Abstract
Entomopathogenic nematodes (EPNs) are small worms whose ecological behaviour consists to invade, kill insects and feed on their cadavers thanks to a species-specific symbiotic bacterium belonging to any of the genera Xenorhabdus or Photorhabdus hosted in the gastro-intestinal tract of EPNs. The symbiont provides a number of biological functions that are essential for its EPN host including the production of entomotoxins, of enzymes able to degrade the insect constitutive macromolecules and of antimicrobial compounds able to prevent the growth of competitors in the insect cadaver. The question addressed in this study was to investigate whether a mammalian pathogen taxonomically related to Xenorhabdus was able to substitute for or “hijack” the symbiotic relationship associating Xenorhabdus and Steinernema EPNs. To deal with this question, a laboratory experimental model was developed consisting in Galleria mellonella insect larvae, Steinernema EPNs with or without their natural Xenorhabdus symbiont and Yersinia pseudotuberculosis brought artificially either in the gut of EPNs or in the haemocoel of the insect larva prior to infection. The developed model demonstrated the capacity of EPNs to act as an efficient reservoir ensuring exponential multiplication, maintenance and dissemination of Y. pseudotuberculosis.
Introduction
Entomopathogenic nematodes (EPNs) are microscopic soil worms exclusively feeding on insect preys. They have the ability to cause death in a huge variety of insects, making them powerful candidate biopesticides in agriculture and horticulture [1,2]. EPNs owe their insecticidal properties to symbiotic bacteria belonging to two genera of Enterobacteriaceae, namely Xenorhabdus and Photorhabdus. These bacteria are hosted in the gastro-intestinal tract of the nematode–located in an intestinal vesicle in the case of Xenorhabdus [3] – at the infectious free-living stage, called infective juveniles (IJs). Upon invasion of an insect prey, the symbiotic bacteria are expelled from the IJ’s digestive tract. These bacteria multiply in the insect haemocoel and release insecticidal toxins as well as degradative enzymes able to digest the insect macromolecules, thereby feeding their EPN partners which mature to the adult stage through 4 larval stages named J1 to J4 and undergo several reproduction cycles [4]. Moreover, the symbiont prevents microbial competitors growth inside the insect’s cadaver by releasing antibiotic and antifungal compounds [5]. After all insect macromolecules have been exhausted, a few symbiotic bacteria enter the intestinal tract of the mature IJs just before they emerge from the dead larva and seek another prey [6,7].
While a few EPNs are generally sufficient to kill an insect prey, up to half a million of IJs can emerge from a single infected host upon completion of their reproductive life cycle inside the insect cadaver [8]. Each of these freshly emerged IJs is able to infect a new insect prey. IJs can survive in the soil for several months thanks to their protective cuticle and a huge lipid supply they can store [9].
In 2008 Heermann and Fuchs have shown that Photorhabdus luminescens, the bacterial symbiont of Heterorhabditis bacteriophora, shares a number of unique genes with the taxonomically related, yet ecologically different, Yersinia enterocolitica [10]. The shared genes are for some of them clustered in so-called “High Pathogenicity Islands” described in Enterobacteriaceae including Yersinia [11]. Many of these genes are either involved in pathogenicity toward insects, like the insecticidal toxin complex (Tc) [12], or in colonisation of eukaryotic cells, like the YplA phospholipase [13]. The recently discovered type 6 secretion system (T6SS) involved in toxin secretion and in mutualism between bacteria [14] is also conserved between P. luminescens and Y. enterocolitica [10]. Unlike P. luminescens which can cause casual infection in humans [15], Y. enterocolitica as well as Y. pseudotuberculosis are mammalian pathogens causing gastro-intestinal diseases in infected hosts. These two Yersiniae are regularly isolated from meat–especially pork meat–and root vegetables [16,17]. However, they have also been found in the gut lumen of adult flies and fly larvae, suggesting that they can use insects as passive vectors [18,19,20]. In addition, in vitro experiments have shown that both Y. enterocolitica and Y. pseudotuberculosis are able to colonize insect cells [21] and even to kill insect larvae like Galleria mellonella [22]. It is well known that Yersinia pestis, the third mammalian pathogenic Yersinia and etiological agent of plague, is able to colonize insects since it uses fleas as vectors. Hinnebusch et al. demonstrated the essential implication of the Yersinia murine toxin (Ymt) in flea colonisation [23]. Interestingly, it has been suggested that Y. pestis acquired ymt gene from P. luminescens or from a close relative [24]. Moreover, Photorhabdus asymbiotica which can infect either insects or humans, possesses a plasmid related to pMT-1 found in Y. pestis [25].
Besides their animal hosts, Y. enterocolitica as well as Y. pseudotuberculosis are commonly found in water, soil and vegetables [16,17,26]. Several studies have shown that Y. pestis can also be found in soil [27,28]. Moreover, several experiments have highlighted the survival of Y. enterocolitica, Y. pseudotuberculosis and Y. pestis in free living soil amoeba [29,30]. Since pathogenic Yersiniae are able to persist in soil and are phylogenetically very close to the bacterial symbionts of EPNs, we wondered whether Yersiniae would be able to intrude the symbiotic relationship associating EPNs and their natural symbiont. In order to test this hypothesis, we used an experimental model consisting of insect larvae of the species Galleria mellonella used as prey for an African species of entomopathogenic Steinernema hosting its natural Xenorhabdus symbiont as well as a Y. pseudotuberculosis field isolate naturally resistant to the anti-microbial compounds produced by Xenorhabdus. We show that Y. pseudotuberculosis can be successfully transmitted by the EPN carrier inside an insect larva in which it persists and multiplies. Moreover, EPNs emerging from the insect cadaver after 10 to 15 days where found to host large numbers of Y. pseudotuberculosis cells in their gastro-intestinal tract. These EPNs were in turn able to transmit Y. pseudotuberculosis to a new insect larva and so on for at least 7 successive infectious cycles (14 weeks). If they turn out to have an ecological significance, these findings may reveal an unexpected biotic reservoir for the long-term persistence and dissemination of pathogenic Yersiniae in the environment.
Material and Methods
Bacterial strains, plasmids and growth conditions
Enterobacteriaceae were grown in LB liquid broth with strong agitation (150rpm) or on LB agar or McConkey agar plates. The NBTA plates (nutrient agar supplemented with 25 mg l−1 bromothymol blue and 40 mg l−1 triphenyltetrazolium chloride) [31] were also used to check the phase I of the Xenorhabdus species used in this study. The incubation temperature was 37°C except for Yersiniae and Xenorhabdus sp. which were grown at 28°C. Antibiotics were added at the following concentrations: Kanamycin (Km): 30μg ml−1, Nalidixic acid (Nal): 25μg ml−1, Streptomycin (Sm): 50μg ml−1; Ampicillin (Ap): 100μg ml−1. Nalidixic-acid resistant (NalR) bacteria were obtained in three consecutive steps by plating 107 to 109 CFU per agar plate supplemented with increasing concentrations of Nalidixic acid (5μg/ml; 20μg/ml; 50μg/ml). Bacterial strains used in this study are listed in Table 1.
Table 1. List of bacterial strains used in this study.
| Strains | Origin (Reference) | Description |
|---|---|---|
| Escherichia coli S17-1λPir | NCCB * (Simon, R. et al., Biotechnol. (1983) 1: 784–791, McFarlane, G.J.B. et al, J. Microbiol. Methods (1987) 6: 301–305)[51,52] | λ lysogenic S17-1 derivative expressing the π protein required for replication of plasmids carrying oriR6K; SmR |
| E. coli 17WP | This work (de Lorenzo, V et al., J. Bacteriol. (1990) 172(11):6568–72)[53] | E. coli S17-1 λPir carrying pUT-miniTn5-gfpmut2, a transposon delivery suicide vector. GFP mini-transposon delivery strain; SmR/ApR/KmR |
| E. coli SM10 λPir | (Miller & Mekalanos, J. Bacteriol. (1988) 170(6):2575–83)[34] | λ lysogenic E. coli derivative expressing the π protein required for replication of plasmids carrying oriR6K; KmR |
| E. coli 10WP | This work | E. coli SM10 λPir carrying mCherry flanked by gfp-mut2 moieties and cloned into pKNG101. Fluorescence cassette replacement vector termed pSGCG; KmR, SmR, sacBR + |
| E. coli strain EC26-KH-2010 | VAR§ | Vero-toxigenic Escherichia coli of the O157 serogroup isolated from a Belgian calf in 2010 |
| E. coli strain VT02 | This work | Nalidixic-acid resistant mutant of EC26-KH-2010 |
| E. coli strain VT03 | This work | VT02 carrying a randomly inserted Gfpmut2-transposon; NalR/KmR |
| Xenorhabdus sp. strain TZ01 | Anne Laudisoit, This work (Mwaitulo et al., Int. J. Trop. Insect Sci. (2011) 26(4):214–226)[37] | Symbiotic bacterium retrieved from Steinernema tanzaniensis nematodes isolated from Tanzanian soil |
| X. sp. strain TZ02 | This work | Nalidixic-acid resistant mutant of TZ01 |
| X. sp. strain TZ03 | This work | TZ02 carrying a randomly inserted Gfpmut2-transposon; NalR/KmR |
| Yersinia pseudotuberculosis strain IP2777 | Institut Pasteur Lille (Derbise et al., J. Infect. Dis. (2013) 207(10):1535–43)[54] | Human clinical isolate, serotype O1 |
| Y. pseudotuberculosis strain 2008/04429 | VAR§ | Isolated from a rabbit cadaver (Belgium) |
| Y. pseudotuberculosis strain 4N1 | This work | Nalidixic acid-resistant mutant of 2008/04429 |
| Y. pseudotuberculosis strain 4N1G | This work | 2008/00429 4N1 carrying a Gfpmut2-transposon inserted in the fimbrial A protein A gene (see M&M); NalR/KmR |
| Y. pseudotuberculosis strain 4N1C | This work | 4N1G with mcherry replacing gfpmut2 in the mini-transposon following allelic exchange using pSGCG. |
| Y. enterocolitica strain VAR08/02 | VAR§ | Pig Isolate belonging to serotype O3 |
| Y. enterocolitica strain YE02 | This work | Nalidixic-acid resistant mutant of VAR08/02 |
| Y. enterocolitica strain YE03 | This work | YE02 carrying a randomly inserted Gfpmut2-transposon; NalR/KmR |
| Salmonella enterica subsp. enterica sv. Enteritidis strain 2011/03561 | VAR§ | Field isolate of poultry origin (Belgium) |
| S.Enteritidis strain SE02 | This work | Nalidixic-acid resistant mutant of 2011/03561 |
| S.Enteritidis strain SE03 | This work | SE02 carrying a randomly inserted Gfpmut2-transposon; NalR/KmR |
| Serratia marcescens strain EE016 | This work | Isolated from a Steinernema sp. MW8B-infected G. melonella larva |
| Ochrobactrum tritici strain EE10.1 | This work | Isolated from a Steinernema sp. MW8B-infected G. melonella larva |
* NCCB, The Netherlands Culture Collection of Bacteria, Utrecht, The Netherlands.
§ VAR, Veterinary and Agrochemical Research Center, Brussels, BELGIUM.
Some Enterobacteria, listed in table 1, were fluorescently labelled with GFP-mut2 [32] using a mini-Tn5 transposon [33]. Mini-transposon labelling was conducted by conjugating a nalidixic-acid resistant variant of the target bacterium with E. coli S17/1 λ pir hosting a transposon delivery suicide vector [34]. Transconjugants were isolated on selective agar plates and tested for GFP fluorescence. Integration of gfp-mut2 was further confirmed by PCR with primers mut2-GFP_F (GGG ATC TTT CGA AAG GGC AGA TTG TGT GG) and mut2-GFP_R (GGA GAG GGT GAA GGT GAT GCA ACA TAC GG). The size of the amplified fragment was 543 bp. For dual labelling experiments, the gfp-mut2 gene of Y. pseudotuberculosis 4N1G was substituted for mCherry, encoding a red-fluorescent protein, by allelic exchange. The replacement cassette consisted in mCherry flanked by the beginning and the end of the gfp-mut2 nucleotide sequence. The upstream and downstream flanking parts consisted of 244 and 223 base pairs of gfp-mut2, respectively, obtained by PCR amplification. A ribosome binding site was added upstream of the mCherry open reading frame to ensure optimal translation. The replacement cassette was cloned into the mobilizable suicide vector pKNG101, which confers resistance to streptomycin and carries the counter-selectable marker sacBR [35]. The recombinant suicide plasmid termed pSGCG was then transferred to Y. pseudotuberculosis 4N1G from the conjugative strain E. coli SM10λpir. Allelic exchange was conducted in two steps. Initial integration of pSGCG was first selected on specific agar plates containing streptomycin (100μg ml−1). After purification of the recombinant strain, allelic exchange was selected on agar plates containing sucrose (100μg ml−1) and recombinant colonies expressing mCherry but not GFP were controlled by both epifluorescence microscopy and PCR.
Mini-Tn5 transposon insertion mapping
The mini-Tn5 transposon used here to tag the Yersinia pseudotuberculosis derivatives 4N1G and 4N1C was mapped by TAIL-PCR using the method of Liu and Wittier [36] and by sequencing the amplified fragment. The mini-Tn5-gfp was found inserted in the chromosome at codon 60 of the fimbrial A protein gene in the same transcriptional orientation (ORF YPK_0694 as described in the annotated genome of Y. pseudotuberculosis YPIII, Accession number NC_010465.1). The transposon-specific primers used for TAIL-PCR were the following: SP1: CGC GAA AGT AGT GAC AAG TGT TGG CCA TGG; SP2: GTA TAA CAT GTC TTA TAC GCC CGT GTC AAC C; SP3: AGA TCC CCG GGT ACC GAG CTC GAA TTC GCG. The arbitrary degenerated primers used here were the same described by Liu and Whittier [36]. Final confirmation of the insertion point of the mini-Tn5 transposon was obtained by amplifying chromosomal fragments covering part of the transposon and part of the fimbrial A protein gene using the PCR primers SP1, SP2 or SP3 together with the fimbrial-specific primer CCG GTT CTA TCA TTG AAG CAC CTT GTT C.
Growth and maintenance of nematodes
Steinernema sp. MW8B isolated from Tanzanian soil [37] was used as model nematode allover the experiments. Steinernema sp. MW8B is symbiotically associated with Xenorhabdus sp. strain TZ01. Nucleotide sequence of the TZ01 16S ribosomal RNA gene (GenBank accession JQ687358.1) is equally similar, though not 100% identical, to that of X. ehlersii, X. budapestensis, X. griffiniae and X. kozodoii. Nematode stocks (Infective juvenile stage) were maintained by successive passages through the last larval stage of the greater wax moth, Galleria mellonella. Infection of the larvae was conducted by incubating 500 to 1000 Steinernema sp. MW8B IJs suspended in 1ml physiological water (NaCl 9g L−1) with four to six larvae confined in a closed Petri dish. Upon emergence from the dead larvae which occurred after 10 ± 2 days later, IJs were collected and stored at room temperature in physiological water.
Galleria mellonella in vitro infection model
For the first infection cycle, 6 G. mellonella larvae were injected with 106 CFU of the studied bacterium (Yersinia sp., GFP-labelled or not) using sterile 1-ml syringes bearing 0.3 × 13mm needles (Becton Dickinson). Injection was performed on the side of the larvae at the basis of the 8th segment. After incubation with ±750 Steinernema sp. MW8B IJs (125 IJs/larva) associated with their natural symbiont Xenorhabdus sp. TZ01, G. mellonella larvae died at day 1 or 2 post-infection and new IJs, named IJs1, emerged at day 10 ± 2. IJs1 were collected and washed thrice with physiological water prior to a new infection cycle started by transferring these IJs1 to plates containing naive (Yersinia-free) G. mellonella larvae. Ten days later, a new generation of IJs, named IJs2, emerged from the dead larva and so on for up to 7 consecutive infection cycles. The Xenorhabdus symbiont remained associated with Steinernema sp. MW8B throughout all infection cycles.
Gnotoxenic EPN engineering
Axenic EPNs were obtained by manually collecting eggs from gravid Steinernema sp. MW8B females recovered from infected G. mellonella larvae prior to the term of the infectious cycle. Such axenic eggs (min 3,000) were surface sterilized with a fresh sterilization solution obtained by diluting 1ml of a 15% NaClO solution and 1ml of a 4M NaOH solution in 10ml of distilled water. The sterile axenic eggs were then suspended in YS liquid medium at 25°C during 3 days and checked for J1 larval stage development. YS medium was prepared by dissolving the following components in 1L of distilled water: 5g yeast extract (Oxoid, Basingstoke, United Kingdom); 5g NaCl (Merck, Darmstadt, Germany); 0.5g NH4H2PO4 (Merck); 0.5g K2HPO4 (Merck); and 0.2g MgSO4.7H2O (Merck). If no contaminants were present, IJ1 were deposited onto a Wouts agar plate [38] that had been freshly inoculated with 108 CFU of the target bacterium in the absence of selective antibiotics. In the following days, EPNs matured to the adult stage and completed their reproductive cycle. After one week, monoxenic EPNs (IJ stage) were collected in physiological water and stored for later Galleria infection experiments. A similar procedure was followed to engineer polyxenic EPNs, i.e. EPNs harbouring two or more bacterial species.
Microscopic observations
A 10-μl suspension of infective juveniles (IJs) was observed on a microscope slide (covered with a 18×18mm coverslip) with an epifluorescence optical microscope (Olympus BX-40-FX) with objectives 10x/0.25 (for EPNs) and 40x/0.75 (for EPNs and bacteria). Samples were observed under visible and UV light (Hg) adjusted for optimal GFP or mCherry fluorescence.
Bacterial counts
To quantify the amount of bacteria contained in one IJs pool, and to avoid any contamination (either from the passage in G. mellonella or from the environment), IJs were surface sterilized following a standardized procedure. In brief, IJs were immersed in a 1.5ml eppendorf for 3 minutes with 1ml of a sterilization solution (as described previously in the M&M) with gentle agitation. After 1min centrifugation at 4000rpm in a minicentrifuge, supernatant was discarded and IJs were rinsed thrice with physiological water (0.9% NaCl). Finally, surface sterilised IJs were crushed and plated on selective agar. The number of IJs present in the pool was estimated by microscopic counting of a representative sample (50μl). Alternatively, a non-sterile method was used to assess the number of targeted bacteria associated with the IJs: G. mellonella cadavers were rinsed with physiological water to collect the freshly emerged IJs in suspension. The number of IJs per larva was estimated by microscopical count on 50-μl drops from this suspension. Serial dilutions of the supernatant were then plated on selective agar medium and bacteria were enumerated.
Theoretical count of Y. pseudotuberculosis
In order stress out Y. pseudotuberculosis multiplication throughout the successive infection cycles, theoretical counts (TY) that would be observed starting from the same inoculum if no bacterial division would occur have been calculated. For this, theoretical volumes of 0.5ml (VGm) and 0.8nl (VIJ) have been assigned per G. mellonella larva and Steinernema sp MW8B IJ, respectively, and a mean EPN emergence yield of 50,000 EPNs (NIJ) per larva was considered. The infection dose was fixed to 125 IJs per G. mellonella larva. The dilution factor is the ratio VGm/(VIJ × NIJ). TY is calculated by dividing the number of Y. pseudotuberculosis CFUs infecting a larva by the dilution factor.
Susceptibility testing towards Xenorhabdus sp. antimicrobial compounds
To estimate the resistance of enterobacteria towards Xenorhabdus sp. TZ01 antibiotic compound production, growth of the tested bacteria was monitored every 30 minutes during 6 hours by optical density (OD) measurement in the presence of Xenorhabdus culture extracts. For this purpose, LB liquid medium was inoculated with an inoculum of the target bacterium derived from a fresh culture to reach an initial OD600 of 0.05–0.1. Prior to inoculation, LB was supplemented with 4% or 8% (v/v) of 0.2-μm filtered supernatant of a 48h liquid culture of X. sp. TZ01 grown at 28°C with shaking at 150rpm (Cell Free Supernatant). X. sp. TZ01 liquid cultures used for supernatant preparation were stopped when OD600 reached 11–13. For growth curve analysis, OD600 was plotted into a log2 scale in order to obtain a linear graph for the exponential phase of the curve. The slope of this line (calculated with GraphPad Prism 6) defines the growth rate of a given bacterium in the defined culture conditions.
Results
Yersinia pseudotuberculosis resists to antimicrobial compounds produced by Xenorhabdus sp.
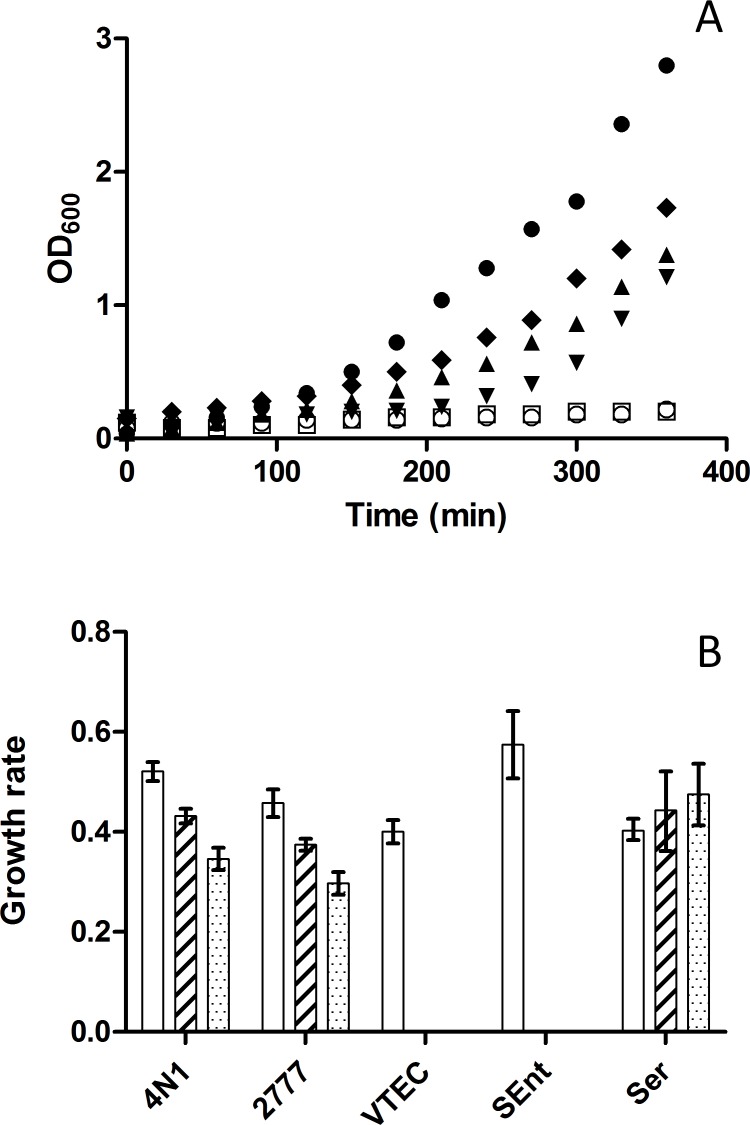
Susceptibility of several strains of Y. pseudotuberculosis, pathogenic E. coli, Salmonella enterica subsp. enterica serovar Enteritidis (S. Enteritidis) and Serratia marcescens towards antimicrobial compounds produced by X. sp. TZ01 was tested by growth curve analysis. Growth of Y. pseudotuberculosis (strains 4N1 and IP2777) was slightly delayed with 4% X. sp. TZ01 supernatant compared to a control growth without X. sp. TZ01 supernatant, but subsequent growth was merely unaffected when up to 8% X. sp. TZ01 supernatant was added. Likewise, S. marcescens EE016 was characterized by a delayed growth while its capacity to grow with up to 8% X. sp. TZ01 supernatant was unaffected. To the contrary, both S. Enteritidis SE01 and Vero-toxigenic E. coli VT01 strains were drastically inhibited with 4% X. sp. TZ01 supernatant (data not showed) and totally unable to grow with 8% added supernatant (Fig. 1A). Growth rates of Y. pseudotuberculosis 4N1 and IP2777 were both slightly affected when X. sp. TZ01 supernatant was added. At 4% supernatant, slopes decreased by 12% and 18% for Y. pseudotuberculosis 4N1 and IP2777, respectively. These values dropped respectively by 30% and 35% when 8% of X. sp. TZ01 supernatant was added. S. marcescens EE016 growth was unaffected with either 4% or 8% added X. sp. TZ01 supernatant (Fig. 1B).
Figure 1. Susceptibility of various enterobacteria to antimicrobial substances produced by X. sp. TZ01.
A: Growth curves in liquid broth of Y. pseudotuberculosis 4N1 (closed circles), Y. pseudotuberculosis 4N1 supplemented with 8% of X. sp. TZ01 culture supernatant (closed triangles), Y. pseudotuberculosis IP2777 supplemented with 8% of X. sp. TZ01 culture supernatant (closed diamonds), Serratia marcescens EE016 supplemented with 8% of X. sp. TZ01 culture supernatant (closed upside down triangles), E. coli VT01 supplemented with 8% X. sp. TZ01 culture supernatant (open circles) and S. Enteritidis SE01 supplemented with 8% of X. sp. TZ01 culture supernatant (opened squares). OD600 values were plotted every 30 minutes during 6 hours. B: Growth rates of Y. pseudotuberculosis 4N1 (4N1), Y. pseudotuberculosis IP2777 (2777), Vero-toxigenic E. coli VT01 (VTEC), Salmonella Enteritidis SE01 (SEnt) and Serratia marcescens EE016 (Ser) in liquid broth supplemented with either 0% (white bars), 4% (hatched bars) or 8% (dotted bars) of X. sp. TZ01 culture supernatant. Growth rates were calculated by plotting experimental OD600 values in log2 scale and taking the slope of the adjusted linear regression curve. Few or no growth was observed for Vero-toxigenic E. coli VT01 and Salmonella Enteritidis SE01 grown with either 4% or 8% of X. sp. TZ01 culture supernatant.
Yersinia pseudotuberculosis colonizes the gastro-intestinal tract of EPNs and survives long-term EPN storage
To assess the ability of Y. pseudotuberculosis to colonize Steinernema sp. MW8B, 7 independent Galleria mellonella infection experiments were conducted with a Y. pseudotuberculosis GFPmut2-labelled strain (4N1G strain) (Table 2).
Table 2. Summary of G. mellonella infection experiments.
| Infection Cycle | IJ emergence | IJ fluorescence | IJ infectivity | IJs/larva | Yp CFU/IJ | Total Yp count |
|---|---|---|---|---|---|---|
| 1 | 7/7 | 7/7 | 5/7 | 50000 +/− 7500 | 5,0 × 103 +/− 0,8 × 103 | 2,5 × 108 +/− 0,3 × 108 |
| 2 | 5/7 | 3/7 | 4/7 | ND | ND | ND |
| 3 | 4/7 | 2/7 | 3/7 | ND | ND | ND |
| 4 | 3/7 | 2/7 | 3/7 | 40800 +/− 6366 | 8,6 × 103 +/− 1,6 × 103 | 3,5 × 108 +/− 0,5 × 108 |
| 5 | 3/7 | 1/7 | 2/7 | ND | ND | ND |
| 6 | 2/7 | 1/7 | 1/7 | ND | ND | ND |
| 7 | 1/7 | 1/7 | NA | 1022 +/− 247 | 5,6 × 103 +/− 1,8 × 103 | 5,7 × 106 +/− 1,4 × 106 |
Seven G. mellonella larvae were injected with 106 CFU of the GFP-labelled Y. pseudotuberculosis strain 4N1G (Yp) and incubated with ca. 125 Steinernema sp. MW8B nematodes (Infective Juvenile stage, IJ) associated with their natural Xenorhabdus sp. TZ01 symbiont as described in M&M. After completion of the first infectious cycle, Steinernema sp. MW8B progeny emerging from one death larva was collected, characterized according to several parameters and used to infect naïve (Yp-free) G. mellonella larvae thereby initiating a new infection cycle, and so on for 7 successive cycles. Column 2–4, number of experiments in which emerged IJs fitted the property featured on the top row; column 5, mean number of IJs emerged from one dead larva; column 6, mean count of Yp CFU retrieved from one crushed IJ; column 7, total count of Steinernema sp. MW8B-associated Yp CFU generated from one G. mellonella larva after successful cycle completion. ND, not determined; NA, not available. The Xenorhabdus sp. TZ01 symbiont was still present after each successful cycle completion.
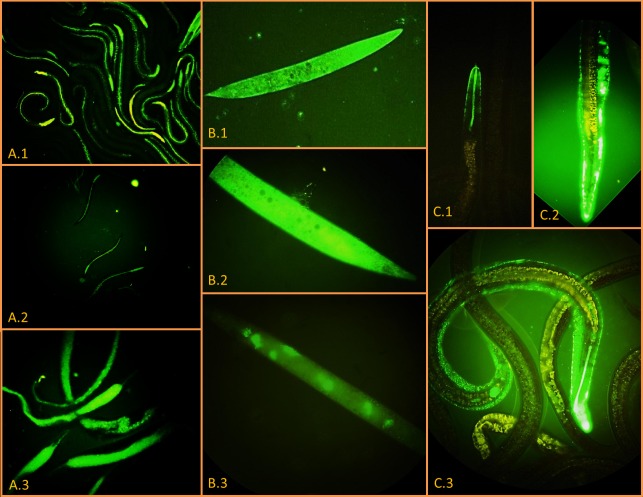
In all experiments, Steinernema sp. MW8B IJs1 exhibited GFPmut2 fluorescence along the entire length of their gut (Fig. 2A.1). However, in 2 out of 7 experiments (29%), IJs1 emerged from Y. pseudotuberculosis 4N1G-infected G. mellonella larvae failed to invade and kill new naive G. mellonella larvae in spite of the fact that they were massively colonized by Y. pseudotuberculosis 4N1G as attested by the bright GFP fluorescence they displayed. In 2 out of 7 experiments (29%), IJs that were both fluorescent and infective emerged from dead G. mellonella cadavers after 4 consecutive infection cycles (Fig. 2A.2). One experiment (14%) led to the emergence of fluorescent/infective IJs after the fifth infection cycle and were so even after 7 consecutive infection cycles which lasted 14 weeks (Fig. 2A.3).
Figure 2. Epifluorescence microscope pictures of GFP-labelled Y. pseudotuberculosis 4N1G in Steinernema sp; MW8B EPNs.
A. EPNs emerging from dead moth larvae after 1 (A.1), 4 (A.2) and 7 (A.3) consecutive infection cycles (100× magnification). B. IJs collected after the first infection cycle and stored at 4°C in physiological water for either 8 (B.1, B.2) or 42 days (B.3) (400× magnification). C. IJs collected after the first infection cycle and stored at 28°C in physiological water for 98 days. C.1, enlarged view of the mouth; C.2, enlarged view of the anus; C.3, whole IJ body (400× magnification, 800× magnification for enlarged view).
Directly after the first emergence, freshly emerged IJs1 were stored at 4°C, 16°C and 28°C in physiological water. IJs1 were observed in epifluorescence microscopy to monitor the presence of Y. pseudotuberculosis 4N1G. These observations were made every day during the first week post emergence (PE) then once a week during 13 weeks. At 4°C, stored IJs1 did not survive a week and were all dead by day 8 PE (Fig. 2B.1 and 2B.2). Nevertheless, Y. pseudotuberculosis 4N1G was still alive—and did even multiply slowly inside the IJs cadavers—since GFP fluorescence was still observed 6 weeks PE (Fig. 2B.3). No differences were observed between IJs1 stored either at 16°C or at 28°C. In these samples, microscopic observations showed that Y. pseudotuberculosis 4N1G was still present inside IJs1 of Steinernema sp. MW8B, either in the gut or in the inter-cuticular space, after 14 weeks of storage at either 16°C or 28°C (Fig. 2C).
To check the ability of Y. pseudotuberculosis 4N1G to remain associated with Steinernema sp. MW8B after several infection cycles, IJs4 were also kept at 28°C. At 3 weeks PE, 23 +/− 3 IJs4 were crushed and counted on selective agar plates. An average of 5.0 × 103 CFUs of Y. pseudotuberculosis 4N1G per IJs4 was measured. Compared to IJs4 at 0 day PE (8.6 × 103 CFU/IJs4), the number of Y. pseudotuberculosis 4N1G CFUs per IJ decreased by 41%. However, quantitative results obtained for IJs4 at week 3 PE and IJs1 at day 0 PE are comparable.
Similar G. mellonella infection experiments were conducted three times with a GFPmut2-labelled Y. enterocolitica O:3 strain (YE03). This strain is more sensitive towards antimicrobials produced by X. sp. since Y. enterocolitica O:3’s growth is totally inhibited with the presence of 8% of X. sp. supernatant (data not shown). Microscopic observations showed EPN colonization by Y. enterocolitica YE03 during at least 2 consecutive infection cycles for one out of the three experiments conducted. Confocal microscopic observations localized Y. enterocolitica YE03 in the gut lumen after 2 infection cycles (Fig. 3). However, no GFPmut2 fluorescence was observed after 3 consecutive infection cycles with Y. enterocolitica YE03. GFPmut2-labelled Escherichia coli VT03 (vero-toxigenic O157 strains), GFPmut2-labelled Salmonella Enteritidis SE03 and an unlabelled tetracycline resistant S. marcescens EE016 strain were subjected to similar G. mellonella infection cycle experiments. None of these 4 Enterobacteriaceae demonstrated Steinernema sp. MW8B colonisation capacity. This was evidenced by the lack of GFPmut2 fluorescence in IJs1 in the E. coli VT03 and S. Enteritidis SE03 experiments. No single IJ1 emerged from G. mellonella larvae injected with S. marcescens EE016.
Figure 3. Localization of Y. enterocolitica YE03 in Steinernema sp. MW8B EPNs emerged from an infected larva.
Confocal microscope slides in Z-axis (numbered from 1 to 8) of a Steinernema sp. MW8B EPN colonized by Y. enterocolitica YE03 emerged from the second infection cycle. GFP-labeled bacteria localize in the mouth and in the gut lumen. EPN borders are drawn in white (800× magnification).
EPNs support dramatic multiplication and dissemination of Yersinia pseudotuberculosis
To confirm quantitatively the maintenance of Y. pseudotuberculosis 4N1G in the experimental model, CFU counts were determined at different time points (Table 2). After the first infection cycle, an average of 5.0 × 103 Y. pseudotuberculosis 4N1G CFUs per Steinernema sp. MW8B IJ were found. Similar counts were determined during 7 consecutive infection cycles, with an average of 8.6 × 103 CFUs of Y. pseudotuberculosis 4N1G per IJ still found after the 4th infection cycle and 5.6 × 103 CFUs after the 7th infection cycle (Table 2). Knowing the number of CFU per IJ and the number of IJs emerged from dead larvae, we calculated the total increase in Y. pseudotuberculosis 4N1G counts after the various infection cycles. Starting with 1.9 × 106 CFUs of Y. pseudotuberculosis 4N1G directly injected in the larva, Y. pseudotuberculosis 4N1G counts after one cycle increased by two orders of magnitude and reached 2.5 × 108 CFUs. These counts were similar after the fourth infection cycle (3.5 × 108 CFU) and started to decrease from the 7th infection cycle (5.67 × 106 CFU). This confirms our model predictions which suggest that in the absence of active multiplication, Y. pseudotuberculosis 4N1G counts would drastically decrease and would become undetectable after two infection cycles (Fig. 4). The experimental counts hence reflect an active multiplication of Y. pseudotuberculosis 4N1G in the studied laboratory model.
Figure 4. Growth of Y. pseudotuberculosis 4N1G during EPN’s infection cycles.
The hatched bars show the total counts of Y. pseudotuberculosis 4N1G CFUs retrieved from IJs emerged from a dead moth larva after 1, 4 and 7 consecutive infection cycles (data from table 2). The straight line shows the theoretical counts that would be observed starting from the same inoculum if no bacterial division would occur. For this calculation, theoretical volumes of 0.5ml and 0.8nl have been assigned per G. mellonella larva and Steinernema sp MW8B IJ, respectively, and a mean EPN emergence yield of 50,000 EPNs per larva has been considered (see M&M).
Yersinia pseudotuberculosis cannot replace Xenorhabdus sp. TZ01 as EPN symbiont
After having demonstrated the colonisation and multiplication capacity of Y. pseudotuberculosis 4N1G in the gut of Steinernema sp. MW8B IJs, we wondered whether Y. pseudotuberculosis 4N1G could substitute for X. sp. TZ01 as a bacterial symbiont in this EPN species. To address this question, we obtained axenic Steinernema sp. MW8B EPNs by collecting surface sterilised eggs from gravid females. Prior to G. mellonella infection, axenic EPNs were incubated with the mCherry-labelled Y. pseudotuberculosis 4N1C strain on Wouts Agar plates in order to obtain IJs exclusively colonised by Y. pseudotuberculosis 4N1C. A pool of such “monoxenic” IJs displaying red fluorescence (Fig. 5A) was divided into 4 equal groups. Two groups were incubated separately with 6 G. mellonella larvae in empty containers. One group was deposited onto a sterile Wouts Agar plate with 4 G. mellonella larvae and the last group was deposited onto a Wouts Agar plate without any larva.
Figure 5. Differential localization of Y. pseudotuberculosis 4N1C and X. sp. TZ03 in Steinernema sp. MW8B nematodes.
Epifluorescence microscope pictures showing axenic EPNs artificially fed on (A) plate-grown red fluorescent Y. pseudotuberculosis 4N1C localizing in the gut (100× magnification) or (B) plate-grown green fluorescent X. sp. TZ03 localizing in a symbiotic vesicle (400× magnification). The latter was still localized in the symbiotic vesicle after 2 consecutive infection cycles on G. mellonella larvae (C) (800× magnification)
At day 3 post infection (PI), only G. mellonella larvae grown on Wouts Agar were found dead. At day 8 PI, all G. mellonella were dead. Three G. mellonella larvae recovered from Wouts agar plates showed emergence of EPNs. The emerged EPNs displayed no mCherry fluorescence when observed microscopically. When crushed and plated onto selective agar plates, no Y. pseudotuberculosis 4N1C could be retrieved from these EPNs neither. At day 10 PI, emergence of EPNs could be observed in 2 larvae incubated in empty containers, but again none of the emerged EPNs exhibited red fluorescence and no Y. pseudotuberculosis 4N1C could be retrieved after EPN crushing and plating on selective agar. In contrast, EPNs grown freely on Wouts agar still exhibited red mCherry fluorescence 10 days after plating. The same experiment was conducted with the GFPmut2-labelled X. sp. TZ03. Microscopic observations showed not only that X. sp. TZ03 was able to colonise the symbiotic vesicle of Steinernema sp. MW8B axenic EPNs (Fig. 5B), but also that X. sp. TZ03 maintained in its host after 2 consecutive cycles (Fig. 5C) and probably much more (not tested).
Discussion, Conclusion, and Perspectives
Compared to other enterobacteriaceae tested so far, the capacity of Y. pseudotuberculosis 4N1 to colonize Steinernema sp. MW8B is remarkably efficient and suggests that a number of biological functions required for its successful dissemination through this host during and between infection cycles are present and functional in this bacterium. We showed that enterobacteria sensitive to the antibiotics secreted by Xenorhabdus. sp. TZ01 have no ability to colonize the EPN gut. Two strains of Y. pseudotuberculosis (4N1 and IP2777) as well as one S. marcescens strain (EE016) isolated from a Steinernema sp. MW8B-infected G. mellonella larva were found naturally resistant to X. sp. TZ01 secreted antibiotics and were tested for their ability to colonize Steinernema sp. MW8B EPNs with the model developed herewith. The growth rate of both Y. pseudotuberculosis strains was slightly affected by the presence of X. sp. TZ01 supernatant, while the S. marcescens EE016 was not. Likewise, Ochrobactrum tritici strain EE10.1 isolated from a Steinernema sp. MW8B-infected G. mellonella larva in our laboratory displayed a similar capacity to resist to X. sp. TZ01 antibiotics (data not shown). Despite this capacity, S. marcescens EE016 was unable to sustain EPNs life cycle completion since no IJ emergence occurred from S. marcescens-injected G. mellonella larvae. This suggests that Serratia and Ochrobactrum may accidentally reach the gut of Steinernema sp. MW8B but are unlikely able to colonize and multiply within the EPN gut as Yersinia pseudotuberculosis does. It has been shown that Serratia marcescens uses the type VI secretion system to neutralize bacterial competitors [39]. Injection of 106 S. marcescens CFUs may therefore impair Xenorhabdus growth in G. mellonella larvae. This could explain why Steinernema sp. MW8B cannot complete its reproductive cycle within S. marcescens-infected G. mellonella. S. marcescens has been shown to be pathogenic towards the free-living nermatode Caenorhabditis elegans but beneficial to the entomopathogenic nematode C. briggsae [40,41]. Zhang et al. reported the isolation of a new Serratia species (S. nematodiphila) from the EPN species Heterorhabditidoides chongmingensis and proposed that S. nematodiphila may have evolved to a symbiotic species, possibly after horizontal gene transfer [42,43]. Dixenic associations have been described, such as P. luminescens and Ochrobactrum spp. found together in tropical species of Heterorhabditis [44]. Genomic comparison between S. nematodiphila and other (non-symbiotic) Serratia spp. could provide interesting insights in the discovery of genes involved in the symbiotic association with EPNs.
In this study we showed that Y. pseudotuberculosis 4N1G is able to colonize and maintain for several generations inside a Steinernema species for long-term periods (14 weeks). Quantitative data showed that EPNs support efficient multiplication of Y. pseudotuberculosis 4N1G during this period. Indeed, counts of Y. pseudotuberculosis CFUs carried away by EPNs emerged from dead larvae are roughly multiplied by a factor 103 at the term of each infection cycle, a number which is probably underestimated as it does not take into account Y. pseudotuberculosis bacteria left over in the dead cadaver. Y. pseudotuberculosis 4N1G colonizes mainly the gut of Steinernema sp. MW8B but can be found in the inter-cuticular space as well after 3-month storage in physiological water. The localisation of Y. pseudotuberculosis 4N1G in Steinernema sp. MW8B IJs’ gut is quite different from the normal localisation of the symbiotic Xenorhabdus sp. TZ03. Indeed, the natural niche of Xenorhabdus inside its Steinernema host–before infecting an insect prey–is a so-called symbiotic vesicle located along and separated from the EPN gut [3]. Our observations on EPN colonization are consistent with axenic EPNs experiments, which demonstrated that Y. pseudotuberculosis 4N1G does not replace the X. sp. TZ01 symbiont during EPN infection cycle but more likely hijack the symbiotic relationship between Xenorhabdus and EPNs. Indeed, when the natural symbiont of Steinernema sp. MW8B is absent, EPNs colonized by Y. pseudotuberculosis 4N1C alone are unable to develop properly in a G. mellonella larva. In control experiments where axenic EPNs are supplemented with GFP-labelled X. sp. TZ03, EPNs recover their ability to indefinitely multiply and feed on G. mellonella larvae. Moreover, unpublished results showed that IJs colonized by both X. sp. and Y. pseudotuberculosis strains labelled with two different fluorescent protein markers do contain both bacteria.
The mini-Tn5 transposon used to tag Y. pseudotuberculosis 4N1G and 4N1C in our experiments was mapped in the fimbrial A protein gene. This gene is found in two intact copies in the Y. pseudotuberculosis genome meaning that the protein is probably still expressed in the GFP-tagged strains. Fimbrial proteins are known to act as colonization factors for Y. pseudotuberculosis and are involved in the attachment to epithelial cells [45]. The capacity of the 4N1G and 4N1C tagged strains to colonise the EPN’s gut, as demonstrated throughout our study, argue in favour of a non-detrimental effect of the transposon insertion compared to the wild-type strain.
Interestingly, it has been shown that Y. pseudotuberculosis and Y. pestis – the etiological agent of plague – can infect or form a biofilm mainly around the head of Caenorhabditis elegans, a well-studied nematode laboratory model [46,47,48]. Given the fact that Y. pestis evolved quite recently from Y. pseudotuberculosis [49], it would be interesting to know whether Y. pestis can also resist to antimicrobial substances produced by Xenorhabdus/Photorhabdus spp. and colonize EPNs. If these findings turn to have an environmental significance, it would provide new insights in the understanding of long-term persistence of Y. pestis in plague endemic areas worldwide [28,50].
Future work should focus on the identification of Yersinia genetic determinants required to colonize and maintain inside EPNs. Several genes shared by Yersinia and the EPN’s natural symbionts are good candidates to play this role such as the phospholipase A encoded by yplA, structural genes of the type 6 secretion system (T6SS) and possibly others [10]. Likewise, genome comparisons between Serratia, Ochrobactrum, Yersinia and Xenorhabdus should help deciphering the critical genetic determinants required for EPN colonization.
Acknowledgments
We thank Dr. Ralf-Udo Ehlers (Kiel University, Germany) for teaching the methodology and tips for proper axenic EPNs preparation, Dr. Guy R. Cornelis (FUNDP, Namur, Belgium) for his gift of E. coli SM10 λPir and pKNG101, Dr. Florent Sebbane (Institut Pasteur de Lille, France) for his gift of Y. pseudotuberculosis IP2777, Michaël Abraham (VAR) for his help in the GFP-labeling of enterobacteria used in this study and Mieke Van Hessche and Jordane Lebrun (VAR) for susceptibility testing experiments.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was funded by the “Fonds pour la Formation à la Recherche dans l’Industrie et dans l’Agriculture”; http://www.fnrs.be/. SG received the funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Brusselman E, Steurbaut W, Sonck B (2006) Optimizing the Application of Entomopathogenic Nematodes. Communications in agricultural and applied biological sciences 71: 701–705. [PubMed] [Google Scholar]
- 2. Wilson M, Ivanova E (2004) Neutral density liquid formulations for nematode-based biopesticides. Biotechnology letters 26: 1167–1171. 10.1023/B:BILE.0000035492.25214.3b [DOI] [PubMed] [Google Scholar]
- 3. Poinar GO Jr., Thomas GM (1966) Significance of Achromobacter nematophilus Poinar and Thomas (Achromobacteraceae: Enbacteriales) in the development of the nematode, DD-136 (Neoaplectana sp. Steinernematidae). Parasitology. pp. 385–390. 10.1017/S0031182000070980 [DOI] [PubMed] [Google Scholar]
- 4. Waterfield NR, Ciche T, Clarke D (2009) Photorhabdus and a host of hosts. Annual Review of Microbiology 63: 557–574. 10.1146/annurev.micro.091208.073507 [DOI] [PubMed] [Google Scholar]
- 5. Li J, Chen G, Webster JM (1997) Nematophin, a novel antimicrobial substance produced by Xenorhabus nematophilus (enterobactereaceae). Canadian Journal of Microbiology. pp. 770–773. 10.1139/m97-110 [DOI] [PubMed] [Google Scholar]
- 6. Emelianoff V, Chapuis E, Le Brun N, Chiral M, Moulia C, et al. (2008) A survival-reproduction trade-off in entomopathogenic nematodes mediated by their bacterial symbionts. Evolution; international journal of organic evolution 62: 932–942. 10.1111/j.1558-5646.2008.00319.x [DOI] [PubMed] [Google Scholar]
- 7. Goodrich-Blair H, Clarke DJ (2007) Mutualism and pathogenesis in Xenorhabdus and Photorhabdus: two roads to the same destination. Molecular Microbiology 64: 260–268. 10.1111/j.1365-2958.2007.05671.x [DOI] [PubMed] [Google Scholar]
- 8. Forst S, Dowds B, Boemare N, Stackebrandt E (1997) Xenorhabdus and Photorhabdus spp.: bugs that kill bugs. Annual Review of Microbiology 51: 47–72. 10.1146/annurev.micro.51.1.47 [DOI] [PubMed] [Google Scholar]
- 9. Hatab Ma, Gaugler R, Ehlers R-U (1998) Influence of culture method on Steinernema galseri lipids. The Journal of parasitology 84: 215–221. 10.2307/3284473 [DOI] [PubMed] [Google Scholar]
- 10. Heermann R, Fuchs TM (2008) Comparative analysis of the Photorhabdus luminescens and the Yersinia enterocolitica genomes: uncovering candidate genes involved in insect pathogenicity. BMC Genomics 9: 40–40. 10.1186/1471-2164-9-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schubert S (2004) The high-pathogenicity island (HPI): evolutionary and functional aspects. International Journal of Medical Microbiology 294: 83–94. 10.1016/j.ijmm.2004.06.026 [DOI] [PubMed] [Google Scholar]
- 12. Sheets JJ, Hey TD, Fencil KJ, Burton SL, Ni W, et al. (2011) Insecticidal toxin complex proteins from Xenorhabdus nematophilus: structure and pore formation. The Journal of biological chemistry 286: 22742–22749. 10.1074/jbc.M111.227009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmiel DH, Wagar E, Karamanou L, Weeks D, Miller VL (1998) Phospholipase A of Yersinia enterocolitica Contributes to Pathogenesis in a Mouse Model. Infection and Immunity 66: 3941–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jani AJ, Cotter Pa (2010) Type VI secretion: not just for pathogenesis anymore. Cell Host & Microbe 8: 2–6. 10.1016/j.chom.2010.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farmer JJ, Jorgensen JH, Grimont PA, Akhurst RJ, Poinar GO, et al. (1989) Xenorhabdus luminescens (DNA hybridization group 5) from human clinical specimens. Journal of clinical microbiology 27: 1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bari ML, Hossain MA, Isshiki K, Ukuku D (2011) Behavior of Yersinia enterocolitica in Foods. Journal of Pathogens 2011 10.4061/2011/420732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jalava K, Hakkinen M, Valkonen M, Nakari U-M, Palo T, et al. (2006) An outbreak of gastrointestinal illness and erythema nodosum from grated carrots contaminated with Yersinia pseudotuberculosis . The Journal of infectious diseases 194: 1209–1216. 10.1086/508191 [DOI] [PubMed] [Google Scholar]
- 18. Rahuma N, Ghenghesh KS, Ben Aissa R, Elamaari A (2005) Carriage by the housefly (Musca domestica) of multiple-antibiotic-resistant bacteria that are potentially pathogenic to humans, in hospital and other urban environments in Misurata, Libya. Annals of tropical medicine and parasitology 99: 795–802. 10.1179/136485905X65134 [DOI] [PubMed] [Google Scholar]
- 19. Zurek L, Denning SS, Schal C, Watson DW (2001) Vector Competence of Musca domestica (Diptera: Muscidae) for Yersinia pseudotuberculosis . Journal of Medical Entomology 38: 333–335. 10.1603/0022-2585-38.2.333 [DOI] [PubMed] [Google Scholar]
- 20. Zurek L, Schal C, Watson DW (2000) Diversity and contribution of the intestinal bacterial community to the development of Musca domestica (Diptera: Muscidae) larvae. J Med Entomol 37: 924–928. 10.1603/0022-2585-37.6.924 [DOI] [PubMed] [Google Scholar]
- 21. Pinheiro VB, Ellar DJ (2007) Expression and insecticidal activity of Yersinia pseudotuberculosis and Photorhabdus luminescens toxin complex proteins. Cellular Microbiology 9: 2372–2380. 10.1111/j.1462-5822.2007.00966.x [DOI] [PubMed] [Google Scholar]
- 22. Champion OL, Cooper IaM, James SL, Ford D, Karlyshev A, et al. (2009) Galleria mellonella as an alternative infection model for Yersinia pseudotuberculosis . Microbiology (Reading, England) 155: 1516–1522. 10.1099/mic.0.026823-0 [DOI] [PubMed] [Google Scholar]
- 23. Hinnebusch BJ, Rudolph AE, Cherepanov P, Dixon JE, Schwan TG, et al. (2002) Role of Yersinia murine toxin in survival of Yersinia pestis in the midgut of the flea vector. Science (New York, NY) 296: 733–735. 10.1126/science.1069972 [DOI] [PubMed] [Google Scholar]
- 24. Duchaud E, Rusniok C, Frangeul L, Buchrieser C, Givaudan A, et al. (2003) The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens . Nature Biotechnology 21: 1307–1313. 10.1038/nbt886 [DOI] [PubMed] [Google Scholar]
- 25. Wilkinson P, Waterfield NR, Crossman L, Corton C, Sanchez-Contreras M, et al. (2009) Comparative genomics of the emerging human pathogen Photorhabdus asymbiotica with the insect pathogen Photorhabdus luminescens . BMC Genomics 10: 302–302. 10.1186/1471-2164-10-302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buzoleva LS, Somov GP (2003) Adaptation variability of Yersinia pseudotuberculosis during long-term persistence in soil. Bulletin of experimental biology and medicine 135: 456–459. 10.1023/A:1024915409187 [DOI] [PubMed] [Google Scholar]
- 27. Ayyadurai S, Houhamdi L, Lepidi H, Nappez C, Raoult D, et al. (2008) Long-term persistence of virulent Yersinia pestis in soil. Microbiology (Reading, England) 154: 2865–2871. 10.1099/mic.0.2007/016154-0 [DOI] [PubMed] [Google Scholar]
- 28. Eisen RJ, Petersen JM, Higgins CL, Wong D, Craig E, et al. (2008) Persistence of Yersinia pestis in soil under natural conditions. Emerging Infectious Diseases 14: 941–942. 10.3201/eid1406.080029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lambrecht E, Baré J, Van Damme I, Bert W, Sabbe K, et al. (2013) Behavior of Yersinia enterocolitica in the presence of the bacterivorous Acanthamoeba castellanii . Applied and Environmental Microbiology 79: 6407–6413. 10.1128/AEM.01915-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nikul’shin SV, Onatskaia TG, Lukanina LM, Bondarenko AI (1992) [Associations of the soil amoeba Hartmannella rhysodes with the bacterial causative agents of plague and pseudotuberculosis in an experiment]. Zhurnal mikrobiologii, epidemiologii, i immunobiologii: 2–5. [PubMed] [Google Scholar]
- 31. Akhurst RJ (1980) Morphological and Functional Dimorphism in Xenorhabdus spp., Bacteria Symbiotically Associated with the Insect Pathogenic Nematodes Neoaplectana and. Journal of General Microbiology 124: 303–309. [DOI] [PubMed] [Google Scholar]
- 32. Cormack BP, Valdivia RH, Falkow S (1996) FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173: 33–38. 10.1016/0378-1119(95)00685-0 [DOI] [PubMed] [Google Scholar]
- 33. Reznikoff WS (2008) Transposon Tn5. Annual Review of Genetics 42: 269–286. 10.1146/annurev.genet.42.110807.091656 [DOI] [PubMed] [Google Scholar]
- 34. Miller VL, Mekalanos JJ (1988) A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR . Journal of Bacteriology 170: 2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaniga K, Delor I, Cornelis GR (1991) A wide-host-range suicide vector for improving reverse genetics in Gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica . Gene 109: 137–141. 10.1016/0378-1119(91)90599-7 [DOI] [PubMed] [Google Scholar]
- 36. Liu YG, Whittier RF (1995) Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25: 674–681. 10.1016/0888-7543(95)80010-J [DOI] [PubMed] [Google Scholar]
- 37. Mwaitulo S, Haukeland S, Saethre MG, Laudisoit A, Maerere AP (2011) First report of entomopathogenic nematodes from Tanzania and their virulence against larvae and adults of the banana weevil Cosmopolites sordidus (Coleoptera: Curculionidae). International Journal of Tropical Insect Science 31: 154–161. [Google Scholar]
- 38. Wouts WM (1981) Mass Production of the Entomogenous Nematode Heterorhabditis heliothidis (Nematoda: Heterorhabditidae) on Artificial Media. Journal of nematology 13: 467–469. [PMC free article] [PubMed] [Google Scholar]
- 39. Murdoch SL, Trunk K, English G, Fritsch MJ, Pourkarimi E, et al. (2011) The opportunistic pathogen Serratia marcescens utilizes type VI secretion to target bacterial competitors. J Bacteriol 193: 6057–6069. 10.1128/JB.05671-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lancaster JD, Mohammad B, Abebe E (2012) Effect of the bacterium Serratia marcescens SCBI on the longevity and reproduction of the nematode Caenorhabditis briggsae KT0001. BMC Res Notes 5: 688 10.1186/1756-0500-5-688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mallo GV, Kurz CL, Couillault C, Pujol N, Granjeaud S, et al. (2002) Inducible antibacterial defense system in C. elegans. Curr Biol 12: 1209–1214. 10.1016/S0960-9822(02)00928-4 [DOI] [PubMed] [Google Scholar]
- 42. Zhang C, Liu J, Xu M, Sun J, Yang S, et al. (2008) Heterorhabditidoides chongmingensis gen. nov., sp. nov. (Rhabditida: Rhabditidae), a novel member of the entomopathogenic nematodes. Journal of Invertebrate Pathology 98: 153–168. 10.1016/j.jip.2008.02.011 [DOI] [PubMed] [Google Scholar]
- 43. Zhang C, Yang S-Y, Xu M-X, Sun J, Liu H, et al. (2009) Serratia nematodiphila sp. nov., symbiotically associated with entomopathogenic nematode Heterorhabditidoides chongmingensis (Rhabditida: Rhabditidae). International journal of systematic and evolutionary microbiology: 1603–1608. 10.1099/ijs.0.65718-0 [DOI] [PubMed] [Google Scholar]
- 44. Babic I, Fischer-Le Saux M, Giraud E, Boemare N (2000) Occurrence of natural dixenic associations between the symbiont Photorhabdus luminescens and bacteria related to Ochrobactrum spp. in tropical entomopathogenic Heterorhabditis spp. (Nematoda, Rhabditida). Microbiology (Reading, England) 146 (Pt 3): 709–718. [DOI] [PubMed] [Google Scholar]
- 45. Collyn F, Lety MA, Nair S, Escuyer V, Ben Younes A, et al. (2002) Yersinia pseudotuberculosis harbors a type IV pilus gene cluster that contributes to pathogenicity. Infect Immun 70: 6196–6205. 10.1128/IAI.70.11.6196-6205.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Darby C, Hsu JW, Ghori N, Falkow S (2002) Caenorhabditis elegans: plague bacteria biofilm blocks food intake. Nature 417: 243–244. 10.1038/417243a [DOI] [PubMed] [Google Scholar]
- 47. Joshua GWP, Karlyshev AV, Smith MP, Isherwood KE, Titball RW, et al. (2003) A Caenorhabditis elegans model of Yersinia infection: biofilm formation on a biotic surface. Microbiology (Reading, England) 149: 3221–3229. 10.1099/mic.0.26475-0 [DOI] [PubMed] [Google Scholar]
- 48. Tan L, Darby C (2004) A Movable Surface: Formation of Yersinia sp. Biofilms on Motile Caenorhabditis elegans . Journal of Bacteriology 186: 5087–5092. 10.1128/JB.186.15.5087-5092.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Achtman M, Zurth K, Morelli G, Torrea G, Guiyoule a, et al. (1999) Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis . Proceedings of the National Academy of Sciences of the United States of America 96: 14043–14048. 10.1073/pnas.96.24.14043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bertherat E, Bekhoucha S, Chougrani S, Razik F, Duchemin JB, et al. (2007) Plague reappearance in Algeria after 50 years, 2003. Emerging infectious diseases 13: 1459–1462. 10.3201/eid1310.070284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McFarlane GJB, Machray GC, Stewart WDP (1987) A simplfied method for conjugal gene transfer into the filamentous cyanobacterium Anabaena sp. ATCC 27893. Journal of Microbiological Methods 6: 301–305. [Google Scholar]
- 52. Simon R, Priefer U, Pühler A (1983) A Broad Host Range Mobilization System for In Vivo Genetic Engineering: Transposon Mutagenesis in Gram Negative Bacteria. Nature Biotechnology 1: 784–791. [Google Scholar]
- 53. De lorenzo V, Herrero M, Jakubzik U, Timmis KN (1990) Mini-TnS Transposon Derivatives for Insertion Mutagenesis, Promoter Probing, and Chromosomal Insertion of Cloned DNA in Gram-Negative Eubacteria. Journal of Bacteriology 172: 6568–6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Derbise A, Pierre F, Merchez M, Pradel E, Laouami S, et al. (2013) Inheritance of the lysozyme inhibitor Ivy was an important evolutionary step by Yersinia pestis to avoid the host innate immune response. The Journal of infectious diseases 207: 1535–1543. 10.1093/infdis/jit057 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.