Abstract
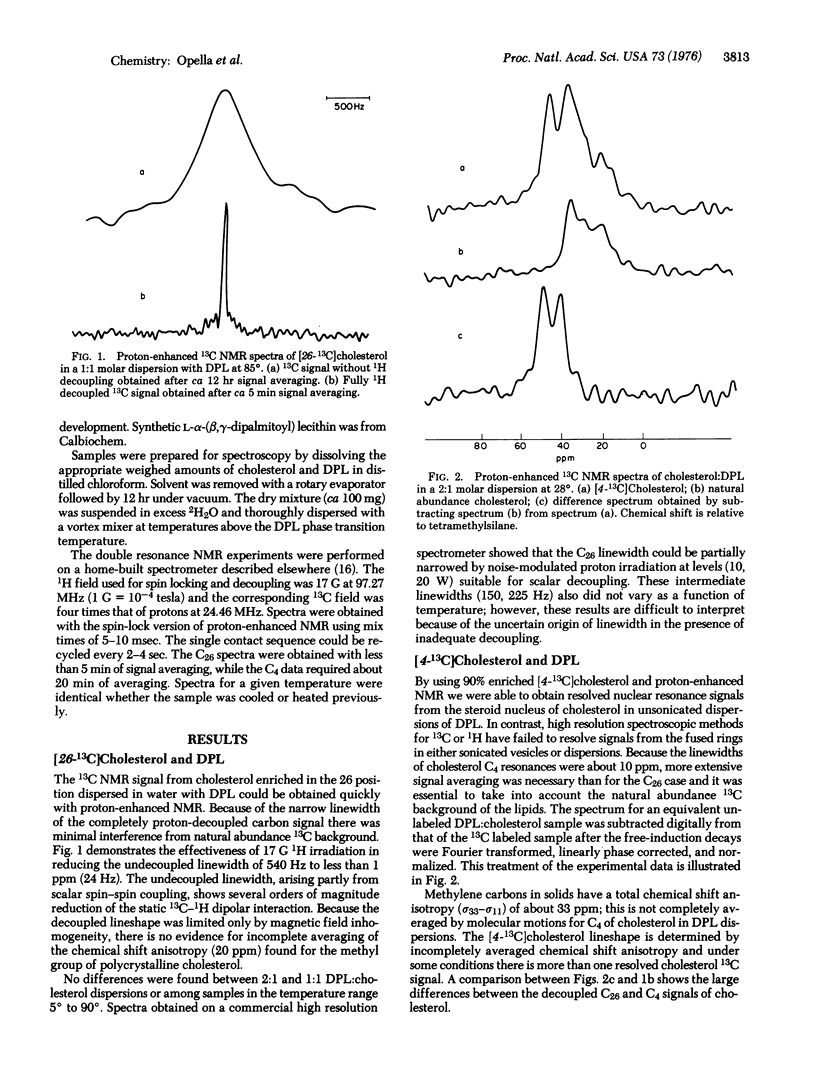
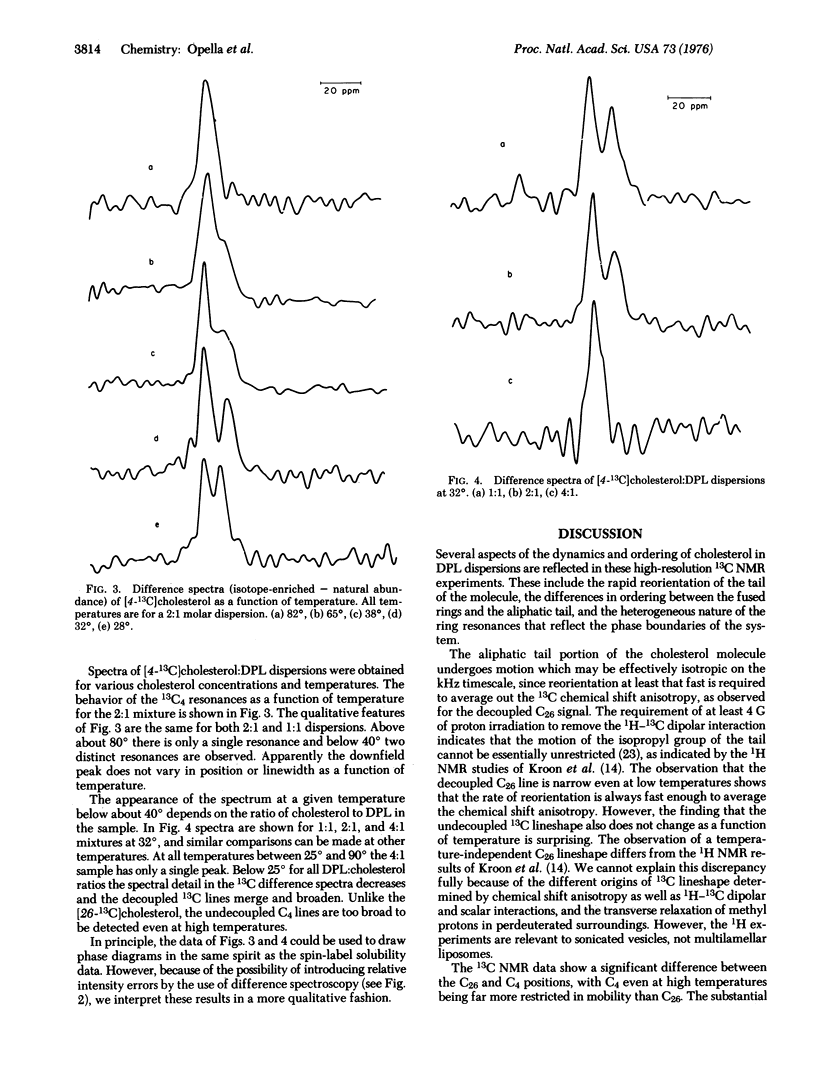
Proton-enhanced 13C nuclear magnetic resonance is used to obtain signals from labeled cholesterols in lecithin dispersions. The [26-(13)C]cholesterol resonance indicates that the aliphatic tail of the molecule undergoes reorientation fast enough to average completely the chemical shift anisotropy. In contrast, [4-(13)C]cholesterol signals are characteristic of limited anisotropic reorientation. The resonances from the 4 position are sensitive to the temperature-concentration phase diagram. A phase boundary is observed at about 20 mole percent cholesterol.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chapman D., Penkett S. A. Nuclear magnetic resonance spectroscopic studies of the interaction of phospholipids with cholesterol. Nature. 1966 Sep 17;211(5055):1304–1305. doi: 10.1038/2111304a0. [DOI] [PubMed] [Google Scholar]
- Darke A., Finer E. G., Flook A. G., Phillips M. C. Nuclear magnetic resonance study of lecithin-cholesterol interactions. J Mol Biol. 1972 Jan 28;63(2):265–279. doi: 10.1016/0022-2836(72)90374-9. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric investigation of the influence of cholesterol on the transition properties of bilayers formed from synthetic L- -lecithins in aqueous suspension. J Biol Chem. 1972 Jun 10;247(11):3697–3700. [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Hui S. W., Parsons D. F. Direct observation of domains in wet lipid bilayers. Science. 1975 Oct 24;190(4212):383–384. doi: 10.1126/science.1179216. [DOI] [PubMed] [Google Scholar]
- Kleemann W., McConnell H. M. Interactions of proteins and cholesterol with lipids in bilayer membranes. Biochim Biophys Acta. 1976 Jan 21;419(2):206–222. doi: 10.1016/0005-2736(76)90347-3. [DOI] [PubMed] [Google Scholar]
- Kroon P. A., Kainosho M., Chan S. I. State of molecular motion of cholesterol in lecithin bilayers. Nature. 1975 Aug 14;256(5518):582–584. doi: 10.1038/256582a0. [DOI] [PubMed] [Google Scholar]
- Ladbrooke B. D., Williams R. M., Chapman D. Studies on lecithin-cholesterol-water interactions by differential scanning calorimetry and X-ray diffraction. Biochim Biophys Acta. 1968 Apr 29;150(3):333–340. doi: 10.1016/0005-2736(68)90132-6. [DOI] [PubMed] [Google Scholar]
- Lee A. G., Birdsall N. J., Levine Y. K., Metcalfe J. C. High resolution proton relaxation studies of lecithins. Biochim Biophys Acta. 1972 Jan 17;255(1):43–56. doi: 10.1016/0005-2736(72)90006-5. [DOI] [PubMed] [Google Scholar]
- Oldfield E., Chapman D., Derbyshire W. Deuteron resonance: A novel approach to the study of hydrocarbon chain mobility in membrane systems. FEBS Lett. 1971 Aug 1;16(2):102–104. doi: 10.1016/0014-5793(71)80343-5. [DOI] [PubMed] [Google Scholar]
- Oldfield E., Chapman D. Dynamics of lipids in membranes: Heterogeneity and the role of cholesterol. FEBS Lett. 1972 Jul 1;23(3):285–297. doi: 10.1016/0014-5793(72)80300-4. [DOI] [PubMed] [Google Scholar]
- Oldfield E., Chapman D. Effects of cholesterol and cholesterol derivatives on hydrocarbon chain mobility in lipids. Biochem Biophys Res Commun. 1971 May 7;43(3):610–616. doi: 10.1016/0006-291x(71)90658-9. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Finer E. G. The stoichiometry and dynamics of lecithin-cholesterol clusters in bilayer membranes. Biochim Biophys Acta. 1974 Jul 31;356(2):199–206. doi: 10.1016/0005-2736(74)90283-1. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separations in binary mixtures of cholesterol and phospholipids. Biochem Biophys Res Commun. 1973 Jul 17;53(2):446–451. doi: 10.1016/0006-291x(73)90682-7. [DOI] [PubMed] [Google Scholar]
- Urbina J., Waugh J. S. Application of proton-enhanced nuclear induction spectroscopy to the study of membranes. Ann N Y Acad Sci. 1973 Dec 31;222:733–739. doi: 10.1111/j.1749-6632.1973.tb15300.x. [DOI] [PubMed] [Google Scholar]
- Urbina J., Waugh J. S. Proton-enhanced 13C nuclear magnetic resonance of lipids and biomembranes. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5062–5067. doi: 10.1073/pnas.71.12.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkleij A. J., Ververgaert P. H., de Kruyff B., Van Deenen L. M. The distribution of cholesterol in bilayers of phosphatidylcholines as visualized by freeze fracturing. Biochim Biophys Acta. 1974 Dec 24;373(3):495–501. doi: 10.1016/0005-2736(74)90029-7. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., Ververgaert P. H., van Deenen L. L., Elbers P. F. Phase transitions of phospholipid bilayers and membranes of Acholeplasma laidlawii B visualized by freeze fracturing electron microscopy. Biochim Biophys Acta. 1972 Nov 2;288(2):326–332. doi: 10.1016/0005-2736(72)90253-2. [DOI] [PubMed] [Google Scholar]


