Abstract
Background
The combination of major congenital heart disease (CHD) and prematurity is associated with poor prognosis, but previous studies have not fully characterized morbidity and mortality in this population. We conducted a retrospective cohort study of very low birth weight (VLBW) infants with major CHD to describe outcomes, including mortality, over time.
Methods
We included all infants <1500 g birth weight with major CHD discharged from Pediatrix Medical Group neonatal intensive care units from 1997–2012. We report incidences of major CHD in VLBW infants and compare mortality and morbidity by infant birth weight, type of major CHD, and time period.
Results
Of 105,539 VLBW infants, 299 (0.3%) were diagnosed with 15 different major CHDs. Coarctation of the aorta (n=67, 22%), atrioventricular septal defect (n=58, 19%), and tetralogy of Fallot (n=53, 18%) were the most common major CHDs identified. Overall mortality was 163/299 (55%). Mortality was ≥70% for 10 lesions and <30% for isolated aortic valve stenosis (6/30, 20%). Mortality in infants with major CHD did not significantly change over time: 76/133 (57%) in 1997–2005, 49/95 (52%) in 2006–2009, and 38/71 (54%) in 2010–2012 (p=0.70). The majority of infants suffered ≥1 comorbidity or died (218/299, 73%).
Conclusion
Major CHD is associated with high morbidity and mortality. While mortality varies by lesion, overall survival and incidence of major morbidity have not improved over time.
Keywords: prematurity, very low birth weight, congenital heart defect, morbidity, mortality
1. Introduction
Very low birth weight (VLBW, <1500 g birth weight) infants are at higher risk of complex congenital heart disease (major CHD) compared with term infants [1,2]. VLBW infants are also at increased risk of other prematurity-related morbidities and mortality [3]. In 2012, 1.4% of all live births in the United States were VLBW [4].
Although major CHD is recognized as an independent risk factor for poor outcome in premature infants, the overall prevalence and spectrum of morbidity and mortality in infants born with both major CHD and VLBW have not been well characterized [2,5,6]. Mortality in VLBW infants with major CHD is high, but previous studies were limited by inclusion of infants with isolated atrial septal defect (ASD) or ventricular septal defect (VSD), missing information on neonatal morbidities, or inability to examine trends in mortality over time [2,6,7]. The pathophysiologic mechanisms responsible for higher mortality of VLBW infants with congenital heart defects are unknown, but comorbidities associated with the need for surgical intervention and prolonged hospitalization likely play a significant role. No prior analyses have evaluated the prevalence of comorbidities in VLBW infants with major CHD or described recent trends over time.
Using data from a nationally representative cohort of neonatal intensive care units (NICUs) in the United States, we sought to describe the morbidity and mortality of VLBW infants with major CHD and examined their trends over time.
2. Methods
2.1. Study population
We included all infants <1500 g birth weight and <32 weeks gestational age (GA) with a diagnosis of major CHD discharged from 348 NICUs managed by the Pediatrix Medical Group between 1997 and 2012. Data are generated by treating clinicians for the purpose of medical documentation and billing in a shared electronic record. Data are then extracted, de-identified, and consolidated into the Pediatrix Clinical Data Warehouse. We excluded infants with missing information on survival at discharge, including transfers.
2.2. Definitions
We defined major CHD as the postnatal diagnosis of any of the lesions typically considered to be moderate-to-severe forms of congenital defect (listed in Table 2). We categorized lesions into cyanotic (any single ventricle, Ebstein’s anomaly, tetralogy of Fallot, pulmonary atresia with intact ventricular septum [PA-IVS], pulmonary atresia with ventricular septal defect [PA-VSD], truncus arteriosus, transposition of the great arteries [TGA], and total anomalous pulmonary venous return [TAPVR]) and acyanotic lesions. We defined intraventricular hemorrhage (IVH) as a diagnosis of IVH grades 3 or 4. We defined necrotizing enterocolitis (NEC) as any diagnosis of NEC requiring medical or surgical therapy. We defined retinopathy of prematurity (ROP) as ROP requiring medical (bevacizumab) or surgical (cryotherapy, laser therapy, vitrectomy) therapy. We defined bronchopulmonary dysplasia (BPD) as previously described [8]. We defined exposure to prostaglandin or inotropes (amrinone, dopamine, dobutamine, epinephrine, milrinone, or norepinephrine) as any exposure to these drugs during the hospitalization. We defined weight at discharge as the weight recorded on the day of discharge or 1 day prior to discharge if no record was available on day of discharge. We categorized discharge year into tertiles based on the distribution of all VLBW infants over the study period: 1997–2005, 2006–2009, and 2010–2012.
Table 2.
Mortality by heart defect and birth weight
| <750 g N=84 |
750–999 g N=87 |
1000–1499 g N=128 |
Overall N=299 |
|
|---|---|---|---|---|
| Aortic valve stenosis | 3/9 (33%) | 1/8(13%) | 2/13 (15%) | 6/30 (20%) |
| AVSD | 15/16 (94%) | 8/16 (50%) | 12/26 (46%) | 35/58 (60%) |
| Coarctation of the aorta | 15/23 (65%) | 4/17 (24%) | 5/27 (36%) | 24/67 (36%) |
| Ebstein’s anomaly | 0/0 | 0/1 (0%) | 3/4 (75%) | 3/5 (60%) |
| HLHS | 3/3 (100%) | 6/7 (86%) | 8/10 (80%) | 17/20 (85%) |
| IAA | 1/1 (100%) | 0/0 | 1/1 (100%) | 2/2 (100%) |
| IAA + VSD | 2/2 (100%) | 1/1 (100%) | 0/0 | 3/3 (100%) |
| Other single ventricle | 0/0 | 1/1 (100%) | 4/5 (80%) | 5/6 (83%) |
| Pulmonary atresia | 3/4 (75%) | 3/4 (75%) | 1/1 (100%) | 7/9 (78%) |
| Pulmonary atresia + VSD | 1/1 (100%) | 1/1 (100%) | 0/0 | 5/7 (71%) |
| TAPVR | 3/3 (100%) | 0/0 (0%) | 2/4 (50%) | 5/7 (71%) |
| Tetralogy of Fallot | 10/12 (83%) | 10/17 (59%) | 6/24 (25%) | 26/53 (49%) |
| TGA | 4/4 (100%) | 6/9 (67%) | 3/5 (60%) | 13/18 (72%) |
| Tricuspid atresia | 3/3 (100%) | 1/2 (50%) | 3/5 (60%) | 7/10 (70%) |
| Truncus arteriosus | 3/3 (100%) | 2/3 (67%) | 3/3 (100%) | 8/9 (90%) |
AVSD: atrioventricular septal defect; HLHS: hypoplastic left heart syndrome; IAA: interrupted aortic arch; TAPVR: total anomalous pulmonary venous return; TGA: transposition of the great arteries; VSD: ventricular septal defect.
2.3. Statistical analysis
We summarized categorical variables as counts and percentages, and continuous variables as medians and interquartile ranges. We compared distributions of categorical and continuous variables across demographic characteristics and by survival at hospital discharge using chi-square tests, Fisher’s exact tests, and unadjusted odds ratios for categorical variables, and Wilcoxon rank sum tests for continuous variables. We conducted all analyses using Stata 12.0 (College Station, TX) and considered a p<0.05 statistically significant. This study was approved by the Duke University Institutional Review Board with waiver of informed consent.
3. Results
3.1. Study cohort and incidence
Out of 105,539 VLBW infants, 299 (0.3%) were diagnosed with major CHD. The median GA and birth weight of all VLBW infants with major CHD were 28 weeks (interquartile range 26, 29) and 930 g (725, 1180), respectively. More than half of the cohort (57%) had a birth weight <1000 g, and 64/299 of infants (21%) were diagnosed with a genetic syndrome (Table 1).
Table 1.
Demographics of infants with cardiac defects
| All infants N=299 n (%) |
Alive N=136 n (%) |
Died N=163 n (%) |
p | ||
|---|---|---|---|---|---|
| Gestational age, weeks | <0.001 | ||||
| ≤25 | 56 (19) | 9 (7) | 47 (29) | ||
| 26–28 | 134 (45) | 56 (41) | 78 (48) | ||
| 29–32 | 109 (37) | 71 (52) | 38 (23) | ||
| Birth weight, g | <0.001 | ||||
| <750 | 84 (28) | 18 (13) | 66 (41) | ||
| 750–999 | 87 (29) | 43 (32) | 44 (27) | ||
| 1000–1499 | 128 (43) | 75 (55) | 53 (33) | ||
| Male | 160 (54) | 80 (59) | 80 (50) | 0.12 | |
| Race/ethnicity | 0.03 | ||||
| Black | 52 (18) | 16 (12) | 36 (24) | ||
| White | 144 (51) | 77 (59) | 67 (44) | ||
| Hispanic | 76 (27) | 34 (26) | 42 (28) | ||
| Other | 12 (4) | 4 (3) | 8 (5) | ||
| Inborn | 231 (79) | 101 (77) | 130 (80) | 0.44 | |
| Cesarean delivery | 221 (75) | 100 (75) | 121 (75) | 0.92 | |
| 5-minute APGAR score | <0.001 | ||||
| 0–3 | 25 (9) | 1 (1) | 24 (15) | ||
| 4–6 | 73 (25) | 23 (17) | 50 (32) | ||
| 7–10 | 192 (66) | 108 (82) | 84 (53) | ||
| Antenatal steroids | 211 (71) | 100 (76) | 111 (68) | 0.31 | |
| Genetic syndrome | 64 (21) | 22 (16) | 42 (26) | 0.04 | |
The mean annual incidence of major CHD in VLBW infants over our study period was 2.9 per 1000 live births per year (range 1.8/1000 in 1998, 4.9/1000 in 2003). The incidence of major CHD in VLBW infants remained unchanged over time—133/41,672 (0.3%) in 1997–2005, 95/36,700 (0.3%) in 2006–2009, and 71/27,161 (0.3%) in 2010–2012 (p=0.21).
3.2. Congenital heart defects and mortality
We identified 15 different major CHDs in the 299 infants (Table 2). The most common defects were coarctation of the aorta (n=67, 22%), atrioventricular septal defect (n=58, 19%), and tetralogy of Fallot (n=53, 18%). The most common single-ventricle major CHD was hypoplastic left heart syndrome (HLHS) (n=20, 7%). A cyanotic major CHD was diagnosed in 139/299 (47%) infants.
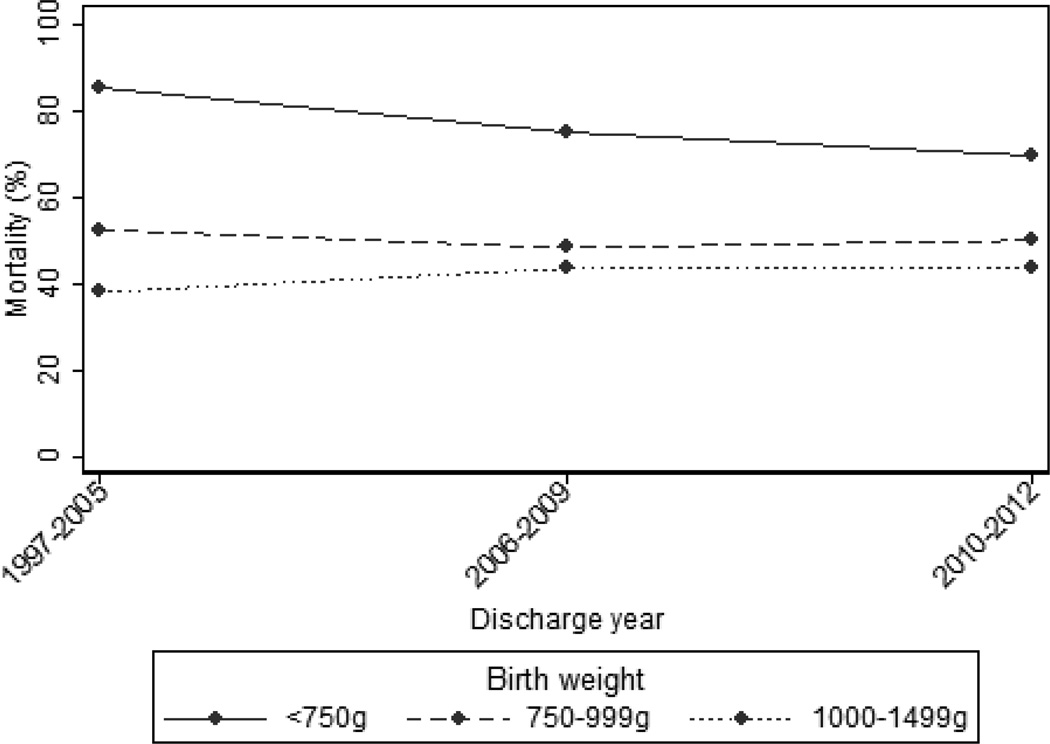
Overall mortality in our cohort was 163/299 (55%). Mortality was ≥70% for 10/15 lesions and was <30% only for isolated aortic valve stenosis (6/30, 20%) (Table 2). Mortality was almost 4-fold higher in infants with major CHD compared with VLBW infants without major CHD—163/299 (55%) vs. 12,911/90,400 (14%), respectively (p<0.001). Infants with major CHD most commonly died in the first 3 weeks of life (median day of life 10 [interquartile range; 2, 21]). The median duration of hospitalization in survivors was 64 days (18, 89). Mortality in infants with major CHD did not change over time: 76/133 (57%) in 1997–2005, 49/95 (52%) in 2006–2009, and 38/71 (54%) in 2010–2012 (p=0.70) (Figure 1). Mortality for VLBW infants without major CHD decreased from 5596/35,650 (16%) in 1997–2005 to 4417/31,235 (14%) in 2006–2009 and 2898/23,515 (12%) in 2010–2012 (p<0.001).
Fig. 1.
Mortality over time by birth weight.
Mortality in VLBW infants with major CHD was higher with lower GA: 47/56 (84%) ≤25 weeks, 78/134 (58%) 26–28 weeks, and 38/109 (35%) 29–32 weeks (p<0.001). Mortality decreased with each 1-week increase in GA (odds ratio=0.68; 95% confidence interval 0.60, 0.78). Mortality was also higher in infants with an associated genetic defect versus those without—42/64 (66%) vs. 121/235 (52%) (p=0.04).
3.3. Interventions
Mechanical ventilation was used in 262/299 infants (88%). Inotropic support and diuretic therapy were used in approximately half of all infants (147/299 [49%] and 150/299 [50%], respectively), while prostaglandin infusion was administered in one quarter (76/299 [25%]). Mortality was higher in infants requiring prostaglandins (62/76 [82%] vs. 101/223 [45%]; p<0.001) or inotropic support (105/147 [71%] vs. 58/152 [38%]; p<0.001).
3.4. Morbidity
Non-cardiac comorbidities were common in our cohort (103/299 [35%]). The proportion of infants with at least 1 non-cardiac comorbidity increased with decreasing birth weight—35/128 (27%) 1000–1499 g, 33/87 (38%) 750–999 g, and 35/84 (42%) <750 g (p=0.03). This trend was affected by the increasing proportion of infants with lower birth weights who were diagnosed with IVH (7/128 [6%], 11/87 [13%], and 15/84 [18%]; p=0.004) and ROP (1/128 [1%], 1/87 [1%], and 7/84 [8%]; p=0.003). There was no significant trend in the proportion of infants with chronic lung disease (21/128 [16%], 18/87 [21%], and 20/84 [24%]; p=0.19) or those with NEC (11/128 [9%], 13/87 [15%], and 10/84 [12%]; p=0.38). The proportion of infants with at least 1 non-cardiac comorbidity was similar in infants with cyanotic vs. acyanotic major CHD (46/139 [33%] vs. 57/160 [36%]; p=0.65). The majority of infants in our cohort had at least 1 comorbidity or died (218/299, 73%) (Table 3).This proportion increased with decreasing birth weight (76/128 [59%] 1000–1499 g, 62/87 [71%] 750–999 g, and 80/84 [95%] <750 g; p<0.001). Infants with cyanotic major CHD were more likely to suffer at least 1 comorbidity or die compared to infants with acyanotic major CHD (112/139 [81%] vs. 106/160 [66%]; p=0.005). Comorbidity or death was also more common in cyanotic infants when truncus arteriosus was included as an acyanotic major CHD, given the more common infantile presentation of congestive heart failure rather than cyanosis (103/130 [79%] vs. 115/169 [68%], p=0.04). The proportion of all VLBW infants with major CHD who suffered at least 1 comorbidity or died did not change significantly over time—105/133 (79%) in 1997–2005, 62/95 (65%) in 2006–2009, and 51/71 (72%) in 2010–2012 (p=0.07).
Table 3.
Comorbidities and mortality by birth weight
| <750 g N=84 n (%) |
750–999 g N=87 n (%) |
1000–1499 g N=128 n (%) |
Overall N=299 n (%) |
|
|---|---|---|---|---|
| Death | 66 (79) | 44 (51) | 53 (41) | 163 (55) |
| Death or bronchopulmonary dysplasia | 78 (93) | 58 (67) | 70 (55) | 206 (69) |
| Death or necrotizing enterocolitis | 68 (81) | 49 (56) | 58 (45) | 175 (59) |
| Death or intraventricular hemorrhage | 68 (81) | 48 (55) | 54 (42) | 170 (57) |
| Death or retinopathy of prematurity | 70 (83) | 45 (52) | 54 (42) | 169 (57) |
| Death or any comorbidity | 80 (95) | 62 (71) | 76 (59) | 218 (73) |
4. Discussion
We conducted a large, retrospective, multicenter cohort study of all VLBW infants with major CHD discharged from NICUs managed by the Pediatrix Medical Group between1997 and 2012. Overall mortality was 55% and did not change significantly during the study period, and the majority of infants died or suffered from at least 1 comorbidity (73%). This was in contrast to outcomes in VLBW infants without major CHD, for whom mortality declined during the study period.
Major CHD was more common in our cohort of VLBW infants compared with the general population, but lower than in prior VLBW studies [2,6,7]. The overall proportion of VLBW infants with major CHD in our cohort was 299/105,539 (0.3%) and remained unchanged for the duration of the study. Other studies have found congenital heart defects in 6–10/1000 VLBW infants [2,9–11]. The lower proportion reported here may be partially explained by our exclusion of VLBW infants with isolated ASD and VSDs. However, a recent multicenter study conducted through the Vermont Oxford Network (VON) between 2006 and 2007 identified 893 cases of major CHD in 99,786 VLBW infants (0.89%) [2]. Excluding patients who were identified after manual review of medical records, which we did not have access to in our cohort, the incidence of major CHD still remained higher (0.7%) than in our study. This higher incidence may be related to referral bias in the VON study, which consisted of a voluntary consortium of NICUs that may include a higher proportion of tertiary care centers [2]. In contrast, the Pediatrix Clinical Data Warehouse used in our study is populated directly via a shared electronic medical record and includes a wide variety of NICUs managed by the Pediatrix Medical Group [12].
Mortality in VLBW infants with major CHD is high; in our cohort, overall mortality was 55%. This is higher than the mortality reported in other studies, which ranged from 26–44% [2,6]. Our mortality most closely resembles that of the VON study (44%), which also focused on major CHD and excluded infants with isolated ASDs and VSDs [2]. Mortality in VLBW infants with major CHD remains significantly higher than in the general VLBW population, which ranges from 12–23% in prior reports and was 14% in our study [3,9]. We did not find a change in mortality of VLBW infants with major CHD over time. This is in contrast to the 3% decrease in mortality in all VLBW infants we found between the earliest and latest years of our study. Of note, this improvement in all VLBW infant mortality was more pronounced than the 1% previously reported by the Neonatal Research Network [13]. Differences in infant severity of illness, as well as the successful implementation of several quality improvement initiatives within the Pediatrix Medical Group, might explain this difference [12].
The mortality of VLBW infants with major CHD in our cohort decreased with increasing gestational age. Mortality in all infants born before 28 weeks GA is high, and prior studies have found that, in infants with or without congenital heart defects, a 1–2-week increase in GA increases an infant’s odds of survival [5,14]. This is likely due to increased organ system maturation, particularly pulmonary maturation, and increased likelihood of a successful surgery to correct the heart defect [15,16].
Several different types of cyanotic and acyanotic major CHDs are seen in VLBW infants, and their associated mortality differs. The most common congenital heart defects in our cohort were coarctation of the aorta, atrioventricular septal defect, and tetralogy of Fallot. This is consistent with findings of previous multicenter studies. In the VON study of 893 VLBW infants with major CHD, for example, the most common lesions were tetralogy of Fallot (166/893, 19%) and coarctation of the aorta (103/893, 11%) [2]. In a retrospective study of 110 infants <1000 g birth weight with congenital heart defects of variable severity cared for at centers participating in the Neonatal Research Network, the most common lesions were VSD (20/110, 18%), tetralogy of Fallot (15/110, 14%), and ASD (11/110, 10%) [7]. The same study saw the highest mortality in infants with HLHS (85%), other single-ventricle defects (84.0%), and TAPVR (71.4%). Mortality in infants with single-ventricle defects was similarly high in our cohort: those with HLHS had a mortality of 85%, and infants with other single-ventricle defects had a mortality of 83%. Mortality in our cohort was 100% in infants with interrupted aortic arch, interrupted aortic arch with ventricular septal defect, and PA- VSD, but this may be due to the small number of infants with these lesions. Mortality was lowest (36%) in infants with coarctation of the aorta. Without knowledge of the severity of coarctation, it is possible that this increased survival was due to lower disease severity, as suggested by the fact that only 24% of infants with coarctation required PGE therapy. Indeed, mortality was higher in infants with coarctation who required PGE therapy compared to those who did not (10/16 [63%] versus 14/51 [28%], p=0.02).
Non-cardiac comorbidities are common in VLBW infants with major CHD; NEC and IVH were common in our cohort. The proportion of infants suffering from either complication varied slightly by weight and ranged between 9% and 15% for NEC, and 6% and 18% for IVH. These proportions are comparable to those reported by the Neonatal Research Network study, where NEC was reported in 12% of infants, and severe intracranial hemorrhage was seen in 14% of the 99 infants who had a head ultrasound in the first 28 days of life [7]. All infants in this study were <1000 g, but the study did include some infants with isolated ASDs and VSDs. Both NEC and IVH are more common in VLBW infants with major CHD compared to VLBW infants without major CHD, where the reported incidences in the long-term follow-up of a cohort of 355,806 VLBW infants without CHD were only 4–6% and 6%, respectively [3]. It is noteworthy that the prevalence of NEC was similar in infants with and without coarctation of the aorta (12% vs. 11%, p=0.83). Again, this may be due to severity of the coarctation, and there was a trend towards a higher prevalence of NEC in infants with coarctation requiring PGE compared to those with coarctation not requiring PGE (3/16 [19%] vs. 5/51 [9%], p=0.39). The small sample size might explain the lack of statistical significance of this finding. BPD was the most common non-cardiac comorbidity in our cohort, present in 21% of all infants. Further, 69% of infants in our cohort either died or suffered from BPD. This is comparable to the findings of the Neonatal Research Network study, where 41/71 survivors (58%) at 36 weeks postmenstrual age suffered from BPD, and 80/110 of infants (72%) either died or suffered from BPD [7]. This is significantly higher than the expected incidence of 26–30% in VLBW infants without CHD [3]. The incidence of BPD only includes surviving infants and has been found to be inversely proportional to GA and birth weight, regardless of whether a heart defect is present [17]. One large study of 3684 VLBW infants, of whom 71 had congenital heart defects, found that those born with congenital heart defects were nearly 4 times more likely than those without congenital heart defects to develop BPD [6]. Mechanical ventilation, which was required in 88% of infants in our study, as well as unfavorable cardio-pulmonary interactions resulting in pulmonary edema, may be responsible for the high incidence of BPD seen in our cohort.
The strengths of our study include the large sample size representing a diverse NICU population, the length of time covered in the study, and the level of detail of data collected in the Pediatrix Clinical Data Warehouse. By including data from more than 300 NICUs over a 15-year period, we were able to determine whether trends were present in the frequency of major CHD in VLBW infants or in the mortality of VLBW infants with major CHD. Our findings are representative of a large proportion of all NICU admissions in the United States and are less likely to suffer from referral bias than prior studies. We were also able to report on interventions received by VLBW infants with major CHD. Lastly, our data allowed a detailed description of several important non-cardiac morbidities, which are encountered more frequently in VLBW infants with major CHD compared with the general VLBW population and likely play an important role in short- and longer-term outcomes. The limitations of our study are primarily related to the limitations of the database. Most importantly, we do not have detailed information about surgical or interventional procedures. We also lack details of anatomic diagnoses, which would allow us to better describe the severity of major CHD and confirm findings such as that of interrupted aortic arch without ventricular septal defect, a very rare cardiac defect. Lastly, we were unable to comment on the impact of prenatal diagnosis of the prevalence and outcomes of major CHD, as this is information was not captured in the database.
In conclusion, we found that incidence of major CHD and associated mortality remained relatively unchanged over time. Mortality in VLBW infants with major CHD remains significantly higher than mortality in VLBW infants without major CHD, and major comorbidities are common. An increase in GA was associated with improved survival.
This retrospective cohort study of VLBW infants with major CHD describes outcomes, including mortality, over time.
Of 105,539 VLBW infants, 299 (0.3%) were diagnosed with 15 different major CHDs.
The majority of infants suffered ≥1 comorbidity or died (218/299, 73%).
Mortality in infants with major CHD did not significantly change over time.
Acknowledgments
Funding source
This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The sponsor played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Dr. Smith receives salary support for research from the National Institutes of Health (NIH) and the National Center for Advancing Translational Sciences of the NIH (HHSN267200700051C, HHSN275201000003I, and UL1TR001117); he also receives research support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). Dr. Hill receives salary support from the U.S. Department of Health and Human Services Food and Drug Administration (1UO1FD004858-01). Dr. Hornik receives salary support for research from the National Center for Advancing Translational Sciences of the NIH (UL1TR001117).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest statement
The remaining authors have no potential conflicts to disclose.
References
- 1.Godfrey M, Schimmel MS, Hammerman C, Farber B, Glaser J, Nir A. The incidence of congenital heart defects in very low birth weight and extremely low birth weight infants. Isr Med Assoc J. 2010;12:36–38. [PubMed] [Google Scholar]
- 2.Archer JM, Yeager SB, Kenny MJ, Soll RF, Horbar JD. Distribution of and mortality from serious congenital heart disease in very low birth weight infants. Pediatrics. 2011;127:293–299. doi: 10.1542/peds.2010-0418. [DOI] [PubMed] [Google Scholar]
- 3.Horbar JD, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012;129:1019–1026. doi: 10.1542/peds.2011-3028. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2012. Natl Vital Stat Rep. 2013;62:1–20. [PubMed] [Google Scholar]
- 5.Costello JM, Polito A, Brown DW, McElrath TF, Graham DA, Thiagarajan RR, et al. Birth before 39 weeks' gestation is associated with worse outcomes in neonates with heart disease. Pediatrics. 2010;126:277–284. doi: 10.1542/peds.2009-3640. [DOI] [PubMed] [Google Scholar]
- 6.Polito A, Piga S, Cogo PE, Corchia C, Carnielli V, Da Fre M, et al. Increased morbidity and mortality in very preterm/VLBW infants with congenital heart disease. Intensive Care Med. 2013;39:1104–1112. doi: 10.1007/s00134-013-2887-y. [DOI] [PubMed] [Google Scholar]
- 7.Pappas A, Shankaran S, Hansen NI, Bell EF, Stoll BJ, Laptook AR, et al. Outcome of extremely preterm infants (<1,000 g) with congenital heart defects from the National Institute of Child Health and Human Development Neonatal Research Network. Pediatr Cardiol. 2012;33:1415–1426. doi: 10.1007/s00246-012-0375-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trembath A, Hornik CP, Clark R, Smith PB, Daniels J, Laughon M. Comparative effectiveness of surfactant preparations in premature infants. J Pediatr. 2013;163:955–960. doi: 10.1016/j.jpeds.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoyert DL, Xu JQ. Deaths: Preliminary data for 2011. [Accessed April 16, 2014];Natl Vital Stat Rep. 2012 61(6) Available from: http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_06.pdf. [PubMed] [Google Scholar]
- 10.Adams-Chapman I, Hansen NI, Shankaran S, Bell EF, Boghossian NS, Murray JC, et al. Ten-year review of major birth defects in VLBW infants. Pediatrics. 2013;132:49–61. doi: 10.1542/peds.2012-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 12.Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement project system—tools for "meaningful use" in continuous quality improvement. Clin Perinatol. 2010;37:49–70. doi: 10.1016/j.clp.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birth weight infants. Am J Obstet Gynecol. 2007;196:e141–e148. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Cnota JF, Gupta R, Michelfelder EC, Ittenbach RF. Congenital heart disease infant death rates decrease as gestational age advances from 34 to 40 weeks. J Pediatr. 2011;159:761–765. doi: 10.1016/j.jpeds.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Azakie A, Johnson NC, Anagnostopoulos PV, Egrie GD, Lavrsen MJ, Sapru A. Cardiac surgery in low birth weight infants: current outcomes. Interact Cardiovasc Thorac Surg. 2011;12:409–413. doi: 10.1510/icvts.2010.253823. discussion 414. [DOI] [PubMed] [Google Scholar]
- 16.Tyson JE, Kennedy K, Broyles S, Rosenfeld CR. The small for gestational age infant: accelerated or delayed pulmonary maturation? Increased or decreased survival? Pediatrics. 1995;95:534–538. [PubMed] [Google Scholar]
- 17.Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183:1715–1722. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]