Abstract
The tyrosine kinase receptor anaplastic lymphoma kinase (ALK) and its ligand, the growth factor pleiotrophin (PTN), are highly expressed during the development of the nervous system and have been implicated in the malignant progression of different tumor types. Here, we describe human single-chain variable fragment (scFv) antibodies that target the ligand-binding domain (LBD) in ALK and show the effect in vitro and in vivo. The ALK LBD was used as a bait in a yeast two-hybdrid system to select human scFv from a library with randomized complementarity-determining region 3 domains. Surface plasmon resonance showed high-affinity binding of the selected scFv. The anti-ALK scFv competed for binding of PTN to ALK in intact cells and inhibited PTN-dependent signal transduction through endogenous ALK. Invasion of an intact endothelial cell monolayer by U87MG human glioblastoma cells was inhibited by the anti-ALK scFv. In addition, the growth of established tumor xenografts in mice was reversed after the induction of the conditional expression of the anti-ALK scFv. In archival malignant brain tumors expression levels of ALK and PTN were found elevated and appear correlated with poor patient survival. This suggests a rate-limiting function of the PTN/ALK interaction that may be exploited therapeutically.
Keywords: ALK, growth factor, PTN, single-chain antibody, tyrosine kinase receptor
Introduction
The tyrosine kinase receptor anaplastic lymphoma kinase (ALK) was first identified in 1994 as an oncogenic fusion protein with nucleophosmin resulting from a t(2,5) translocation in some lymphoma (Morris et al., 1994). A number of different fusion partners of the intracellular ALK portion were described over the past decade (Duyster et al., 2001), most recently as a transforming oncogene in lung cancers (Soda et al., 2007) (reviewed in Chiarle et al., 2008; Li and Morris, 2007), and activating mutations in the ALK receptor were found associated with familial neuroblastoma (summarized in McCarthy, 2008). Full-length ALK was cloned as a transmembrane receptor and its highest expression was found in the developing central and peripheral nervous system (Iwahara et al., 1997; Morris et al., 1997). The intracellular kinase domain is closely related to the insulin receptor tyrosine kinase, but the extracellular domain of ALK, which comprises ~60% of the 220 kDa protein, is unique (Duyster et al., 2001). We identified ALK as a receptor for the growth factor pleiotrophin (PTN) (Li et al., 1990; Fang et al., 1992) from an unbiased panning of a phage display cDNA library from the fetal brain against PTN as the bait protein, and defined a small region in the extracellular domain of ALK as the ligand-binding domain (LBD) (Stoica et al., 2001). Binding of PTN and the related growth factor midkine (MK) to ALK (Stoica et al., 2002) control cell survival and proliferation, although additional receptors that can modulate signaling of PTN and MK have been described (see ‘Discussion’).
The developmental expression pattern of the ligand PTN is similar to that of the ALK receptor with the highest levels found in the central and peripheral nervous system during late gestation (Li et al., 1990). In contrast to the limited expression in normal adult tissue, full-length ALK and PTN are overexpressed in a number of human cancers, in particular brain tumors (Schulte and Wellstein, 1997; Mentlein and Held-Feindt, 2002; Powers et al., 2002; Peria et al., 2007). We have previously reported that the ALK protein and mRNA are overexpressed in some tumors of glial origin and shown that ribozyme-mediated depletion of ALK mRNA from U87MG glioblastoma cells resulted in apoptosis of xenograft tumors in mice (Powers et al., 2002). Different laboratories have shown the significance of PTN as a growth and survival factor for different solid tumors, including melanoma (Czubayko et al., 1996), pancreatic cancer (Weber et al., 2000), glioblastoma (Grzelinski et al., 2006) and multiple myeloma (Chen et al., 2007).
Here, we describe human single-chain variable fragment (scFv) antibodies targeted against the LBD of ALK and show their ability to inhibit the PTN/ALK interaction in intact cells as well as PTN signaling. We also show the inhibition of invasion of U87MG cells into an intact endothelial monolayer and report that the growth of established tumors in mice was inhibited by the anti-ALK scFv. This shows a rate-limiting function of the PTN/ALK axis. ALK and PTN were expressed at significantly higher levels in human brain tumors in comparison to normal brain tissues, and analysis of published data corroborated this finding and showed that PTN, MK and ALK, but not PTPz, overexpression correlate with poor outcome of the disease.
Results
Predicted structure of the ALK ligand-binding domain
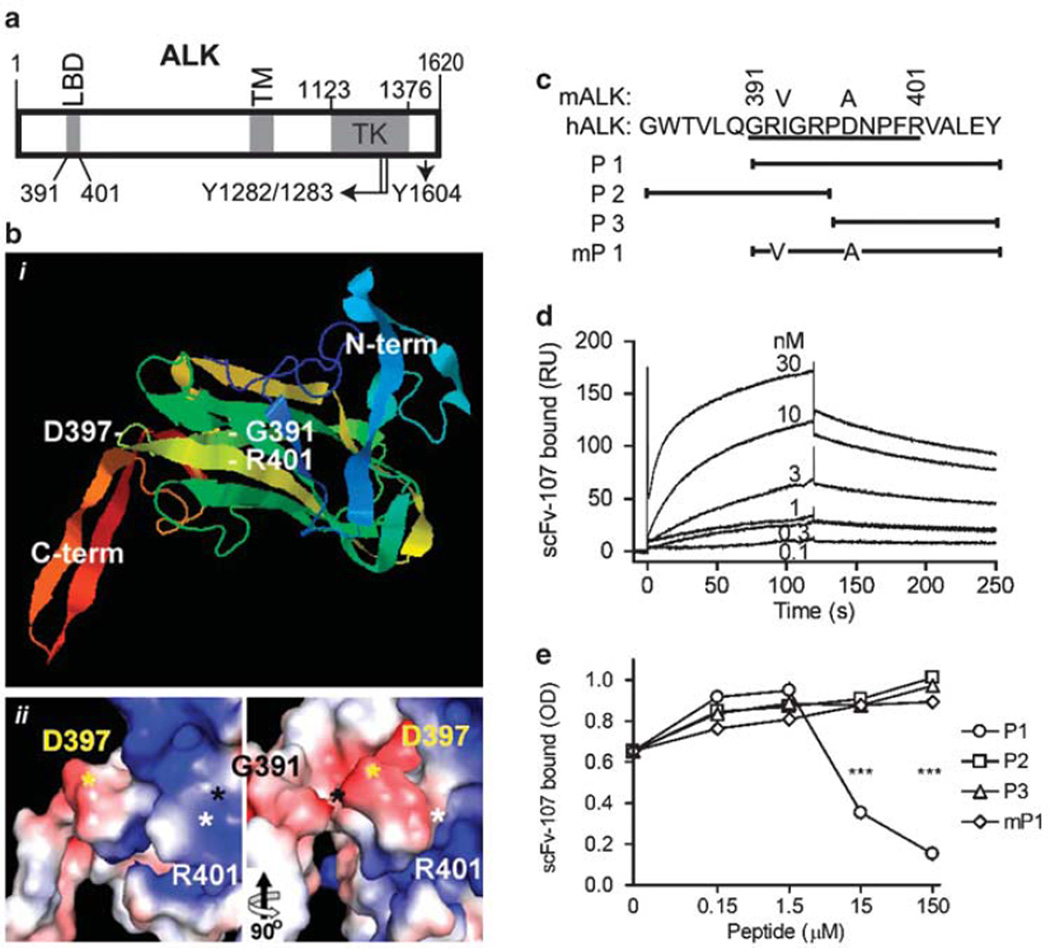
The LBD comprises amino acids 391–401 in the extracellular domain of ALK (Figure la) (Stoica et al., 2001). To gain more insight into the structure surrounding the LBD, we used the Protein Homology/analogY Recognition Engine. The search engine threaded ALK amino acids 261–468 onto chain A of receptor-type protein tyrosine phosphatase mu (RPTPµ; c2c9aA) with high confidence (E-value of 2.4 × 1012; 100% precision). On the basis of this analysis, the LBD is exposed on the protein surface (Figure 1bi) and positioned within the protein–protein interaction domain of RPTPµ (Aricescu et al., 2007). The residues surrounding D397 are predicted to be exposed on the surface, whereas G391 appears hidden (Figure 1bii).
Figure 1.
Anaplastic lymphoma kinase (ALK) domains and binding of single-chain variable fragment (scFv)-107. (a) Human ALK protein domains with the amino-acid boundaries (GenBank # NP004295). Y1282/1283 are consensus phosphorylation sites also present in the insulin-receptor family (Miura et al., 1995), and Y1604 is in the PLC-γ binding site in ALK. (b) Structure prediction of the ligand-binding domain (LBD) using the Protein Homology/analogY Recognition Engine (PHYRE). Amino acid R261 to S468 of ALK were threaded by the search engine onto chain A of receptor-type protein tyrosine phosphataseµ (c2c9aA) (Aricescu et al., 2007). The predicted structure is shown: (i) ribbon diagram; (ii) PyMOL software calculated surface at two 90° rotated viewing angles. The boundaries of the LBD (G391–R401) as well as the N- and C-terminus are indicated. R401 (white) and D397 (yellow) are positioned on the surface in contrast to G391 (black). (c) Amino-acid sequences of the human ALK LBD (underlined) and of peptides used to characterize scFv-107 binding; mALK and murine P1 (mP1) refer to the mouse sequence of ALK (GenBank # NP031465). (d) LBD binding to scFv-107 by surface plasmon resonance. scFv-107 was used as the analyte from 1 nm to 1 µm. The KD was determined as 1.14 nm. (e) Competition of different peptides for binding of scFv-107 to immobilized glutathione S-transferase-Pl. One representative of two independent experiments with mean ± s.e. of triplicate values is shown. ***P<0.001 relative to scFv bound in the presence of P2, P3 or mPl. OD, optical density.
Selection of anti-ALK scFvs
The LBD identified from a cDNA library phage display against immobilized PTN indicated that at least one binding site of the ligand was a linear epitope. Thus, we used a yeast two-hybrid approach to select scFvs that recognize this LBD epitope and hypothesized that such scFvs may show inhibitory effects on the ligand/receptor interaction. scFvs were initially selected from a library of randomized complementarity-determining region 3 (CDR-3) of the heavy chain (Auf der Maur et al., 2002). The library was constructed within an scFv framework (FW) (Auf der Maur et al., 2004) that served as the negative control in the experiments reported here. The bait was a C-terminal fusion of the LBD of ALK (P1; Figure 1c) with LexA. After the initial selection, the scFv with the strongest binding (scFv-14) was affinity matured by random mutagenesis in the CDR-3 of the light chain and scFv-107 was derived from this screen.
Binding affinity of scFv-107
The binding kinetics of C-terminally His-tagged scFv-107 to a glutathione S-transferase (GST)-P1 fusion protein (see Figure 1c) was monitored by surface plasmon resonance. This analysis gave an equilibrium dissociation constant (KD) of 1.14 nm (Figure 1d) with the negative control scFv-FW showing no detectable binding. In addition, GST without the P1 fragment did not bind to scFv-107 (data not shown). This nanomolar KD is within the range of affinities reported for scFvs used as receptor blocking agents (Tang et al., 2007) as well as scFvs used as chimeric partners in immunotoxins (Ho et al., 2006).
Binding epitope of scFv-107 on ALK
To narrow down the binding epitope for scFv-107 in the LBD, enzyme-linked immunosorbent assays with four synthetic peptides were used: P1, the 16 amino-acid LBD from human ALK that served as the bait during screening, two 11-mer peptides that contain either the five N-terminal or the 11 C-terminal amino acids of P1 and the mouse homolog of P1 (mP1) (Figure 1c). scFv-107 bound to the immobilized P1 but not to the other peptides (data not shown). Competition of the different peptides for binding of scFv-107 to immobilized GST-P1 showed that the P1 peptide inhibited scFv-107 binding dose-dependently (Figure 1e). Murine P1 (mPl), which differs from the human sequence by two amino acids (I393V and D397A; Figure 1c), did not compete for antibody binding to the human P1. This suggests that the scFv epitope in the human ALK LBD is present in the sequence portion that is distinct from the murine ALK. In addition, the P2 and P3 peptides did not compete for scFv-107 binding to GST-P1, suggesting that the epitope overlaps with the junction of P2 and P3. We conclude that the scFv-107 epitope is between R392 and P399 of the human ALK LBD on the protein’s surface (Figure 1b). Interestingly, sequence analysis of orthologs showed a conservative amino acid replacement of human and monkey D397 in canine, bovine, equine and rat ALK by E397 in contrast to the murine A397.
Antigen recognition by scFv produced in bacteria and mammalian cells
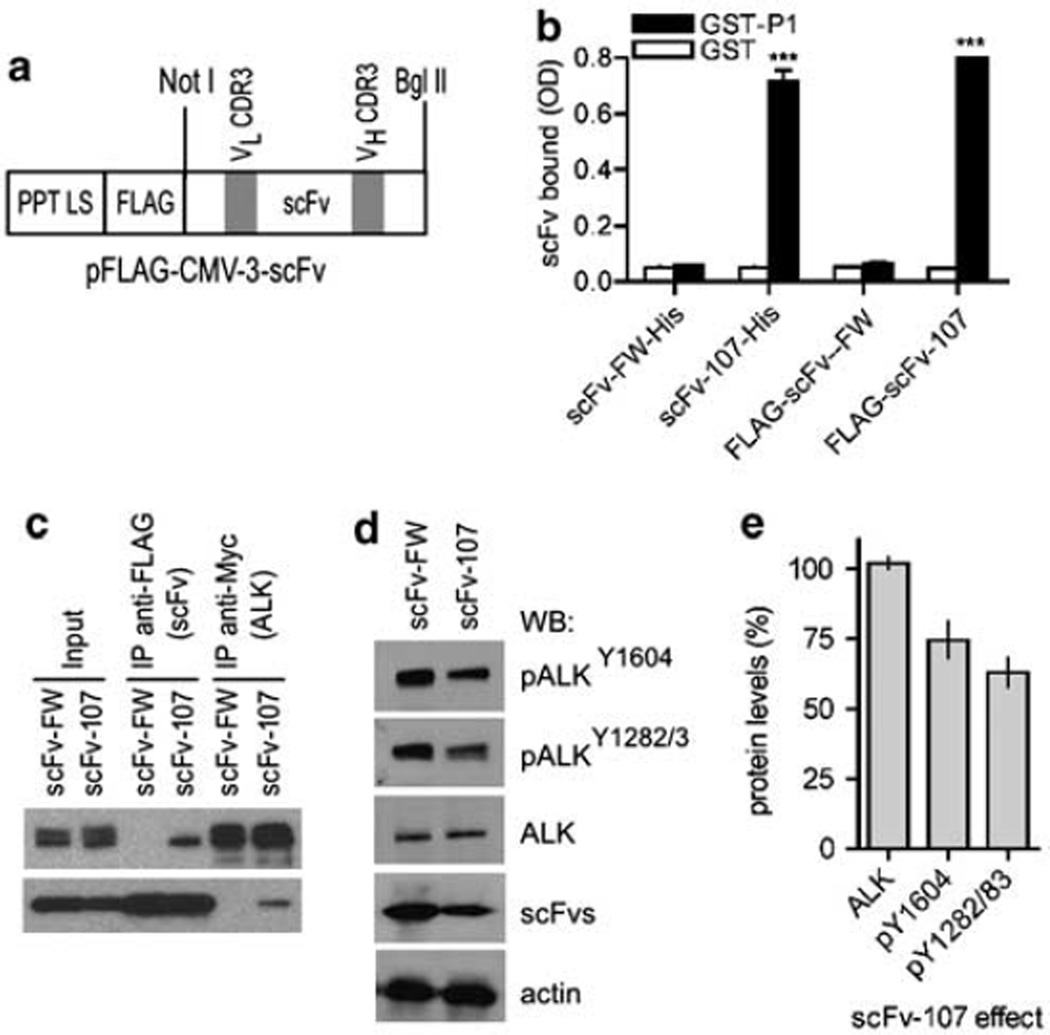
For further mechanistic studies in mammalian cells, scFv-107 and the control scFv-FW were subcloned into a eukaryotic expression vector that includes an N-terminal FLAG tag and secretory signal sequence (Figure 2a). A comparison of FLAG-scFv-107 harvested from supernatants of transiently transfected HEK293T cells and the bacterially produced scFv-107-His showed that both scFvs bound to immobilized GST-P1 but not to GST (Figure 2b). Neither of the control scFv-FWs bound to immobilized GST-P1 or GST (Figure 2b). In addition, scFv-107-His competed with the FLAG-scFv-107 for binding to GST-P1, whereas the control scFv-FW-His did not compete (not shown). Thus, scFv-107 from mammalian cells or bacteria is indistinguishable in their antigen recognition.
Figure 2.
Single-chain variable fragment (scFv) interaction with anaplastic lymphoma kinase (ALK). (a) scFv domains and eukaryotic expression vector. The N-terminal preprotrypsin leader sequence (PPT LS) and FLAG tag are indicated. VL and VH complementarity-determining region 3 (CDR-3) are variable light and heavy chain CDR-3. scFv-107 is targeted to ALK, whereas scFv-framework (FW) is the negative framework control. (b) Comparison of mammalian and bacterially produced scFvs. Binding of scFvs generated in mammalian cells (FLAG-tag) or bacteria (His-tag) to the P1 epitope in ALK is shown. Glutathione S-transferase (GST) or GST-P1 served as the immobilized bait (mean + s.e. Optical density (OD) readings from triplicate wells; ***P<0.001 relative to GST). (c–e) Interaction of scFv and ALK in intact human cells. (c) HEK293T cells co-transfected with C-terminally Myc-tagged ALK (Kuo et al., 2007) and FLAG-scFv were immunoprecipitated for scFV (anti-FLAG) or ALK (anti-Myc) and immunoblotted to detect ALK and scFvs, respectively. Quantitation of the blots from two independent experiments showed the relative amount of ALK immunoprecipitated as 0.356 ±0.085 for scFv-107 versus 0.062±0.039 for the negative control scFv-FW. The relative amounts of scFv pulled down by the immunoprecipitated ALK was 0.640±0.041 for scFv-107 and 0.038±0.008 for the negative control scFv-FW (P<0.01 for both). (d and e) ALK phosphorylation in lysates from HEK293T cells transfected with ALK and scFv-107 or the negative control scFv-FW. A representative blot and quantitation from duplicate experiments is shown.
Interaction of scFv-107 and ALK in intact cells
In an initial series of experiments, lysates of HEK293T cells co-transfected with expression vectors for ALK and scFv were immunprecipitated for either the scFv or for ALK using agarose-conjugated antibodies to the respective tags in scFv (FLAG) or in ALK (C-terminal Myc tag). Immunoblot analysis showed that ALK pulled down scFv-107 and scFv-107 immunoprecipitated full-length ALK (Figure 2c). The control scFv-FW did not bind to ALK in these assays. ALK is autopho-sphorylated when the receptor is overexpressed in cells (Kuo et al., 2007). Co-transfection of scFv-107 with ALK decreased ALK phosphorylation at Y1604 (the PLC-γ binding site) and Y1282/3 (in the catalytic domain) relative to the control scFv-FW (Figures 2d and e).
Competition of PTN and scFv-107 for binding to ALK in intact cells
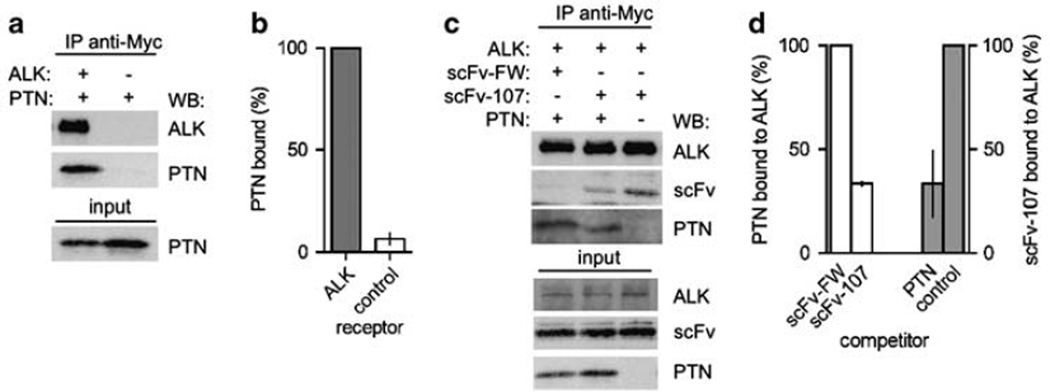
Earlier studies showed binding of radiolabeled PTN to ALK expressed in 32D cells (Stoica et al., 2001), and we first evaluated the binding of PTN to ALK in the HEK293T cell system used here. Expression of ALK and PTN with subsequent immunoprecipitation for ALK (through the C-terminal Myc tag) and immuno-blots for PTN confirmed the ligand–receptor interaction (Figures 3a and b). Competition between PTN and scFv-107 for binding to ALK was then tested in co-transfection assays of ALK, PTN and scFv-107 or the negative control scFv-FW. For this, ALK was immu-noprecipitated from transfected cells and the amounts of PTN or scFv captured by the immunoprecipitations were evaluated by immunoblotting. A representative blot (Figure 3c) and quantitation of independent repeat experiments (Figure 3d) show that scFv-107 and PTN mutually compete for binding to ALK. We conclude from this that scFv-107 binds to the ALK LBD and inhibits the binding of PTN to ALK in intact cells.
Figure 3.
Anaplastic lymphoma kinase (ALK), pleiotrophin (PTN) and single-chain variable fragment (scFv) interaction in transfected HEK293T cells. (a, b) Binding of PTN to ALK. A representative blot (a) and quantitation (b) from duplicate experiments is shown. (c and d) Competition of PTN and scFv for binding to ALK. A representative blot (c) and quantitation (d) of PTN (white bars) or scFv-107 (gray bars) bound to immunoprecipitated ALK is shown from duplicate experiments. Lysates of cells co-transfected with ALK, FLAG-scFv (see Figure 2c) or PTN were immunoprecipitated with the antibodies indicated and immunoblotted to detect ALK, scFvs or PTN, respectively.
Inhibition of ALK signaling by scFv-107
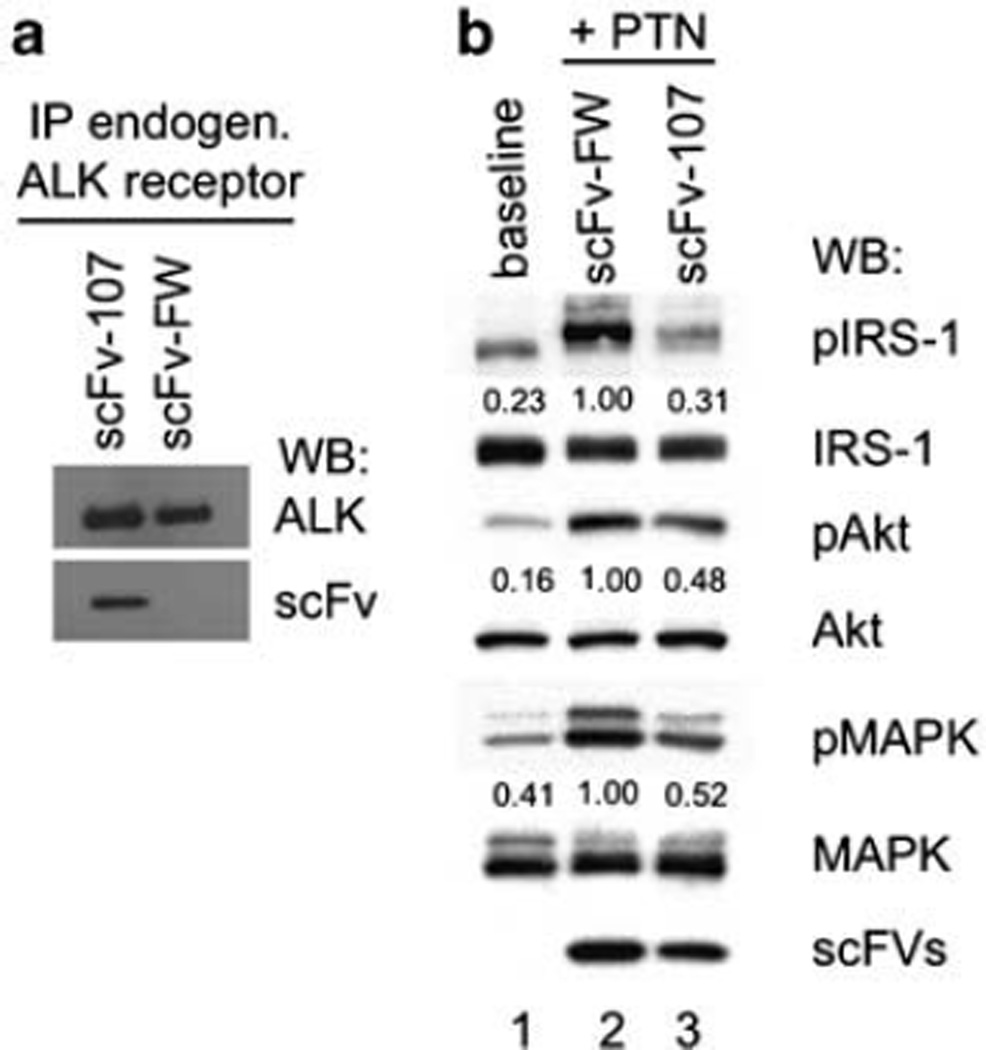
Downstream signaling of PTN through the ALK receptor was evaluated in U138MG cells that express ALK but not PTPz (see below) as described by others (Lu et al., 2005). These cells were incubated with scFv proteins and ALK immunoprecipitates from the cells blotted for scFv and for ALK. ALK only pulled down the anti-ALK scFv-107 and not the control scFv-FW demonstrating the binding of scFv-107 to endogenous ALK (Figure 3a). We found that U138MG cells have low baseline level of pIRSl, pAkt and pMAPK (Figure 4b, lane 1) that were not altered by scFv-FW or scFv-107 (not shown). Addition of PTN to the cells induced phosphorylation of IRS-1, Akt and MAPK, and the presence of the anti-ALK scFv-107 inhibited this PTN signaling (Figure 4b, lane 2 versus lane 3). We conclude from these data that the anti-ALK scFv-107 inhibits signaling by PTN.
Figure 4.
Effect of single-chain variable fragment (scFv) on pleiotrophin (PTN) signaling through endogenous anaplastic lymphoma kinase (ALK) in U138MG cells. (a) Binding of scFv to endogenous ALK in intact cells. U138MG cells were incubated with scFvs and ALK was immunoprecipitated. ALK and scFvs in the precipitates were detected by immunoblots as indicated. (b) PTN signaling. U138MG cells were transiently transfected with the anti-ALK scFv-107 or the control scFv-framework (FW), starved for 48 h and stimulated with exogenously added PTN for 15 min. Cell lysates were immunoblotted for the phospho-proteins pIRS-1Y612, pAktS473 and pMAPKT202/Y204 as well as the respective total proteins. Quantitation of phospho-proteins by densitometry is given relative to PTN stimulated cells transfected with the control scFv-FW.
Effect of scFv-107 on glioblastoma cell invasion of an endothelial monolayer
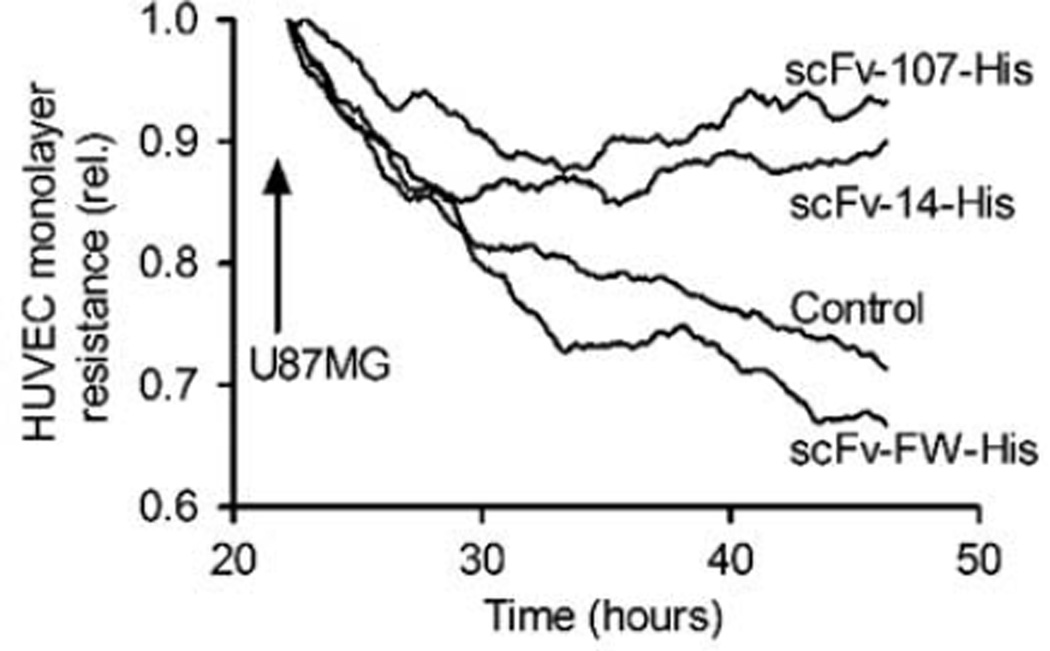
The invasive capability of cells is a hallmark of malignancy and we thus studied whether antibody targeting of ALK would affect tumor cell invasion by U87MG human glioblastoma cells. In a co-culture system, a confluent endothelial cell monolayer was exposed to tumor cells (Figure 5) and electrical resistance of the monolayer was monitored as an indicator of invasion (Keese et al., 2002). U87MG cells showed a continuous invasion reflected in decreasing resistance of the endothelial monolayer over a 10-h period (Figure 5, control). Inclusion of either anti-ALK scFv-14 or scFv-107 prevented this invasion, whereas the control scFv-FW had no significant effect. The low-affinity scFv-14 was less effective than the high-affinity scFv-107. The anti-ALK scFv-107 treatments had no significant effect on cell growth of cultured U87MG cells (data not shown) We conclude that U87MG tumor cell invasion of stromal tissues can be inhibited by targeting of the PTN/ALK interaction.
Figure 5.
Effects of single-chain variable fragments (scFvs) on U87MG tumor cell invasion of a human endothelial cell (HUVEC) monolayer. Electrical resistance of endothelial cells grown to a confluent monolayer was followed after addition of U87MG cells in the presence or absence (control) of different purified scFvs: Non-specific scFv-framework (FW), anti-anaplastic lymphoma kinase low-affinity scFv-14 and high-affinity scFv-107. One representative of two independent experiments, each done in duplicate, is shown.
Conditional expression of scFv
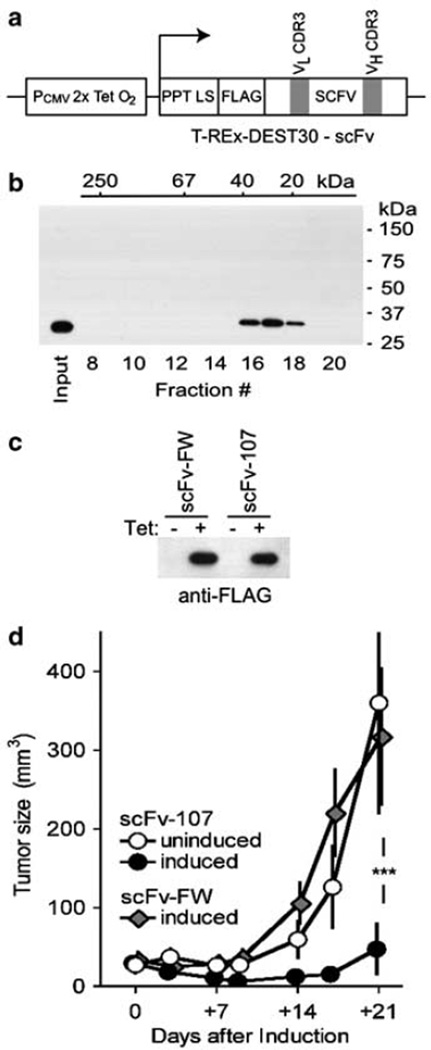
For tumor growth studies in mice, we generated U87MG tumor cells that express the scFvs under the control of a tetracycline-inducible promoter (Figure 6a). As scFvs secreted from cells may form aggregates that affect the activity, native gel filtration was used to analyse an aliquot of scFv-107 protein harvested from the growth media of transfected cells (Figure 6b). The scFv protein eluted as a single peak at ~ 30 kDa close to the predicted molecular weight of a monomeric scFv and the apparent mass observed in the SDS-polyacry-lamide gel elactrophoresis. The elution of the scFv from the gel filtration column at the predicted mass also suggests that the protein does not bind to serum proteins that were present at high abundance in the sample.
Figure 6.
Single-chain variable fragment (scFv)-107 expression inhibits anaplastic lymphoma kinase (ALK)-dependent tumor growth in vivo. (a) Diagram of the tetracycline-inducible scFv expression vector containing either the anti-ALK scFv-107 or the negative control scFv-framework (FW). (b) Size fractionation of supernatants from scFv-107 expressing cells. Immunoblot for anti-FLAG of aliquots of fractions eluted from a Superose 12 gel filtration column. The molecular mass calibration is indicated along the top abscissa. (c) Inducible scFv protein secretion into the supernatants of stably transfected U87MG cell lines by anti-FLAG blot of cell supernatants −/+ tetracycline (Tet) treatment (1 µg/ml). (d) Xenograft tumor growth of U87MG cells. Tumors were allowed to establish and tumor volumes (mm3) followed without and after tetracycline treatment of animals to induce expression of scFV-107 or scFV-FW in the tumor cells. One of two independent experiments with mean±s.e. is shown. ***P<0.001 for ‘induced scFv-107’ (n = 18) versus ‘induced scFv-FW (n = 16) or versus ‘uninduced scFv-107’ (n = 9). No significant difference (P > 0.05) was found between ‘uninduced scFv-107’ versus ‘induced scFv-FW’.
Mass transfected U87MG tumor cell lines were found to secrete equal amounts of control scFv-FW or anti-ALK scFv-107 into the extracellular milieu on induction with tetracycline (Figure 6c). The concentration of scFvs detected in the supernatants was 4–10 nm, and thus > 3-fold of the KD of scFv-107 for its target. The U87MG cell lines were also examined for their proliferation rates and colony formation in soft agar, and no significant differences were observed without or with the expression of either of the scFvs (data not shown) in agreement with earlier observations with depletion of endogenous ALK from these cells (Powers et al., 2002).
Inhibition of ALK-dependent tumor growth by scFv-107
To monitor the effect of ALK targeting on tumor growth, U87MG cells containing the inducible scFv expression vectors were grown as xenografts in athymic nude mice. After measurable tumors were established, expression of the scFvs was induced in a subset of animals by switching them to a diet containing doxycycline. Tumor sizes in the different groups of animals relative to the time point of initiation of scFv expression are shown in Figure 6d. Induction of expression of the control scFv-FW had no effect on tumor growth when compared with the uninduced U87MG cells carrying the anti-ALK scFv-107. Tumor sizes increased exponentially in both groups by approximately 10-fold over time. In contrast, tumors induced to express scFv-107 initially shrank (P<0.05) and were significantly smaller than the controls at all time points monitored (P<0.001). Commensurate with this, analysis of tumor extracts harvested at 3 and 6 days after the induction of scFv expression showed increased markers of apoptosis in the anti-ALK group (not shown).
ALK and PTN mRNA levels in archival human brain tissues
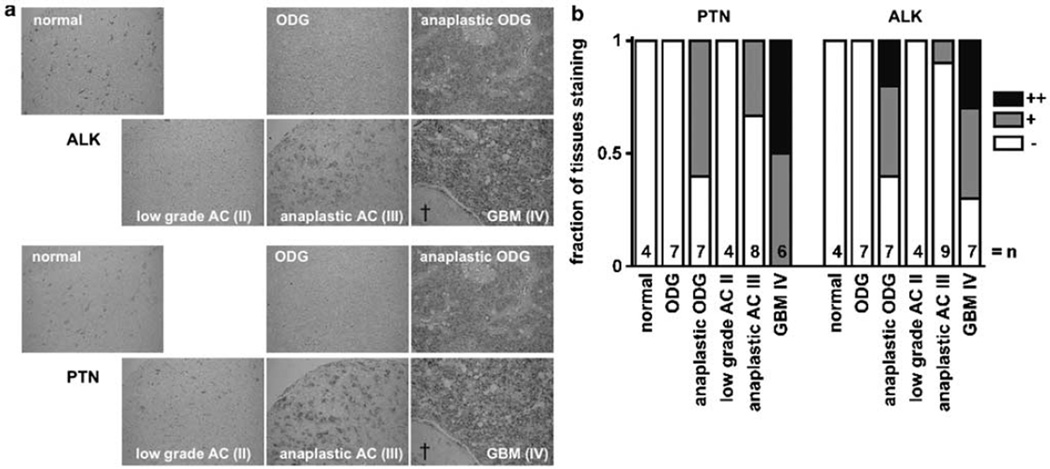
To assess the abundance in tumor versus normal tissues, we evaluated the expression of ALK and PTN mRNA in the normal human brain and 34 human glial tumor tissues (Figure 7). The specimens were serial sections of tissue microarrays that allow for simultaneous staining of a series of samples and thus a direct comparison of staining intensities (Henke et al., 2006). We detected ALK expression in five of seven and PTN in all glioblastoma multiforme (GBM) samples. In addition, in four out of seven anaplastic oligodendroglioma ALK and PTN mRNA were clearly detectable (Figure 7b). Overall, only the most aggressive high-grade tumors, that is GBM and anaplastic oligodendroglioma, showed significantly increased expression of PTN and ALK mRNA relative to normal tissues and to low-grade tumors (P<0.01). There was a positive correlation between ALK and PTN mRNA expression (P< 0.001) that is also visible when superimposing the serial sections of the tissues (see Figure 7a; the ‘†’ indicates a necrotic area that is pathognomonic for the diagnosis of GBM).
Figure 7.
Anaplastic lymphoma kinase (ALK) and pleiotrophin (PTN) expression in archival normal human brain and glial tumor tissues. (a) ALK and PTN mRNA were detected by in situ hybridization of serial sections. The ALK probe for in situ hybridization recognizes only the extracellular domain of ALK. Note the necrotic area (†) pathognomonic for glioblastoma multiforme (GBM). (b) Frequency and level of expression in a series of samples. Protocols and probes used for in situ hybridization were previously described (Powers et al., 2002; Henke et al., 2006). ODG, oligodendroglioma; AC, astrocytoma; II, III, IV = WHO grade.
In silico analysis of PTN, MK, ALK and PTPz expression in brain tumors
In addition to our experimental data (Figure 7), we analysed published data sets and include the results in Table 1. One study reports gene expression levels in normal brain in comparison with different brain tumor tissues (Sun et al., 2006) and shows that PTN, MK and PTPz are significantly upregulated in all brain tumor tissues. ALK was found upregulated in GBM and not in astrocytoma or oligodendroglioma. This corroborates the data in Figure 7. Three additional studies that report survival of patients with brain tumors show significant correlations of high levels of expression of MK, PTN or ALK with poor survival (P-values of 0.03–0.0001; Table 1) (Shai et al., 2003; Freije et al., 2004; Phillips et al., 2006). It is tempting to speculate that inhibition of their activity would improve patient survival. In contrast, the upregulation of PTPz in brain tumors relative to normal brain (P<0.0001; Table 1) was not correlated with the outcome in these studies, suggesting that it may not be a significant driver of tumor progression.
Table 1.
Expression of PTN, MK, ALK and PTPz mRNA in normal brain versus brain tumor tissues and correlation with patient survival
|
Expression levels relative to normal brain (n = 23) (Sun et al., 2006;) |
Correlation survival and brain tumor gene expression (n: alive vs dead) |
|||||
|---|---|---|---|---|---|---|
| AC | ODG | GBM | Shai et al. (2003) | Freije et al. (2004) | Phillips et al. (2006) | |
| n | 26 | 50 | 77 | 9 vs 20 | 13 vs 56 | 13 vs 58 |
| PTN | 0.0004 | <0.0001 | <0.0001 | NS | 0.002 | 0.0001 |
| MK | <0.0001 | 0.0001 | <0.0001 | 0.03 | 0.0003 | 0.014 |
| ALK | NS | NS | 0.013 | 0.003 | NS | NS |
| PTPz | <0.0001 | <0.0001 | <0.0001 | NS | NS | NS |
Abbreviations: AC, astrocytoma; ALK, anaplastic lymphoma kinase; GBM, glioblastoma multiforme; MK, midkine; NS, not significant; ODG, oligodendroglioma; PTN, pleiotrophin.
Data from published studies were analysed using the Oncomine database (Rhodes et al., 2004). The number of patient samples in each group and the P-value for the respective comparisons are indicated.
Discussion
Pleiotrophin and ALK are highly expressed in the nervous system during development (see Introduction), and the increased expression of PTN and ALK with more advanced and undifferentiated stages in cancers of glial origin reported here indicates a reversion of tumor cells to a precursor phenotype seen during development. Recent studies also show a significant function of the PTN/ALK interaction during neuronal injury (Mi et al., 2007) and thus connect this growth factor/receptor pathway to adult tissue repair. Interestingly, germ-line inactivation of ALK, PTN or MK did not affect animal survival, although a more detailed analysis showed alterations in animal behavior and fertility (Muramatsu et al., 2006; Zou et al., 2006; Bilsland et al., 2007; reviewed in Li and Morris, 2007). Overall, this suggests that therapeutic targeting of the PTN/ALK pathway in cancers may not cause severe pathologies in adults.
In addition to the binding to the ALK tyrosine kinase, PTN and its relative MK also were reported to bind to a receptor-type protein tyrosine phosphatase PTPz, PTPRZ1 or RPTβ/ζ (Maeda et al., 1996, 1999; Maeda and Noda, 1998). The study from TF Deuel’s laboratory showed that PTN inactivates PTPz thereby controlling the activity of tyrosine kinases (Meng et al., 2000; Perez-Pinera et al., 2006), including ALK signaling (Perez-Pinera et al., 2007). Furthermore, the PTPz/MK interaction induces osteoblast cell migration and neuronal survival (Owada et al., 1999; Qi et al., 2001; Sakaguchi et al., 2003). Interestingly, two isoforms of PTN (18 and 15 kDa) were identified more recently and seem to have distinct functions and signaling paths with respect to ALK and PTPz (Lu et al., 2005). A Höke’s laboratory evaluated the contribution of PTN and potential receptors to neuronal repair (Mi et al., 2007), and observed an upregulation of PTN and ALK during spinal motor neuron denervation. Antibody blockade showed that ALK is the mediator of trophic activities of PTN in the motor neurons. Overall, these reports suggest a crucial function of the PTN/ ALK axis in the repair of nervous system tissues and a potential cross talk with the receptor tyrosine phospatase PTPz.
Several monoclonal anti-ALK antibodies have been reported to function as agonists and stimulate signaling as well as biologic effects through ALK (Moog-Lutz et al., 2005; Mathivet et al., 2007). These reports did not find a competition with receptor binding or inhibition of signaling by the PTN ligand and suggested that receptor dimerization induced the signaling. We had used an ALK fragment that contains the LBD to generate inhibitory antibodies and reported that polyclonal anti-ALK LBD antibodies generated in rabbits inhibit PTN binding to the ALK receptor (Figure 2c, Stoica et al., 2001). The polyclonal antibody partially inhibited the effect of PTN in NIH3T3 cells and functioned as a partial agonist (Bowden et al., 2002). In contrast, mouse monoclonal antibodies generated against the ALK LBD inhibited the binding of MK to ALK and effects of MK in cells but showed no agonist effect (Stoica et al., 2002). The scFv reported here had no discernible agonist activity in different assays and we show the inhibition of PTN signaling and of PTN binding to the ALK receptor. For the current scFv, we have defined the epitope very closely (see Figure 1) and found that it overlaps with antagonist mAbs (AW unpublished data). We conclude, that the epitope recognized by a particular antibody is a significant contributing factor to its function as an agonist or inhibitor of receptor signaling.
Pleiotrophin and MK are heparin-binding growth factors and their interaction with proteoglycans affects their biologic activity (Li et al., 2007; Sugahara and Mikami, 2007) reminiscent of the effect of glycosami-noglycans fibroblast growth factor (FGF) signaling (reviewed in Powers et al., 2000). Complementary to this, a recent study showed that a peptide fragment of PTN containing amino acids 65–97 binds to the glycosaminoglycan co-receptor of FGF2 and PTN and inhibits the mitogenic and tumorigenic activities of both growth factors (Hamma-Kourbali et al., 2008). This finding may explain the dominant-negative effect of PTN fragments described earlier (Zhang et al., 1997; Chang et al., 2006). Chondroitin and dermatan sulfates (CS/DS) can also capture PTN and present it to high-affinity receptors (Bao et al., 2005), and more recent studies demonstrate that PTN is dependent on CS/DS binding for its neurite outgrowth activity (Li et al., 2007; Sugahara and Mikami, 2007). Antibodies against PTN or ALK inhibited the neuritogenesis induced by growth factors bound to the CS/DS chains and showed the involvement of PTN and ALK. It is noteworthy that the aforementioned receptor tyrosine phosphatase PTPz is a CS proteoglycan, which may explain the tight binding of PTN or MK and its function in growth factor signaling.
In conclusion, antibody targeting of the ALK receptor interaction with its ligands PTN or MK may provide a new, mechanism-based approach to the treatment of tumors that show high expression levels of the growth factor/receptor pairs.
Materials and methods
Cell lines
SW13, U87MG, U138MG and HEK293T were from the American Type Culture Collection and HUVEC from Cambrex Biosciences (Walkersville, MD, USA).
Computer modeling
The protein sequence of ALK covering amino acids 201–600 (GenBank # NP004295) was submitted to the Protein Homology/analogY Recognition Engine (PHYRE; Imperial College, London, UK). The structure model generated by PHYRE is displayed as a ribbon as well as calculated surface (PyMOL; DeLano Scientific, Palo Alto, CA, USA). Annotations are provided in the legend to Figure 1b.
scFv generation
Residues in the complementarity-determining region 3 of the heavy chain (CDR-H3) generally contribute the most substantial contacts to the antigen (Chothia et al., 1985; Chothia and Lesk, 1987; Padlan, 1994). We thus applied our yeast two-hybrid, antigen–antibody interaction screening technology to isolate antigen-binding scFvs by screening a human scFv library of randomized synthetic CDR-H3 sequences (Auf der Maur et al., 2002). The library was constructed on an scFv-FW that had been previously isolated by a yeast screening system (Auf der Maur et al., 2001, 2004). This antigen-independent intrabody selection system identifies from a natural pool of human variable-light (VL) and variable-heavy (VH) chains combinations with favorable stability, solubility and expression yield. FW consists of a VL domain (lambda1) connected by a flexible glycine-serine linker (GGGGS)4 to a VH3 domain. The CDR-H3 of FW comprises 13 amino acids (DAGIA-VAGTGFDY). To construct the library, the central part of the CDR-H3 (DAXXXXXXXGFDY) was randomized by PCR using a degenerated oligonucleotide. The last two residues were kept constant because of their structural importance (Chothia and Lesk, 1987). The scFv library was cloned in a yeast expression vector (pLibl) as a C-terminal fusion to the transcriptional activation domain of Gal4 (Auf der Maur et al., 2002).
A 16 amino acid peptide (P1; see Figure 1c) containing the LBD of ALK (Stoica et al., 2001) was cloned into a yeast expression vector (pBaitl) as a C-terminal fusion to the DNA-binding protein LexA. The reporter yeast strain YDE173 (Auf der Maur et al., 2002) containing the stably integrated reporter genes HIS3 and lacZ under the control of six LexA-binding sites was transformed with the bait vector together with the random CDR-H3 scFv library. Transformed cells were selected on plates lacking histidine and containing 2.5 mm 3-amino-triazole. Growing colonies were picked over a period of 6 days and the library plasmids were isolated. The same reporter strain was transformed with the rescued plasmids to confirm antigen-dependent gene activation.
To obtain an scFv with higher affinity, this primary binder was subjected to an affinity maturation process by mutagenesis and a second screening round in yeast. Mutagenesis of the primary binder for affinity maturation was accomplished by randomizing parts of the CDR-3 within the variable light chain. This was performed directly in yeast by homologous recombination (Schaerer-Brodbeck and Barberis, 2004). The CDR-L3 of FW comprises 11 amino acids (GTWDSSLSGVV). The first two positions were partially randomized such that the first position either encodes Y, A or Q and the second position encodes T, S or A. At the positions 5–8, all amino-acid residues were allowed. The remaining positions were kept constant. Randomization was introduced by PCR. The resulting PCR product had a size of 356 bp and comprised the randomized CDR cassette with 267 bp upstream and 27 bp downstream framework sequences. scFv-107 resulted from this screen.
Surface plasmon resonance
Glutathione S-transferase-P1 was coupled to CM5 sensor chips (Biacore/GE Healthcare, Piscataway, NJ, USA) with a surface density of 500 RU by standard amine coupling. scFvs were used as analyte in 10 mm Hepes, 0.15 m NaCl, 3.4 mm EDTA, 0.005% surfactant P20, pH 7.4. Regeneration of the protein-binding surface was done with 10 mm glycine pH 2.5 + 0.05% SDS. All samples were run in duplicate in two independent experiments.
Invasion assays
HUVECs at 1.6 × 106 cells/ml were plated in wells of the electrical cell-substrate impedance sensor system (Applied Biophysics, Troy, NY, USA; 8W10E). After a confluent monolayer was established, 105 U87MG cells were added to each well ± scFvs at 0.052mg/ml in duplicate wells for each. Results are shown as normalized resistance over time from the time the U87MG cells were added.
Plasmid constructs, transfections and immunodetection
For scFv protein production in mammalian cells, the scFvs cDNAs were cloned into the Not I and Bgl II sites of pFLAG-CMV-3 vector (see Figure 2a; Sigma-Aldrich, St Louis, MO, USA) without the C-terminal His-tag of the scFvs used for bacterial expression. For tetracycline-inducible expression, the scFvs were cloned into pTREx-DEST30 (see Figure 4a; Invitrogen, Carlsbad, CA, USA). To obtain tetracycline-inducible expression of control or anti-ALK scFv, U87MG cells were first transfected with pcDNA6/TR (Invitrogen) and selected in blasticidin (25 mg/ml) and then with pTRex-scFv (selection with Geneticin, 75 mg/ml). HEK293T or U138MG cells were transiently transfected for 24 h with FLAG-tagged scFv and Myc-tagged ALK (Kuo et al., 2007) or other expression vectors as indicated. Cells lysates and blots were prepared as described (Powers et al., 2002). Antibodies were anti-FLAG M2-horseradish peroxidase (HRP) and mouse mAb anti-Myc clone 9E10 (Sigma-Aldrich), rabbit anti-ALK (Zymed, South San Francisco, CA, USA), mouse mAb anti-actin (Chemicon, Los Angeles, CA, USA), donkey anti-mouse HRP and anti-rabbit-HRP (Amersham, Pittsburg, PA, USA).
Gel filtration analysis
Single-chain variable fragment protein harvested from super-natants of transfected cells was subjected to gel filtration under native, isotonic conditions without detergent. A Superose 12 column (Pharmacia/GE Heatlthcare, Piscataaway, NJ, USA) equilibrated with phosphate-buffered saline (150 mm, pH 7.4) was used as described earlier (Wellstein et al., 1992).
Tumor xenograft studies
Tumor cells were implanted and tumor growth was monitored as described (Powers et al., 2002). Mass-transfected U87MG cell lines expressing tetracycline-regulated scFv expression vectors were used; U87MG with control scFv-FW or with anti-ALK scFv-107. For each tumor cell line, at least 15 animals were inoculated with tumor cells. Tumor growth was followed till measurable tumors were detected, and animals were then separated into two subsets of equal tumor sizes without or with the induction of scFv expression by switching to a doxycycline-supplemented diet (Bio-Serv, Frenchtown, NJ, USA).
Acknowledgements
We thank Drs Emma T Bowden, Angera H Kuo and Gerald E Stoica (Georgetown University) for help with some of the experiments as well as discussions and suggestions. This work was supported by RO1 CA108440 from the National Institute of Health (to AW).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Aricescu AR, Siebold C, Choudhuri K, Chang VT, Lu W, Davis SJ, et al. Structure of a tyrosine phosphatase adhesive interaction reveals a spacer-clamp mechanism. Science. 2007;317:1217–1220. doi: 10.1126/science.1144646. [DOI] [PubMed] [Google Scholar]
- Auf der Maur A, Escher D, Barberis A. Antigen-independent selection of stable intracellular single-chain antibodies. FEBS Lett. 2001;508:407–412. doi: 10.1016/s0014-5793(01)03101-5. [DOI] [PubMed] [Google Scholar]
- Auf der Maur A, Tissot K, Barberis A. Antigen-independent selection of intracellular stable antibody frameworks. Methods. 2004;34:215–224. doi: 10.1016/j.ymeth.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Auf der Maur A, Zahnd C, Fischer F, Spinelli S, Honegger A, Cambillau C, et al. Direct in vivo screening of intrabody libraries constructed on a highly stable single-chain framework. J Biol Chem. 2002;277:45075–45085. doi: 10.1074/jbc.M205264200. [DOI] [PubMed] [Google Scholar]
- Bao X, Mikami T, Yamada S, Faissner A, Muramatsu T, Sugahara K. Heparin-binding growth factor, pleiotrophin, mediates neuritogenic activity of embryonic pig brain-derived chondroitin sulfate/dermatan sulfate hybrid chains. J Biol Chem. 2005;280:9180–9191. doi: 10.1074/jbc.M413423200. [DOI] [PubMed] [Google Scholar]
- Bilsland JG, Wheeldon A, Mead A, Znamenskiy P, Almond S, Waters KA, et al. Behavioral and neurochemical alterations in mice deficient in anaplastic lymphoma kinase suggest therapeutic potential for psychiatric indications. Neuropsychopharmacology. 2007;33:685–700. doi: 10.1038/sj.npp.1301446. 2008. [DOI] [PubMed] [Google Scholar]
- Bowden ET, Stoica GE, Wellstein A. Anti-apoptotic signaling of pleiotrophin through its receptor, anaplastic lymphoma kinase. J Biol Chem. 2002;277:35862–35868. doi: 10.1074/jbc.M203963200. [DOI] [PubMed] [Google Scholar]
- Chang Y, Berenson JR, Wang Z, Deuel TF. Dominant negative pleiotrophin induces tetraploidy and aneuploidy in U87MG human glioblastoma cells. Biochem Biophys Res Commun. 2006;351:336–339. doi: 10.1016/j.bbrc.2006.09.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Gordon MS, Campbell RA, Li M, Wang CS, Lee HJ, et al. Pleiotrophin is highly expressed by myeloma cells and promotes myeloma tumor growth. Blood. 2007;110:287–295. doi: 10.1182/blood-2006-08-042374. [DOI] [PubMed] [Google Scholar]
- Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8:11–23. doi: 10.1038/nrc2291. [DOI] [PubMed] [Google Scholar]
- Chothia C, Lesk AM. Canonical structures for the hypervariable regions of immunoglobulins. J Mol Biol. 1987;1196:901–917. doi: 10.1016/0022-2836(87)90412-8. [DOI] [PubMed] [Google Scholar]
- Chothia C, Novotny J, Bruccoleri R, Karplus M. Domain association in immunoglobulin molecules. The packing of variable domains. J Mol Biol. 1985;186:651–663. doi: 10.1016/0022-2836(85)90137-8. [DOI] [PubMed] [Google Scholar]
- Czubayko F, Schulte AM, Berchem GJ, Wellstein A. Melanoma angiogenesis and metastasis modulated by ribozyme targeting of the secreted growth factor pleiotrophin. Proc Natl Acad Sci USA. 1996;93:14753–14758. doi: 10.1073/pnas.93.25.14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyster J, Bai RY, Morris SW. Translocations involving anaplastic lymphoma kinase (ALK) Oncogene. 2001;20:5623–5637. doi: 10.1038/sj.onc.1204594. [DOI] [PubMed] [Google Scholar]
- Fang W, Hartmann N, Chow DT, Riegel AT, Wellstein A. Pleiotrophin stimulates fibroblasts and endothelial and epithelial cells and is expressed in human cancer. J Biol Chem. 1992;267:25889–25897. [PubMed] [Google Scholar]
- Freije WA, Castro-Vargas FE, Fang Z, Horvath S, Cloughesy T, Liau LM, et al. Gene expression profiling of gliomas strongly predicts survival. Cancer Res. 2004;64:6503–6510. doi: 10.1158/0008-5472.CAN-04-0452. [DOI] [PubMed] [Google Scholar]
- Grzelinski M, Urban-Klein B, Martens T, Lamszus K, Bakowsky U, Hobel S, et al. RNA interference-mediated gene silencing of pleiotrophin through polyethylenimine-complexed small interfering RNAs in vivo exerts antitumoral effects in glioblastoma xenografts. Hum Gene Ther. 2006;17:751–766. doi: 10.1089/hum.2006.17.751. [DOI] [PubMed] [Google Scholar]
- Hamma-Kourbali Y, Bernard-Pierrot I, Heroult M, Dalle S, Caruelle D, Milhiet PE, et al. Inhibition of the mitogenic, angiogenic and tumorigenic activities of pleiotrophin by a synthetic peptide corresponding to its C-thrombospondin repeat-I domain. J Cell Physiol. 2008;214:250–259. doi: 10.1002/jcp.21191. [DOI] [PubMed] [Google Scholar]
- Henke RT, Eun Kim S, Maitra A, Paik S, Wellstein A. Expression analysis of mRNA in formalin-fixed, paraffin-embedded archival tissues by mRNA in situ hybridization. Methods. 2006;38:253–262. doi: 10.1016/j.ymeth.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Ho M, Nagata S, Pastan I. Isolation of anti-CD22 Fv with high affinity by Fv display on human cells. Proc Natl Acad Sci USA. 2006;103:9637–9642. doi: 10.1073/pnas.0603653103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, et al. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14:439–449. doi: 10.1038/sj.onc.1200849. [DOI] [PubMed] [Google Scholar]
- Keese CR, Bhawe K, Wegener J, Giaever I. Real-time impedance assay to follow the invasive activities of metastatic cells in culture. Biotechniques. 2002;33:842–850. doi: 10.2144/02334rr01. [DOI] [PubMed] [Google Scholar]
- Kuo AH, Stoica GE, Riegel AT, Wellstein A. Recruitment of insulin receptor substrate-1 and activation of NF-kappaB essential for midkine growth signaling through anaplastic lymphoma kinase. Oncogene. 2007;26:859–869. doi: 10.1038/sj.onc.1209840. [DOI] [PubMed] [Google Scholar]
- Li F, Shetty AK, Sugahara K. Neuritogenic activity of chondroitin/dermatan sulfate hybrid chains of embryonic pig brain and their mimicry from shark liver. Involvement of the pleiotrophin and hepatocyte growth factor signaling pathways. J Biol Chem. 2007;282:2956–2966. doi: 10.1074/jbc.M609296200. [DOI] [PubMed] [Google Scholar]
- Li R, Morris SW. Development of anaplastic lymphoma kinase (ALK) small-molecule inhibitors for cancer therapy. Med Res Rev. 2007;28:372–412. doi: 10.1002/med.20109. 2008. [DOI] [PubMed] [Google Scholar]
- Li YS, Milner PG, Chauhan AK, Watson MA, Hoffman RM, Kodner CM, et al. Cloning and expression of a developmentally regulated protein that induces mitogenic and neurite outgrowth activity. Science. 1990;250:1690–1694. doi: 10.1126/science.2270483. [DOI] [PubMed] [Google Scholar]
- Lu KV, Jong KA, Kim GY, Singh J, Dia EQ, Yoshimoto K, et al. Differential induction of glioblastoma migration and growth by two forms of pleiotrophin. J Biol Chem. 2005;280:26953–26964. doi: 10.1074/jbc.M502614200. [DOI] [PubMed] [Google Scholar]
- Maeda N, Ichihara-Tanaka K, Kimura T, Kadomatsu K, Muramatsu T, Noda M. A receptor-like protein-tyrosine phosphatase PTPzeta/RPTPbeta binds a heparin-binding growth factor midkine. Involvement of arginine 78 of midkine in the high affinity binding to PTPzeta. J Biol Chem. 1999;274:12474–12479. doi: 10.1074/jbc.274.18.12474. [DOI] [PubMed] [Google Scholar]
- Maeda N, Nishiwaki T, Shintani T, Hamanaka H, Noda M. 6B4 proteoglycan/phosphacan, an extracellular variant of receptorlike protein-tyrosine phosphatase zeta/RPTPbeta, binds pleiotro-phin/heparin-binding growth-associated molecule (HB-GAM) J Biol Chem. 1996;271:21446–21452. doi: 10.1074/jbc.271.35.21446. [DOI] [PubMed] [Google Scholar]
- Maeda N, Noda M. Involvement of receptor-like protein tyrosine phosphatase zeta/RPTPbeta and its ligand pleiotrophin/ heparin-binding growth-associated molecule (HB-GAM) in neuronal migration. J Cell Biol. 1998;142:203–216. doi: 10.1083/jcb.142.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathivet T, Mazot P, Vigny M. In contrast to agonist monoclonal antibodies, both C-terminal truncated form and full length form of Pleiotrophin failed to activate vertebrate ALK (anaplastic lymphoma kinase)? Cell Signal. 2007;19:2434–2443. doi: 10.1016/j.cellsig.2007.07.011. [DOI] [PubMed] [Google Scholar]
- McCarthy N. ALK takes the rap—editorial. Nat Rev Cancer. 2008;8:1. [Google Scholar]
- Meng K, Rodriguez-Pena A, Dimitrov T, Chen W, Yamin M, Noda M, et al. Pleiotrophin signals increased tyrosine phosphorylation of beta beta-catenin through inactivation of the intrinsic catalytic activity of the receptor-type protein tyrosine phosphatase beta/zeta. Proc Natl Acad Sci USA. 2000;97:2603–2608. doi: 10.1073/pnas.020487997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentlein R, Held-Feindt J. Pleiotrophin, an angiogenic and mitogenic growth factor, is expressed in human gliomas. J Neurochem. 2002;83:747–753. doi: 10.1046/j.1471-4159.2002.01179.x. [DOI] [PubMed] [Google Scholar]
- Mi R, Chen W, Hoke A. Pleiotrophin is a neurotrophic factor for spinal motor neurons. Proc Natl Acad Sci USA. 2007;104:4664–4669. doi: 10.1073/pnas.0603243104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Surmacz E, Burgaud JL, Baserga R. Different effects on mitogenesis and transformation of a mutation at tyrosine 1251 of the insulin-like growth factor I receptor. J Biol Chem. 1995;270:22639–22644. doi: 10.1074/jbc.270.38.22639. [DOI] [PubMed] [Google Scholar]
- Moog-Lutz C, Degoutin J, Gouzi JY, Frobert Y, Brunet-de Carvalho N, Bureau J, et al. Activation and inhibition of anaplastic lymphoma kinase receptor tyrosine kinase by monoclonal antibodies and absence of agonist activity of pleiotrophin. J Biol Chem. 2005;280:26039–26048. doi: 10.1074/jbc.M501972200. [DOI] [PubMed] [Google Scholar]
- Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non- Hodgkin’s lymphoma [published erratum appears in Science 1995 Jan 20;267(5196):316–7] Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- Morris SW, Naeve C, Mathew P, James PL, Kirstein MN, Cui X, et al. ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin’s lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK) Oncogene. 1997;14:2175–2188. doi: 10.1038/sj.onc.1201062. [DOI] [PubMed] [Google Scholar]
- Muramatsu H, Zou P, Kurosawa N, Ichihara-Tanaka K, Maruyama K, Inoh K, et al. Female infertility in mice deficient in midkine and pleiotrophin, which form a distinct family of growth factors. Genes Cells. 2006;11:1405–1417. doi: 10.1111/j.1365-2443.2006.01028.x. [DOI] [PubMed] [Google Scholar]
- Owada K, Sanjo N, Kobayashi T, Mizusawa H, Muramatsu H, Muramatsu T, et al. Midkine inhibits caspase-dependent apoptosis via the activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase in cultured neurons. J Neurochem. 1999;73:2084–2092. [PubMed] [Google Scholar]
- Padlan EA. Anatomy of the antibody molecule. Mol Immunol. 1994;31:169–217. doi: 10.1016/0161-5890(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Perez-Pinera P, Alcantara S, Dimitrov T, Vega JA, Deuel TF. Pleiotrophin disrupts calcium-dependent homophilic cell-cell adhesion and initiates an epithelial-mesenchymal transition. Proc Natl Acad Sci USA. 2006;103:17795–17800. doi: 10.1073/pnas.0607299103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pinera P, Zhang W, Chang Y, Vega JA, Deuel TF. Anaplastic lymphoma kinase is activated through the pleiotrophin/ receptor protein-tyrosine phosphatase beta/zeta signaling pathway: an alternative mechanism of receptor tyrosine kinase activation. J Biol Chem. 2007;282:28683–28690. doi: 10.1074/jbc.M704505200. [DOI] [PubMed] [Google Scholar]
- Peria FM, Neder L, Marie SK, Rosemberg S, Oba-Shinjo SM, Colli BO, et al. Pleiotrophin expression in astrocytic and oligodendroglial tumors and it’s correlation with histological diagnosis, microvascular density, cellular proliferation and overall survival. J Neurooncol. 2007;84:255–261. doi: 10.1007/s11060-007-9379-2. [DOI] [PubMed] [Google Scholar]
- Phillips H, Kharbanda S, Chen R, Forrest W, Soriano R, Wu T, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Powers C, Aigner A, Stoica GE, McDonnell K, Wellstein A. Pleiotrophin signaling through anaplastic lymphoma kinase (ALK) is rate-limiting for glioblastoma growth. J Biol Chem. 2002;277:14153–14158. doi: 10.1074/jbc.M112354200. [DOI] [PubMed] [Google Scholar]
- Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- Qi M, Ikematsu S, Maeda N, Ichihara-Tanaka K, Sakuma S, Noda M, et al. Haptotactic migration induced by midkine. Involvement of protein-tyrosine phosphatase zeta. Mitogen-activated protein kinase, and phosphatidylinositol 3-kinase. J Biol Chem. 2001;276:15868–15875. doi: 10.1074/jbc.m005911200. [DOI] [PubMed] [Google Scholar]
- Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N, Muramatsu H, Ichihara-Tanaka K, Maeda N, Noda M, Yamamoto T, et al. Receptor-type protein tyrosine phosphatase zeta as a component of the signaling receptor complex for midkine-dependent survival of embryonic neurons. Neurosci Res. 2003;45:219–224. doi: 10.1016/s0168-0102(02)00226-2. [DOI] [PubMed] [Google Scholar]
- Schaerer-Brodbeck C, Barberis A. Coupling homologous recombination with growth selection in yeast: a tool for construction of random DNA sequence libraries. Biotechniques. 2004;37:202–206. doi: 10.2144/04372BM05. [DOI] [PubMed] [Google Scholar]
- Schulte AM, Wellstein A. In: Tumour Angiogenesis. Bicknell R, Lewis Cm, Ferrara N, editors. Oxford, New York, Tokyo: Oxford University Press; 1997. pp. 273–289. [Google Scholar]
- Shai R, Shi T, Kremen T, Horvath S, Liau L, Cloughesy T, et al. Gene expression profiling identifies molecular subtypes of gliomas. Oncogene. 2003;22:4918–4923. doi: 10.1038/sj.onc.1206753. [DOI] [PubMed] [Google Scholar]
- Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- Stoica GE, Kuo A, Aigner A, Sunitha I, Souttou B, Malerczyk C, et al. Identification of ALK (anaplastic lymphoma kinase) as a receptor for the growth factor pleiotrophin. J Biol Chem. 2001;276:16772–16779. doi: 10.1074/jbc.M010660200. [DOI] [PubMed] [Google Scholar]
- Stoica GE, Kuo A, Powers C, Bowden ET, Buchert-Sale E, Riegel AT, et al. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. JBiol Chem. 2002;277:35990–35998. doi: 10.1074/jbc.M205749200. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Mikami T. Chondroitin/dermatan sulfate in the central nervous system. Curr Opin Struct Biol. 2007;17:536–545. doi: 10.1016/j.sbi.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S, et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9:287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Tang Y, Lou J, Alpaugh RK, Robinson MK, Marks JD, Weiner LM. Regulation of antibody-dependent cellular cytotoxicity by IgG intrinsic and apparent affinity for target antigen. J Immunol. 2007;179:2815–2823. doi: 10.4049/jimmunol.179.5.2815. [DOI] [PubMed] [Google Scholar]
- Weber D, Klomp HJ, Czubayko F, Wellstein A, Juhl H. Pleiotrophin can be rate-limiting for pancreatic cancer cell growth. Cancer Res. 2000;60:5284–5288. [PubMed] [Google Scholar]
- Wellstein A, Fang WJ, Khatri A, Lu Y, Swain SS, Dickson RB, et al. A heparin-binding growth factor secreted from breast cancer cells homologous to a developmentally regulated cytokine. J Biol Chem. 1992;267:2582–2587. [PubMed] [Google Scholar]
- Zhang N, Zhong R, Wang ZY, Deuel TF. Human breast cancer growth inhibited in vivo by a dominant negative pleiotrophin mutant. J Biol Chem. 1997;272:16733–16736. doi: 10.1074/jbc.272.27.16733. [DOI] [PubMed] [Google Scholar]
- Zou P, Muramatsu H, Sone M, Hayashi H, Nakashima T, Muramatsu T. Mice doubly deficient in the midkine and pleiotrophin genes exhibit deficits in the expression of beta-tectorin gene and in auditory response. Lab Invest. 2006;86:645–653. doi: 10.1038/labinvest.3700428. [DOI] [PubMed] [Google Scholar]