1-AGG3) genes [4,5]. Upon perception of a cognate ligand by its transmembrane G protein coupled receptor (GPCR) on the cell surface, the heterotrimeric G-protein complex dissociates to form an activated Gα-subunit and an obligate dimer Gβγ, which further transmits signaling into the cytoplasm by interaction with various downstream effector proteins [6]. Loss-of-function mutants and gain-of-function lines overexpressing G-protein subunits and signaling components showed that G-proteins play a vital role in regulating diverse growth and developmental processes, hormonal and stress responses [6,7]. AGB1 functions in many facets of development and signal transduction in Arabidopsis, for example, agb1 mutants display diverse phenotypes with highly branched root systems, rounder leaves as well as shorter siliques [8], and have altered sensitivity to brassinosteroid (BR) and ABA during seed germination, and altered sugar sensing and stomate closure [9–13].
G proteins are involved in signal transduction through interaction with their effector proteins and regulate their activities [14]. Many G protein effectors have been functionally identified in animals, but few effectors for canonical G proteins were characterized, especially for AGB1 in plants. Currently, some genes involved in physical and genetic AGB1-interaction have been identified, such as ARD1 (ACI-reductone dioxygenase-like protein), a protein interacting with Gβ exhibited higher enzymatic activity by the involvement of Gβγ [15], NDL1 (N-MYC DOWNREGULATED-LIKE1), a protein physically interacting with AGB1 that regulates auxin distribution in roots [16], and a Golgi-localized hexose transporter, SGB1 [17], and an AGB1-interactome have been reported in Arabidopsis [18]. However, these studies have not explained the divergent functions of AGB1 in plants, and the molecular mechanisms underlying G protein-mediated ABA signaling remain to be investigated.
ABA is a crucial mediator in plant response to both biotic [19] and abiotic stresses, such as dehydration, salinity, low temperature [20,21], and in plant developmental processes such as seed development, dormancy, germination, and seedling growth [22]. Components of the heterotrimeric G-protein complex are involved in the ABA signaling pathway and play an important role in seed germination, early seedling development, stomate opening and closure in Arabidopsis [9,10,13,23,24]. In addition, ABA was shown to bind to GTG1 and GTG2 on the plasma membrane [25], and a quantitative proteomics-based analysis showed that many ABA-responsive proteins depend on the presence of functional GTG1/GTG2 proteins. This indicates the importance of G proteins in ABA signaling [26]. On the other hand, agb1-2 mutant has greater ABA sensitivity than gpa1-4 or gcr1-2 mutants during seed germination and post-germination development, indicating that AGB1 is a primary regulator of the G-protein complex in ABA signaling [7,11]. However, the putative downstream effectors of AGB1 have not been assessed, therefore, the putative molecular mechanism underlying the involvement of AGB1 in ABA signaling pathway remains unclear.
In this research, the role of AGB1 in the ABA-related signaling pathway and its interaction proteins and/or downstream genes was investigated using yeast two-hybrids, and ABA-treated Arabidopsis cDNA library was screened using AGB1 as bait. AtMPK6 (mitogen-activated protein kinase 6) was found to interact with AGB1 [27]. Furthermore, the expression profiles of downstream genes in agb1-2 mutant lines upon ABA and drought treatments were investigated. The results showed that a subset of genes involved in the ABA signaling pathway and in drought tolerance were up-regulated in agb1-2 lines. Finally, the performance of transgenic Arabidopsis plants overexpressing AtVIP1 (VirE2-interacting protein1), a gene encoding a bZIP transcription factor protein (a downstream target of AtMPK6), was investigated. Our results demonstrated that AGB1 interacts with AtMPK6 and may negatively regulate the ABA signaling pathway and drought tolerance by down-regulating the AtMPK6, AtVIP1, and AtMYB44 cascade in Arabidopsis.
Materials and Methods
Plant materials and growth conditions
The mutants agb1-1 and agb1-2 in the Col-0 background have been described previously [8,9]. agb1-2 (At4g34460) homozygous mutants were confirmed by genomic PCR using gene-specific primers P1: 5′ -TCATTAGATTGGACACCGGAG- 3′; P3: 5′ -TGTGAATCCTGCTGTAATCCC- 3′ and T-DNA border primer P2 (LBb1.3): 5′ -ATTTTGCCGATTTCGGAAC- 3′ (S1B Fig.). RT-PCR was performed using primers P1 and P3 (S1C Fig. and S2 Table). Homozygous mutant and wild-type seeds kept at identical conditions were surface sterilized with 30% bleach for 10 min and washed five times with sterile water. Seeds were plated on MS medium plates containing 3% sucrose in darkness for 3 d at 4°C, and then transferred to a growth chamber with a 16-h-light (20°C)/8-h-darkness (22°C) cycle.
Yeast two-hybrid screening
For screening of proteins interacting with AGB1, the full coding region of AGB1 was amplified using primers AtAGB1-BD-FULL-NdeI-F and AtAGB1-BD-FULL-EcoRI-R (S2 Table), and digested/ligated into the C-terminal of the Gal4 DNA binding domain in pGBKT7. The recombined vectors were co-transformed along with the ABA-treated Arabidopsis cDNA library into the yeast strain AH109 as described by the manufacturer (Clontech, USA; http://www.clontech.com/). To confirm interaction between AGB1 and AtMPK6, the full-length coding sequences of AtMPK6 and AGB1 were amplified with primers AtMPK6-BD-FULL-NdeI-F and AtMPK6-BD-FULL-SalI-R, and AtAGB1-AD-FULL-NdeI-F and AtAGB1-AD-FULL-EcoRI-R (S2 Table). AtMPK6 and AGB1 were inserted into pGBKT7 and pGADT7 respectively. The two recombined vectors were co-transformed into the yeast strain AH109. Transformed yeast was cultivated in liquid SD/–Leu/–Trp to OD600 = 0.6, and then 5 μl of yeast suspension was added to each of the control SD/-Leu/-Trp and screening SD/–Leu/–Trp/–His/–Ade medium. The growth of yeast cells was photographed after 3 d of incubation at 30°C.
Bimolecular fluorescence complementation (BiFC) assay
Full-length coding sequences of AGB1 and AtMPK6 were amplified from a cDNA library of Arabidopsis using gene-specific primers AtAGB1-YFPN-FULL-BamHI-F and AtAGB1-YFPN-FULL-SalI-R, and primers AtMPK6-YFPC-FULL-BamHI-F and AtMPK6-YFPC-FULL-SalI-R (S2 Table). AGB1 sequence was inserted into the pSPYNE vector (fusing AGB1 to the N-terminal side of YFP) and the AtMPK6 to the pSPYCE vector (fusing MPK6 to the C-terminal side of YFP) [28]. The empty vectors pSPYNE and pSPYCE, expressing split YFP domains were used as a negative controls, and the combination of bZIP63-YFPN and bZIP63-YFPC was used for a positive controls [28]. Both pSPYNE-AGB1 and pSPYCE-MPK6 were mixed, and co-transformed into protoplasts of Arabidopsis ecotype Col-0. Samples were incubated at 23°C for 16–20 h before observation. YFP fluorescence was recorded using a confocal laser-scanning microscope (Zeiss LSM700) and the nuclei of protoplasts were stained with 4’,6-diamidino-2-phenylindole (DAPI, Sigma).
GST pull-down assay in vitro
The GST-AGB1 and His-MPK6 constructs were introduced into E. coli strain BL21 (DE3), inducing the expression of the fusion proteins by adding 1 mM of isopropylthio-β-galactoside (IPTG) at 37°C. The GST-AGB1 and His-MPK6 fusion proteins were purified using glutathione-agarose 4B (GE Healthcare) beads and Ni-agarose, respectively, according to the manufacturer’s instructions (GE Healthcare). For protein pull-down assays, the GST fusion proteins were bound to a glutathione sepharose 4B column and the loaded matrix was then incubated with purified His fusion protein in binding buffer [20 mM HEPES, pH 7.4, 1 mM EDTA, 5 mM MgCl2, 1 mM DTT, 0.1% Triton X-100, 1 mg ml−1 BSA and 1 mM PMSF] for 4 h at 4°C. The beads were then centrifuged at 2000 g for 1 min at 4°C and washed four times with protein pull-down wash buffer (1 × PBS: 137 mM NaCl, 8.1 mM Na2HPO4·12H2O, 2.68 mM KCl, 1.47 mM KH2PO4, 1 mM PMSF, pH 7.4). Bound proteins were eluted, and then fractionated by 10% SDS-PAGE, and analyzed by Western blotting using antibodies of HisProbe-HRP (Invitrogen).
Co-immunoprecipitation (Co-IP) assay in vivo
AGB1, fused to Flag-tag (AtAGB1-Flag-FULL-F and AtAGB1-Flag-FULL-R, containing SalI and SpeI restriction sites respectively), and the AtMPK6, fused to Myc-tag (AtMPK6-Myc-FULL-F and AtMPK6-Myc-FULL-R, containing SalI and SpeI restriction sites respectively) were digested/ligated into pCAMBIA1300 vector (S2 Table). CsCl gradient centrifugation was used to purify the plasmids and the plasmids were transformed transiently into Arabidopsis mesophyII protoplasts (Clo-0). The protoplasts were homogenized overnight in buffer (10 mM Tris-HCl [pH 7.6], 150 mM NaCl, 2 mM EDTA, 0.5% [v/v] Nonidet P-40, 2 × protease inhibitor [Roche]) and then were centrifuged at 13,000 g for 15 min at 4°C. A 30 μl volume of anti-Myc agarose beads (Sigma-Aldrich) for immunoprecipitation of Myc-tag were incubated with the extraction supernatant for 3 h at 4°C. After the samples were washed five times with 1 ml of 1 × PBS buffer, the immunoprecipitated products were analyzed by Western blot. Antibodies of Anti-Myc (Sigma-Aldrich) or anti-Flag (Sigma-Aldrich) were used, and the chemiluminescence signal was detected by autoradiography.
Subcellular localization analysis of proteins
Full-length cDNA fragments coding AtMPK6 and AGB1 were PCR-amplified with gene-specific primers AtMPK6-GFP-FULL-SalI-F and AtMPK6-GFP-FULL-BamHI-R, and AtAGB1-GFP-FULL-SalI-F and AtAGB1-GFP-FULL-BamHI-R (S2 Table), and inserted into the N-terminal side of 163h-GFP vector, respectively. Subcellular localization analysis of two fused proteins was completed in protoplasts of Arabidopsis ecotype Col-0 [29] and agb1-2. Localizations of MPK6-GFP and AGB1-GFP were recorded 15–18 h after transformation using a confocal laser-scanning microscope (Zeiss LSM700) and the nuclei of protoplasts were stained with 4’,6-diamidino-2-phenylindole (DAPI, Sigma).
Generation of 35S::AtVIP1 transgenic Arabidopsis
The full-length coding sequence of AtVIP1 was amplified with gene-specific primers AtVIP1-FULL-SmaI-F and AtVIP1-FULL-SpeI-R (S2 Table), and inserted into the binary vector pBI121 under control of the Cauliflower Mosaic Virus (CaMV) 35S promoter. The construct was confirmed by sequencing, and transformed into Agrobacterium tumefaciens strain (GV3101), and then transformed into wild-type Arabidopsis (Columbia-0 ecotype) by the floral dip method [30]. The seeds collected were then selected on MS medium containing 50 mgl−1kanamycin for three generations. Transgenic lines (T3 generation) with different expression levels of AtVIP1 were used for further analysis.
Stress tolerance analyses of Arabidopsis plants
Germination assay was performed using 60 seeds of the WT and OE-VIP1–4, 5, 6 lines (identical storage condition). Seeds were surface sterilized and plated on 0.8% agar (Sigma) containing MS salts (Sigma) and 3% (w/v) sucrose with or without ABA. Plated seeds were then chilled at 4°C in darkness for 3 d, and finally germinated at 22°C in 16 h light/8 h darkness. After 4 days, germination rates were scored based on emergence of the radical through the seed coat. This experiment was repeated three times.
For ABA sensitivity and PEG tolerance analyses, seeds of WT and OE-VIP1–4, 5, 6 lines were germinated on MS medium for 3 d, and then transferred to normal medium or medium contained ABA or PEG. Root lengths of seedlings grown in 16 h light/8 h darkness were recorded after 14 d. For drought tolerance analyses, three-week-old plants in soil were withheld from watering for 21 d and then were re-watered at the 21st day of drought. Observations were recorded after 3 d or 5 d of re-watering.
RNA extraction and gene expression analyses
21-d-old Arabidopsis plants (Col-0) and agb1-2 mutant were removed from the soil and treated with 200 μM ABA (stock solution in ethanol) and intact plants were harvested 0.5, 1, 2, 4, 6, 8, 12, 16, and 24 h later. For dehydration stress, 21-d-old Arabidopsis plants and agb1-2 mutant were removed from the soil, and placed on filter paper at room temperature, and intact plants were harvested 0.5, 1, 2, 4, 6, 8, 12, 16, and 24 h later. Total RNA was isolated and 2 μg of total RNA was used for first strand cDNA synthesis with a PrimeScript 1st Strand cDNA Synthesis kit (Takara). Quantitative expression assays were performed with the SYBR Premix Ex Taq (Takara) and an ABI 7300 according to the manufacturer’s protocols (Applied Biosystem). The PCR program was 95°C for 2 min followed by 40 cycles of denaturation for 15 s at 95°C and annealing/extension at 60°C for 1 min. Expressions of all genes were assayed for triplicated. Gene expression was calculated using the Delta-Delta cycle threshold method [31]. Relative quantitative results were calculated by normalization to ACT2 (GenBank accession number: AT3G18780). The primer pairs used for real-time PCR are listed in S2 Table.
Determination of proline content
The proline contents of wild type and agb1-2 plants were determined as previously described [32,33].
Results
AGB1 negatively regulates ABA sensitivity and drought tolerance in Arabidopsis
Loss of function of AGB1 increased ABA sensitivity either at seed germination and post-germination growth stages [11]. In the present study, the effects of ABA on the G-protein β subunit mutants agb1-1 and agb1-2 were examined. Under normal conditions without ABA treatment, the mutant agb1-1, agb1-2 and WT lines showed 86–97% germination rates at 48 h (S2A Fig.) with no significant difference. Compared to the 72% germination rate of wild-type plants at 48 h, lower germination rates were observed in the agb1-1 and agb1-2 mutants, approximately 45% (for agb1-1) and 49% (for agb1-2), respectively, in the presence of 1.0 μM ABA (S2B Fig.). The cotyledon greening rates of WT, agb1-1 and agb1-2 seedlings were likewise similar under normal conditions, but those of WT were significantly higher than those in agb1-1 and agb1-2 seedlings growing on the medium supplemented with 1.0 μM ABA (*P<0.05) (S2 Fig. C and D), indicating that AGB1 plays a negative role in ABA signaling, similar to what was found previously [11,34]. Meantime, it was clear that the root lengths of agb1 mutants were significantly shorter than that of wild-type under 2.0 μM ABA (*P<0.05) (S3 Fig. A, D and F), indicating that AGB1 may have a negative role in the ABA-mediated response. On the other hand, AGB1 transcript was significantly decreased after treatment with 200 μM ABA (Fig. 1A) and also was drastically decreased after drought treatment with a slightly recovery at later stages (8 h and 12 h) (Fig. 1A), suggesting that AGB1 was down-regulated under ABA and drought treatments.
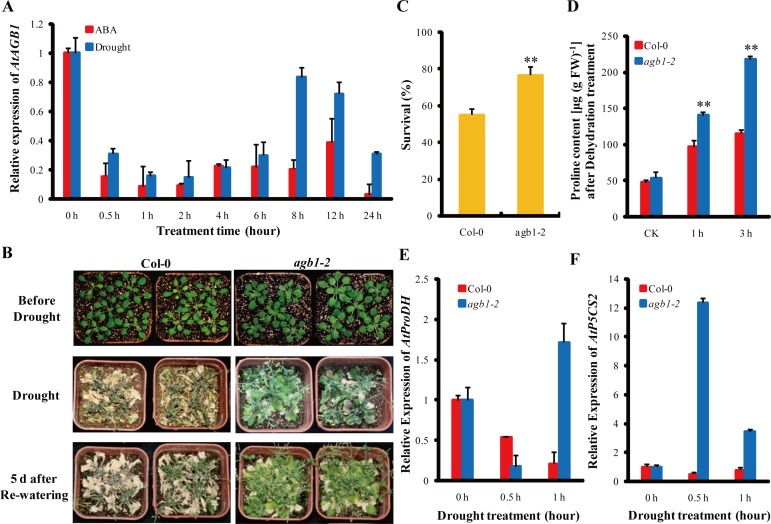
Figure 1. Expression pattern analysis of AGB1 in Arabidopsis and drought tolerance assay of agb1.
(A) Expression patterns of AGB1 were identified after treatments with 200 μM ABA and drought stress for 0, 0.5, 1, 2, 4, 6, 8, 12, and 24 h. The expression value of AGB1 at 0 h was normalized as 1; expression values at other time points were relative to the value at 0 h. Values represent means ± SD with three biological replicates. (B) Drought tolerance assay of WT (Col-0) and agb1-2 plants. WT and agb1-2 plants (before drought) were subjected to water stress by withholding water for 21 days (drought) and were then re-watered for 5 days (5 d after re-watering). (C) Survival rates of the WT and agb1-2 in (B) after drought stress. Data represent means ± SD (n = 60) from three independent experiments. Asterisks indicate significant differences (Student’s t test, **P<0.01) between WT and agb1-2. (D) Proline contents of WT and agb1-2 plants after dehydration treatments. For the proline assay, 14-day-old seedlings were subjected to drought treatment for 0, 1 and 3 h. Proline contents of WT and agb1-2 plants at the same time points were significantly different at 1 and 3 h. Data represent means ± SD from three independent experiments. Asterisks indicate significant differences (Student’s t test, **P<0.01) between WT and agb1-2. (E) and (F) Expression analysis of the proline biosynthesis genes ProDH (E) and P5CS2 (F) in WT and agb1-2 seedlings after drought treatment for 0, 0.5 and 1 h. All results are means ± SD for three independent experiments.
To evaluate drought stress tolerance of G-protein β subunit mutant lines, we selected a knockout mutant agb1-2, and drought treated by withholding water from 3-week-old plants in pots for 3 weeks; 54.76% of WT plants survived for 5 d after re-watering, significantly lower than the 76.19% survival rate of agb1-2 plants (**P<0.01) (Fig. 1B and C). Additionally, analysis showed that there were no differences in root length between wild-type (ecotype, Col-0) and agb1 mutants on MS medium, however, in wild-type seedlings, roots length was significantly shorter than that of agb1-1 and agb1-2 mutant lines when the MS medium contained 4% and 8% PEG (*P<0.05) (S3 Fig. A, B, C and E). Physiological measurements showed that, under normal conditions, there was no significant difference in proline contents between WT and agb1-2 (Fig. 1D). However, the proline contents in agb1-2 plants were significantly higher at 1 h (31% higher) and 3 h (47% higher) than those in WT under dehydration at the same time point (**P<0.01) (Fig. 1D). Moreover, the expression levels of two key regulators of proline biosynthesis, AtProDH and AtP5CS2, were increased in agb1-2 in comparison to WT plants after drought treatment (Fig. 1E and F), leading to proline accumulation and enhancement of drought resistance in agb1-2 lines.
AtMPK6 interacts with AGB1 in Y2H, pull-down, BiFC and CO-IP assays
To identify proteins interacting with AGB1 in regulating the ABA signaling pathway, we performed a yeast two-hybrid (Y2H) screening of the ABA-treated Arabidopsis cDNA library by using the full-length AGB1 as bait. Yeast cells with both AtMPK6 (GenBank accession: AEC10325; AT2G43790) and AGB1 grew on the selection medium, but not when either was absent, indicating that AtMPK6 interacts with AGB1 in yeast cells (Fig. 2A). The interaction between these two proteins was further confirmed using a BiFC assay based on split YFP [28]. The N- and C-terminal domains of YFP were fused to AGB1 and AtMPK6, respectively, and transiently co-expressed in protoplasts of Arabidopsis ecotype Col-0. Fluorescence from reconstituted YFP indicated that interaction between AGB1 and AtMPK6 occurred in the nucleus (Fig. 2B). Interaction of these two proteins was also identified by GST pull down assays. Recombinant GST-AGB1 and His-AtMPK6 fusion proteins were purified and co-incubated with GST-binding beads. Western blot analysis further confirmed the interaction between AGB1 and AtMPK6 in vitro (Fig. 2C).
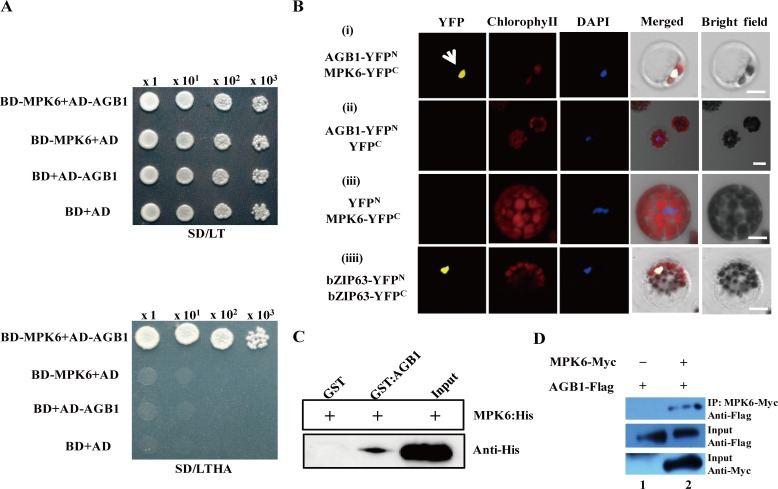
Figure 2. AtMPK6 interacted with AGB1 in Y2H, Pull-down, BiFC and Co-IP assays.
(A) Interaction tests using yeast two-hybrid assays between AGB1 and AtMPK6. Yeast cells with AGB1 (AD-AGB1) and AtMPK6 (BD-MPK6) were placed in different liquid concentrations on control medium SD/-Trp/-Leu (SD/LT) and selection medium SD/-Trp/-Leu/-His/-Ade (SD/LTHA). For negative controls, pGBKT7 without insert (BD alone), pGADT7 without insert (AD alone), and the empty vectors AD and BD were used. Experiments were performed three times and a representative result is shown. (B) AGB1 interacted with AtMPK6 by BiFC assays in Arabidopsis protoplast. The recombinant constructs AGB1-YFPN (YFP N-terminal) and MPK6-YFPC (YFP C-terminal) were co-transformed into protoplast cells of Arabidopsis WT (Col-0). For negative controls, pSPYNE without insert AGB1 (YFPN) (iii) and pSPYCE without insert MPK6 (YFPC) (ii) were used. The combination of bZIP63-YFPN and bZIP63-YFPC was used as a positive control (iiii), Fluorescence was recorded 20 h after transformation. Experiments were performed 10 times and a representative result is shown. Bars = 40 μm in (i), (iii) and (iiii), and 20 μm in (ii). (C) Interaction assay using pull-down analysis. AGB1 and AtMPK6 fused with GST and His tags, respectively were mixed and passed through a glutathione column (binding GST tag). After elution with pull-down binding buffer, samples were separated by SDS-PAGE, and His-MPK6 was detected by immunoblotting with anti-His antibody. GST alone was used as the negative control. Experiments were performed three times and a representative result is shown. (D) Co-IP assay of AGB1 with AtMPK6. AGB1-Flag and MPK6-Myc or AGB1-Flag and empty pCAMBIA1300 vector (contained Myc-tags) were transiently co-transformed into Arabidopsis protoplast. After 16 h, the total Arabidopsis cell lysates were prepared for Co-IP with anti-Myc agarose. Then, anti-Myc immunoprecipitates were subjected to Western blot analysis with anti-Flag antibody (top). Meanwhile, the total cell lysates were also subjected to Western blot analysis with anti-Flag (middle), and anti-Myc (bottom, for MPK6-Myc expression) antibodies. Experiments were performed three times and a representative result is shown.
The interaction between AGB1 and AtMPK6 in vivo was also verified by the Co-IP assay in Arabidopsis protoplasts. Both MPK6-Myc and AGB1-Flag constructs were transiently co-expressed in Arabidopsis protoplasts. The anti-Myc agarose beads were used to immunoprecipitate Myc-tag from the Arabidopsis cell lysates when co-expressed with MPK6-Myc and AGB1-Flag. Antibodies for Flag-tag were used to detect the AGB1-Flag fusion protein in immunoprecipitation products. Western blot analysis indicated that AGB1-Flag fusion protein was detected in the immunoprecipitated samples, but not detected in the control samples co-expressing the empty vector pCAMBIA1300 (contained Myc-tag only) and the recombination vector AGB1-Flag (compare lanes 1 and 2, top panel, Fig. 2D). This suggest that AtMPK6 could interact with AGB1 in Arabidopsis cells.
ABA treatment and mutation of AGB1 did not affect the subcellular localization of AtMPK6
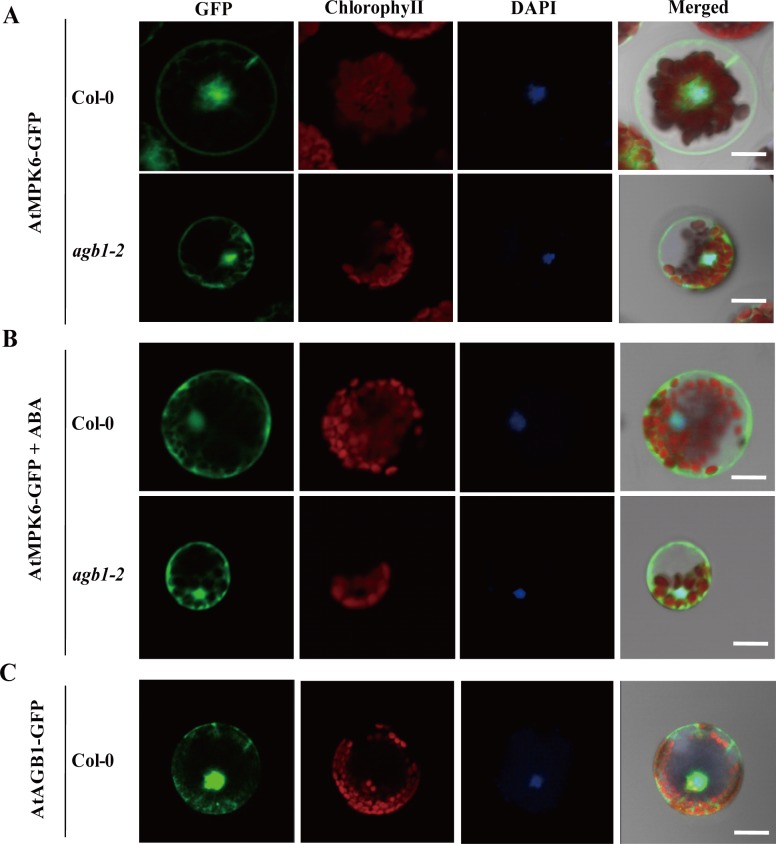
When transiently expressed in Arabidopsis protoplasts (ecotype Col-0), GFP-fused AtMPK6 (AtMPK6-GFP) was detected in the cytosol and nucleus. Similar results were obtained with agb1-2 (Fig. 3A). When the protoplasts of both the mutant and wild type lines were treated with ABA, the localization of AtMPK6-GFP showed no difference (Fig. 3B), indicating that subcellular localization of AtMPK6 was unaffected by ABA treatment or mutation in AGB1. Additionally, our observation showed that AGB1 is present in the plasma membrane and nucleus (Fig. 3C), and it is identical with previous reports [34–36], suggesting that AtMPK6 and AGB1 co-localization in the nucleus might provide a novel insight into the interaction shown above by BiFC (Fig. 2B).
Figure 3. Subcellular localization analysis of AtMPK6 and AtAGB1.
35S:AtMPK6-GFP was transiently expressed in protoplast cells of Arabidopsis strains WT (Col-0) and the agb1-2 mutant in (A) and (B). Subcellular localizations of AtMPK6-GFP were detected without ABA treatment (A) and with 10 μM ABA (B). Results were visualized by confocal microscopy. (C) Subcellular localization of AGB1. Each experiment was performed three times, and thirty cells were observed for each construct and a representative result is shown (Bars = 20 μm).
AtVIP1, AtMYB44 and AtMPK6 were up-regulated in the agb1-2 mutant after ABA or drought treatments
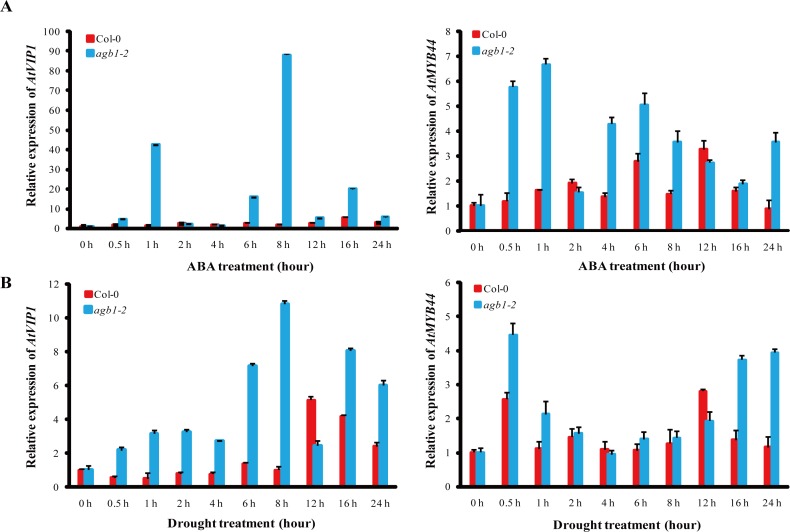
Both AtVIP1 and AtMYB44 can be phosphorylated by AtMPK6 [37,38]. Moreover, AtVIP1 regulates expression of transcription factor AtMYB44 [39] and overexpression of AtMYB44 results in increased sensitivity of seed germination to ABA and enhances drought tolerance [40]. Therefore, expression of AtVIP1 and AtMYB44 were compared in agb1-2 and WT plants subjected to ABA or drought treatments. Transcript levels of AtVIP1 in agb1-2 lines were significantly higher than those in WT, and reached nearly 35-fold and 51-fold at 1 h and 8 h, respectively, and that of AtMYB44 reached nearly 5-fold and 4-fold at 0.5 h and 1 h in agb1-2 lines under ABA treatment compared with WT (Fig. 4A). Except at 12 h, transcripts of AtVIP1 in agb1-2 lines were also higher than those in WT, and reached 10-fold at 8 h in agb1-2 relative to WT, and transcripts of AtMYB44 in agb1-2 lines under drought treatment were 2.7-fold and 3.5-fold compared with that of WT at 16 h and 24 h, respectively (Fig. 4B). On the other hand, transcripts of AtMPK6 in agb1-2 lines were significantly increased at 0.5 h and 1 h after ABA treatment compared to those in WT (S4 Fig.). These results indicated that AGB1 might negatively regulate the ABA signaling pathway in Arabidopsis by down-regulating expression of AtMPK6, AtVIP1 and AtMYB44 under ABA or drought treatments.
Figure 4. Expression of AtVIP1 and AtMYB44 in WT and agb1-2 after ABA and drought treatments.
(A) and (B) Expression analysis of AtVIP1 and AtMYB44 in WT and agb1-2 plants after ABA (A) and drought (B) treatments for 0, 0.5, 1, 2, 4, 6, 8, 12, 16, and 24 h. Results are means ± SD with three biological replicates.
A set of stress-responsive genes and ABA-biosynthesis genes were up-regulated in agb1-2 after drought treatment
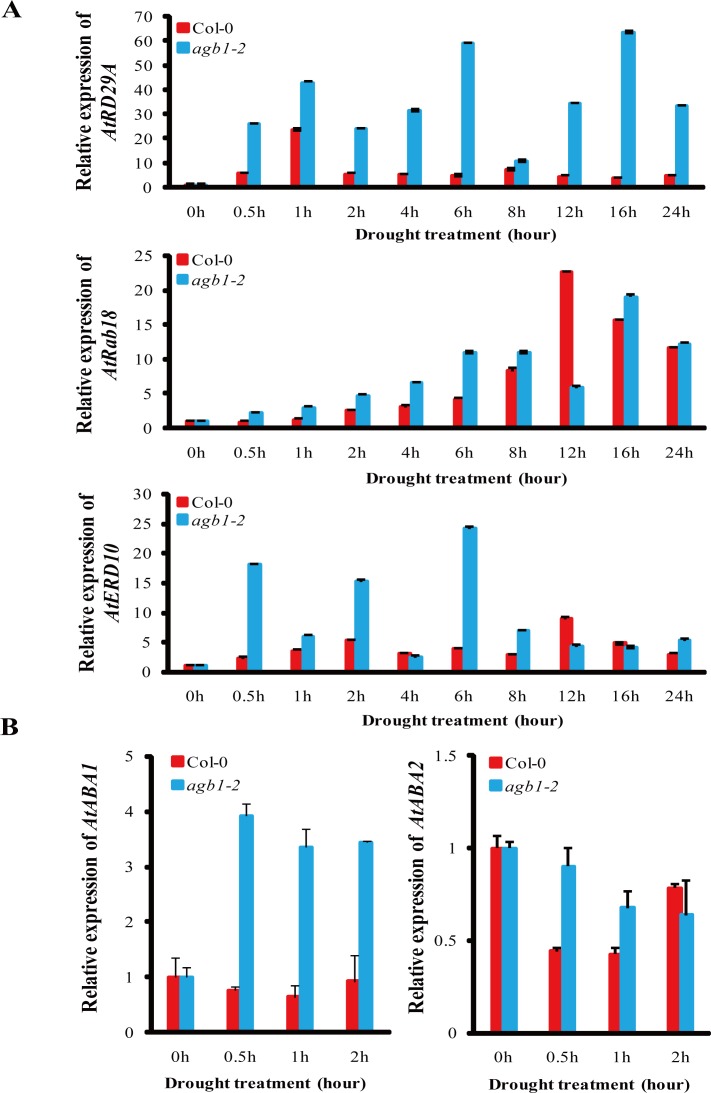
Real-time PCR was performed to analyze the expression of RD29A, a downstream gene of AtMYB44 [40], and stress-responsive genes RAB18 and ERD10 [41,42] in agb1-2 and WT plants. When subjected to dehydration stress treatments, a set of stress-responsive genes, such as RD29A, RAB18 and ERD10 in WT and agb1-2 were induced. For example, the expression of RD29A in agb1-2 was higher than those in WT, reaching nearly 17-fold at 16 h after drought treatment compared with WT (Fig. 5A). Except at 12 h, the transcript levels of RAB18 and ERD10 in agb1-2 were higher than those in WT, and the transcript levels of RAB18 reached nearly 3-fold and that of ERD10 nearly 6.5-fold higher at 6 h in agb1-2 compared with WT (Fig. 5A). ABA-biosynthesis genes, such as ABA1 and ABA2, were also up-regulated in agb1-2 after drought treatment, and the expression of ABA1 in agb1-2 reached nearly 5.2-fold compared with that of WT at 1 h (Fig. 5B). These results indicated that AGB1 negatively regulated the expression of a subset of drought-responsive genes involved in ABA signaling pathway, including RD29A, RAB18, ERD10, ABA1 and ABA2.
Figure 5. Expression of stress-responsive and ABA-biosynthesis genes in WT and agb1-2 plants after drought treatment.
(A) Expression of RD29A, RAB18, and ERD10 in WT and agb1-2 after drought treatment for 0, 0.5, 1, 2, 4, 6, 8, 12, 16, and 24 h. (B) Expression of ABA biosynthesis genes ABA1 and ABA2 in WT and agb1-2 after drought treatment for 0, 0.5, 1, and 2 h. Results are means ± SD with three biological replicates.
Transgenic 35S:AtVIP1 plants were hypersensitive to ABA treatment and enhanced drought tolerance
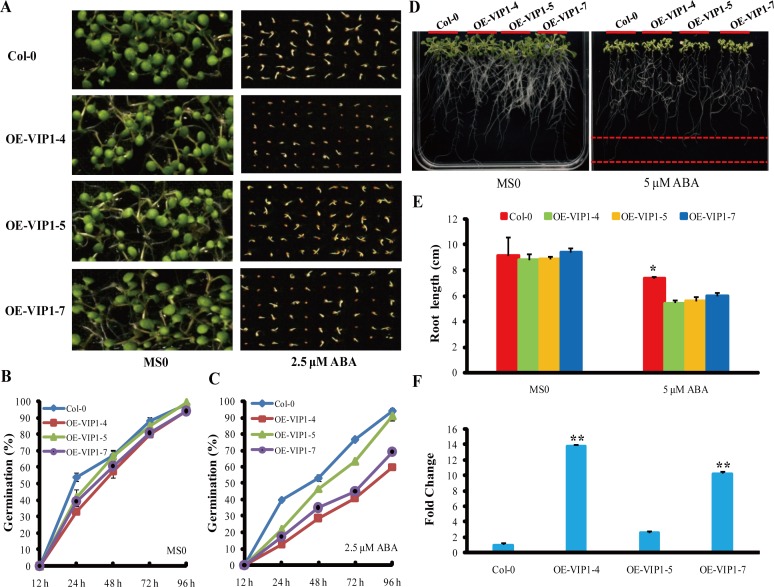
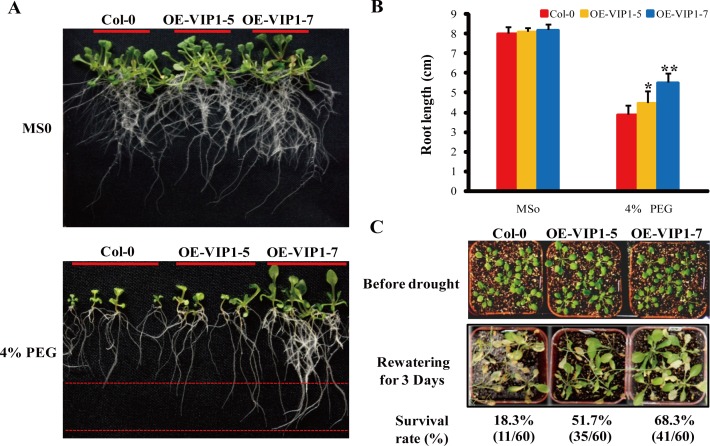
ABA functions to inhibit seed germination and seedling growth. Under normal conditions without ABA treatment, the 35S:AtVIP1 and WT lines showed similar germination rates (80–88%) at 72 h. However, the germination rates of 35S:AtVIP1 lines were 40–63% compared to 76% in WT at the same time point following treatment with 2.5 μM ABA (Fig. 6A, B and C). The root lengths of WT and 35S:AtVIP1 seedlings were likewise similar under normal conditions, but those of WT were significantly longer than 35S:AtVIP1 seedlings when grown on medium supplemented with 5 μM ABA (*P<0.05) (Fig. 6D and E). Germination rate of transgenic lines OE-VIP1–4 (40%) and OE-VIP1–7 (45%) were lower than that of line OE-VIP1–5 (63%) (Fig. 6C) after treatment with 2.5 μM ABA and the phenotypes of the transgenic lines were related to the expression levels of AtVIP1 (Fig. 6F), indicating that the expression level of the AtVIP1 transgene was positively related with sensitivity to ABA treatment in transgenic plants during germination and early seedling growth. With regard to osmotic stress, there were no differences between WT and 35S:AtVIP1 lines on MS medium, but the root lengths of WT seedlings were significantly shorter than those of 35S:AtVIP1 lines OE-VIP1–5 (P<0.05) and OE-VIP1–7 (P<0.01) when grown on MS medium contained 4% PEG (Fig. 7A and B). For plants grown in soil, 35S:AtVIP1 lines OE-VIP1–5 (51.7% survival) and OE-VIP1–7 (68.3% survival) retained better appearance and higher survival rates than WT (18.3% survival) at 3 d after re-watering following 21 d of water deprivation (Fig. 7C), suggesting that overexpression of AtVIP1 conferred drought tolerance in transgenic plants.
Figure 6. Overexpression of AtVIP1 inhibited seed germination and root length under ABA treatment.
Identically stored seeds were surface sterilized and washed extensively with water and plated on MS0 medium plates containing 3% Suc in the absence or presence of 2.5 μM ABA. Plates were kept at 4°C in darkness for 3 d and then transferred to growth chambers (16 h light/8 h darkness regime) at 22°C. (A) Seed germination analysis of WT and 35S:AtVIP1 transgenic plants containing OE-VIP1–4, -5 and -7 under normal conditions (MS0) and ABA treatment (MS0 plus 2.5 μM ABA). (B) and (C) Seed germination rates of different 35S:AtVIP1 transgenic lines under normal conditions (B), and 2.5 μM ABA treatment (C) at different time points. 35S:AtVIP1 transgenic lines displayed lower germination rates than WT. Values for each time point are means of three experiments, and each experiment comprised 60 plants. (D) Root growth WT and 35S:AtVIP1 transgenic plants under normal conditions (MS0) and 5 μM ABA treatment for 14 d. (E) Primary root lengths (cm) shown in (D) were measured 14 days after treatment. Values are means ± SD (n = 30) from three independent experiments. Asterisk indicates significant differences (Student’s t test,*P<0.05) between the Col-0 and 35S:AtVIP1 transgenic lines. (F) Relative expression analysis (fold change) of AtVIP1 in WT and transgenic lines. Expression of VIP1 in WT was normalized as 1. Results are means ± SD from three independent experiments, and asterisks indicate significant differences (Student’s t test,**P<0.01) between Col-0 and transgenic lines.
Figure 7. Drought tolerance analysis of 35S:AtVIP1 transgenic plants.
(A) Growth of WT and 35S:AtVIP1 transgenic lines containing OE-VIP1–5 and -7 under normal conditions (MS0) and drought stress (MS0 plus 4% PEG). (B) Primary root lengths (cm) were measured at 14 days after PEG treatment. Values are means ± SD (n = 30) from three independent experiments and asterisks indicate significant differences (Student’s t test,*P<0.05 or **P<0.01) between WT and 35S::AtVIP1 transgenic plants. (C) Drought tolerance assay of the WT and 35S::AtVIP1 transgenic lines in soil. WT plants and 35S:AtVIP1 transgenic lines (21 days old, before drought treatment) were withheld from watering for 21 days and then re-watered for 3 days. Values under the figures are survival rates. Three independent experiments were conducted.
Discussion
AGB1 negatively regulates the ABA response by down-regulating AtMPK6-AtVIP1-AtMYB44 pathway in Arabidopsis
The downstream effectors of G proteins and associated signaling pathways were not well-defined. AGB1 was previously considered to be a primary regulator of the G-protein complex in ABA signaling [7,11], whereas the regulatory mechanism of AGB1 in ABA responses in Arabidopsis remained unclear [11]. Recently, although several AGB1 interaction proteins in Arabidopsis have been identified in the G-signaling Interactome Database (AGIdb, http://bioinfolab.unl.edu/AGIdb), few protein kinases have been shown to interact with AGB1 [43,44]. In the present study, we used Y2H, Pull-down (in vitro), BiFC and Co-IP assay (in vivo) to demonstrate that AGB1 interacts with AtMPK6, a key protein kinase involved in stress responses (Fig. 2). These results established the link between G-proteins and the MAP-like kinase cascade. Protein kinases are major components in cellular signal transduction pathways, and mediate responses to various biotic and abiotic stresses, including hormone signaling pathways [45]. For example, AtMPK6 was transiently activated after ABA application and overexpression of AtMPK6 enhanced ABA response during germination. However, the atmpk6 mutant was insensitive to ABA during germination and under drought stress condition [27,46,47], suggesting that the interaction between MPK6 and AGB1 may play a role in ABA signaling. In addition, both AtVIP1 and AtMYB44 can be phosphorylated by AtMPK6 [37,38]. AtMYB44 is regulated by AtVIP1 [39], and the dehydration-responsive gene, RD29A, is a downstream target gene of AtMYB44 [40]. Furthermore, overexpression of AtMYB44 also enhanced ABA sensitivity and drought tolerance in Arabidopsis [40]. The evidence suggested that a signaling cascade that involves AtMPK6, AtVIP1, AtMYB44, and RD29A might be involved in the ABA signaling pathway in Arabidopsis. In this report, the agb1 mutants showed hypersensitivity to ABA (S2 Fig.), and enhanced drought tolerance (Fig. 1B and C). On the other hand, the target genes involved in this pathway were up-regulated in agb1-2 under ABA or drought treatments (Fig. 4, Fig. 5A and S4 Fig.). Overexpression of AtVIP1 enhanced the sensitivity of transgenic plants to ABA treatment (Fig. 6A-E). These results indicating that a novel role of AGB1 may be involved in transcriptional regulation through the AtMPK6, AtVIP1, AtMYB44, and RD29A cascade in Arabidopsis in response to ABA or dehydration.
With respect to the mechanism of AtMPK6 regulation by AGB1, it was reported that the activity of the kinase was regulated by affecting trafficking in plant cells [48]. However, the results showed that the subcellular localization of AtMPK6 was not affected in agb1-2 (Fig. 3A), suggesting that trafficking changes were not involved in the regulation of AtMPK6 by AGB1. On the other hand, although the activity of AtMPK6 is reported to be independent of ABA [49], other studies have suggested that AtMPK6 was transiently activated after ABA treatment in a AtMKK1-dependent manner in seedlings [27,46]. In the present study, the expression of AtMPK6 in the agb1-2 mutant was up-regulated after ABA application (S4 Fig.), implying that AGB1 might be involved in transcriptional regulation of AtMPK6 in response to ABA. Whilst, other components might be involved in response to ABA signaling through interaction between AGB1 and AtMPK6. Recently, AtVIP1 was shown to interact with AGB1 in Arabidopsis [50] and modulated the osmo-sensing of Arabidopsis plants [51], and AtVIP1 or AtMYB44 acts as a downstream gene that can be regulated by AtMPK6 [37,38]. Thus, AGB1 probably mediates the interaction of AtMPK6 and other components existing in the signaling complex in plant cells. This was due to WD40 repeats are thought to mediate protein-protein interactions, and AGB1 is one of these interaction proteins (http://smart.embl-heidelberg.de/). Additionally, some WD40 proteins in Arabidopsis are involved in protein degradation. Whilst, AGB1 interacts with an E3 ubiquitin ligase PUB20 and DDB1 [50,52,53]. So this does not rule out the possibility that the stability of AtMPK6 or its downstream substrates is regulated by AGB1. Previous reports showed that GPA1 interacted with PLDα1 and inhibited its activity by coupling with GDP [54]. ACI-reductone dioxygenase 1 (ARD1) interacted with AGB1, and was activated by AGB1 in vitro [15]. Although AGB1 did not affect the kinase activity of AtMPK6, and was not phosphorylated by AtMPK6 (data not shown), it will be interesting to examine whether AGB1 regulates the AtMPK6-dependent phosphorylation of MPK6 substrates.
In this study, we found that, except for RD29A, some stress-responsive genes such as RAB18 and ERD10 (Fig. 5A), ABA-biosynthesis genes containing ABA1 and ABA2 (Fig. 5B), and proline-biosynthesis genes including AtProDH and AtP5CS2 (Fig. 1E and F) in agb1-2 were significantly up-regulated after drought treatment, suggesting that in addition to the AtMPK6, AtVIP1, AtMYB44, and RD29A cascade, other factors might also be involved in ABA response and drought tolerance of plants regulated by AGB1. It was reported that Gβ interacts with MAPK-like protein, PsMPK3, and responds to ABA treatment in Pisum sativum [43]. As PsMPK3 is highly homologous with AtMPK3 and AtMPK6, two MAPKs that are key nodal and functionally redundant factors in the ABA signaling pathway [55], we hypothesized that AGB1 acts as a molecular switch in regulating the ABA signaling pathway in plants by interacting simultaneously with AtMPK3 and AtMPK6. However, further studies are necessary to confirm this hypothesis.
AGB1 negatively regulates a drought tolerance in Arabidopsis
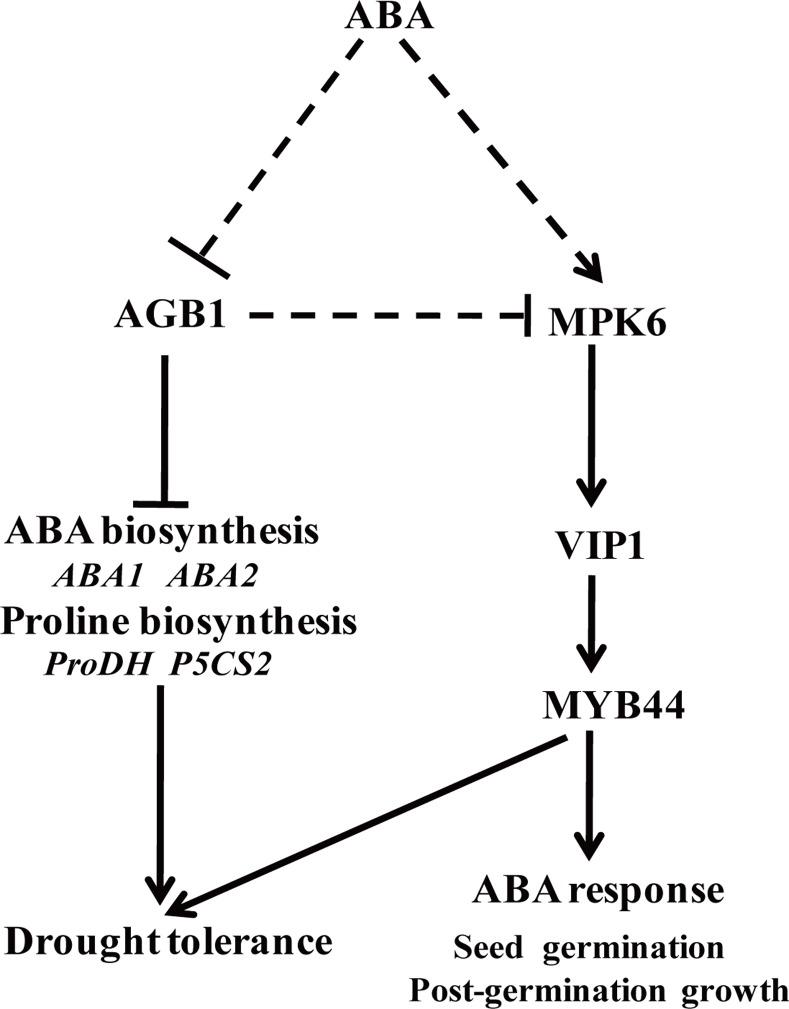
AGB1 not only affects the adaptability of plants to the environment, but also alters seed weight under water deficit [56]. However, the physiological and molecular mechanisms involved in the regulation of drought response are not completely understood. In this report, AGB1 was demonstrated to negatively affect drought tolerance in Arabidopsis (Fig. 1B, C and S3E Fig.). Stress-responsive genes RAB18 and ERD10 (Fig. 5A), ABA1 and ABA2 (Fig. 5B), and AtProDH and AtP5CS2 (Fig. 1E and 1F) in agb1-2 were significantly up-regulated after drought, along with proline accumulation in the agb1-2 mutant (Fig. 1D). It has been reported that overexpression of AtMPK6 increased expression of the ABA biosynthesis genes NCED3 and ABA2 [47], and overexpression of stress-responsive genes, including RD29A and RAB18, which resulted in enhanced dehydration tolerance in transgenic plants [57,58]. Accumulation of proline is an important indicator in determining salt/drought tolerance, and overproduction of proline led to increased tolerance against abiotic stress in transgenic tobacco plants [59]. In this study, overexpression of AtVIP1 enhanced drought tolerance of transgenic plants (Fig. 7A, B and C), indicating that inducible expression of stress-responsive genes contributed to enhanced drought tolerance in agb1-2. Moreover, we detected some cis-acting elements associated with abiotic stress responses, such as DRE (drought response element), LTRE (low temperature response element) and ABRE (ABA response element) in the promoter region of AGB1 using the database PLACE Signal Scan Search (S1 Table) [60], suggesting that AGB1 is indeed involved in drought responses in plants. Interestingly, AGB1 expression was down-regulated by ABA and drought treatment (Fig. 1A). Therefore, it was hypothesized that AGB1 negatively regulated expression of some ABA response and drought tolerance genes in wild type plants under normal conditions [40,61]. Whereas under stress conditions, expression of AGB1 was inhibited (Fig. 1A), thereby expression of ABA response genes and drought tolerance genes were activated (Fig. 4A, B and Fig. 5A, B), thus contributing to enhanced stress tolerance and better survival under drought conditions. In brief, AGB1 interacts with AtMPK6 (Fig. 2A-D), and regulates ABA response and drought tolerance by down-regulating the ABA responsive transcriptional factor AtMPK6 in Arabidopsis (Fig. 8).
Figure 8. A mode for AGB1 functions in ABA- and drought stress response through affecting AtMPK6-related pathway.
AGB1 negatively modulates ABA and drought response in Arabidopsis by down-regulating AtMPK6, AtVIP1 and AtMYB44 cascades and suppresses ABA biosynthesis and proline accumulation through regulating expression of ABA1, ABA2, ProDH and P5CS2. Positive effects are indicated by arrows and bars indicate repression.
Conclusions
Suppression of AtAGB1 enhanced ABA sensitivity and conferred drought tolerance in Arabidopsis, and AGB1 interacted with ABA-response protein kinase AtMPK6 in vivo. Moreover, the expression of three ABA responsive genes, AtMPK6, AtVIP1 (downstream gene of AtMPK6) and AtMYB44 (downstream gene of AtVIP1) were up-regulated in agb1-2 mutant, and overexpression AtVIP1 enhanced drought tolerance. All of these indicated that AGB1 regulated the AtMPK6-related pathway, but further studies are needed to determine the roles of AtMPK6 in AGB1-mediated intracellular signaling.
Supporting Information
(A) Positions of T-DNA insertion in agb1-2 mutant. Black boxes represent exons. The positions of T-DNA insertions in agb1-2 is indicated by arrowheads. Primer pairs used in genomic PCR and primer pairs used in RT-PCR to assess AGB1 transcripts are indicated. Annealing sites of the primers used in (B) and (C) are indicated by arrows. (B) Genomic PCR analyses verified homozygosity for the T-DNA alleles. (C) RT-PCR analysis of AGB1 transcript levels in agb1-2 mutant. Actin was used as an internal control.
(TIF)
Identically stored seeds were surface sterilized and washed extensively with water and plated on MS0 media plates containing 3% Suc in the absence or presence of 1.0 μM ABA. Plates were kept at 4°C in darkness for 3 d and then transferred to growth chambers (16 h light/8 h darkness regime) at 22°C. (A) and (B) Seed germination rates of wild-type (Col-0), agb1-1 and agb1-2 mutant lines under normal conditions (A), and 1.0 μM ABA treatment (B) at different time points. Values for each time point are means of three experiments, and each experiment comprised 80 plants. (C) Photographs of greening seedlings of the wild type and atagb1 grown on medium with or without 1.0 μM ABA after 21 d. (D) Cotyledon greening rates were calculated under normal condition and 1.0 μM ABA treatment for (C) experiment. Three experiments were performed with similar results. Values are means ± SD (n = 45). Asterisks indicate significant differences (Student’s t test,*P<0.05) between the Col-0 and atagb1 mutant lines.
(TIF)
Identically stored wild-type and agb1 mutants seeds were surface sterilized and washed extensively with water and plated on MS0 medium plates containing 3% Suc. Plates were kept at 4°C in darkness for 3 d and then transferred to growth chambers (16 h light/8 h darkness regime) at 22°C. Seeds were germinated on MS0 medium for 3 d, and then transferred to normal medium (A), MS0 medium plus 4% PEG (B) and 8% PEG (C), and MS0 medium plus 2.0 μM ABA (D) for 11 d. (E) The length of primary roots was measured at 11 d after transfer corresponding to (A, B, C). At least three experiments were done with similar results. Values presented are the mean ± SD (n = 20). Asterisks indicate a significant difference (Student’s t test, *p<0.05) between wild-type and agb1 mutants. (F) The length of primary roots was measured at 11 d after transfer corresponding to (D). Three experiments were done with similar results. Values presented are the mean ± SD (n = 20). Asterisks indicate a significant difference (Student’s t test, *p<0.05) between wild-type and agb1 mutants.
(TIF)
Expression patterns of AtMPK6 after 200 μM ABA treatment for 0, 0.5, and 1 h. The expression value of AtMPK6 at 0 h was normalized as 1 for WT and agb1-2. Results are means ± standard deviation (SD, n = 3) and asterisks indicate significant differences (Student’s t test,*P<0.05 or **P<0.01) between WT and agb1-2 at same time points.
(TIF)
(DOC)
(DOC)
Acknowledgments
We are grateful to Dr R.A. McIntosh (Plant Breeding Institute, University of Sydney, Australia), Dr. Lan-Qin Xia (Institute of Crop Sciences, Chinese Academy of Agricultural Sciences), Dr. Jose Fernandez (Plants and Crop Sciences Division, School of Biosciences at University of Nottingham), and Dr. Malcolm J Hawkesford (Plant Biology and Crop Science, Rothamsted Research) for critical review of this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Key Project for Research on Transgenic Biology (2013ZX08002-002) and Natural Science Foundation of China (31271715). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Temple BRS, Jones AM (2007) The plant heterotrimeric G-protein complex. Annu Rev Plant Biol 58: 249–266. 10.1146/annurev.arplant.58.032806.103827 [DOI] [PubMed] [Google Scholar]
- 2. Ma H, Yanofsky MF, Meyerowitz EM (1990) Molecular cloning and characterization of GPA1, a G protein alpha subunit gene from Arabidopsis thaliana. Proc Natl Acad Sci U S A 87: 3821–3825. 10.1073/pnas.87.10.3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weiss CA, Garnaat CW, Mukai K, Hu Y, Ma H (1994) Isolation of cDNAs encoding guanine nucleotide-binding protein beta-subunit homologues from maize (ZGB1) and Arabidopsis (AGB1). Proc Natl Acad Sci U S A 91: 9554–9558. 10.1073/pnas.91.20.9554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mason MG, Botella JR (2000) Completing the heterotrimer: isolation and characterization of an Arabidopsis thaliana G protein γ-subunit cDNA. Proc Natl Acad Sci U S A 97: 14784–14788. 10.1073/pnas.97.26.14784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chakravorty D, Trusov Y, Zhang W, Acharya BR, Sheahan MB, et al. (2011) An atypical heterotrimeric G-protein γ-subunit is involved in guard cell K+-channel regulation and morphological development in Arabidopsis thaliana. Plant J 67: 840–851. 10.1111/j.1365-313X.2011.04638.x [DOI] [PubMed] [Google Scholar]
- 6. Jones AM, Assmann SM (2004) Plants: the latest model system for G-protein research. EMBO Rep 5: 572–578. 10.1038/sj.embor.7400174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen J-G (2007) Heterotrimeric G-proteins in plant development. Frontiers in bioscience: a journal and virtual library 13: 3321–3333. 10.2741/2928 [DOI] [PubMed] [Google Scholar]
- 8. Ullah H, Chen J-G, Temple B, Boyes DC, Alonso JM, et al. (2003) The β-subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell 15: 393–409. 10.1105/tpc.006148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen J-G, Pandey S, Huang J, Alonso JM, Ecker JR, et al. (2004) GCR1 can act independently of heterotrimeric G-protein in response to brassinosteroids and gibberellins in Arabidopsis seed germination. Plant Physiol 135: 907–915. 10.1104/pp.104.038992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ullah H, Chen J-G, Wang S, Jones AM (2002) Role of a heterotrimeric G protein in regulation of Arabidopsis seed germination. Plant Physiol 129: 897–907. 10.1104/pp.005017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pandey S, Chen J-G, Jones AM, Assmann SM (2006) G-protein complex mutants are hypersensitive to abscisic acid regulation of germination and postgermination development. Plant Physiol 141: 243–256. 10.1104/pp.106.079038 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12. Booker KS, Schwarz J, Garrett MB, Jones AM (2010) Glucose attenuation of auxin-mediated bimodality in lateral root formation is partly coupled by the heterotrimeric G protein complex. PloS One 5: e12833 10.1371/journal.pone.0012833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang X-Q, Ullah H, Jones AM, Assmann SM (2001) G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 292: 2070–2072. 10.1126/science.1059046 [DOI] [PubMed] [Google Scholar]
- 14. Pierce KL, Premont RT, Lefkowitz RJ (2002) Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3: 639–650. 10.1038/nrm908 [DOI] [PubMed] [Google Scholar]
- 15. Friedman EJ, Wang HX, Jiang K, Perovic I, Deshpande A, et al. (2011) Acireductone dioxygenase 1 (ARD1) is an effector of the heterotrimeric G protein β subunit in Arabidopsis. J Biol Chem 286: 30107–30118. 10.1074/jbc.M111.227256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mudgil Y, Uhrig JF, Zhou J, Temple B, Jiang K, et al. (2009) Arabidopsis N-MYC DOWNREGULATED-LIKE1, a positive regulator of auxin transport in a G protein–mediated pathway. Plant Cell 21: 3591–3609. 10.1105/tpc.109.065557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang HX, Weerasinghe RR, Perdue TD, Cakmakci NG, Taylor JP, et al. (2006) A Golgi-localized hexose transporter is involved in heterotrimeric G protein-mediated early development in Arabidopsis. Mol Biol Cell 17: 4257–4269. 10.1091/mbc.E06-01-0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klopffleisch K, Phan N, Augustin K, Bayne RS, Booker KS, et al. (2011) Arabidopsis G-protein interactome reveals connections to cell wall carbohydrates and morphogenesis. Mol Syst Biol 7: 532 10.1038/msb.2011.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan J, Hill L, Crooks C, Doerner P, Lamb C (2009) Abscisic acid has a key role in modulating diverse plant-pathogen interactions. Plant physiol 150: 1750–1761. 10.1104/pp.109.137943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiong L, Zhu J-K (2003) Regulation of abscisic acid biosynthesis. Plant Physiol 13 3:29–36. 10.1104/pp.103.025395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61: 651–679. 10.1146/annurev-arplant-042809-112122 [DOI] [PubMed] [Google Scholar]
- 22. Finkelstein RR, Gampala SSL, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14: S15–S45. 10.1105/tpc.010441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lapik YR, Kaufman LS (2003) The Arabidopsis cupin domain protein AtPirin1 interacts with the G protein α-Subunit GPA1 and regulates seed germination and early seedling development. Plant Cell 15: 1578–1590. 10.1105/tpc.011890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perfus-Barbeoch L, Jones AM, Assmann SM (2004) Plant heterotrimeric G protein function: insights from Arabidopsis and rice mutants. Curr Opin Plant Biol 7: 719–731. 10.1016/j.pbi.2004.09.013 [DOI] [PubMed] [Google Scholar]
- 25. Pandey S, Nelson DC, Assmann SM (2009) Two novel GPCR-type G proteins are abscisic acid receptors in Arabidopsis. Cell 136: 136–148. 10.1016/j.cell.2008.12.026 [DOI] [PubMed] [Google Scholar]
- 26. Alvarez S, Roy Choudhury S, Hicks LM, Pandey S (2013) Quantitative proteomics-based analysis supports a significant role of GTG proteins in regulation of ABA response in Arabidopsis roots. J Proteome Res 12: 1487–1501. 10.1021/pr301159u [DOI] [PubMed] [Google Scholar]
- 27. Ichimura K, Mizoguchi T, Yoshida R, Yuasa T, Shinozaki K (2000) Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J 24: 655–665. 10.1046/j.1365-313x.2000.00913.x [DOI] [PubMed] [Google Scholar]
- 28. Walter M, Chaban C, Schütze K, Batistic O, Weckermann K, et al. (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40: 428–438. 10.1111/j.1365-313X.2004.02219.x [DOI] [PubMed] [Google Scholar]
- 29. Ludwig AA, Saitoh H, Felix G, Freymark G, Miersch O, et al. (2005) Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proc Natl Acad Sci U S A 102: 10736–10741. 10.1073/pnas.0502954102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743. 10.1046/j.1365-313x.1998.00343.x [DOI] [PubMed] [Google Scholar]
- 31. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25: 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 32. Shi H, Wang Y, Cheng Z, Ye T, Chan Z (2012) Analysis of natural variation in bermudagrass (Cynodon dactylon) reveals physiological responses underlying drought tolerance. PLoS One 7: e53422 10.1371/journal.pone.0053422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shi H, Ye T, Chen F, Cheng Z, Wang Y, et al. (2013) Manipulation of arginase expression modulates abiotic stress tolerance in Arabidopsis: effect on arginine metabolism and ROS accumulation. J Exp Bot 64: 1367–1379. 10.1093/jxb/ers400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kansup J, Tsugama D, Liu S, Takano T (2013) The Arabidopsis adaptor protein AP-3μ interacts with the G-protein β subunit AGB1 and is involved in abscisic acid regulation of germination and post-germination development. J Exp Bot 64: 5611–5621. 10.1093/jxb/ert327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anderson D, Botella J (2007) Expression analysis and subcellular localization of the Arabidopsis thaliana G-protein β-subunit AGB1. Plant Cell Rep 26: 1469–1480. 10.1007/s00299-007-0356-1 [DOI] [PubMed] [Google Scholar]
- 36. Tsugama D, Liu H, Liu S, Takano T (2012) Arabidopsis heterotrimeric G protein β subunit interacts with a plasma membrane 2C-type protein phosphatase, PP2C52. Biochim Biophys Acta 1823: 2254–2260. 10.1016/j.bbamcr.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 37. Nguyen XC, Hoang MHT, Kim HS, Lee K, Liu X-M, et al. (2012) Phosphorylation of the transcriptional regulator MYB44 by mitogen activated protein kinase regulates Arabidopsis seed germination. Biochem Biophys Res Commun 423: 703–708. 10.1016/j.bbrc.2012.06.019 [DOI] [PubMed] [Google Scholar]
- 38. Pitzschke A, Djamei A, Teige M, Hirt H (2009) VIP1 response elements mediate mitogen-activated protein kinase 3-induced stress gene expression. Proc Natl Acad Sci U S A 106: 18414–18419. 10.1073/pnas.0905599106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pitzschke A, Hirt H (2010) Mechanism of MAPK-targeted gene expression unraveled in plants. Cell Cycle 9: 18–19. 10.4161/cc.9.1.10329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jung C, Seo JS, Han SW, Koo YJ, Kim CH, et al. (2008) Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol 146: 623–635. 10.1104/pp.107.110981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamaguchi-Shinozaki K, Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6: 251–264. 10.1105/tpc.6.2.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang JF, Yuan LJ, Shao Y, Du W, Yan DW, et al. (2008) The disturbance of small RNA pathways enhanced abscisic acid response and multiple stress responses in Arabidopsis. Plant Cell Environ 31: 562–574. 10.1111/j.1365-3040.2008.01786.x [DOI] [PubMed] [Google Scholar]
- 43. Bhardwaj D, Sheikh AH, Sinha AK, Tuteja N (2011) Stress induced beta subunit of heterotrimeric G-proteins from Pisum sativum interacts with mitogen activated protein kinase. Plant Signal Behav 6: 287–292. 10.4161/psb.6.2.14971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsugama D, Liu S, Takano T (2013) Arabidopsis heterotrimeric G protein β subunit, AGB1, regulates brassinosteroid signalling independently of BZR1. J Exp Bot 64: 3213–3223. 10.1093/jxb/ert159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mishra NS, Tuteja R, Tuteja N (2006) Signaling through MAP kinase networks in plants. Arch Biochem Biophys 452: 55–68. 10.1016/j.abb.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 46. Xing Y, Jia W, Zhang J (2008) AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J 54: 440–451. 10.1111/j.1365-313X.2008.03433.x [DOI] [PubMed] [Google Scholar]
- 47. Xing Y, Jia W, Zhang J (2009) AtMKK1 and AtMPK6 are involved in abscisic acid and sugar signaling in Arabidopsis seed germination. Plant Mol Biol 70: 725–736. 10.1007/s11103-009-9503-0 [DOI] [PubMed] [Google Scholar]
- 48. Rodriguez M, Petersen M, Mundy J (2010) Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol 61: 621–649. 10.1146/annurev-arplant-042809-112252 [DOI] [PubMed] [Google Scholar]
- 49. Tsugama D, Liu S, Takano T (2012) Drought-induced activation and rehydration-induced inactivation of MPK6 in Arabidopsis. Biochem Biophys Res Commun 426: 626–629. 10.1016/j.bbrc.2012.08.141 [DOI] [PubMed] [Google Scholar]
- 50. Tsugama D, Liu S, Takano T (2013) A bZIP protein, VIP1, interacts with Arabidopsis heterotrimeric G protein β subunit, AGB1. Plant Physiol Biochem 71: 240–246. 10.1016/j.plaphy.2013.07.024 [DOI] [PubMed] [Google Scholar]
- 51. Tsugama D, Liu S, Takano T (2012) A bZIP protein, VIP1, is a regulator of osmosensory signaling in Arabidopsis. Plant Physiol 159: 144–155. 10.1104/pp.112.197020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kobayashi S, Tsugama D, Liu S, Takano T (2012) A U-Box E3 ubiquitin ligase, PUB20, interacts with the Arabidopsis G-protein β subunit, AGB1. PLoS One 7: e49207 10.1371/journal.pone.0049207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee J-H, Terzaghi W, Gusmaroli G, Charron J-BF, Yoon H-J, et al. (2008) Characterization of Arabidopsis and Rice DWD proteins and their roles as substrate receptors for CUL4-RING E3 ubiquitin ligases. Plant Cell 20: 152–167. 10.1105/tpc.107.055418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhao J, Wang X (2004) Arabidopsis phospholipase Dα1 interacts with the heterotrimeric G-protein α-subunit through a motif analogous to the DRY motif in G-protein-coupled receptors. J Biol Chem 279: 1794–1800. 10.1074/jbc.M309529200 [DOI] [PubMed] [Google Scholar]
- 55. Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S (2007) Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 19: 63–73. 10.1105/tpc.106.048298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nilson SE, Assmann SM (2010) Heterotrimeric G proteins regulate reproductive trait plasticity in response to water availability. New Phytol 185: 734–746. 10.1111/j.1469-8137.2009.03120.x [DOI] [PubMed] [Google Scholar]
- 57. Lång V, Palva ET (1992) The expression of a rab-related gene, rab18, is induced by abscisic acid during the cold acclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mol Biol 20: 951–962. 10.1007/BF00027165 [DOI] [PubMed] [Google Scholar]
- 58. Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57: 781–803. 10.1146/annurev.arplant.57.032905.105444 [DOI] [PubMed] [Google Scholar]
- 59. Kishor PK, Hong Z, Miao G-H, Hu C-AA, Verma DPS (1995) Overexpression of [delta]-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108: 1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27: 297–300. 10.1093/nar/27.1.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mizoi J, Ohori T, Moriwaki T, Kidokoro S, Todaka D, et al. (2013) GmDREB2A; 2, a canonical dehydration-responsive element-binding protein2-type transcription factor in soybean, is posttranslationally regulated and mediates dehydration-responsive element-dependent gene expression. Plant Physiol 161: 346–361. 10.1104/pp.112.204875 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Positions of T-DNA insertion in agb1-2 mutant. Black boxes represent exons. The positions of T-DNA insertions in agb1-2 is indicated by arrowheads. Primer pairs used in genomic PCR and primer pairs used in RT-PCR to assess AGB1 transcripts are indicated. Annealing sites of the primers used in (B) and (C) are indicated by arrows. (B) Genomic PCR analyses verified homozygosity for the T-DNA alleles. (C) RT-PCR analysis of AGB1 transcript levels in agb1-2 mutant. Actin was used as an internal control.
(TIF)
Identically stored seeds were surface sterilized and washed extensively with water and plated on MS0 media plates containing 3% Suc in the absence or presence of 1.0 μM ABA. Plates were kept at 4°C in darkness for 3 d and then transferred to growth chambers (16 h light/8 h darkness regime) at 22°C. (A) and (B) Seed germination rates of wild-type (Col-0), agb1-1 and agb1-2 mutant lines under normal conditions (A), and 1.0 μM ABA treatment (B) at different time points. Values for each time point are means of three experiments, and each experiment comprised 80 plants. (C) Photographs of greening seedlings of the wild type and atagb1 grown on medium with or without 1.0 μM ABA after 21 d. (D) Cotyledon greening rates were calculated under normal condition and 1.0 μM ABA treatment for (C) experiment. Three experiments were performed with similar results. Values are means ± SD (n = 45). Asterisks indicate significant differences (Student’s t test,*P<0.05) between the Col-0 and atagb1 mutant lines.
(TIF)
Identically stored wild-type and agb1 mutants seeds were surface sterilized and washed extensively with water and plated on MS0 medium plates containing 3% Suc. Plates were kept at 4°C in darkness for 3 d and then transferred to growth chambers (16 h light/8 h darkness regime) at 22°C. Seeds were germinated on MS0 medium for 3 d, and then transferred to normal medium (A), MS0 medium plus 4% PEG (B) and 8% PEG (C), and MS0 medium plus 2.0 μM ABA (D) for 11 d. (E) The length of primary roots was measured at 11 d after transfer corresponding to (A, B, C). At least three experiments were done with similar results. Values presented are the mean ± SD (n = 20). Asterisks indicate a significant difference (Student’s t test, *p<0.05) between wild-type and agb1 mutants. (F) The length of primary roots was measured at 11 d after transfer corresponding to (D). Three experiments were done with similar results. Values presented are the mean ± SD (n = 20). Asterisks indicate a significant difference (Student’s t test, *p<0.05) between wild-type and agb1 mutants.
(TIF)
Expression patterns of AtMPK6 after 200 μM ABA treatment for 0, 0.5, and 1 h. The expression value of AtMPK6 at 0 h was normalized as 1 for WT and agb1-2. Results are means ± standard deviation (SD, n = 3) and asterisks indicate significant differences (Student’s t test,*P<0.05 or **P<0.01) between WT and agb1-2 at same time points.
(TIF)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.