Abstract
During transport and storage, vaccines may be exposed to temperatures outside of the range recommended for storage, potentially causing efficacy losses. To better understand and prevent such losses, Dominant Negative Inhibitor (DNI), a recombinant protein antigen for a candidate vaccine against anthrax, was formulated as a liquid and as a glassy lyophilized powder with the adjuvants aluminum hydroxide and glycopyranoside lipid A (GLA). Freeze-thawing of the liquid vaccine caused the adjuvants to aggregate and decreased its immunogenicity in mice. Immunogenicity of liquid vaccines also decreased when stored at 40 °C for 8 weeks, as measured by decreases in neutralizing antibody titers in vaccinated mice. Concomitant with efficacy losses at elevated temperatures, changes in DNI structure were detected by fluorescence spectroscopy and increased deamidation was observed by capillary isoelectric focusing (cIEF) after only 1 week of storage of the liquid formulation at 40 °C. In contrast, upon lyophilization, no additional deamidation after 4 weeks at 40 °C and no detectable changes in DNI structure or reduction in immunogenicity after 16 weeks at 40 °C was observed. Vaccines containing aluminum hydroxide and GLA elicited higher immune responses than vaccines adjuvanted with only aluminum hydroxide, with more mice responding to a single dose.
Keywords: Lyophilization, Anthrax, Dominant Negative Inhibitor, Vaccine Adjuvants, Aluminum, Glycopyranoside Lipid A (GLA), Vaccines, Freeze-thaw, Stability, Formulation
1. Introduction
The recommended storage temperature range for vaccines is typically very narrow1 and exposure to temperatures either above or below the recommended storage window may be detrimental with respect to vaccine potency2. For example, 75–100% of vaccines are exposed to freezing temperatures during transport through the cold chain3, which may cause vaccine formulations to experience at least one freeze-thaw cycle. Freeze-thawing of vaccines has been shown to cause aggregation of aluminum salt adjuvant particles4,5,6, perturbations in protein antigen structure4,7, and/or losses in immunogenicity4,8.
In addition to experiencing inadvertent freeze-thawing, vaccines may also be exposed to elevated temperatures causing protein antigens to experience physical9,10,11,12 or chemical13,14 degradation, resulting in a loss in vaccine potency11,15. To study the thermal sensitivity of vaccines, accelerated stability studies are typically conducted at temperatures significantly higher than the recommended storage temperatures. Accelerated stability studies are also commonly used as a predictor of long term stability and shelf life at optimal storage temperatures16.
Maintaining proper cold-chains is challenging, especially in developing countries. To alleviate this challenge, vaccines should be formulated to withstand a broad range of temperatures. Lyophilization is one strategy that can be applied to protect proteins and other therapeutic agents against temperature extremes, thereby relieving the constraints of the cold chain17. Formulation of live, attenuated measles vaccines in dry powders9 represents an example of this approach. Degradation in lyophilized formulations is inhibited in part because of the low water content and high viscosities (>1015 centipoise) found in glassy lyophilized formulations17.
Many vaccines require administration of multiple doses to confer adequate protection. Especially in developing countries, this requirement is problematic, and often patients do not complete multidose regimens18, 19. Presumably, better patient compliance would be obtained if vaccines required fewer doses. Adjuvants are often added to vaccines to increase vaccine potency, and offer the potential to decrease the required number of vaccine doses20. Aluminum salts, and aluminum hydroxide combined with monophosphoryl lipid A (MPL) have been approved for use as adjuvants in FDA-approved vaccines21. However, no FDA-approved vaccines that contain adjuvants currently are marketed in lyophilized formulations22, in part because of the loss of vaccine efficacy that may occur during the requisite freezing step in the lyophilization process. Recent work has shown that by controlling the kinetics of freezing and glass formation through judicious choice of formulation and process conditions, highly stable, efficacious lyophilized vaccines containing aluminum salt adjuvants may be produced23, 24, 25,15.
Aluminum salt adjuvants are known to stimulate primarily a humoral response. To produce a more robust cellular immune response to a vaccine, other adjuvants typically must be added26. One such co-adjuvant is monophosphoryl lipid A (MPL), a non-toxic derivative of lipopolysaccharide (LPS) that can act as a toll-like receptor-4 agonist26. Glycopyranoside lipid A (GLA) is a synthetic version of MPL that is more homogenous and active than MPL26. To date, there are no reports of commercial lyophilized vaccine formulations that combine both an aluminum hydroxide adjuvant and a cellular immunity stimulant such as GLA.
To examine the possibility of creating stable lyophilized vaccines containing both aluminum hydroxide and GLA, the anthrax vaccine candidate, Dominant Negative Inhibitor (DNI), was used as a model recombinant protein antigen. During Bacillus anthracis infection, bacteria secrete protective antigen (PA), lethal factor (LF) and edema factor (EF)27. PA forms complexes on the surface of host cells with LF (a zinc protease) and EF (an adenylate cyclase), giving rise to lethal toxin (LT) and edema toxin (ET), respectively. LT exerts its cytotoxic effects by interrupting mitogen-activated protein kinase kinase signaling, while ET influences intracellular cAMP levels. DNI is a recombinant version of PA (rPA) that contains the two point mutations: K397D and D425K. These mutations do not affect heptemerization or subunit binding, but do impair translocation of EF and LF into the cytoplasm of host cells28, 29. Previous studies have shown DNI to be an effective candidate vaccine antigen with respect to eliciting high PA antibody titers30, and the biophysical and immunological stability properties of the DNI antigen have been evaluated31. In addition, rPA is known to undergo chemical degradation via deamidation of specific Asn residues, including six labile sites out of the 68 total Asn residues in rPA33, which leads to loss of the antigen’s biological activity and immunogenicity32, 33, 34.
In this study we first tested the hypothesis that both heat and freeze-thaw stresses damage adjuvanted liquid vaccine formulations of DNI, decreasing their immunogenicity due to losses in protein structure and/or agglomeration of aluminum hydroxide adjuvant particles. Second, we evaluated the possibility that glassy-state, lyophilized formulations of DNI-based vaccines are more robust against thermal stress, especially as reflected in slower rates of Asn deamidation, a known major chemical degradation pathway for rPA32, 33, 34. Finally, we tested the hypothesis that incorporation of the Toll-like receptor-4 (TLR4) agonist GLA together with microparticulate aluminum hydroxide in DNI vaccine formulations will confer additional potency, and that this additional functionality can also be protected against thermal stresses through lyophilization.
2. Materials and Methods
2.1 Materials
High purity α,α-trehalose dihydrate and sulfuric acid were purchased from Mallinckrodt Baker (Phillipsburg, NJ). Ammonium acetate, triethanolamine, and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (St. Louis, MO). Two percent Alhydrogel® (aluminum hydroxide adjuvant, “alum”) was obtained from Accurate Chemicals and Scientific Corp (Westbury, NY). Lyophilized synthetic monophosphoryl lipid A (glycopyranoside Lipid A (GLA) adjuvant) was purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Three mL 13 mm glass lyophilization vials, caps and seals were from West Pharmaceutical Services (Lititz, PA). Concentrated 10× phosphate buffered saline (PBS), and Tween 20 were from Fischer Scientific (Fair Lawn, NJ). Water for injection was purchased from Baxter Healthcare Corporation (Deerfield, IL). Peroxidase-conjugated affinipure donkey anti-mouse IgG (H+L) was from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). 3,3’,5,5’tetramentylbenzidine (Ultra TMB) was from Thermo Scientific (Rockford, IL).
2.2 Vaccine formulation
Dominant negative inhibitor (DNI) protein manufactured by Baxter Pharmaceutical Solutions LLC (Bloomington, IN) was received as a lyophilized formulation containing 25 mg DNI, 113 mg mannitol, 33 mg sucrose, and 2.4 mg dibasic phosphate. Lyophilized DNI was reconstituted in 3 mL of filtered DI water and dialyzed overnight with three buffer exchanges in a 10 mM ammonium acetate buffer pH 7 using 3,500 MWCO Slide-A-Lyzer dialysis cassettes from Thermo Scientific (Rockford, IL).
All vaccines were formulated to contain 10 mM ammonium acetate pH 7 with 0.2 mg/mL DNI and 0.5 mg/mL aluminum as Al(OH)3 (Alhydrogel). For isotonicity, 9.5 w/v% trehalose was added. In addition to aluminum hydroxide, 0.05 mg/mL GLA was added as a second adjuvant to half of the vaccine formulations. GLA was prepared at 1 mg/mL by suspending lyophilized GLA in a 0.5% triethanolamine pH 7 solution using probe sonication35. To create the vaccine formulations containing GLA, suspended GLA was added to Alhydrogel suspensions, vortexed for 5 seconds and then rotated end over end for 30 minutes at 4 °C. 0.2 mg/mL DNI protein antigen was added to buffered adjuvant solutions and rotated end over end for 30 minutes to allow protein to adsorb completely to adjuvant particles.
2.3 Protein adsorption
Protein adsorption was measured by mass balance after centrifuging the vaccine formulation at 9,000×g for 4 min at 4 °C to remove particles and adsorbed protein, and measuring the unbound protein concentration in the supernatant through use of the Bradford assay. A standard curve was created using known concentrations of DNI. The amount of protein adsorbed to adjuvant was calculated by subtracting the amount of unbound protein from the known amount of protein in the vaccine.
2.4 Lyophilization
Vaccine formulations were lyophilized with 1 mL of formulation per vial. Lyophilizer shelves were pre-cooled to −10 °C (FTS Systems Lyophilizer, Warminster, PA) and vials were placed on the shelves. Vaccine formulations were surrounded by vials filled with DI water to minimize radiative heat transfer effects for vials near the edge of the lyophilizer shelves. The shelf temperature was decreased at a rate of 0.5 °C/min to −40 °C and then held at −40 °C for 1 hour to allow the samples to freeze completely. Primary drying was initiated by decreasing the chamber pressure to 60 mTorr and increasing the shelf temperature to −20 °C at a rate of 2 °C/min. Samples were held at −20 °C for 20 hours. Secondary drying was conducted by increasing the shelf temperature to 0 °C at a rate of 0.2 °C/min followed by an increase to 30 °C at a rate of 0.5 °C/min and holding the shelf temperature at 30 °C for 5 hours. After drying, the shelf temperature was returned to 25 °C and the chamber was back-filled with nitrogen until atmospheric pressure was reached. Chlorobutyl rubber stoppers were inserted into vials under a nitrogen atmosphere. Vials were sealed with aluminum caps, and then were stored at −80 °C. Lyophilized vaccines were stored at this low temperature to minimize molecular mobility and any resulting degradation in the formulations prior to their analysis or their use in accelerated stability studies.
2.5 Freeze-thaw study
Freeze-thaw stability was examined for liquid vaccine formulations. Formulations were cycled between −20 °C and 4 °C, leaving formulations at each temperature for one day to permit complete freezing or thawing. Vaccines experienced 0, 1, or 5 freeze-thaw cycles.
2.6 Elevated temperature incubation study
To test the stability of vaccines at elevated temperatures, liquid and lyophilized vaccines were stored at 4, 40 or 70 °C for 0, 1, 2, 4, 8, or 16 weeks. Time 0 lyophilized vaccines refer to vaccines reconstituted and used immediately after removal from storage at −80 °C.
2.7 Particle size analysis
Particle size distributions from 0.04–2,000 µm were measured using laser diffraction particle size analysis (LS 230, Beckman Coulter, Miami, FL). Initial liquid, and reconstituted lyophilized placebo vaccine formulations with and without GLA were measured. For each run, laser diffraction intensities were recorded three times for 90-sec each and averaged. Triplicate samples of each formulation were analyzed.
Particles in the size range of 2–2,000 µm were measured and counted by microflow image analysis (FlowCAM®, Fluid Imaging Technologies, Yarmouth, ME). Particle levels in the initial liquid formulations and in reconstituted formulations of lyophilized vaccines that had been incubated at 40 °C were measured in triplicate. 0.2 mL of samples diluted 10 times were run with a 100-µm flow cell using a 10× objective and collimator. Dark and light settings of 15 and 16 were used, respectively. For freeze-thaw studies, triplicate 1 mL of vaccine formulation diluted 100 times were analyzed with a 300-µm flow cell with a 4× objective. Dark and light settings of 20 were used.
2.8 Differential interference contrast microscopy
A Zeiss Axiovert 200M widefield microscope was used to take differential interference contrast images of vaccine formulations after 0, 1, or 5 freeze-thaw cycles. A 20× objective was used.
2.9 Differential scanning calorimetry (DSC)
Onset glass transition temperatures of placebo lyophilized formulations were obtained using differential scanning calorimetry (Diamond DSC, Perkin Elmer, Waltham, MA). Triplicate samples were prepared inside an aluminum pan under dry nitrogen. The temperatures in the pans were cycled twice between 25 °C and 150 °C at a scan rate of 10 °C/min. The second heating scan was used to determine the onset glass transition temperature.
2.10 Fluorescence analysis
Intrinsic tryptophan fluorescence was measured by adding seven hundred µL of vaccine formulations from the freeze-thaw and incubation studies to 2×10 mm pathlength quartz cuvettes. The vaccine formulations were left in the cuvettes overnight at 4 °C to allow settling so that intrinsic fluorescence measurements could be performed in a fluorometer (Photon Technology International, Birmingham, NJ). For all vaccines, an excitation wavelength of 295 nm was used for fluorescence measurements, and the emission spectra were collected from 305–410 nm in 1 nm increments while the temperature was ramped from 10–70 °C in 2.5 °C increments. An equilibration time of 1 min was used at each temperature. Slit widths were set at 3 nm for excitation and emission. The intrinsic fluorescence peak positions as a function of temperature were calculated by a mean spectral center of mass method using in-house data analysis software (Middaugh Suite).
Extrinsic SYPRO orange fluorescence was measured by adding eighty nine µL of each vaccine formulation and one µL of 350× SYPRO Orange dye (Molecular Probes, Inc., Eugene, OR) to PCR tubes. PCR tubes were transferred to a Stratagene RT-PCR instrument (Agilent Technologies, Inc., Santa Clara, CA) and SYPRO Orange fluorescence was measured at 610 nm upon excitation at 492 nm while the temperature was ramped from 25 to 70 °C in 1 °C intervals. An equilibrium time of 90 s was used at each temperature. The fluorescence intensity was normalized using a maxima-minima method using Microsoft Excel where the results are generated by fitting the data equal to one at the maxima and to zero at the minima.
All intrinsic and extrinsic fluorescence experiments were performed in duplicate and the signals of the samples were corrected for their respective blanks. The transition temperatures (Tm) were calculated using the second-order derivative of the peak position (intrinsic fluorescence) or SYPRO orange fluorescence intensity (extrinsic fluorescence) versus temperature data. Only the major transition (Tm) was analyzed for vaccine formulations, which showed more than one transition. Due to some irreversibility, these values are not thermodynamic Tm’s, but should be referred to as apparent Tm’s and used in a comparative manner only.
2.11 Deamidation study
Vaccine formulations subjected to freezing and thawing, and high temperature incubation were tested for changes in charge heterogeneity profiles, presumably due to Asn deamidation. Each formulation contained 1 mg/mL DNI protein in 10 mM ammonium acetate pH 7 with 9.5 w/v% trehalose. Formulations contained either 0 or 0.5 mg/mL aluminum as Al(OH)3 from Alhydrogel®. Liquid vaccine formulations from the freeze-thaw study were frozen and thawed in the presence of aluminum hydroxide adjuvant. Vaccine formulations for the thermal incubation study were in liquid or lyophilized forms during incubation at 40 °C with or without aluminum hydroxide adjuvant.
Analysis of charge heterogeneity profiles of DNI protein formulated with aluminum adjuvant required the DNI protein to be desorbed from aluminum hydroxide particles. The adjuvant-DNI complexes first were pelleted by centrifugation at 10,000×g for 3 minutes (Sorvall Centrifuge, Thermo Scientific). The supernatant was removed and assayed for protein content by UV-visible absorption spectroscopy to confirm the absence of unbound protein. The pellet was resuspended in 1 mL of desorption media (10 mM ammonium acetate, 1 M phosphate and 5 M guanidine hydrochloride, pH 7.0). The resulting suspension was incubated at room temperature for 3 hours, followed by centrifugation at 10,000×g for 3 min. The supernatant was collected and assayed for DNI content. These steps were repeated 2 more times. The percent desorption was calculated by dividing the total content of protein in the collected supernatant by the initial amount of protein initially bound to the aluminum hydroxide particles. The supernatants were combined and buffer exchanged into 10 mM ammonium acetate, pH 7.0 using Amicon centrifugation filters (10 kDa MWCO). The protein content was again determined by UV-visible absorption spectroscopy to measure the final protein concentration prior to cIEF analysis.
To characterize changes in the distribution of DNI charge variants, which presumably result from asparagine deamidation events known to occur with recombinant PA32, 33, 34, cIEF experiments were performed on an iCE280 instrument from Protein-Simple (Toronto, Canada). All experiments were performed with duplicate samples at 4 °C using a temperature-controlled auto-sampler, with each sample measured in triplicate. Samples of DNI protein (final concentration 0.1 mg/ml) were mixed with Pharmalyte® 3.0–10.0 (final concentration of 4%, obtained from GE Healthcare), acidic and basic pI markers of 4.65 and 8.18 (Protein-Simple, Canada), and methyl cellulose (final concentration of 0.35%, Protein-Simple, Canada). In addition, 6M urea was added since it was found to provide better separation of the rPA charge variants. The optimized separation conditions included pre-focusing at 1500V for 1 minute followed by 8 minutes of focusing at 3000V. Quantification of charge variants was performed using Chrom Perfect® software. The total number of deamidated residues per protein molecule was calculated by (1) determining the fraction of the total area for each cIEF peak, (2) multiplying each peak by the presumed number of deamidated Asn residues represented by each of the peaks, and (3) summing the obtained values for each peak in the cIEF electropherogram profile to determine the total number of deamidation events, as described in detail elsewhere36 using an analogous IEF gel system.
2.12 Vaccine immunogenicity
Murine studies were conducted under the University of Colorado at Boulder Institutional Animal Care and Use Committee (IACUC) protocol #1209.02. Female BALB/c mice 5–6 weeks old from Taconic (Hudson, NY) were allowed to acclimate at least one week before use. Ten mice were in each group. Blood samples were collected from the mice under isofluorane anesthesia on days 0, 14 and 28 through the retro orbital cavity. The collected serum was separated by centrifugation at 10,000 rpm for 14 minutes at 4 °C and stored at −80 °C until analysis. On days 0 and 14, mice were injected subcutaneously behind the neck with various formulations. To study the effects of freeze-thawing on the immunogenicity of DNI vaccines, formulations in the presence or absence of GLA were subjected to 1 or 5 freeze thaw cycles prior to administration to mice. To study the effects of incubation of DNI vaccine formulations at elevated temperatures, mice were injected with liquid vaccine formulations as positive controls, placebo lyophilized formulations as negative controls, liquid vaccine formulations that had been stored for 8 weeks at 40 °C, and lyophilized vaccine formulations that had been incubated at 40 °C for 0, 1, 4, 8 and 16 weeks prior to reconstitution.
2.13 Total antibody enzyme linked immunosorbent assay (ELISA)
Nunc MaxiSorb 96 well plates (Thermo Fischer Scientific, Rochester, NY) were coated with 50 µL/well of 1µg/mL DNI diluted in PBS and incubated at 2–8 °C overnight. Plates were washed 3 times with PBS containing 0.05% Tween 20. Plates were blocked with 300 µL/well of PBS with 1% BSA, incubated at room temperature for 2 h, and washed again. Serum was initially diluted in PBS with 1% BSA, 0.05% Tween 20. 50-fold dilutions were used for serum collected on days 0 and 14, and 750-fold or 250-fold dilutions were used for serum collected on Day 28 for mice injected with or without GLA, respectively. A series of in-plate 2-fold dilutions were made for each sample. Plates were incubated for 1.5 h at room temperature and washed. Forty µL of HRP-conjugated donkey anti-mouse antibody diluted 10,000 times was added to each well and incubated for 1.5 h at room temperature with shaking, followed by washing. 40 µL TMB was added to each well and incubated for 15 min, followed by quenching with 40 µL of 2N sulfuric acid. Plates were measured at 450 nm on a Molecular Devices Kinetic Microplate Reader (Sunnyvale, CA). Each serum sample was analyzed in triplicate.
To determine titers, average OD 450 values as a function of dilution were fit to a 4-parameter logistic equation using SigmaPlot software (Systat Software Inc., San Jose, CA). The constraints 0 < min < 0.15 and max < 3.3 were used. The cutoff value was calculated individually for each mouse as five times the value given on day 0 at a dilution of 100. To evaluate statistically significant differences between groups, a t-test was used for normally distributed groups and a Mann-Whitney Rank Sum Test on non-normally distributed groups.
2.14 Neutralizing antibodies
J774 cells grown in DMEM plus 10% FBS were seeded (5×103 per well) in white 96 well cell culture plates and incubated at 37 °C overnight. Serum samples were mixed at a 1:100 dilution into media containing lethal toxin (300 ng/mL, 1:1 PA:LF), then diluted two-fold in a separate dilution plate into toxin-containing media, down to a 1:12,800 dilution. The media was removed from the cell wells, and toxin-serum mixtures were transferred into them and incubated for 24 h at 37 °C. Some cells received media or toxin-containing media only, and served as live and dead controls, respectively. Cell viability was assessed using Cell Titer Glo (Promega, Madison, WI) and a Spectramax L Luminometer (Molecular Devices, Sunnyvale, CA). Neutralizing titers were defined as the inverse titer that protected at least 50% of the cells from lethal toxin.
3. Results
3.1 Freeze thaw studies – Vaccine characterization
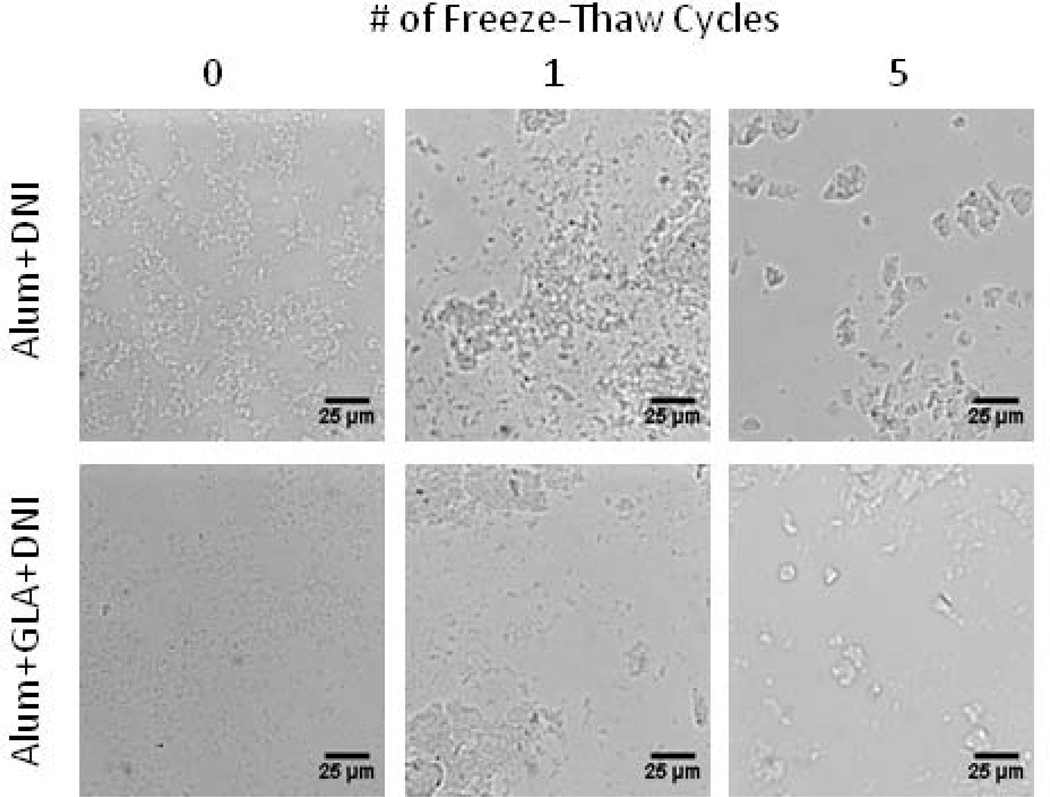
Initially, all liquid vaccine formulations appeared identical based on differential interference contrast microscopy regardless of adjuvant present (Figure 1). After one freeze-thaw cycle loose clumping of adjuvant particles was observed. After five freeze-thaw cycles, large particles (>10 µm) were seen in all formulations, irrespective of the presence or absence of GLA.
Figure 1.
Aluminum hydroxide adjuvant particles aggregated during freezing and thawing as seen by differential interference contrast microscopy images after 0, 1, and 5 freeze-thaw cycles. More particle aggregation was observed with increasing the number of freeze-thaw cycles.
The concentration of particles of size greater than 5 microns in each formulation was measured using a FlowCAM microflow imaging instrument (Figure 2). All vaccine formulations started with particles of similar mean particle diameters (~7–10 µm) and concentrations (~1 million particles/mL). After one freeze-thaw cycle, a small increase in mean particle size (to ~12 µm) was observed. After five freeze-thaw cycles, the mean particle size in each of the formulations was approximately 20 µm. Concomitant with the formation of larger particles, there were decreases in the number of smaller particles found in the formulations. Both formulations had particle concentrations that were of the same order of magnitude throughout the freeze-thaw study.
Figure 2.
Particle concentrations of formulations after 0, 1, 3, and 5 freeze-thaw cycles. With increasing numbers of freeze-thaw cycles, a decrease in 5–10 µm particles was detected and an increase in larger particles was seen. Particle size ranges are 5–10 µm (black), 10–20 µm (dark gray), 20–30 µm (light gray), 30+ µm (white).
DNI was found to be adsorbed completely to adjuvant in all vaccine formulations tested, both initially and after 1 or 5 freeze-thaw cycles (data not shown). After formulations containing DNI adsorbed to aluminum hydroxide particles were pelleted by centrifugation and resuspended in PBS for 1 hour at 37 °C, ~20% of the DNI desorbed.
3.2 Effect of freeze-thawing on antigen structure
Intrinsic tryptophan and extrinsic SYPRO Orange fluorescence studies were conducted to examine protein structure after 0, 1, and 5 freeze-thaw cycles. DNI in all formulations, regardless of the number of freeze-thaw cycles, or the presence of adjuvants, exhibited a cooperative thermal transition at approximately 45 °C suggesting negligible effect of freeze thaw stress on conformational/structural stability of DNI. In addition, no increase in deamidation events was detected by cIEF after 1 or 5 freeze thaw cycles in vaccine formulations (data not shown).
3.3 Freeze thaw studies - Immunogenicity
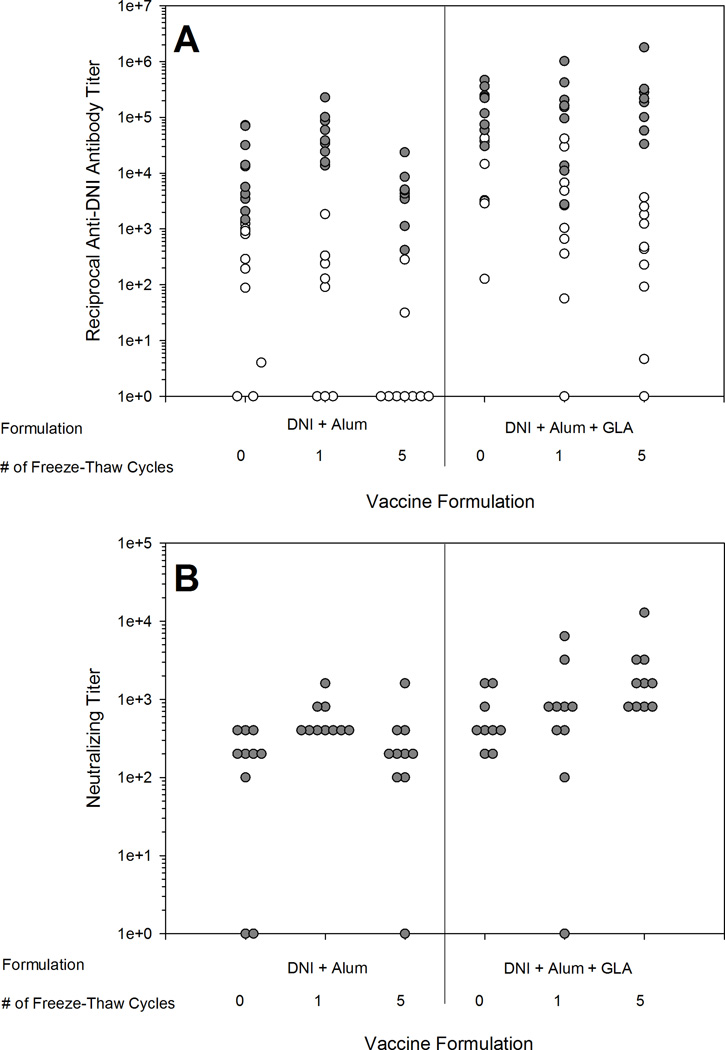
Liquid vaccine formulations were subjected to 0, 1 or 5 freeze-thaw cycles and injected into mice. All mice responded with anti-DNI antibodies after two injections of the vaccine regardless of the number of freeze-thaw cycles, but more non-responders were seen after one injection of the Alum+DNI formulation that had been subjected to five freeze-thaw cycles. After one injection, a significant decrease in titer was seen for both the Alum+DNI and Alum+GLA+DNI vaccines exposed to 5 freeze-thaw cycles when compared to vaccines not exposed to freezing and thawing (p=0.007 and p=0.011, respectively) (Figure 3A). Neutralizing titers in sera collected after the second immunization elicited by the various formulations were not significantly reduced as compared to the initial vaccine for any of the formulations (Figure 3B). Neutralizing titers were not measured after the first immunization.
Figure 3.
Total anti-DNI antibody titers (A) and neutralizing antibody titers (B) after one vaccine injection (white circles) and after two vaccine injections (gray circles) for liquid vaccine after 0, 1, and 5 freeze-thaw cycles. Reduced immunogenicity was detected with 5 freeze-thaw cycles after one injection. Toxin-neutralizing endpoint titers were only determined using serum samples collected after the second immunization.
3.4 Elevated temperature studies – Vaccine characterization
To ensure that lyophilized vaccines were stored in a glassy state, glass transition temperatures were measured in lyophilized placebo formulations (without protein). The glass transition temperature for placebo vaccine formulations without and with GLA were 115.5 ± 1.6 °C and 117.3 ± 3.8 °C, respectively. The glass transition temperatures of the formulations were very similar to that of pure trehalose, 110–120 °C37, showing that the water content of the formulations was minimal. Previous work with similar formulations and lyophilization cycles suggests that the water content was below 1%15. If water were present in the formulation, the glass transition temperature would be drastically reduced because water acts as a potent plasticizer37. Since the glass transition temperatures were significantly higher than the storage temperatures tested in this study (4, 40 and 70 °C), the lyophilized vaccine formulations remained in a glassy state during storage. Due to the associated large increases in molecular mobility, degradation rates typically increase dramatically when lyophilized formulations are stored above their glass transition temperatures.
Previous work showed that formulations containing aluminum salt adjuvants can be lyophilized and reconstituted without significant changes to the initial liquid particle size distribution, provided that sufficient amounts of trehalose are used in combination with rapid cooling methods before lyophilization23,15. Particle size distributions were determined for initial liquid and reconstituted lyophilized placebo formulations with and without GLA (Figure 4). Vaccine formulations without GLA had similar particle size distributions before and after lyophilization and reconstitution, whereas vaccine formulations containing GLA exhibited slight increases in particle size after lyophilization and reconstitution.
Figure 4.
Particle size distributions of placebo vaccine (gray) and placebo vaccine with GLA (black) before (solid line) and after lyophilization and reconstitution (dashed line). Initial liquid particle size distributions were very similar for both formulations. After lyophilization and reconstitution, only a slight increase in particle size distribution was seen in the formulation containing GLA.
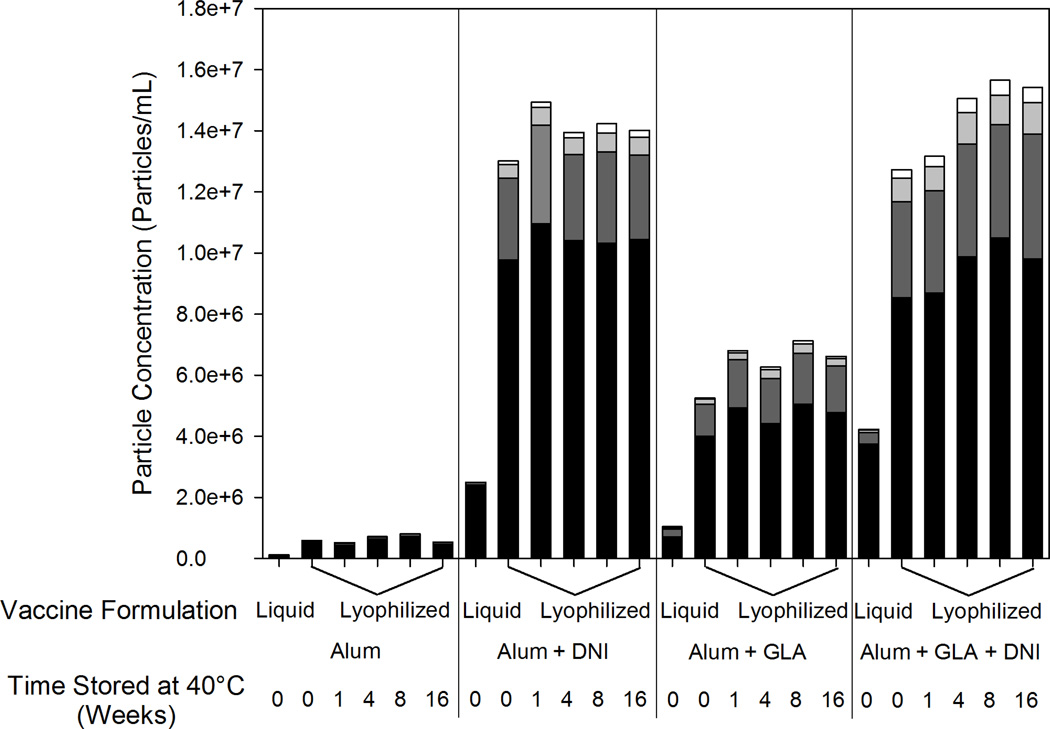
For each lyophilized and reconstituted formulation, a significant increase compared to the initial liquid formulations in the number of particles greater than 2 microns in size was observed by FlowCAM analysis (Figure 5). A greater number of particles were detected in formulations containing GLA, and even more particles were observed when DNI was added to the formulation. After the initial increase in particles following lyophilization, no further increase in particle counts could be detected after incubation at 40 °C for up to 16 weeks.
Figure 5.
Particle size and concentration for particles greater than 2 microns in liquid and reconstituted lyophilized vaccines formulations. Particles 2–5 µm (black), 5–10 µm (dark gray), 10–15 µm (light gray) and greater than 15 µm (white). An increase in particle number was seen when the formulations were lyophilized and reconstituted but no change was seen when the vaccine was incubated at 40 °C for up to 16 weeks.
DNI adsorption to adjuvant particles was measured in liquid formulations prior to lyophilization, in reconstituted lyophilized formulations, and in lyophilized and reconstituted vaccine formulations that had been incubated at 40 °C for up to 16 weeks. Essentially complete (90–100%) adsorption of the DNI protein to aluminum hydroxide adjuvant was observed for all conditions tested (data not shown). When aluminum hydroxide particles with adsorbed DNI were collected by centrifugation, resuspended in PBS and incubated at 37 °C for one hour, only 70% of DNI remained adsorbed, suggesting that DNI may at least partially desorb in vivo after injection.
3.5 Effect of elevated temperatures on antigen structure
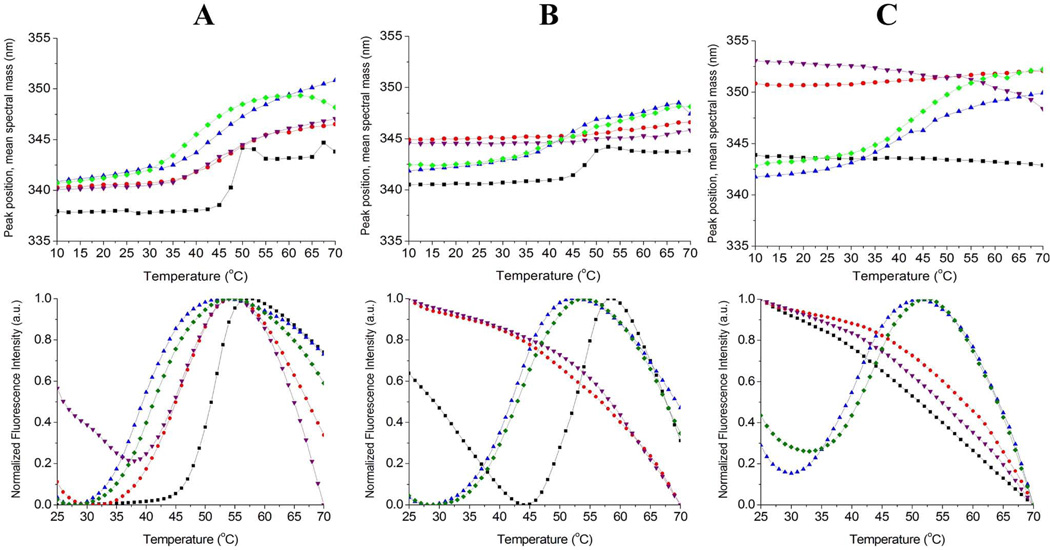
Prior to storage at elevated temperatures, cooperative thermal transitions were observed in all DNI samples when analyzed by either intrinsic tryptophan or extrinsic SYPRO Orange fluorescence spectroscopy. In all of the formulations, the main structural transition for DNI occurred at a temperature of ~45 °C. Intrinsic fluorescence spectra of DNI adsorbed to adjuvant in liquid suspensions showed ~2 nm red-shift in peak positions compared to those for DNI in solution, potentially indicating conformational perturbation in DNI upon adsorption to the adjuvant surface (data not shown). Examples of thermal scans of intrinsic and extrinsic fluorescence of DNI samples used to calculate thermal melting temperature (Tm) values are shown in Figure 6.
Figure 6.
Examples of intrinsic Trp (top row) and extrinsic SYPRO Orange (bottom row) fluorescence thermal melting curves for DNI vaccine formulations after being stored at 4 °C (A), 40 °C (B), and 70°C (C) for 4 weeks. DNI samples were liquid formulation (black), liquid aluminum formulation (red), lyophilized aluminum formulation (blue), liquid aluminum formulation with GLA (purple) and lyophilized aluminum formulation with GLA (green). Lyophilized samples were reconstituted prior to analysis.
After storage at 4 °C for 1, 2, 4, 8, and 16 weeks, analysis of the protein’s conformational stability showed thermal transitions at ~45 °C for all DNI samples (Figure 6 and Table 1). However, when liquid samples (with adjuvant) were stored at 40 or 70 °C, no thermal transitions were observed by fluorescence analysis, suggesting that unfolding of the protein had occurred during storage at these elevated temperatures. In liquid samples containing DNI (without adjuvant), clear thermal transitions were observed after incubation at 40 °C for up to 4 weeks, a weak transition was detected after 8 weeks of incubation, and no transitions could be detected after 16 weeks (Table 1). These results demonstrate the loss of structural integrity of DNI during storage in solution at 40 °C over several months. No structural transitions were detected for liquid DNI samples (with or without adjuvant) that had been stored at 70 °C for any length of time. In contrast, after storage at any of the tested temperatures for a period of up to 16 weeks, lyophilized vaccine formulations displayed cooperative thermal transitions, indicating improved storage stability, as measured by conformational stability analysis, of the DNI in the lyophilized dosage form compared to the liquid formulations.
Table 1.
DNI-containing vaccines incubated at 4, 40 or 70 °C for 0–16 weeks exhibiting fluorescent melting temperatures at 40–50 °C as measured by intrinsic and extrinsic SYPRO Orange methods (●) or only the intrinsic method (○). Sample without detectable transitions are denoted with (○) symbols indicating loss of structural integrity. Lyophilized vaccines maintained DNI thermal melting temperatures during incubation at the three temperatures, where liquid vaccines did not exhibit detectable melting temperatures when stored at temperatures above 4 °C.
| Storage Temperature | ||||
|---|---|---|---|---|
| Sample | Storage Length (weeks) |
4 °C | 40 °C | 70 °C |
| Liquid Unbound DNI | 0 | ● | ||
| 1 | ● | ● | ○ | |
| 2 | ● | ● | ○ | |
| 4 | ● | ● | ○ | |
| 8 | ● | ○ | ○ | |
| 16 | ● | ○ | ○ | |
| Liquid Alum+DNI | 0 | ● | ||
| 1 | ● | ○ | ○ | |
| 2 | ● | ○ | ○ | |
| 4 | ● | ○ | ○ | |
| 8 | ● | ○ | ○ | |
| 16 | ● | ○ | ○ | |
| Liquid Alum+GLA+DNI | 0 | ● | ||
| 1 | ● | ○ | ○ | |
| 2 | ● | ○ | ○ | |
| 4 | ● | ○ | ○ | |
| 8 | ● | ○ | ○ | |
| 16 | ● | ○ | ○ | |
| Lyophilized Alum+DNI | 0 | ● | ||
| 1 | ● | ● | ● | |
| 2 | ● | ● | ● | |
| 4 | ● | ● | ● | |
| 8 | ● | ● | ● | |
| 16 | ● | ● | ● | |
| Lyophilized Alum+GLA+DNI | 0 | ● | ||
| 1 | ● | ● | ● | |
| 2 | ● | ● | ● | |
| 4 | ● | ● | ● | |
| 8 | ● | ● | ● | |
| 16 | ● | ● | ● | |
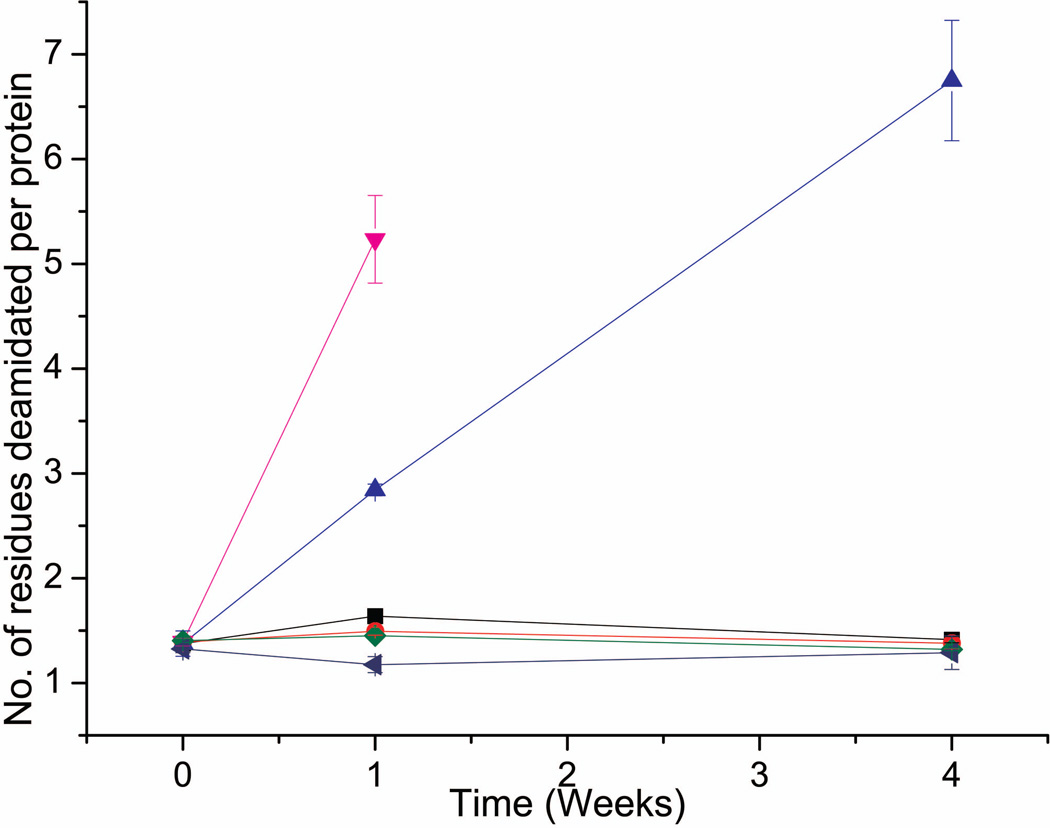
Deamidation of the DNI protein was significantly slowed in the lyophilized state compared to the liquid state at elevated temperatures as shown in Figure 7 (with representative electropherograms shown in Supplemental Figure S1). Both liquid and lyophilized formulations showed no increase in new DNI deamidation events during storage at 4 °C for up to 4 weeks. However, when liquid formulations were stored at 40 °C, increased charge heterogeneity with additional acidic peaks, consistent with the known Asn deamidation events in rPA protein32,33,34, was detected by cIEF analysis after 1 week of storage. In contrast, as shown in Figure 7, no increase in deamidation events was detected in lyophilized formulations (with or without aluminum adjuvant) for up to 4 weeks at 40 °C. Moreover, liquid formulations containing aluminum hydroxide adjuvant showed significantly faster deamidation than DNI formulations without adjuvant at 40 °C over 1–2 weeks. Interestingly, for liquid formulations stored at either 4°C or 40 °C for 4 weeks, the charged isoforms of DNI displayed a reduced total area by cIEF analysis in the presence vs. the absence of aluminum adjuvant (Supplemental Figure S1B). Although the nature of this effect requires more study (e.g., it may reflect more extensive deamidation of the multiple Asn residues, or other structural alterations, in the DNI protein), it was not observed with lyophilized DNI formulations further supporting the stabilizing effects of lyophilization. In summary, we conclude that lyophilized DNI formulations were highly resistant to deamidation (as measured by formation of new acidic peaks by cIEF) compared to the corresponding liquid formulations.
Figure 7.
Extent of DNI deamidation as measured by cIEF increased during storage at higher temperatures and in the presence of aluminum hydroxide adjuvant when stored as liquid formulations. Lyophilized DNI vaccines showed no increase in deamidation events during storage, even at higher temperatures. DNI samples include: liquid formulation at 4 °C (black) and 40 °C (blue), liquid aluminum formulation at 4 °C (red) and 40 °C (pink), lyophilized formulation at 40 °C (green), and lyophilized aluminum formulation at 40 °C (navy). Representative cIEF electropherograms are shown in Supplemental Figure S1. See methods section for calculation of deamidation events; duplicate samples were analyzed in triplicate (n=6) with error bars showing standard deviation values.
3.6 Elevated temperature studies – Immunogenicity
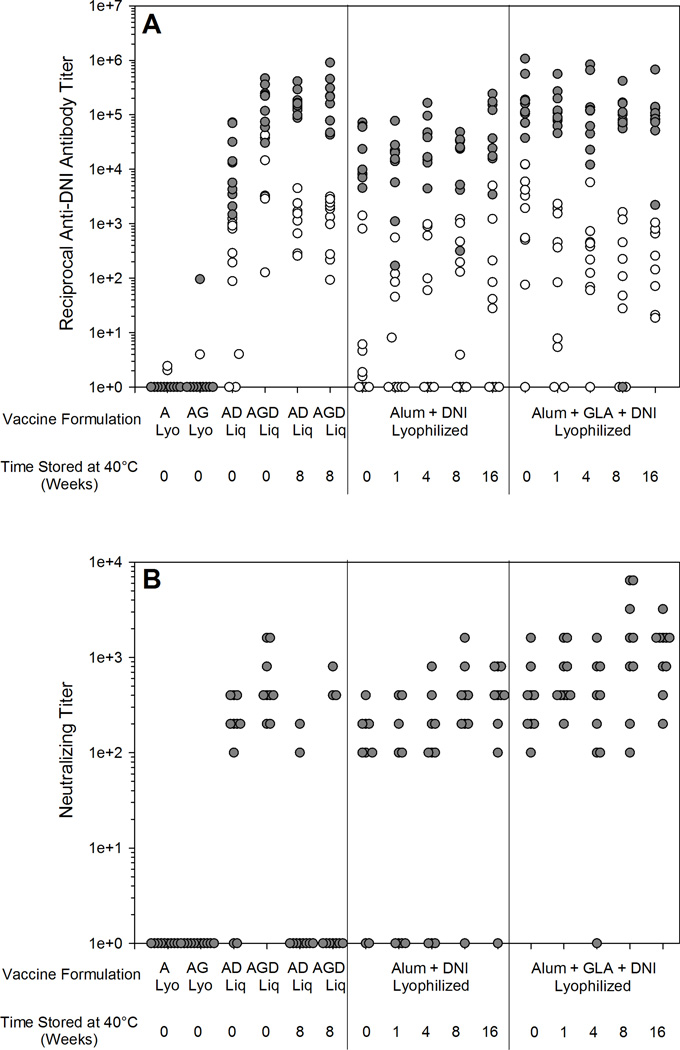
The immunogenicity of the vaccine formulations were evaluated based on serum anti-DNI IgG titers as well as serum LT-neutralizing activity in a mouse macrophage cytotoxicity assay. Immunization of mice with liquid vaccines that had been stored at 40 °C resulted in the production of anti-DNI antibodies, but very few mice responded with neutralizing titers (Figure 8). Although the protein antigen in the liquid vaccine stored for 8 weeks at 40 °C was able to produce antibodies that recognize native DNI by ELISA, these antibodies were not effective at neutralizing LT, which is consistent with the structural data indicating the loss of antigen physicochemical structure when stored at 40 °C.
Figure 8.
Total anti-DNI antibody titers (A) and neutralizing antibody titers (B) after one vaccine injection (white circles) and after two vaccine injections (gray circles) for liquid and reconstituted lyophilized Alum (A), Alum+GLA(AG), Alum+DNI (AD) and Alum+GLA+DNI (AGD) vaccines stored at 40 °C for 0–16 weeks. Lyophilized vaccines remain immunogenic even after storage at 40 °C for 16 weeks, where liquid vaccines showed a decrease in immunogenicity after 8 weeks of incubation.
Lyophilization did not acutely affect the immunogenicity of the vaccines. Liquid vaccines and vaccines reconstituted immediately after lyophilization produced equivalent immune responses, both in the presence and absence of GLA (p=0.307 and p=0.775, respectively). However, unlike the liquid formulations, lyophilized formulations stored at 40 °C up to 16 weeks with (p=0.793) and without GLA (p=0.347) were as effective as unstressed liquid vaccine formulations at eliciting DNI antibodies and LT neutralizing activity. Moreover, after two injections, the liquid vaccine formulation containing GLA produced significantly higher anti-DNI serum IgG antibody titers and toxin-neutralizing activities than the formulations without GLA (p≤0.02). In addition, a single injection of vaccine formulations containing GLA resulted in significant levels of DNI-specific antibodies, whereas formulation without GLA often typically required two injections in order to elicit a significant antigen-specific response.
4. Discussion
Since all lyophilized vaccines experience freezing once during the lyophilization process and a large fraction of vaccines experience freezing temperatures at least once during passage through the cold chain, freeze-thaw studies were conducted. Vaccine formulations were first exposed to one freeze-thaw cycle to mimic damage that might be caused due to freezing during the lyophilization process. The structure of DNI within these formulations appeared to be unaffected by freeze-thawing, or by lyophilization and reconstitution based on structural (Tm values for DNI determined from fluorescence scanning) and chemical (DNI charge heterogeneity profile by cIEF to monitor deamidation) integrity of the protein. Freezing and thawing the vaccine formulations one time caused an increase in the number of particles as well as an increase in larger sized particles. Since the rate of freezing used in the freeze-thaw study was different from the lyophilization cycle, the increase in particle formation was different for each study. After vaccines were frozen and thawed once, their immunogenicities were similar to that of the initial liquid vaccine. Additionally, lyophilized and reconstituted versions of the same vaccine formulation generated immune responses similar to those of the initial liquid form. These results demonstrate that the freezing stage of lyophilization should not cause damage to the vaccine.
Vaccine formulations were frozen and thawed five times to mimic more extensive damage that could happen as a result of thermal excursions during shipping and storage. After five freeze-thaw cycles, vaccine formulations exhibited no physicochemical alterations to protein antigen as measured by protein melting temperatures and charge heterogeneity profiles. Larger particles were formed at the expense of smaller particles with more freeze-thaw cycles. Although no differences in immunogenicity (compared to responses resulting from administration of comparable vaccine formulations that were not subjected to freeze-thawing) were detected after two injections, reduced anti-DNI antibody titers were observed after administration of single doses of both the vaccine formulations containing alum and DNI, and formulations containing alum, GLA and DNI. After five freeze-thaw cycles, the fraction of mice responding with anti-DNI antibodies to the alum-containing DNI vaccine after a single injection decreased from 80% to 20%, although 100% responded after two injections. The exact cause of the reduced immune response to the first dose is still debatable, although it may be associated with changes in aluminum hydroxide particle size resulting from the multiple freeze-thaw cycles.
Liquid vaccine formulations lost potency following exposure to higher temperatures. The protein structure in liquid formulations was perturbed after 1 week of storage at 40 °C. In contrast, DNI structure was preserved in lyophilized vaccine formulations, even after storage at 70 °C for 16 weeks. Additionally, in separate studies at 40 °C over 4 weeks, lyophilization was shown to prevent deamidation of the DNI protein, even in the presence of aluminum hydroxide particles. Previous studies have shown that protein antigens such as rPA and Botulinum neurotoxin deamidate faster in the presence of an aluminum salt adjuvant presumably due to higher surface pH of the adjuvant13, 34. The immunogenicity of liquid vaccines was compromised by 8 weeks of storage at 40 °C, whereas the immunogenicity of lyophilized vaccines was retained after storage at 40 °C for 16 weeks.
To administer the vaccine formulations in as few doses as possible, reducing transportation needs and cost while increasing patient compliance, GLA could be added to vaccine formulations already containing aluminum hydroxide. The immunogenicity of vaccine formulations containing GLA was higher after one injection than formulations without GLA. The response after one injection with GLA was almost as high as two injections without GLA, demonstrating the ability of GLA to increase the immune response and reduce the required number of doses. Also, a higher percentage of mice responded after one injection to vaccines containing GLA. Similar results were seen when the related MPL adjuvant was added to human Papillomavirus vaccines38, 39.
5. Conclusions
Damage can be caused by both elevated and freezing temperatures in liquid vaccine formulations. Freeze-thaw cycles were found to be detrimental to a DNI vaccine’s immunogenicity and aluminum hydroxide particles. Lyophilized DNI formulations showed much better physicochemical stability than the liquid formulations upon incubation for up to 16 weeks at 4, 40 and 70 °C based on fluorescence structural studies and cIEF deamidation measurements. To complement the structural studies, lyophilized vaccines stored at 40 °C did not lose immunogenicity for storage up to 16 weeks whereas liquid vaccines lost immunogenicity prior to 8 weeks. The immunogenicity of vaccines containing GLA was much higher than vaccines that contained only aluminum hydroxide adjuvant. After only one injection, vaccines that contained GLA produced a higher percentage of mice that responded with anti-DNI and neutralizing antibodies.
In lyophilized vaccine formulations, variations in temperature during transport are less detrimental to vaccine potency. In the lyophilized state minimal water is present, avoiding any potential damage by freeze-thaw events. Lyophilized vaccines also permit longer storage at recommended temperatures and can remain immunogenic for short excursions to elevated temperatures if breaks in the cold chain occur.
Supplementary Material
(a) Representative cIEF electropherograms comparing day 0 and 1 week for both liquid and lyophilized formulations of DNI stressed at 40°C. DNI samples (from bottom to top) are liquid formulation at day 0 (blue) and after 1 week at 40 °C (green), liquid aluminum formulation at day 0 (dark green) and after 1 week at 40 °C (red), lyophilized aluminum formulation at day 0 (pink) and after 1 week at 40 °C (yellow) (b) Percent (%) total area of cIEF electropherograms (vs. time zero) calculated for both liquid and lyophilized formulations (with and without adjuvant) of DNI plotted as a function of time. Solid red bars represent formulations without aluminum while shaded red bars represent formulations with aluminum. * indicates percent total area was not calculated due to extensive loss of total peak area.
Acknowledgements
Funding for this project was provided by the NIH grant U01-A1-08-2210.
References
- 1.Temperature sensitivity of vaccines. Geneva: World Health Organization; 2006. Quality, Safety and Standards Team of the Department of Immunizations, Vaccines and Biologicals. [Google Scholar]
- 2.Kumru OS, Joshi SB, Smith DE, Middaugh CR, Prusik T, Volkin DB. Vaccine instability in the cold chain: Mechanisms, analysis and formulation strategies. Biologicals. 2014 doi: 10.1016/j.biologicals.2014.05.007. (in press) [DOI] [PubMed] [Google Scholar]
- 3.Matthias DM, Robertson J, Garrison MM, Newland S, Nelson C. Freezing temperatures in the vaccine cold chain: A systematic literature review. Vaccine. 2007;25:3980–3986. doi: 10.1016/j.vaccine.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 4.Chen D, Tyagi A, Carpenter J, Perkins S, Sylvester D, Guy M, Kristensen DD, Braun LJ. Characterization of the freeze sensitivity of a hepatitis B vaccine. Hum Vaccines. 2009;5:26–32. doi: 10.4161/hv.5.1.6494. [DOI] [PubMed] [Google Scholar]
- 5.Salnikova MS, Davis H, Mensch C, Celano L, Thiriot DS. Influence of formulation pH and suspension state on freezing-induced agglomeration of aluminum adjuvants. J Pharm Sci. 2012;101:1050–1062. doi: 10.1002/jps.22815. [DOI] [PubMed] [Google Scholar]
- 6.Kurzątkowski W, Kartoğlu Ü, Staniszewska M, Górska, Krause A, Wysocki MJ. Structural damages in adsorbed vaccines affected by freezing. Biologicals. 2013;41:71–76. doi: 10.1016/j.biologicals.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Solanki VA, Jain NK, Roy I. Stabilization of tetanus toxoid formulation containing aluminium hydroxide adjuvant against freeze-thawing. Int J Pharm. 2011;414:140–147. doi: 10.1016/j.ijpharm.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Braun LJ, Tyagi A, Perkins S, Carpenter J, Sylvester D, Guy M, Kristensen D, Chen D. Development of a freeze-stable formulation for vaccines containing aluminum salt adjuvants. Vaccine. 2009;27:72–79. doi: 10.1016/j.vaccine.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Kissmann J, Ausar SF, Rudolph A, Braun C, Cape SP, Sievers RE, Federspiel MJ, Joshi SB, Middaugh CR. Stabilization of measles virus for vaccine formulation. Hum Vaccines. 2008;4:350–359. doi: 10.4161/hv.4.5.5863. [DOI] [PubMed] [Google Scholar]
- 10.Hu L, Trefethen JM, Zeng Y, Yee L, Ohtake S, Lechuga-Ballesteros D, Warfield KL, Aman MJ, Shulenin S, Unfer R, Enterlein SG, Truong-Le V, Volkin DB, Joshi SB, Middaugh CR. Biophysical characterization and conformational stability of ebola and marburg virus-like particles. J Pharm Sci. 2011;100:5156–5173. doi: 10.1002/jps.22724. [DOI] [PubMed] [Google Scholar]
- 11.Wagner L, Verma A, Meade BD, Reiter K, Narum DL, Brady RA, Little SF, Burns DL. Structural and immunological analysis of anthrax recombinant protective antigen adsorbed to aluminum hydroxide adjuvant. Clin Vaccine Immunol. 2012;19:1465–1473. doi: 10.1128/CVI.00174-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu L, Joshi SB, Liyanage R, Pansalawatta M, Alderson MR, Tate A, Robertson G, Maisonneuve J, Volkin DB, Middaugh CR. Physical characterization and formulation development of a recombinant pneumolysoid protein-based pneumococcal. J Pharm Sci. 2013;102:387–400. doi: 10.1002/jps.23375. [DOI] [PubMed] [Google Scholar]
- 13.Estey T, Vessely C, Randolph TW, Henderson I, Braun LJ, Nayar R, Carpenter JF. Evaluation of chemical degradation of a trivalent recombinant protein vaccine against botulinum neurotoxin by LysC peptide mapping and MALDI-TOF mass spectrometry. J Pharm Sci. 2009;98:2994–3012. doi: 10.1002/jps.21543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hickey JM, Holtz KM, Manikwar P, Joshi SB, Mcpherson CE, Buckland B, Srivastava IK, Middaugh CR, Volkin DB. Mechanism of a decrease in potency for the recombinant influenza A virus hemagglutinin H3 antigen during storage. J Pharm Sci. 2014;103:821–827. doi: 10.1002/jps.23848. [DOI] [PubMed] [Google Scholar]
- 15.Hassett KJ, Cousins MC, Rabia LA, Chadwick CM, O’Hara JM, Nandi P, Brey RN, Mantis NJ, Carpenter JF, Randolph TW. Stabilization of a recombinant ricin toxin A subunit vaccine through lyophilization. Eur J Pharm Biopharm. 2013;85:279–286. doi: 10.1016/j.ejpb.2013.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasija M, Li L, Rahman N, Ausar SF. Forced degradation studies: An essential tool for the formulation development of vaccines. Vaccine: Development and Therapy. 2013;3:11–33. [Google Scholar]
- 17.Carpenter JF, Pikal MJ, Chang BS, Randolph TW. Rational design of stable lyophilized protein formulations: Some practical advice. Pharm Res. 1997;14:969–975. doi: 10.1023/a:1012180707283. [DOI] [PubMed] [Google Scholar]
- 18.Nelson JC, Bittner RC, Bounds L, Zhao S, Baggs J, Donahue JG, Hambridge SJ, Jacobsen SJ, Klein NP, Naleway AL, Zangwill KM, Jackson LA. Compliance with multiple-dose vaccine schedules among older children, adolescents, and adults: Results from a vaccine safety datalink study. Am J Public Health. 2009;99:S389–S397. doi: 10.2105/AJPH.2008.151332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widdice LE, Bernstein DI, Leonard AC, Marsolo KA, Kahn JA. Adherence to the HPV vaccine dosing intervals and factors associated with completion of 3 doses. Pediatrics. 2011;127:77–84. doi: 10.1542/peds.2010-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Hagan DT, De Gregorio E. The path to a successful vaccine adjuvant--’the long and winding road'. Drug Discov Today. 2009;14:541–551. doi: 10.1016/j.drudis.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011;11:865–872. doi: 10.1038/nri3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. Food and Drug Administration. Complete List of Vaccines Licensed for Immunization and Distribution in the US [Internet] 2012 Available from: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm.
- 23.Clausi AL, Merkley SA, Carpenter JF, Randolph TW. Inhibition of aggregation of aluminum hydroxide adjuvant during freezing and drying. J Pharm Sci. 2008;97:2049–2061. doi: 10.1002/jps.21143. [DOI] [PubMed] [Google Scholar]
- 24.Clausi A, Cummiskey J, Merkley S, Carpenter JF, Braun LJ, Randolph TW. Influence of particle size and antigen binding on effectiveness of aluminum salt adjuvants in a model lysozyme vaccine. J Pharm Sci. 2008;97:5252–5262. doi: 10.1002/jps.21390. [DOI] [PubMed] [Google Scholar]
- 25.Clausi A, Morin A, Carpenter JF, Randolph TW. Influence of protein conformation and adjuvant aggregation on the effectiveness of aluminum hydroxide adjuvant in a model alkaline phosphatase vaccine. J Pharm Sci. 2009;98:114–121. doi: 10.1002/jps.21433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coler RN, Bertholet S, Moutafsi M, Guderian JA, Windish HP, Baldwin SL, Laughlin EM, Duthie MS, Fox CB, Carter D, Friede M, Vedvick TS, Reed SG. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PloS one. 2011;6:e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sweeney DA, Hicks CW, Cui X, Li Y, Eichacker PQ. Anthrax infection. Am J Respir Crit Care Med. 2011;184:1333–1341. doi: 10.1164/rccm.201102-0209CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sellman BR, Mourez M, Collier RJ. Dominant-negative mutants of a toxin subunit: An approach to therapy of anthrax. Science. 2001;292:695–697. doi: 10.1126/science.109563. [DOI] [PubMed] [Google Scholar]
- 29.Yan M, Collier RJ. Characterization of dominant-negative forms of anthrax protective antigen. Mol Med. 2003;9:46–51. [PMC free article] [PubMed] [Google Scholar]
- 30.Aulinger BA, Roehrl MH, Mekalanos JJ, Collier RJ, Wang JY. Combining anthrax vaccine and therapy: A dominant-negative inhibitor of anthrax toxin is also a potent and safe immunogen for vaccines. Infect Immun. 2005;73:3408–3414. doi: 10.1128/IAI.73.6.3408-3414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iver V, Hu L, Schanté CE, Vance D, Chadwick C, Jain NK, Brey RN, Joshi SB, Volkin DB, Andra KK, Bann JG, Mantis NJ, Middaugh CR. Biophysical characterization and immunization studies of dominant negative inhibitor (DNI), a candidate anthrax toxin subunit vaccine. Hum Vaccin Immunother. 2013;9:2362–2370. doi: 10.4161/hv.25852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powell BS, Enama JT, Ribot WJ, Webster W, Little S, Hoover T, Adamovicz JJ, Andrews GP. Multiple asparagine deamidation of Bacillus anthracis protective antigen causes charge isoforms whose complexity correlates with reduced biological activity. Proteins. 2007;68:458–479. doi: 10.1002/prot.21432. [DOI] [PubMed] [Google Scholar]
- 33.Verma A, McNichol B, Domínguez-Castillo RI, Amador-Molina JC, Arciniega JL, Reiter K, Meade BD, Ngundi MM, Stibitz S, Burns DL. Use of site directed mutagenesis to model the effects of spontaneous deamidation on the immunogenicity of Bacillus anthracis protective antigen. Infect Immun. 2013;81:278–284. doi: 10.1128/IAI.00863-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Souza AJM, Mar KD, Huang J, Majumdar S, Ford BM, Dyas B, Ulrich RG, Sullivan VJ. Rapid deamidation of recombinant protective antigen when adsorbed on aluminum hydroxide gel correlates with reduced potency of vaccine. J Pharm Sci. 2013;102:454–461. doi: 10.1002/jps.23422. [DOI] [PubMed] [Google Scholar]
- 35.Baldridge JR, Crane RT. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods. 1999;19:103–107. doi: 10.1006/meth.1999.0834. [DOI] [PubMed] [Google Scholar]
- 36.Tsai PK, Bruner MW, Irwin JI, Ip CC, Oliver CN, Nelson RW, Volkin DB, Middaugh CR. Origin of the isoelectric heterogeneity of monoclonal immunoglobulin h1B4. Pharm Res. 1993;10:1580–1586. doi: 10.1023/a:1018912417607. [DOI] [PubMed] [Google Scholar]
- 37.Ohtake S, Wang YJ. Trehalose: Current use and future applications. J Pharm Sci. 2011;100:2020–2053. doi: 10.1002/jps.22458. [DOI] [PubMed] [Google Scholar]
- 38.Thönes N, Herreiner A, Schädlich L, Piuko K, Müller A direct comparison of human papillomavirus type 16 L1 particles reveals a lower immunogenicity of capsomeres than viruslike particles with respect to the induced antibody response. J Virol. 2008;82:5472–5485. doi: 10.1128/JVI.02482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jagu S, Kwak K, Garcea RL, Roden RBS. Vaccination with multimeric L2 fusion protein and L1 VLP or capsomeres to broaden protection against HPV infection. Vaccine. 2010;28:4478–4486. doi: 10.1016/j.vaccine.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Representative cIEF electropherograms comparing day 0 and 1 week for both liquid and lyophilized formulations of DNI stressed at 40°C. DNI samples (from bottom to top) are liquid formulation at day 0 (blue) and after 1 week at 40 °C (green), liquid aluminum formulation at day 0 (dark green) and after 1 week at 40 °C (red), lyophilized aluminum formulation at day 0 (pink) and after 1 week at 40 °C (yellow) (b) Percent (%) total area of cIEF electropherograms (vs. time zero) calculated for both liquid and lyophilized formulations (with and without adjuvant) of DNI plotted as a function of time. Solid red bars represent formulations without aluminum while shaded red bars represent formulations with aluminum. * indicates percent total area was not calculated due to extensive loss of total peak area.