Abstract
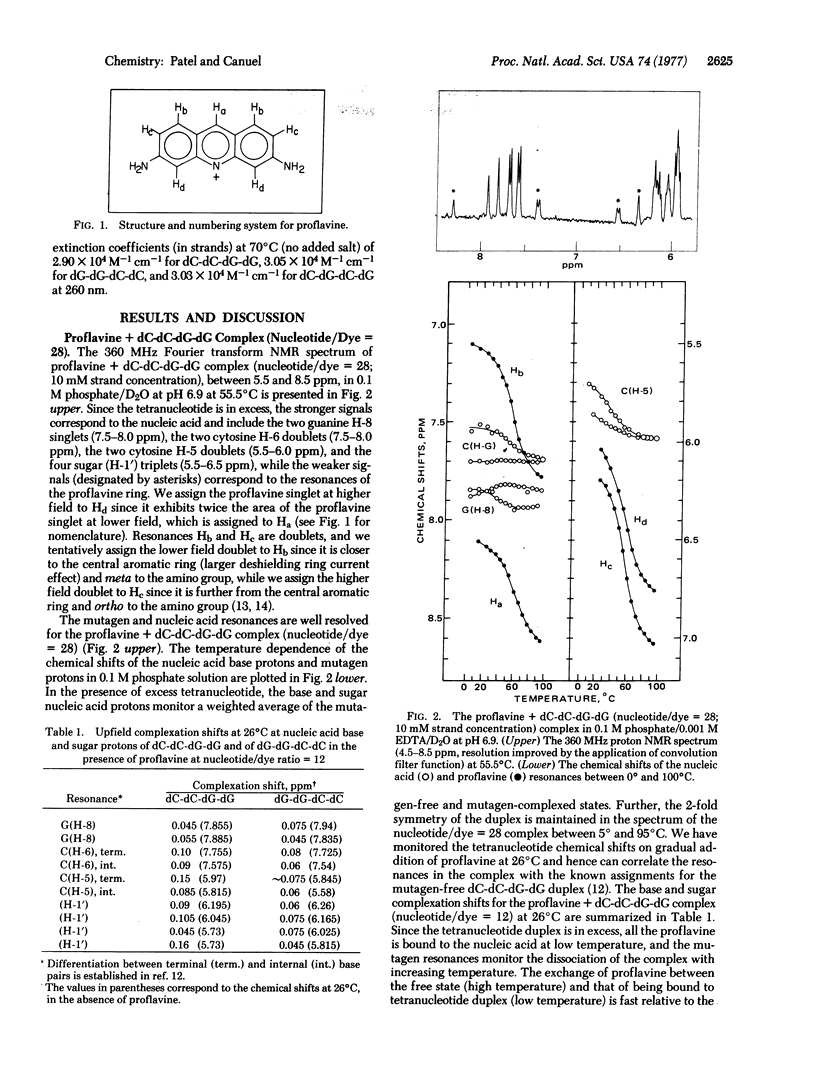
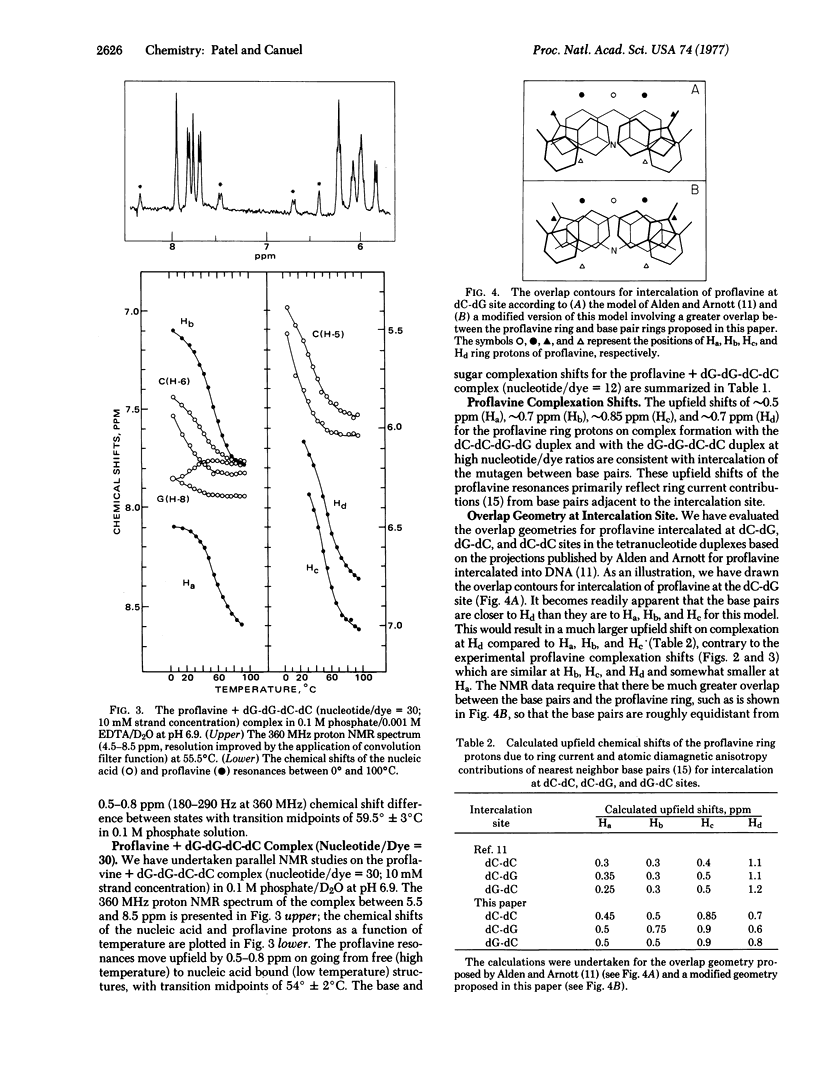
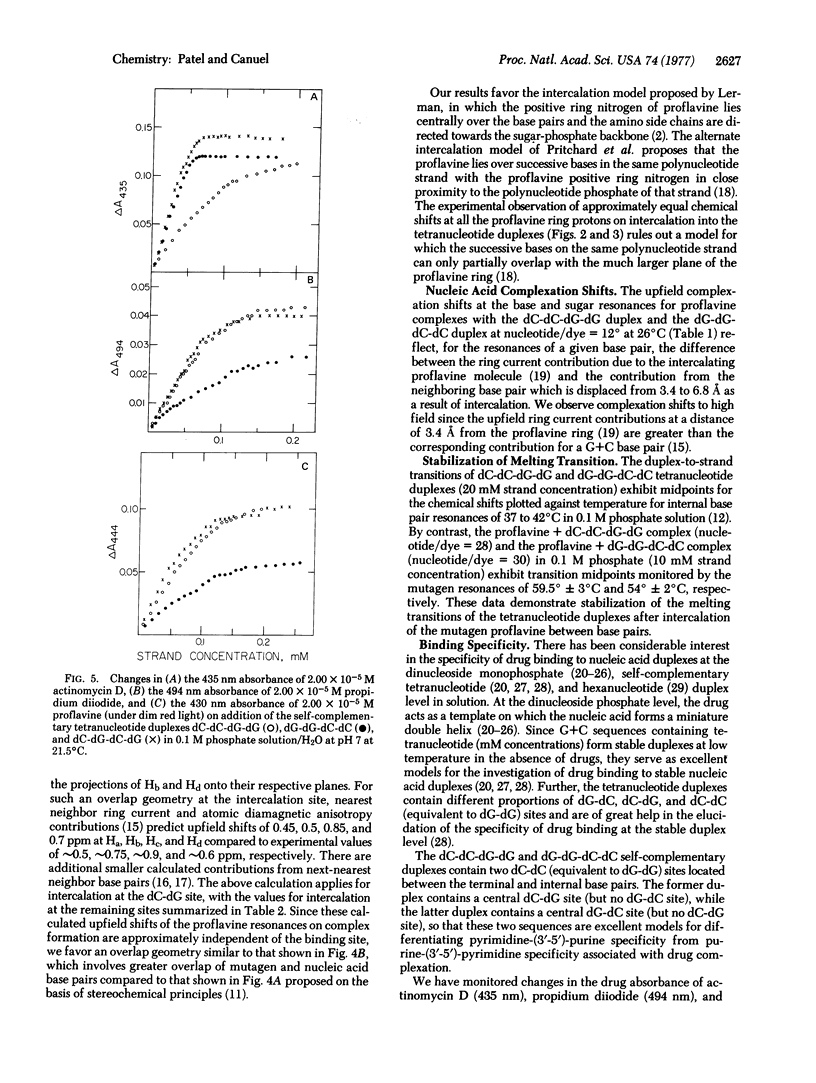
The complex formed between the mutagen proflavine and the dC-dC-dG-dG and dG-dG-dC-dC self-complementary tetranucleotide duplexes has been monitored by proton high resolution nuclear magnetic resonance spectroscopy in 0.1 M phosphate solution at high nucleotide/drug ratios. The large upfield shifts (0.5 to 0.85 ppm) observed at all the proflavine ring nonexchangeable protons on complex formation are consistent with intercalation of the mutagen between base pairs of the tetranucleotide duplex. We have proposed an approximate overlap geometry between the proflavine ring and nearest neighbor base pairs at the intercalation site from a comparison between experimental shifts and those calculated for various stacking orientations. We have compared the binding of actinomycin D, propidium diiodide, and proflavine to self-complementary tetranucleotide sequences dC-dC-dG-dG and dG-dG-dC-dC by UV absorbance changes in the drug bands between 400 and 500 nm. Actinomycin D exhibits a pronounced specificity for sequences with dG-dC sites (dG-dG-dC-dC), while propidium diiodide and proflavine exhibit a specificity for sequences with dC-dG sites (dC-dC-dG-dG). Actinomycin D binds more strongly than propidium diiodide and proflavine to dC-dG-dC-dG (contains dC-dG and dG-dC binding sites), indicative of the additional stabilization from hydrogen bonding and hydrophobic interactions between the pentapeptide lactone rings of actinomycin D and the base pair edges and sugar-phosphate backbone of the tetranucleotide duplex.
Keywords: mutagen-tetranucleotide duplex, helix-coil transition, intercalation specificity at dC-dG sites
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alden C. J., Arnott S. Visualization of planar drug intercalations in B-DNA. Nucleic Acids Res. 1975 Oct;2(10):1701–1717. doi: 10.1093/nar/2.10.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake A., Peacocke A. R. The interaction of aminocridines with nucleic acids. Biopolymers. 1968;6(9):1225–1253. doi: 10.1002/bip.1968.360060902. [DOI] [PubMed] [Google Scholar]
- Davanloo P., Crothers D. M. Kinetic studies of drug-dinucleotide complexes. Biochemistry. 1976 Nov 30;15(24):5299–5305. doi: 10.1021/bi00669a016. [DOI] [PubMed] [Google Scholar]
- Davanloo P., Crothers D. M. Phase partition studies of actinomycin-nucleotide complexes. Biochemistry. 1976 Oct 5;15(20):4433–4438. doi: 10.1021/bi00665a015. [DOI] [PubMed] [Google Scholar]
- Dourlent M., Hogrel J. F. Competitive cooperative bindings of a small ligand to a linear polymer. II. Investigations on the mechanisms of proflavine binding to poly (A) and DNA. Biopolymers. 1976 Jan;15(1):29–41. doi: 10.1002/bip.1976.360150105. [DOI] [PubMed] [Google Scholar]
- Dourlent M., Hélène C. A quantitative analysis of proflavine binding to polyadenylic acid, polyuridylic acid, and transfer RNA. Eur J Biochem. 1971 Nov 11;23(1):86–95. doi: 10.1111/j.1432-1033.1971.tb01595.x. [DOI] [PubMed] [Google Scholar]
- Giessner-Prettre C., Pullman B., Borer P. N., Kan L. S., Ts'o P. O. Ring-current effects in the Nmr of nucleic acids: a graphical approach. Biopolymers. 1976 Nov;15(11):2277–2286. doi: 10.1002/bip.1976.360151114. [DOI] [PubMed] [Google Scholar]
- Giessner-Prettre C., Pullman B. On the atomic or "local" contributions to proton chemical shifts due to the anisotropy of the diamagnetic susceptibility of the nucleic acid base. Biochem Biophys Res Commun. 1976 May 17;70(2):578–581. doi: 10.1016/0006-291x(76)91086-x. [DOI] [PubMed] [Google Scholar]
- Giessner-Prettre C., Pullman B. Sur les courbes d'isoécran des protons dans quelques agents s'intercalant dans les acides nucléliques. C R Acad Sci Hebd Seances Acad Sci D. 1976 Sep 27;283(6):675–677. [PubMed] [Google Scholar]
- Kroon P. A., Kreishman G. P., Nelson J. H., Chan S. I. The effects of chain length on the secondary structure of oligoadenylates. Biopolymers. 1974 Dec;13(12):2571–2592. doi: 10.1002/bip.1974.360131214. [DOI] [PubMed] [Google Scholar]
- Krugh T. R. Association of actinomycin D and deoxyribodinucleotides as a model for binding of the drug to DNA. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1911–1914. doi: 10.1073/pnas.69.7.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugh T. R., Wittlin F. N., Cramer S. P. Ethidium bromide-dinucleotide complexes. Evidence for intercalation and sequence preferences in binding to double-stranded nucleic acids. Biopolymers. 1975 Jan;14(1):197–210. doi: 10.1002/bip.1975.360140114. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. Structural considerations in the interaction of DNA and acridines. J Mol Biol. 1961 Feb;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- Li H. J., Crothers D. M. Relaxation studies of the proflavine-DNA complex: the kinetics of an intercalation reaction. J Mol Biol. 1969 Feb 14;39(3):461–477. doi: 10.1016/0022-2836(69)90138-7. [DOI] [PubMed] [Google Scholar]
- McCall P. J., Bloomfield V. A. Binding of proflavin to T2L bacteriophage and its DNA. Biopolymers. 1976 Jan;15(1):97–111. doi: 10.1002/bip.1976.360150108. [DOI] [PubMed] [Google Scholar]
- McCall P. J., Bloomfield V. A. Kinetics of proflavin binding to bacteriophages T2L and T4D. Biopolymers. 1976 Dec;15(12NA-NA-770103-770104):2323–2336. doi: 10.1002/bip.1976.360151202. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L. Ethidium bromide-(dC-dG-dC-dG)2 complex in solution: intercalation and sequence specificity of drug binding at the tetranucleotide duplex level. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3343–3347. doi: 10.1073/pnas.73.10.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J. NMR studies of the structure and stability of the 1 : 2 actinomycin D-d-pG-C complex in aqueous solution. Biochim Biophys Acta. 1976 Aug 2;442(1):98–108. doi: 10.1016/0005-2787(76)90180-5. [DOI] [PubMed] [Google Scholar]
- Patel D. J. Peptide antibiotic-oligonucleotide interactions. Nuclear magnetic resonance investigations of complex formation between actinomycin D and d-ApTpGpCpApT in aqueous solution. Biochemistry. 1974 May 21;13(11):2396–2402. doi: 10.1021/bi00708a025. [DOI] [PubMed] [Google Scholar]
- Patel D. J. Proton and phosphorus NMR studies of d-CpG(pCpG)n duplexes in solution. Helix-coil transition and complex formation with actinomycin-D. Biopolymers. 1976 Mar;15(3):533–558. doi: 10.1002/bip.1976.360150310. [DOI] [PubMed] [Google Scholar]
- Pritchard N. J., Blake A., Peacocke A. R. Modified intercalation model for the interaction of amino acridines and DNA. Nature. 1966 Dec 17;212(5068):1360–1361. doi: 10.1038/2121360a0. [DOI] [PubMed] [Google Scholar]
- Sakore T. D., Jain S. C., Tsai C. C., Sobell H. M. Mutagen-nucleic acid intercalative binding: structure of a 9-aminoacridine: 5-iodocytidylyl(3'-5')guanosine crystalline complex. Proc Natl Acad Sci U S A. 1977 Jan;74(1):188–192. doi: 10.1073/pnas.74.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schara R., Müller W. Uber die Wechselwirkung des Actinomycin C 3 mit Mono- Di- und Oligonucleotiden. Eur J Biochem. 1972 Sep 18;29(2):210–216. doi: 10.1111/j.1432-1033.1972.tb01977.x. [DOI] [PubMed] [Google Scholar]
- Seeman N. C., Day R. O., Rich A. Nucleic acid-mutagen interactions: crystal structure of adenylyl-3',5'-uridine plus 9-aminoacridine. Nature. 1975 Jan 31;253(5490):324–327. doi: 10.1038/253324a0. [DOI] [PubMed] [Google Scholar]
- Tsai C. C., Jain S. C., Sobell H. M. Drug-nucleic acid interaction: X-ray crystallographic determination of an ethidium-dinucleoside monophosphate crystalline complex, ethidium: 5-iodouridylyl(3'-5')adenosine. Philos Trans R Soc Lond B Biol Sci. 1975 Nov 6;272(915):137–146. doi: 10.1098/rstb.1975.0076. [DOI] [PubMed] [Google Scholar]


