Abstract
Over 20 species of Angiostrongylus have been described from around the world, but only Angiostrongylus cantonensis has been confirmed to cause central nervous system disease in humans. A neurotropic parasite that matures in the pulmonary arteries of rats, A. cantonensis is the most common cause of eosinophilic meningitis in southern Asia and the Pacific and Caribbean islands. The parasite can also cause encephalitis/encephalomyelitis and rarely ocular angiostrongyliasis. The present paper reviews the life cycle, epidemiology, pathogenesis, clinical features, diagnosis, treatment, prevention and prognosis of A. cantonesis infection. Emphasis is given on the spectrum of central nervous system manifestations and disease pathogenesis.
Keywords: eosinophilic meningitis, Angiostrongylus cantonensis, parasitic encephalitis
1. Angiostrongylus spp
Over 20 species of Angiostrongylus have been described worldwide (Prociv et al., 2000; Thiengo et al., 2013). The parasites are nematodes (phylum Nematoda) in the superfamily Metastrongyloidea, with species having carnivores, insectivores, and mainly rodents as their definitive hosts (Drozdz, 1970; Prociv et al., 2000; Thiengo et al., 2013). Transmission of most species involves ingestion of gastropod intermediate hosts. Only two species of Angiostrongylus have been confirmed to cause human infection: A. costaricensis, and A. cantonensis (Prociv et al., 2000). A. costaricensis inhabits the mesenteric arteries, causing abdominal angiostrongyliasis with marked eosinophilic infiltration of the viscera, a zoonosis reported from the southern United States to northern Argentina (Incani et al., 2007; Morera and Cespedes, 1970; Rebello et al., 2012; Rodriguez et al., 2014; Thiengo et al., 2013). A. cantonesis is neurotropic, migrating to neural tissue after infection and resulting in three syndromes: eosinophilic meningitis (also known as meningitic angiostrongyliasis), encephalitis, and ocular angiostrongyliasis (Sawanyawisuth and Chotmongkol, 2013). Eosinophilic meningitis is the most common presentation of neuroangiostrongyliasis and is now recognized as an emerging zoonotic disease (Wang et al., 2012). A. malaysiensis can cause neurological disease in monkeys under experimental conditions (Cross, 1979), and there are reports from Malaysia and Indonesia suggesting that A. malaysiensis also causes human disease (Carney and Stafford, 1979; Lim and Ramachandran, 1979). However, A. malaysiensis has yet to be isolated from humans (Prociv et al., 2000). Since A. costaricensis does not cause neurologic symptoms, this paper will focus on A. cantonensis infection [For more information about A. costaricensis please see additional references (Grisotti and Avila-Pires, 2011; Rebello et al., 2013; Rebello et al., 2012; Rodriguez et al., 2014; Teixeira et al., 1993)].
2. Angiostrongylus cantonensis morphology
Angiostrongylids are small nematodes with thin cylindrical bodies and a reduced bursa in males, which are associated with the vascular system. Mackerras and Sandars were the first to try to describe all life-forms of A. cantonensis (Mackerras and Sandars, 1954) not realizing they were actually describing A. mackerrasae, another neurotropic parasite of rats almost identical to A. cantonensis and not separated as a distinct specie until 14 years later (Bhaibulaya, 1975).
During its life cycle first stage larvae (L1) molts four consecutive times generating second-(L2), third-(L3), fourth-(L4), and fifth-stage (L5) larvae. L3 larvae are the infective form for definitive (rats) and accidental (humans) hosts (Figure 1A). Adult worms reside in the pulmonary arteries and right ventricle of rats, which gave the name “rat lungworm” for the parasite. Adult worms of both sexes are characterized by a long, phylliform body tapering at both ends (Figure 1C). Females are larger and more robust than males reaching a size of 21–35 by 0.30–0.36 mm (females) and 16–25 by 0.25–0.35 mm (males) (Cowie, 2013; Cross, 1997; Orihel and Ash, 1995; Thiengo et al., 2013; Thiengo et al., 2010). The appearance of the adult male bursa, the caudal apparatus used to clasp the female during mating, and of the tip of the tail are important in the differentiation of subgenera and species of Angiostrongylus adult worms and L3 larvae, respectively (Cowie, 2013; Thiengo et al., 2013) (Figure 1D). More detailed morphological descriptions of all developmental stages of A. cantonensis and its differentiation from other Angiostrongylus species have been published elsewhere (Ash, 1970; Bhaibulaya, 1975; Thiengo et al., 2013; Thiengo et al., 2010; Ubelaker, 1986).
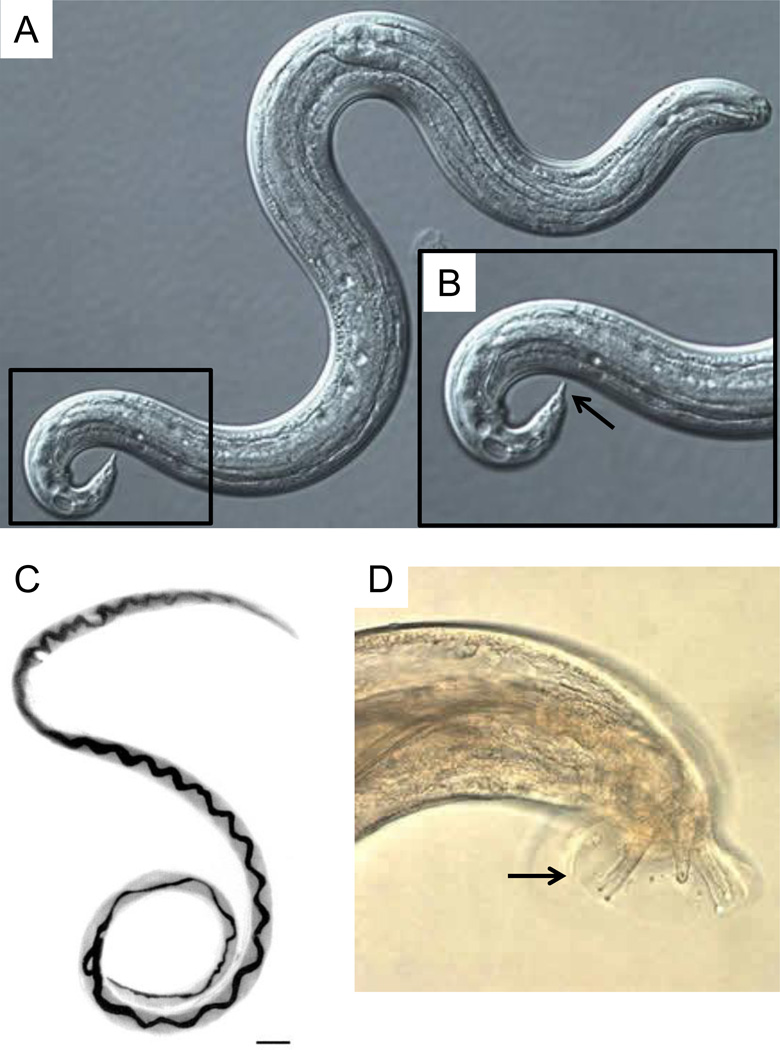
Figure 1.
Angiostrongylus cantonensis developmental stages. (A) Differential interference contrast microscopy image of third-stage (L3) infective larvae recovered from a slug. L3 larvae are about 0.45 by 0.02 mm and present cuticle with faint transverse striations. (B) Higher magnification of demarcated region in A showing terminal projection on the tip of the tail (arrow) which is characteristic of A. cantonensis. Adult female (C) and tail of adult male (D) worms recovered from rat lungs. Note the characteristic barber-pole appearance of female worms and rays (arrow) of males. Scale bar = 1 mm in C. Sources: (A), (B) and (D) = http://www.cdc.gov/dpdx/angiostrongyliasis/gallery.html; (C) = http://wwwnc.cdc.gov/eid/article/8/3/01-0316-f1.htm.
3. Life cycle
A. cantonensis was first described in Guangzhou, China, in 1935 (Chen, 1935). The rat is the definitive host of A. cantonensis and is infected after ingesting L3 larvae (Bhaibulaya, 1975; Mackerras and Sandars, 1954). A few hours after being ingested, L3 larvae penetrate the intestinal wall and enter the bloodstream (Thiengo et al., 2013). Once in the intestinal venous (hepatoportal) circulation, L3 larvae pass through the liver and inferior vena cava, and reach the pulmonary circulation from the right ventricle. L3 larvae then pass through pulmonary capillaries arriving at the left heart where they are dispersed by the arterial circulation to the body. Within several days, many L3 larvae reach the brain, enter the neural parenchyma, grow and molt, becoming L4 larvae. Later, another molt into the subadult stage L5 occurs after migration of L4 larvae to the subarachnoid space. Young adult worms leave the brain and return to the pulmonary arteries, traveling via the venous sinuses, superior vena cava and right heart. Adult worms are found in the pulmonary arteries 26–35 days after L3 infection (Bhaibulaya, 1975; Cross, 1997). Rats (R. norvegicus and R. rattus) are tolerant of A. cantonensis infection, surviving relatively large infective doses (as high as 150 parasites developing in the brain), without significant central nervous system (CNS) abnormalities (Prociv et al., 2000). In addition, it was recently shown that A. cantonensis can also complete its life cycle in Mongolian gerbils (Meriones unguiculatus) under laboratory conditions (Wei et al., 2014).
Approximately 35 days postinfection, worms reach sexual maturity within the pulmonary arteries and copulate. The female worm then lays approximately 15,000 eggs per day in the pulmonary arteries (Wu, 2006). These eggs travel via the blood circulation to the lung parenchyma where they develop in situ in about a week and contain L1 larvae, which then hatch. L1 larvae penetrate alveoli, migrate up the airways to the pharynx, and are swallowed, reaching the gastrointestinal tract (Bhaibulaya, 1975). L1 larvae are then eliminated in the rodent’s feces starting about 42–45 days postinfection with L3s (Thiengo et al., 2013), and proceed to infect mollusks as intermediate hosts. Snails and slugs (generally Pila polita, Pila ampullacea, Pomacea canaliculata, and Achatina fulica), the principal intermediate hosts, become infected either by ingestion of L1 in rat feces or by penetration of these larvae through the body surface(Thiengo, 1996). In the intermediate host, the L1 molts twice in 12–20 days (Chao et al., 1987; Thiengo et al., 2013) and the L3 that eventually develops is directly infective to rats upon ingestion, thus, completing the life cycle. However, a variety of paratenic hosts (transport hosts which eat mollusks) may be interspersed in transmission of L3s, including terrestrial planarians and crabs, freshwater shrimp, frogs, toads, and certain fish (Ash, 1968; Radomyos et al., 1994; Wallace and Rosen, 1966, 1967, 1969). Although the significance of paratenic hosts as a source of infection to rats is an unresolved issue, it is generally believed that these paratenic hosts in which the parasite does not undergo any further development play an important role in transmission, as they increase the opportunities for the parasite to infect definitive hosts as well as humans (Prociv et al., 2000; Thiengo et al., 2013).
Humans are accidental hosts that acquire A. cantonensis after eating undercooked intermediate or paratenic hosts, or vegetables that contain L3 larvae. Once ingested, L3 larvae migrate into the central nervous system (CNS), following a path similar to the one occurring in rats (Wang et al., 2008). The presence of the parasite in the brain results in eosinophilic meningitis, which is the most common presentation of this infection in humans, or encephalitis. The parasite also can migrate into the eye causing ocular angiostrongyliasis. It is a matter of debate if L3 larvae are able to molt inside the human CNS and migrate to the pulmonary arteries. Since eosinophilic meningitis is the predominant clinical finding, some believe that, different from what occurs in the definitive host, L3 larvae do not molt or migrate further but remain in the human CNS for 1–2 months before they die (Sawanyawisuth and Chotmongkol, 2013). However, immature adult worms have been found in the pulmonary arteries of patients with angiostrongyliasis (Orihel and Ash, 1995; Prociv, 1999). In addition, another study showed the presence of abnormalities on chest X-rays of patients who had survived an episode of eosinophilic meningitis (Shih et al., 1992). These studies suggest that, on rare occasions, A. cantonensis can molt in the CNS, develop further, and travel to the lungs of humans (Orihel and Ash, 1995; Prociv et al., 2000; Sawanyawisuth and Chotmongkol, 2013). There is no data regarding the L3 larval dose required to cause symptomatic human infection, and although no studies have determined the possibility of subclinical infections, these would certainly be expected (Prociv et al., 2000).
4. Epidemiology
A. cantonensis is the most common cause of eosinophilic meningitis in southern Asia and the Pacific and Caribbean islands (Murphy and Johnson, 2013; Sawanyawisuth and Chotmongkol, 2013). Human infection was first described in Taiwan in 1945 (Nomura and Lin, 1945). Currently, over 2,800 human cases have been reported from about 30 countries with most records been from tropical and subtropical areas in Southeast Asia and the Pacific Basin (Cowie, 2013; Wang et al., 2008). The parasite endemic in southern Asia, the Pacific and Caribbean islands, and portions of Australia, southeastern USA, Egypt, Nigeria, Côte d’Ivoire, and South America (Brazil and Ecuador) (Caldeira et al., 2007; Morassutti et al., 2014; New et al., 1995; Thiengo et al., 2013; Wang et al., 2008). Human A. cantonensis infection has attracted increasing attention because of outbreaks (Caldeira et al., 2007; Thiengo et al., 2013) and increasing numbers of sporadic cases being reported in travelers returning from endemic regions in Europe (Wang et al., 2012).
The dispersal of A. cantonensis from its presumptive home range in southern Asia is likely due to the transport of infected rats on ships and airplanes, increased migration of human populations and military operations in the endemic areas and the spread of some species of snails to different parts of the world (Kliks and Palumbo, 1992). For example, the recent invasion and spread of the giant African snail, A. fulica, in Brazil, which began in the 1980s and has now been recorded in 25 of the 26 states and in the Federal District, likely contributed to the outbreaks of eosinophilic meningitis caused by A. cantonensis in that country (Caldeira et al., 2007; Cognato et al., 2013; Thiengo et al., 2013; Thiengo et al., 2010).
Due to A. cantonensis promiscuity regarding its intermediate and paratenic hosts the route of infection in human cases varies geographically (Kim et al., 2014; Wang et al., 2008; Wang et al., 2012). The introduction of the native South American snail P. canaliculata to Taiwan and mainland of China in the 1980s led to a replacement of A. fulica as the major intermediate host and has become the main source of human infection in these regions (Wang et al., 2012). The ingestion of raw or undercooked snails (Pila spp.) is the main route of infection in Thailand (Wang et al., 2012). Monitor lizard consumption is a problem in India and Sri Lanka (Noskin et al., 1992) and freshwater prawns and terrestrial crabs are the major source in Tahiti and other Pacific islands (Alicata, 1965; Kliks and Palumbo, 1992; Punyagupta et al., 1975). The ingestion of vegetables contaminated with L3 larvae has been implicated in infections in Australia and in travelers returning from Jamaica (Prociv et al., 2000; Slom et al., 2002). However, it is not completely understood how contamination of vegetables occurs since there is no indication that A. cantonensis infective larvae are released in snail mucus or other secretions (Prociv et al., 2000). Then again, small snails or parts of snails accidentally ingested with various fresh foods are always a possibility. Because of similar accidental or purposeful ingestion, children who play in the dirt in endemic areas are at increased risk for infection.
Humans are not the only hosts that suffer clinical angiostrongyliasis. Infection of the definitive host R. norvegicus by A. cantonesis has a 10 – 20% mortality and causes normocytic hypochromic anemia, thrombocytopenia, eosinophilia, neutrophilia, basophilia, increased cardiac enzymes, acidosis and hypoxia that are associated with necrosis and fibrosis of the lung parenchyma (Garcia et al., 2014). Mice, like humans, are non-permissive hosts developing extensive brain injury and neurological symptoms such as ataxia, tremor and paralysis when infected (OuYang et al., 2012). Fifty-five cases of naturally occurring canine neural angiostrongyliasis were described from Australia (Mason, 1987) and in the southeastern U.S., where infected rats have been found in several places in Louisiana (Campbell and Little, 1988), cases have been seen involving a howler monkey, lemur, gibbon, opossums, and even a miniature horse (Costa et al., 2000; Duffy et al., 2004; Gardiner et al., 1990; Kim et al., 2002).
5. Neurological manifestations
The pathogenesis of human infection was first studied in the early 1960s by extensive epidemiological studies in the Pacific (Punyagupta et al., 1970; Punyagupta et al., 1975). The neurological manifestations of A. cantonensis infection include eosinophilic meningitis, encephalitis/encephalomyelitis, radiculitis, cranial nerve abnormalities and ataxia. Patients with eosinophilic meningitis display at least 10 eosinophils per mm3 of cerebrospinal fluid (CSF), or ≥10% eosinophils in the total CSF leukocyte count (Kuberski, 1981). The proportions of other leukocyte types in the CSF or the presence of concomitant peripheral eosinophilia are not necessary for diagnosis (Diaz, 2009; Sawanyawisuth and Chotmongkol, 2013). Prodromal syndromes due to the passage of L3 larvae through different organs may occur. Hence, enteritis can be associated with invasion of the gastrointestinal tract (Sawanyawisuth et al., 2010; Yii, 1976). Cough, rhinorrhea, and sore throat can develop when worms pass through the lungs and trachea (Cross, 1978). Fever and malaise are nonspecific symptoms of infection and can also occur before the development of CNS disease (Cross, 1978; Yii, 1976).
The incubation period for the development of eosinophilic meningitis is typically about 2 weeks, which coincides with the time it takes for the L3 larvae to migrate into CNS tissue and incite a reaction. However, it can range from one day to several months (Wang et al., 2008). The clinical presentation of neuroangiostrongyliasis varies between adults and children. In adults, once CNS symptoms develop, patients present most commonly with severe headache (95% of cases) (Chau et al., 2003; Wang et al., 2008). The headache is generally all over the head without any specific point of tenderness, frequently described as “exploding” and persists for 1–7 days (Sawanyawisuth and Chotmongkol, 2013). Neck stiffness occurs in over 40% of cases and is associated with severity of the disease (Chau et al., 2003; Slom et al., 2002). Paraesthesias occur in approximately 40% of cases and often persist for less than two weeks (Yii, 1976). Patients with paraesthesia may complain of numbness, itching, or a sensation of worms crawling under their skin. Vomiting (38%) and nausea (28%) can accompany headache and visual disturbances or diplopia can occur in 38–92% of cases (Wang et al., 2012). Fever occurs in 32% of adult patients, with 10% of them presenting high-grade fever (38–39°C) (Wang et al., 2008). Infrequent symptoms include sixth and seventh cranial nerve palsy, hearing abnormalities, intestinal obstruction, and spinal involvement (Chotmongkol et al., 2004; Sawanyawisuth and Chotmongkol, 2013; Sawanyawisuth et al., 2010).
Children with eosinophilic meningitis caused by A. cantonensis present with a higher incidence of nausea and vomiting (82%), fever (80%), somnolence (80%), constipation (76%), abdominal pain (40%), weakness of extremities (20%), and muscle twitching and convulsions than adults (Wang et al., 2008). In addition, over 50% of children have projectile vomiting, which usually disappears within one week (Yii, 1976). On the other hand, children present with a lower incidence of paraesthesia and neck stiffness (Wang et al., 2012).
Encephalitis/encephalomyelitis caused by A. cantonensis is a severe disease characterized by mental status changes including coma and focal neurological signs. Patients often develop eosinophilic meningitis and/or a transient abdominal pain syndrome that evolves with sensory and motor disturbances of the legs with pain, weakness, absent reflexes, bowel/bladder dysfunction, labile hypertension, coma and, in severe cases, death (Kliks et al., 1982). Rarely, seizures can also be present (Sawanyawisuth and Chotmongkol, 2013). The incubation period is shorter than in cases of eosinophilic meningitis. The encephalitis is associated with a large worm burden that can occur following the ingestion of highly permissive intermediate hosts such as A. fulica (Wallace and Rosen, 1969). Encephalitis and severe disease are more common in children and the elderly. These patients present with high fever and headaches of long duration (Hwang and Chen, 1991; Sawanyawisuth et al., 2009).
6. The pathogenesis of neurological dysfunction
Limited necropsy studies of cases of human angiostrongyliasis (Lindo et al., 2004; Lo Re and Gluckman, 2003; Punyagupta et al., 1975; Tangchai et al., 1967) (Figure 2) reveal: a) meningeal infiltration by eosinophils, macrophages, and lymphocytes; b) distinct tracks within the brain parenchyma associated with cell debris, micro thrombi and inflammatory cells; and c) presence of eosinophilic granulomas and sometimes Charcot-Leyden crystals surrounding dead worms (Lindo et al., 2004; Tangchai et al., 1967; Wang et al., 2008). Physical damage caused by the migration of larvae may explain the tracks and micro-cavities found in the brain and spinal cord of patients. However, physical destruction of neural tissue by larvae migration does not explain the entire clinical picture as immune activation and cellular infiltration around living or well-preserved larvae are not prominent (Lindo et al., 2004; Tangchai et al., 1967). These findings indicate that the presence of dead larvae is the main factor inducing an eosinophilic immune response in the brain. This is supported by findings that demonstrate that the inflammatory response is consistent with a T-helper 2 pattern with the synthesis of cytokines and chemokines known to be involved in eosinophil migration such as interleukin(IL)-5, IL-12, IL-33, and eotaxin (CCL11) (Chuang et al., 2010; Li et al., 2012; Peng et al., 2013; Sugaya et al., 1997). The accumulation of cells and fluid within the CNS increases CSF pressure resulting in headache (Punyagupta et al., 1975; Yii, 1976).
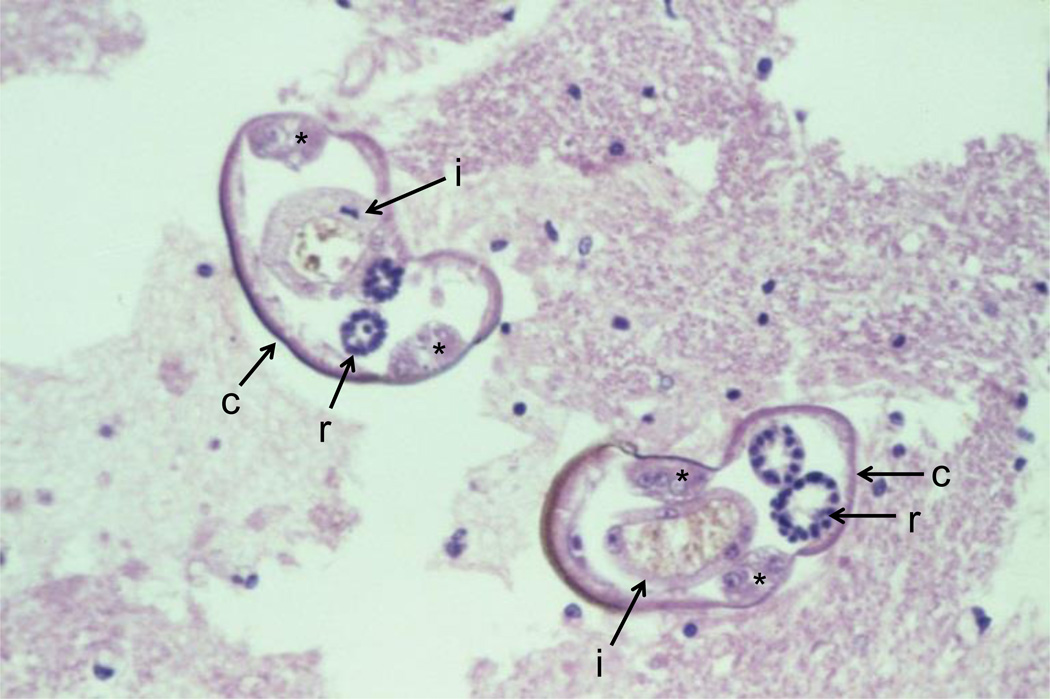
Figure 2.
Cross-section of two Angiostrongylus cantonensis larvae in the spinal cord. Note the smooth thin cuticle (c), lateral cords (*), intestines (i) with few multinucleated cells and reproductive tubes (r). Source: http://www.astmh.org/source/ZaimanSlides/index.cfm?photo=BEA3A648-E0C7-F0E4-AAEC8C1C0EB551C5.
The role of eosinophils in the immune response against A. cantonensis has received intense scrutiny. Employing IL-5 transgenic and IL-5 receptor alpha knockout mice it was demonstrated that A. cantonensis infection results in IL-5 production and CSF eosinophilia (Sugaya et al., 1997). Since IL-5 transgenic mice displayed increased migration of eosinophils to the brain, lower intracranial worm recovery, and smaller female worms than wild type controls whereas the opposite was observed with IL-5 receptor alpha knockout mice, it was suggested that eosinophils are involved in the killing of intracranial worms (Sugaya et al., 1997). More recently, it was demonstrated that treatment of mice with anti-CCR3 monoclonal antibody, which is abundant on the surface of eosinophils and is responsible for their activation and chemotaxis, reduced the infiltration of eosinophils and the severity of eosinophilic meningitis (Chuang et al., 2010). A similar picture is seen when mice are treated with anti-ST2 monoclonal antibodies, which blocks the IL-33/ST-2 pathway and inhibits the production of IL-5 (Chuang et al., 2014). The authors speculated that the central function of eosinophils may involve tissue remodeling, repair, and immune regulation (Chuang et al., 2014; Chuang et al., 2010; Gosnell and Kramer, 2013). Taken together, these observations indicate that eosinophils have a complex role in the disease pathogenesis requiring further studies. Furthermore, the toxicity of worm components, neurotoxins produced by eosinophils, NK cell activation, and the intrathecal synthesis and activation of complement may also contribute to the pathogenesis of neuroangiostrongyliasis (Chen et al., 2014; Dorta-Contreras et al., 2011; Padilla-Docal et al., 2011; Padilla-Docal et al., 2013).
7. Diagnosis
A high level of suspicion is necessary to suggest a clinical diagnosis. Symptoms of eosinophilic meningitis are nonspecific and a history of intermediate or paratenic hosts consumption is not always available. In addition, headaches in patients with neuroangiostrongyliasis can be misdiagnosed as vascular headache or muscle contraction (Sawanyawisuth and Chotmongkol, 2013). Every patient with a history of exposure in an endemic area, with or without a history of eating raw or undercooked intermediate or paratenic hosts, and a compatible clinical presentation should be considered for the diagnosis. Definitive diagnosis is made by detection of A. cantonensis larvae in the CSF or eye of suspected patients (Kuberski et al., 1979; Prociv et al., 2000; Sawanyawisuth and Chotmongkol, 2013; Wang et al., 2008; Wang et al., 2012). However, the detection rate is frequently low (Punyagupta et al., 1975; Yii, 1976) which makes the diagnosis primarily based on clinical history, CSF eosinophilia, and immunological tests.
In the absence of contraindications, a lumbar puncture must be done in all patients with suspected eosinophilic meningitis. The CSF is usually clear or cloudy, but not grossly turbid or xanthochromic, and the CSF leukocyte count is often between 150 and 2000 cells/µL (average of 700 cells/mm3) (Sawanyawisuth and Chotmongkol, 2013; Schmutzhard et al., 1988). Eosinophilic pleocytosis exceeds 10% in more than 95% of patients, and is usually 20–70% (Schmutzhard et al., 1988; Wang et al., 2008). The CSF protein concentration is elevated, whereas the glucose level is normal or slightly reduced (Punyagupta et al., 1975; Wang et al., 2008). Opening CSF pressures are elevated in nearly 40% of cases, and the headache typically is relieved by lumbar puncture (Sawanyawisuth and Chotmongkol, 2013). Peripheral blood eosinophilia is found in at least two thirds of patients (Sawanyawisuth and Chotmongkol, 2013; Schmutzhard et al., 1988). Blood eosinophilia does not correlate with CSF eosinophilia or with the clinical course (Kuberski et al., 1979; Schmutzhard et al., 1988).
The absence of focal lesions on CT or MRI scans of the brain distinguishes A. cantonensis meningitis from other helminthic infections of the CNS (gnathostomiasis or neurocysticercosis) (Diaz, 2009; Wang et al., 2008). However, CT and MRI can also reveal nonspecific alterations. Cerebral edema, ventricular dilatation, and diffuse meningeal enhancing ring or disc lesions, sometimes resembling tuberculoma, can be present on CT scan (Chau et al., 2003; Tsai et al., 2003). MRI may demonstrate high signal intensities over the globus pallidus and cerebral peduncle on T1-weighted imaging, leptomeningeal enhancement, ventriculomegaly, and punctate areas of abnormal enhancement within the cerebral and cerebellar hemisphere on gadolinium-enhancing T1 imaging, and a hyperintense signal on T2-weighted images (Jin et al., 2005; Tsai et al., 2003). It has been stated that T2 signal lesions present in neuroangiostrongyliasis are different from the hemorrhagic lesions present in Gnathostoma spinigerum infections (Grisotti and Avila-Pires, 2011; Kanpittaya et al., 2000; Tsai et al., 2003).
Since larvae are rarely detected in the CSF (Kuberski et al., 1979; Punyagupta et al., 1975), immunologic techniques have been developed to confirm a presumptive diagnosis (Maleewong et al., 2001; Nuamtanong, 1996). ELISA and Western blot testing have been described and both of them detect the presence of antibodies against A. cantonensis. The reaction of serum antibodies to the 31-kd and 29-kd antigens of A. cantonensis by Western blot analysis provides a relatively specific immunodiagnosis (Maleewong et al., 2001; Nuamtanong, 1996). However, antibodies against the 31-kd antigen of A. cantonensis cross-react with A. costaricensis (Morassutti et al., 2012), immunoassays are not widely available and none of them has yet been fully validated. Another limitation with serodiagnosis is that antibodies are not present in the acute stage of infection (Murphy and Johnson, 2013). Angiostrongylus serologies can be performed at a reference laboratory in Thailand (Lo Re and Gluckman, 2003). A recently developed real-time PCR assay to detect circulating antigen holds promise for the timely diagnosis of acute A. cantonensis infection (Qvarnstrom et al., 2010).
8. Ocular disease
Ocular angiostrongyliasis is a rare manifestation of A. cantonensis infection occurring in 1.2% of cases. The first case of human ocular angiostrongyliasis was reported from Bangkok in 1962 (Prommindaroj et al., 1962). A recent review reported a total of 42 cases in 13 different countries. However, almost 50% of the reported cases occurred in Thailand and, except for one case from Jamaica, all the others were recorded in Asia (Feng et al., 2013). The most common symptom of ocular angiostrongyliasis is visual loss (94.3% of cases). Visual loss manifests mainly as blurred vision, but can vary from floaters (8.6%) to perception of light only or total blindness (5.7% of cases). Eye redness and pain occur in 17% of cases and one third of patients present with funduscopic changes. Diplopia and strabismus can also occur (Punyagupta et al., 1975; Sawanyawisuth et al., 2007). Concomitant eosinophilic meningitis occurs in 50% of cases of ocular angiostrongyliasis (Diao et al., 2011).
The presence of a larva in the eye upon funduscopic or slit lamp examination confirms the diagnosis and occurs in 90% of cases. Every patient with suspicion of A. cantonensis infection and visual symptoms requires an ophthalmologic consultation to rule out ocular infection. In the majority of cases, only a single live larva is found in the affected eye in the anterior (37.1%) or vitreous (40%) chamber. The subretinal space is a less common location, occurring in only 14% of cases. Peripheral blood eosinophilia appears to occur more frequently in patients with ocular angiostrongyliasis than in patients with eosinophilic meningitis or encephalitis and this finding helps in the diagnosis (Diao et al., 2011; Liu et al., 2006; Malhotra et al., 2006). Ocular angiostrongyliasis also presented as optic neuritis in 6 out of the 42 cases reported (Feng et al., 2013).
It is not known how A. cantonensis enters the eye. There are several mechanisms that have been suggested. One is that after reaching the brain, larvae migrate along the base of the brain and transverse the optic nerve, traveling between the nerve and sheath reaching the eye (Diao et al., 2011). In support of this mechanism, an experimental study showed the presence of L5 larvae trapped in the optic nerve and periorbital tissue of rats (Kanchanaranya et al., 1972) and in a case report there was a nodule in the optic nerve of a patient with ocular angiostrongyliasis (Qi et al., 2009). The second suggested mechanism is that larvae may reach the eye through the blood stream via the retinal artery. This may explain the cases of ocular angiostrongyliasis without concomitant eosinophilic meningitis (Diao et al., 2011). Finally, it could be argued that the presence of larvae in the optic nerve could occur because larvae traveled from the eye to the brain, hence in the opposite direction.
9. Prevention, treatment and prognosis
Due to the large number of possible definitive, intermediate, and paratenic hosts for A. cantonensis present worldwide, strategies to prevent this infection cannot merely focus on the eradication of the parasite (Sawanyawisuth and Chotmongkol, 2013; Thiengo et al., 2013; Wang et al., 2008). However, it is possible to block disease transmission by educating at-risk populations. Recommended methods for prevention of A. cantonensis infection include: a) educating at-risk populations to be aware of A. cantonensis and the disease it causes; b) eating adequately cooked intermediate and paratenic hosts of A. cantonensis; c) eradicating intermediate hosts near houses and vegetable gardens; and d) not eating unwashed vegetables that may be contaminated. It may be difficult to achieve compliance of some of these recommendations in populations with incompatible habits. For example, eating raw or undercooked snails is a popular and ancient custom in China (Wang et al., 2008). It is also important to educate health care personnel, most importantly physicians, in both non-endemic and endemic regions, to be aware of the existence of A. cantonensis, its common symptoms, and its modes of transmission to promptly diagnose the infection in humans (Wang et al., 2012).
Lumbar puncture is effective in reducing the intensity and duration of headaches (Sawanyawisuth et al., 2004). Steroid therapy is also effective in the treatment of headaches resulting from this infection and decreases the need for repeated lumbar punctures (Chotmongkol et al., 2000). The mean duration of headache was also reduced significantly by using a two week course of albendazole alone (Jitpimolmard et al., 2007). The combination of corticosteroids and albendazole is commonly used for treatment of human angiostrongyliasis (Chotmongkol et al., 2000; Wang et al., 2008), but a recent trial showed that the combination of prednisolone plus albendazole was not superior to prednisolone alone for the treatment of eosinophilic meningitis (Chotmongkol et al., 2009).
In cases of ocular angiostrongyliasis, surgery is necessary to remove the larva. Prior to surgery, laser can be used to kill the worm and prevent further damage (Chotmongkol et al., 2004; Sawanyawisuth et al., 2007; Yii, 1976). Steroids can also be used to reduce intraocular inflammation, but albendazole is not recommended because dead parasites may cause serious intraocular inflammation (Diao et al., 2011; Feng et al., 2013). However, regardless of the treatment method, visual damage is not markedly improved in relation to the time of presentation (Diao et al., 2011; Sawanyawisuth and Chotmongkol, 2013; Wang et al., 2008).
Concerning the prognosis of angiostrongyliasis, patients with eosinophilic meningitis have a good prognosis. Headaches are dramatically improved after treatment with steroids and/or lumbar punctures. Even without any treatment, the disease tends to improve over time (Wang et al., 2008). On the other hand, for patients with encephalitis, prognosis is poor, likely due to the greater severity of CNS damage.
Highlights.
A. cantonensis is the most common cause of eosinophilic meningitis in southern Asia
A. cantonensis can also cause encephalitis, encephalomyelitis and ocular disease
Eosinophilic inflammation caused by dead larvae in the brain explains the pathogenesis
Main clinical features are headache, fever, stiff neck, paraesthesias and vomiting
We also review the treatment, prevention and prognosis of A. cantonesis infection
Acknowledgements
YCM is supported by the Inter-hemispheric Research/NIH Global Infectious Disease Research Training Program (D43 TW007129). The funders had no role in data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yuri C. Martins, Email: yuri.chaves@einstein.yu.edu.
Herbert B. Tanowitz, Email: Herbert.tanowitz@einstein.yu.edu.
Kevin R. Kazacos, Email: kkazacos@purdue.edu.
References
- Alicata JE. Biology and distribution of the rat lungworm, Angiostrongylus cantonensis, and its relationship to eosinophilic meningoencephalitis and other neurological disorders of man and animals. Advances in parasitology. 1965;3:223–248. doi: 10.1016/s0065-308x(08)60366-8. [DOI] [PubMed] [Google Scholar]
- Ash LR. The occurrence of Angiostrongylus cantonensis in frogs of New Caledonia with observations on paratenic hosts of metastrongyles. J Parasitol. 1968;54:432–436. [PubMed] [Google Scholar]
- Ash LR. Diagnostic morphology of the third-stage larvae of Angiostrongylus cantonensis, Angiostrongylus vasorum, Aelurostrongylus abstrusus, and Anafilaroides rostratus (Nematoda: Metastrongyloidea) J Parasitol. 1970;56:249–253. [PubMed] [Google Scholar]
- Bhaibulaya M. Comparative studies on the life history of Angiostrongylus mackerrasae Bhaibulaya, 1968 and Angiostrongylus cantonensis (Chen, 1935) International journal for parasitology. 1975;5:7–20. doi: 10.1016/0020-7519(75)90091-0. [DOI] [PubMed] [Google Scholar]
- Caldeira RL, Mendonca CL, Goveia CO, Lenzi HL, Graeff-Teixeira C, Lima WS, Mota EM, Pecora IL, Medeiros AM, Carvalho Odos S. First record of molluscs naturally infected with Angiostrongylus cantonensis (Chen, 1935) (Nematoda: Metastrongylidae) in Brazil. Memorias do Instituto Oswaldo Cruz. 2007;102:887–889. doi: 10.1590/s0074-02762007000700018. [DOI] [PubMed] [Google Scholar]
- Campbell BG, Little MD. The finding of Angiostrongylus cantonensis in rats in New Orleans. The American journal of tropical medicine and hygiene. 1988;38:568–573. doi: 10.4269/ajtmh.1988.38.568. [DOI] [PubMed] [Google Scholar]
- Carney WP, Stafford EE. Angiostrongyliasis in Indonesia: a review. In: Cross JH, editor. Studies on angiostrongyliasis in eastern Asia and Australia, Special Publication No. 2 (NAMRU-2-SP-44) Taipei: US Naval Medical Research Unit; 1979. pp. 14–25. [Google Scholar]
- Chao D, Lin CC, Chen YA. Studies on growth and distribution of Angiostrongylus cantonensis larvae in Ampullarium canaliculatus. The Southeast Asian journal of tropical medicine and public health. 1987;18:248–252. [PubMed] [Google Scholar]
- Chau TT, Thwaites GE, Chuong LV, Sinh DX, Farrar JJ. Headache and confusion: the dangers of a raw snail supper. Lancet. 2003;361:1866. doi: 10.1016/s0140-6736(03)13506-4. [DOI] [PubMed] [Google Scholar]
- Chen AL, Qiu XY, Wang W, Zhou CL, Zeng X, Liu XJ, Qiu JF, Wang Y. The quantitative and functional changes of NK cells in mice infected with Angiostrongylus cantonensis. Parasitology research. 2014;113:2087–2094. doi: 10.1007/s00436-014-3858-0. [DOI] [PubMed] [Google Scholar]
- Chen HT. A new pulmonary nematode of rats, Pulmonema cantonesis ng, nsp from Canton. Ann Parasitol. 1935;13:5. [Google Scholar]
- Chotmongkol V, Kittimongkolma S, Niwattayakul K, Intapan PM, Thavornpitak Y. Comparison of prednisolone plus albendazole with prednisolone alone for treatment of patients with eosinophilic meningitis. The American journal of tropical medicine and hygiene. 2009;81:443–445. [PubMed] [Google Scholar]
- Chotmongkol V, Sawanyawisuth K, Thavornpitak Y. Corticosteroid treatment of eosinophilic meningitis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2000;31:660–662. doi: 10.1086/314036. [DOI] [PubMed] [Google Scholar]
- Chotmongkol V, Yimtae K, Intapan PM. Angiostrongylus eosinophilic meningitis associated with sensorineural hearing loss. The Journal of laryngology and otology. 2004;118:57–58. doi: 10.1258/002221504322731664. [DOI] [PubMed] [Google Scholar]
- Chuang CC, Chen CW, Huang YT, Du WY. Anti-ST2 monoclonal antibody inhibits eosinophil infiltration in Angiostrongylus cantonensis-infected mice. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2014 doi: 10.1016/j.jmii.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Chuang CC, Su KE, Chen CW, Fan CK, Lin FK, Chen YS, Du WY. Anti-CCR3 monoclonal antibody inhibits eosinophil infiltration in Angiostrongylus cantonensis-infected ICR mice. Acta tropica. 2010;113:209–213. doi: 10.1016/j.actatropica.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Cognato BB, Morassutti AL, Silva AC, Graeff-Teixeira C. First report of Angiostrongylus cantonensis in Porto Alegre, State of Rio Grande do Sul, Southern Brazil. Revista da Sociedade Brasileira de Medicina Tropical. 2013;46:664–665. doi: 10.1590/0037-8682-0073-2013. [DOI] [PubMed] [Google Scholar]
- Costa LRR, McClure JJ, Snider TG, III, Stewart TB. Verminous meningoencephalomyelitis by Angiostrongylus (=Parastrongylus) cantonensis in an American Miniature Horse. Equine Vet Educ. 2000;12:5. [Google Scholar]
- Cowie RH. Biology, Systematics, Life Cycle, and Distribution of Angiostrongylus cantonensis, the Cause of Rat Lungworm Disease. Hawai'i journal of medicine & public health: a journal of Asia Pacific Medicine & Public Health. 2013;72:6–9. [PMC free article] [PubMed] [Google Scholar]
- Cross JH. Clinical manifestations and laboratory diagnosis of eosinophilic meningitis syndrome associated with angiostrongyliasis. The Southeast Asian journal of tropical medicine and public health. 1978;9:161–170. [PubMed] [Google Scholar]
- Cross JH. Experimental studies on Angiostrongylus species and strains in monkeys and laboratory animals. In: Cross JH, editor. Studies on angiostrongyliasis in eastern Asia and Australia, Special Publication No. 2 (NAMRU-2-SP-44) Taipei: US Naval Medical Research Unit; 1979. pp. 118–137. [Google Scholar]
- Cross JH. Angiostrongyliasis. In: Connor DH, Chandler FW, Schwartz DA, Manz HJ, Lack EE, editors. Pathology of infectious diseases. Stamford: Appleton & Lange; 1997. pp. 1307–1314. [Google Scholar]
- Diao Z, Wang J, Qi H, Li X, Zheng X, Yin C. Human ocular angiostrongyliasis: a literature review. Tropical doctor. 2011;41:76–78. doi: 10.1258/td.2010.100294. [DOI] [PubMed] [Google Scholar]
- Diaz JH. Recognizing and reducing the risks of helminthic eosinophilic meningitis in travelers: differential diagnosis, disease management, prevention, and control. Journal of travel medicine. 2009;16:267–275. doi: 10.1111/j.1708-8305.2009.00305.x. [DOI] [PubMed] [Google Scholar]
- Dorta-Contreras AJ, Padilla-Docal B, Moreira JM, Robles LM, Aroca JM, Alarcon F, Bu-Coifiu-Fanego R. Neuroimmunological findings of Angiostrongylus cantonensis meningitis in Ecuadorian patients. Arquivos de neuropsiquiatria. 2011;69:466–469. doi: 10.1590/s0004-282x2011000400011. [DOI] [PubMed] [Google Scholar]
- Drozdz J. [Revision of the classification of the genus Angiostrongylus Kamensky 1905 (Nematoda: Metastrongyloidea)] Annales de parasitologie humaine et comparee. 1970;45:597–603. doi: 10.1051/parasite/1970455597. [DOI] [PubMed] [Google Scholar]
- Duffy MS, Miller CL, Kinsella JM, de Lahunta A. Parastrongylus cantonensis in a nonhuman primate, Florida. Emerging infectious diseases. 2004;10:2207–2210. doi: 10.3201/eid1012.040319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Nawa Y, Sawanyavisuth K, Lv Z, Wu ZD. Comprehensive review of ocular angiostrongyliasis with special reference to optic neuritis. The Korean journal of parasitology. 2013;51:613–619. doi: 10.3347/kjp.2013.51.6.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JS, Dos Santos Bonfim TC, Junior AM, Tunholi VM, Tunholi-Alves VM, Mota EM, Simoes RD, Santana AC, Hooper C, Pinheiro J, Boia MN. Hematological and histopathological changes in Rattus norvegicus (Wistar) experimentally infected by Angiostrongylus cantonensis (Chen, 1935) Parasitology international. 2014;63:631–637. doi: 10.1016/j.parint.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Gardiner CH, Wells S, Gutter AE, Fitzgerald L, Anderson DC, Harris RK, Nichols DK. Eosinophilic meningoencephalitis due to Angiostrongylus cantonensis as the cause of death in captive non-human primates. The American journal of tropical medicine and hygiene. 1990;42:70–74. doi: 10.4269/ajtmh.1990.42.70. [DOI] [PubMed] [Google Scholar]
- Gosnell WL, Kramer KJ. The role of eosinophils in angiostrongyliasis: multiple roles for a versatile cell? Hawai'i journal of medicine & public health: a journal of Asia Pacific Medicine & Public Health. 2013;72:49–51. [PMC free article] [PubMed] [Google Scholar]
- Grisotti M, Avila-Pires FD. Worms, slugs and humans: the medical and popular construction of an emerging infectious disease. Historia, ciencias, saude--Manguinhos. 2011;18:877–891. doi: 10.1590/s0104-59702011000300016. [DOI] [PubMed] [Google Scholar]
- Hwang KP, Chen ER. Clinical studies on angiostrongyliasis cantonensis among children in Taiwan. The Southeast Asian journal of tropical medicine and public health. 1991;22(Suppl):194–199. [PubMed] [Google Scholar]
- Incani RN, Caleiras E, Martin M, Gonzalez C. Human infection by Angiostrongylus costaricensis in Venezuela: first report of a confirmed case. Revista do Instituto de Medicina Tropical de Sao Paulo. 2007;49:197–200. doi: 10.1590/s0036-46652007000300012. [DOI] [PubMed] [Google Scholar]
- Jin E, Ma D, Liang Y, Ji A, Gan S. MRI findings of eosinophilic myelomeningoencephalitis due to Angiostrongylus cantonensis. Clinical radiology. 2005;60:242–250. doi: 10.1016/j.crad.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Jitpimolmard S, Sawanyawisuth K, Morakote N, Vejjajiva A, Puntumetakul M, Sanchaisuriya K, Tassaneeyakul W, Tassaneeyakul W, Korwanich N. Albendazole therapy for eosinophilic meningitis caused by Angiostrongylus cantonensis. Parasitology research. 2007;100:1293–1296. doi: 10.1007/s00436-006-0405-7. [DOI] [PubMed] [Google Scholar]
- Kanchanaranya C, Prechanond A, Punyagupta S. Removal of living worm in retinal Angiostrongylus cantonensis. American journal of ophthalmology. 1972;74:456–458. doi: 10.1016/0002-9394(72)90908-7. [DOI] [PubMed] [Google Scholar]
- Kanpittaya J, Jitpimolmard S, Tiamkao S, Mairiang E. MR findings of eosinophilic meningoencephalitis attributed to Angiostrongylus cantonensis. AJNR. American journal of neuroradiology. 2000;21:1090–1094. [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Stewart TB, Bauer RW, Mitchell M. Parastrongylus (=Angiostrongylus) cantonensis now endemic in Louisiana wildlife. J Parasitol. 2002;88:1024–1026. doi: 10.1645/0022-3395(2002)088[1024:PACNEI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kim JR, Hayes KA, Yeung NW, Cowie RH. Diverse Gastropod Hosts of Angiostrongylus cantonensis, the Rat Lungworm, Globally and with a Focus on the Hawaiian Islands. PloS one. 2014;9:e94969. doi: 10.1371/journal.pone.0094969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliks MM, Kroenke K, Hardman JM. Eosinophilic radiculomyeloencephalitis: an angiostrongyliasis outbreak in American Samoa related to ingestion of Achatina fulica snails. The American journal of tropical medicine and hygiene. 1982;31:1114–1122. doi: 10.4269/ajtmh.1982.31.1114. [DOI] [PubMed] [Google Scholar]
- Kliks MM, Palumbo NE. Eosinophilic meningitis beyond the Pacific Basin: the global dispersal of a peridomestic zoonosis caused by Angiostrongylus cantonensis, the nematode lungworm of rats. Social science & medicine. 1992;34:199–212. doi: 10.1016/0277-9536(92)90097-a. [DOI] [PubMed] [Google Scholar]
- Kuberski T. Eosinophils in cerebrospinal fluid: criteria for eosinophilic meningitis. Hawaii medical journal. 1981;40:97–98. [PubMed] [Google Scholar]
- Kuberski T, Bart RD, Briley JM, Rosen L. Recovery of Angiostrongylus cantonensis from cerebrospinal fluid of a child with eosinophilic meningitis. Journal of clinical microbiology. 1979;9:629–631. doi: 10.1128/jcm.9.5.629-631.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Zhang RL, Fu YC, Wu WP, Chen MX, Geng YJ, Huang DN, Ai L, Yang F, Hu Z. Monoclonal antibody 12D5 inhibits eosinophil infiltration in the brain of Angiostrongylus cantonensis-infected BALB/c mice. Acta tropica. 2012;121:118–124. doi: 10.1016/j.actatropica.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Lim BL, Ramachandran CP. Ecological studies of Angiostrongylus malaysiensis (Nematoda: Metastrongylidae) in Malaysia. In: Cross JH, editor. Studies on angiostrongyliasis in eastern Asia and Australia, Special Publication No. 2 (NAMRU-2-SP-44) Taipei: US Naval Medical Research Unit; 1979. pp. 26–48. [Google Scholar]
- Lindo JF, Escoffery CT, Reid B, Codrington G, Cunningham-Myrie C, Eberhard ML. Fatal autochthonous eosinophilic meningitis in a Jamaican child caused by Angiostrongylus cantonensis. The American journal of tropical medicine and hygiene. 2004;70:425–428. [PubMed] [Google Scholar]
- Liu IH, Chung YM, Chen SJ, Cho WL. Necrotizing retinitis induced by Angiostrongylus cantonensis. American journal of ophthalmology. 2006;141:577–579. doi: 10.1016/j.ajo.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Lo Re V, 3rd, Gluckman SJ. Eosinophilic meningitis. The American journal of medicine. 2003;114:217–223. doi: 10.1016/s0002-9343(02)01495-x. [DOI] [PubMed] [Google Scholar]
- Mackerras MJ, Sandars DF. Lifehistory of the rat lung-worm and its migration through the brain of its host. Nature. 1954;173:956–957. doi: 10.1038/173956a0. [DOI] [PubMed] [Google Scholar]
- Maleewong W, Sombatsawat P, Intapan PM, Wongkham C, Chotmongkol V. Immunoblot evaluation of the specificity of the 29-kDa antigen from young adult female worms Angiostrongylus cantonensis for immunodiagnosis of human angiostrongyliasis. Asian Pacific journal of allergy and immunology / launched by the Allergy and Immunology Society of Thailand. 2001;19:267–273. [PubMed] [Google Scholar]
- Malhotra S, Mehta DK, Arora R, Chauhan D, Ray S, Jain M. Ocular angiostrongyliasis in a child--first case report from India. Journal of tropical pediatrics. 2006;52:223–225. doi: 10.1093/tropej/fmi092. [DOI] [PubMed] [Google Scholar]
- Mason KV. Canine neural angiostrongylosis: the clinical and therapeutic features of 55 natural cases. Australian veterinary journal. 1987;64:201–203. doi: 10.1111/j.1751-0813.1987.tb15181.x. [DOI] [PubMed] [Google Scholar]
- Morassutti AL, Levert K, Perelygin A, da Silva AJ, Wilkins P, Graeff-Teixeira C. The 31-kDa antigen of Angiostrongylus cantonensis comprises distinct antigenic glycoproteins. Vector borne and zoonotic diseases. 2012;12:961–968. doi: 10.1089/vbz.2011.0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morassutti AL, Thiengo SC, Fernandez M, Sawanyawisuth K, Graeff-Teixeira C. Eosinophilic meningitis caused by Angiostrongylus cantonensis: an emergent disease in Brazil. Memorias do Instituto Oswaldo Cruz. 2014;109:399–407. doi: 10.1590/0074-0276140023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morera P, Cespedes R. Angiostrongylus costaricensis n. sp. (Nematoda: Metastrongyloidea), a new lungworm occurring in man in Costa Rica. Revista de biologia tropical. 1970;18:173–185. [PubMed] [Google Scholar]
- Murphy GS, Johnson S. Clinical Aspects of Eosinophilic Meningitis and Meningoencephalitis caused by Angiostrongylus cantonensis, the Rat Lungworm. Hawai'i journal of medicine & public health: a journal of Asia Pacific Medicine & Public Health. 2013;72:35–40. [PMC free article] [PubMed] [Google Scholar]
- New D, Little MD, Cross J. Angiostrongylus cantonensis infection from eating raw snails. The New England journal of medicine. 1995;332:1105–1106. doi: 10.1056/NEJM199504203321619. [DOI] [PubMed] [Google Scholar]
- Nomura S, Lin H. First clinical case of Haemostrongylus ratti. Taiwan No Ikai. 1945;3:4. [Google Scholar]
- Noskin GA, McMenamin MB, Grohmann SM. Eosinophilic meningitis due to Angiostrongylus cantonensis. Neurology. 1992;42:1423–1424. doi: 10.1212/wnl.42.7.1423. [DOI] [PubMed] [Google Scholar]
- Nuamtanong S. The evaluation of the 29 and 31 kDa antigens in female Angiostrongylus cantonensis for serodiagnosis of human angiostrongyliasis. The Southeast Asian journal of tropical medicine and public health. 1996;27:291–296. [PubMed] [Google Scholar]
- Orihel TC, Ash LR. Parasites in human tissues. Chicago: ASCP Press; 1995. [Google Scholar]
- OuYang L, Wei J, Wu Z, Zeng X, Li Y, Jia Y, Ma Y, Zhan M, Lei W. Differences of larval development and pathological changes in permissive and nonpermissive rodent hosts for Angiostrongylus cantonensis infection. Parasitology research. 2012;111:1547–1557. doi: 10.1007/s00436-012-2995-6. [DOI] [PubMed] [Google Scholar]
- Padilla-Docal B, Dorta-Contreras AJ, Moreira JM, Martini-Robles L, Muzzio-Aroca J, Alarcon F, Magraner-Tarrau ME, Bu-Coifiu-Fanego R. Comparison of major immunoglobulins intrathecal synthesis patterns in Ecuadorian and Cuban patients with angiostrongyliasis. The American journal of tropical medicine and hygiene. 2011;84:406–410. doi: 10.4269/ajtmh.2011.10-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Docal B, Iglesias-Gonzalez I, Bu-Coifiu-Fanego R, Socarras-Hernandez CA, Dorta-Contreras AJ. Intrathecal activation as a typical immune response within the central nervous system in angiostrongyliasis. The American journal of tropical medicine and hygiene. 2013;88:230–235. doi: 10.4269/ajtmh.12-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Sun R, Zhang Q, Zhao J, Wei J, Zeng X, Zheng H, Wu Z. Interleukin 33 mediates type 2 immunity and inflammation in the central nervous system of mice infected with Angiostrongylus cantonensis. The Journal of infectious diseases. 2013;207:860–869. doi: 10.1093/infdis/jis682. [DOI] [PubMed] [Google Scholar]
- Prociv P. Parasitic meningitis. The Medical journal of Australia. 1999;170:517–518. doi: 10.5694/j.1326-5377.1999.tb127872.x. [DOI] [PubMed] [Google Scholar]
- Prociv P, Spratt DM, Carlisle MS. Neuro-angiostrongyliasis: unresolved issues. International journal for parasitology. 2000;30:1295–1303. doi: 10.1016/s0020-7519(00)00133-8. [DOI] [PubMed] [Google Scholar]
- Prommindaroj K, Leelawongs N, Pradatsundarasar A. Human angiostrongyliasis of the eye in Bangkok. The American journal of tropical medicine and hygiene. 1962;1:759–761. doi: 10.4269/ajtmh.1962.11.759. [DOI] [PubMed] [Google Scholar]
- Punyagupta S, Bunnag T, Juttijudata P, Rosen L. Eosinophilic meningitis in Thailand. Epidemiologic studies of 484 typical cases and the etiologic role of Angiostrongylus cantonensis. The American journal of tropical medicine and hygiene. 1970;19:950–958. [PubMed] [Google Scholar]
- Punyagupta S, Juttijudata P, Bunnag T. Eosinophilic meningitis in Thailand. Clinical studies of 484 typical cases probably caused by Angiostrongylus cantonensis. The American journal of tropical medicine and hygiene. 1975;24:921–931. [PubMed] [Google Scholar]
- Qi H, Diao Z, Yin C. A case of optic nerve compression caused by Angiostrongylus cantonensis. The American journal of tropical medicine and hygiene. 2009;81:4. [PubMed] [Google Scholar]
- Qvarnstrom Y, da Silva AC, Teem JL, Hollingsworth R, Bishop H, Graeff-Teixeira C, da Silva AJ. Improved molecular detection of Angiostrongylus cantonensis in mollusks and other environmental samples with a species-specific internal transcribed spacer 1-based TaqMan assay. Applied and environmental microbiology. 2010;76:5287–5289. doi: 10.1128/AEM.00546-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomyos P, Tungtrongchitr A, Praewanich R, Khewwatchan P, Kantangkul T, Junlananto P, Ayudhya SI. Occurrence of the infective stage of Angiostrongylus cantonensis in the yellow tree monitor (Varanus bengalensis) in five Provinces of Thailand. The Southeast Asian journal of tropical medicine and public health. 1994;25:498–500. [PubMed] [Google Scholar]
- Rebello KM, Menna-Barreto RF, Chagas-Moutinho VA, Mota EM, Perales J, Neves-Ferreira AG, Oliveira-Menezes A, Lenzi H. Morphological aspects of Angiostrongylus costaricensis by light and scanning electron microscopy. Acta tropica. 2013;127:191–198. doi: 10.1016/j.actatropica.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Rebello KM, Siqueira CR, Ribeiro EL, Valente RH, Mota EM, Perales J, Neves-Ferreira AG, Lenzi HL. Proteolytic activity in the adult and larval stages of the human roundworm parasite Angiostrongylus costaricensis. Memorias do Instituto Oswaldo Cruz. 2012;107:752–759. doi: 10.1590/s0074-02762012000600008. [DOI] [PubMed] [Google Scholar]
- Rodriguez R, da Silva AC, Muller CA, Alves SL, Graeff-Teixeira C, Fornari F. PCR for the diagnosis of abdominal angiostrongyliasis in formalin-fixed paraffin-embedded human tissue. PloS one. 2014;9:e93658. doi: 10.1371/journal.pone.0093658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawanyawisuth K, Chotmongkol V. Eosinophilic meningitis. Handbook of clinical neurology. 2013;114:207–215. doi: 10.1016/B978-0-444-53490-3.00015-7. [DOI] [PubMed] [Google Scholar]
- Sawanyawisuth K, Kitthaweesin K, Limpawattana P, Intapan PM, Tiamkao S, Jitpimolmard S, Chotmongkol V. Intraocular angiostrongyliasis: clinical findings, treatments and outcomes. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007;101:497–501. doi: 10.1016/j.trstmh.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Sawanyawisuth K, Limpawattana P, Busaracome P, Ninpaitoon B, Chotmongkol V, Intapan PM, Tanawirattananit S. A 1-week course of corticosteroids in the treatment of eosinophilic meningitis. The American journal of medicine. 2004;117:802–803. doi: 10.1016/j.amjmed.2004.06.038. [DOI] [PubMed] [Google Scholar]
- Sawanyawisuth K, Pugkhem A, Mitchai J, Intapan PM, Anunnatsiri S, Limpawattana P, Chotmongkol V. Abdominal angiostrongyliasis caused by Angiostrongylus cantonensis: a possible cause of eosinophilic infiltration in human digestive tract. Pathology, research and practice. 2010;206:102–104. doi: 10.1016/j.prp.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Sawanyawisuth K, Takahashi K, Hoshuyama T, Sawanyawisuth K, Senthong V, Limpawattana P, Intapan PM, Wilson D, Tiamkao S, Jitpimolmard S, Chotmongkol V. Clinical factors predictive of encephalitis caused by Angiostrongylus cantonensis. The American journal of tropical medicine and hygiene. 2009;81:698–701. doi: 10.4269/ajtmh.2009.09-0309. [DOI] [PubMed] [Google Scholar]
- Schmutzhard E, Boongird P, Vejjajiva A. Eosinophilic meningitis and radiculomyelitis in Thailand, caused by CNS invasion of Gnathostoma spinigerum and Angiostrongylus cantonensis. Journal of neurology, neurosurgery, and psychiatry. 1988;51:80–87. doi: 10.1136/jnnp.51.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih SL, Hsu CH, Huang FY, Shen EY, Lin JC. Angiostrongylus cantonensis infection in infants and young children. The Pediatric infectious disease journal. 1992;11:1064–1066. [PubMed] [Google Scholar]
- Slom TJ, Cortese MM, Gerber SI, Jones RC, Holtz TH, Lopez AS, Zambrano CH, Sufit RL, Sakolvaree Y, Chaicumpa W, Herwaldt BL, Johnson S. An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. The New England journal of medicine. 2002;346:668–675. doi: 10.1056/NEJMoa012462. [DOI] [PubMed] [Google Scholar]
- Sugaya H, Aoki M, Yoshida T, Takatsu K, Yoshimura K. Eosinophilia and intracranial worm recovery in interleukin-5 transgenic and interleukin-5 receptor alpha chain-knockout mice infected with Angiostrongylus cantonensis. Parasitology research. 1997;83:583–590. doi: 10.1007/s004360050302. [DOI] [PubMed] [Google Scholar]
- Tangchai P, Nye SW, Beaver PC. Eosinophilic meningoencephalitis caused by angiostrongyliasis in Thailand. Autopsy report. The American journal of tropical medicine and hygiene. 1967;16:454–461. doi: 10.4269/ajtmh.1967.16.454. [DOI] [PubMed] [Google Scholar]
- Teixeira CG, Thiengo SC, Thome JW, Medeiros AB, Camillo-Coura L, Agostini AA. On the diversity of mollusc intermediate hosts of Angiostrongylus costaricensis Morera & Cespedes, 1971 in southern Brazil. Memorias do Instituto Oswaldo Cruz. 1993;88:487–489. doi: 10.1590/s0074-02761993000300020. [DOI] [PubMed] [Google Scholar]
- Thiengo SC. Mode of Infection of Sarasinula marginata (Mollusca) with Larvae of Angiostrongylus costaricensis (Nematoda) Memorias do Instituto Oswaldo Cruz. 1996;91:2. [Google Scholar]
- Thiengo SC, de Oliveira Simoes R, Fernandez MA, Junior AM. Angiostrongylus cantonensis and Rat Lungworm Disease in Brazil. Hawai'i journal of medicine & public health : a journal of Asia Pacific Medicine & Public Health. 2013;72:18–22. [PMC free article] [PubMed] [Google Scholar]
- Thiengo SC, Maldonado A, Mota EM, Torres EJ, Caldeira R, Carvalho OS, Oliveira AP, Simoes RO, Fernandez MA, Lanfredi RM. The giant African snail Achatina fulica as natural intermediate host of Angiostrongylus cantonensis in Pernambuco, northeast Brazil. Acta tropica. 2010;115:194–199. doi: 10.1016/j.actatropica.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Tsai HC, Liu YC, Kunin CM, Lai PH, Lee SS, Chen YS, Wann SR, Lin WR, Huang CK, Ger LP, Lin HH, Yen MY. Eosinophilic meningitis caused by Angiostrongylus cantonensis associated with eating raw snails: correlation of brain magnetic resonance imaging scans with clinical findings. The American journal of tropical medicine and hygiene. 2003;68:281–285. [PubMed] [Google Scholar]
- Ubelaker JE. Systematics of species referred to the genus Angiostrongylus. J Parasitol. 1986;72:237–244. [PubMed] [Google Scholar]
- Wallace GD, Rosen L. Studies on eosinophilic meningitis. 2. Experimental infection of shrimp and crabs with Angiostrongylus cantonensis. American journal of epidemiology. 1966;84:120–131. doi: 10.1093/oxfordjournals.aje.a120617. [DOI] [PubMed] [Google Scholar]
- Wallace GD, Rosen L. Studies on eosinophilic meningitis. IV. Experimental infection of fresh-water and marine fish with Angiostrongylus cantonensis. American journal of epidemiology. 1967;85:395–402. doi: 10.1093/oxfordjournals.aje.a120701. [DOI] [PubMed] [Google Scholar]
- Wallace GD, Rosen L. Studies on eosinophilic meningitis. V. Molluscan hosts of Angiostrongylus cantonensis on Pacific Islands. The American journal of tropical medicine and hygiene. 1969;18:206–216. [PubMed] [Google Scholar]
- Wang QP, Lai DH, Zhu XQ, Chen XG, Lun ZR. Human angiostrongyliasis. The Lancet infectious diseases. 2008;8:621–630. doi: 10.1016/S1473-3099(08)70229-9. [DOI] [PubMed] [Google Scholar]
- Wang QP, Wu ZD, Wei J, Owen RL, Lun ZR. Human Angiostrongylus cantonensis: an update. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2012;31:389–395. doi: 10.1007/s10096-011-1328-5. [DOI] [PubMed] [Google Scholar]
- Wei Y, Hong Q, Chen D, Liang C, Liu H, Luo X, Zhu X. Permissibility of Mongolian gerbil for Angiostrongylus cantonensis infection and utility of this animal model for anthelmintic studies. Parasitology research. 2014;113:1687–1693. doi: 10.1007/s00436-014-3813-0. [DOI] [PubMed] [Google Scholar]
- Wu GH. Angiostrongylus cantonensis. In: Tang JQ, editor. Nature-borne diseases. Beijing: Science Press; 2006. pp. 1182–1189. [Google Scholar]
- Yii CY. Clinical observations on eosinophilic meningitis and meningoencephalitis caused by Angiostrongylus cantonensis on Taiwan. The American journal of tropical medicine and hygiene. 1976;25:233–249. doi: 10.4269/ajtmh.1976.25.233. [DOI] [PubMed] [Google Scholar]