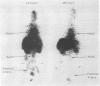
Abstract
Human serum albumin has been conjugated to 1-(p-bnezenediazonium)-(ethylenedinitrilo)tetraacetic acid, a powerful chelating agent, and radioactive 111indium ions have been added specifically to the chelating groups. The product, with a specific radioactivity of about 1 mCi/mg of protein, was employed as a radiotracer in scintillation scanning studies with human volunteers. Results show that 48 hr after injection, practically all of the label remains attached to albumin. This is confirmed by electrophoresis of serum proteins; 7 days after injection, 85% of the radioactivity in the serum is still in the albumin fraction. These observations agree with in vitro studies of the labeled albumin in human serum, where loss of the metal ion from the chelating group to the protein transferrin amounts to less than 3% after 1 week and less than 5% after 2 weeks. Measurements of the distribution of label in mice up to 23 days after injection suggest that metabolism of the labeled protein does not lead to binding of indium ions by transferrin. The binding of indium and other metal ions by transferrin has previously posed a major impediment to the use of metal chelates for in vivo diagnostic procedures. Demonstration of the kinetic inertness of the chelate in these experiments suggests the use of related chelates as physical probes of biological systems.
Full text
PDF
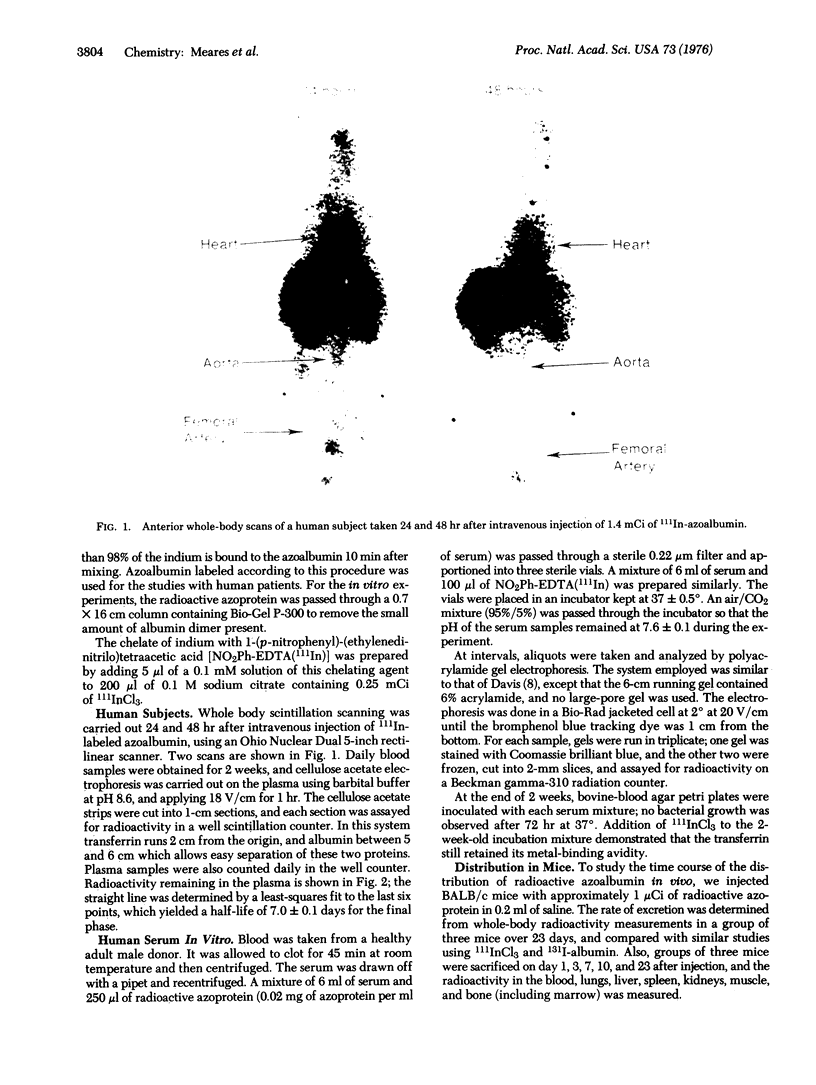
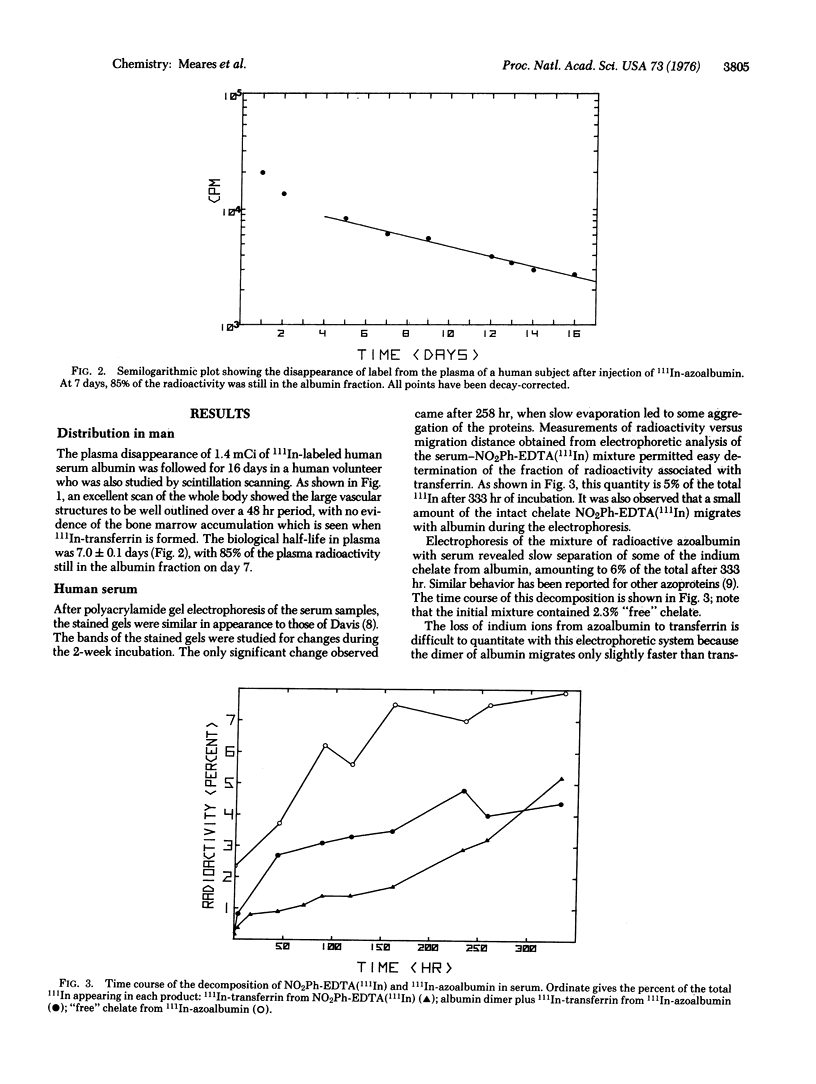
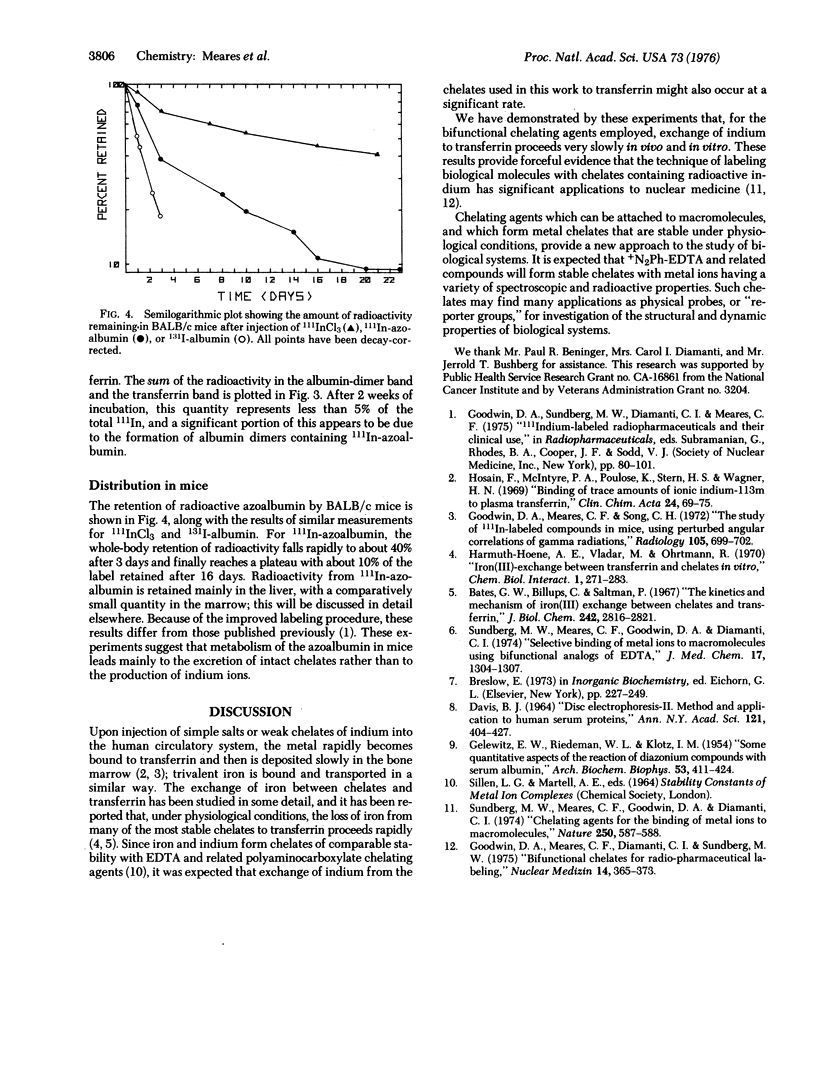
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates G. W., Billups C., Saltman P. The kinetics and mechanism of iron (3) exchange between chelates and transferrin. II. The presentation and removal with ethylenediaminetetraacetate. J Biol Chem. 1967 Jun 25;242(12):2816–2821. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- GELEWITZ E. W., RIEDEMAN W. L., KLOTZ I. M. Some quantitative aspects of the reaction of diazonium compounds with serum albumin. Arch Biochem Biophys. 1954 Dec;53(2):411–424. doi: 10.1016/0003-9861(54)90422-1. [DOI] [PubMed] [Google Scholar]
- Goodwin D. A., Meares C. F., Diamanti C. I., Sundberg M. W. Bifunctional chelates for radio-pharmaceutical labeling. Nucl Med (Stuttg) 1975 Dec 15;14(4):365–373. [PubMed] [Google Scholar]
- Goodwin D. A., Meares C. F., Song C. H. The study of 111 In-labeled compounds in mice, using perturbed angular correlations of gamma radiations. Radiology. 1972 Dec;105(3):699–702. doi: 10.1148/105.3.699. [DOI] [PubMed] [Google Scholar]
- Harmuth-Hoene A. E., Vladár M., Ohrtmann R. Iron(3)-exchange between transferrin and chelates in vitro. Chem Biol Interact. 1970 Feb;1(3):271–283. doi: 10.1016/0009-2797(70)90014-1. [DOI] [PubMed] [Google Scholar]
- Hosain F., McIntyre P. A., Poulose K., Stern H. S., Wagner H. N., Jr Binding of trace amounts of ionic indium-113m to plasma transferrin. Clin Chim Acta. 1969 Apr;24(1):69–75. doi: 10.1016/0009-8981(69)90142-9. [DOI] [PubMed] [Google Scholar]
- Sundberg M. W., Meares C. F., Goodwin D. A., Diamanti C. I. Chelating agents for the binding of metal ions to macromolecules. Nature. 1974 Aug 16;250(467):587–588. doi: 10.1038/250587a0. [DOI] [PubMed] [Google Scholar]
- Sundberg M. W., Meares C. F., Goodwin D. A., Diamanti C. I. Selective binding of metal ions to macromolecules using bifunctional analogs of EDTA. J Med Chem. 1974 Dec;17(12):1304–1307. doi: 10.1021/jm00258a015. [DOI] [PubMed] [Google Scholar]