Abstract
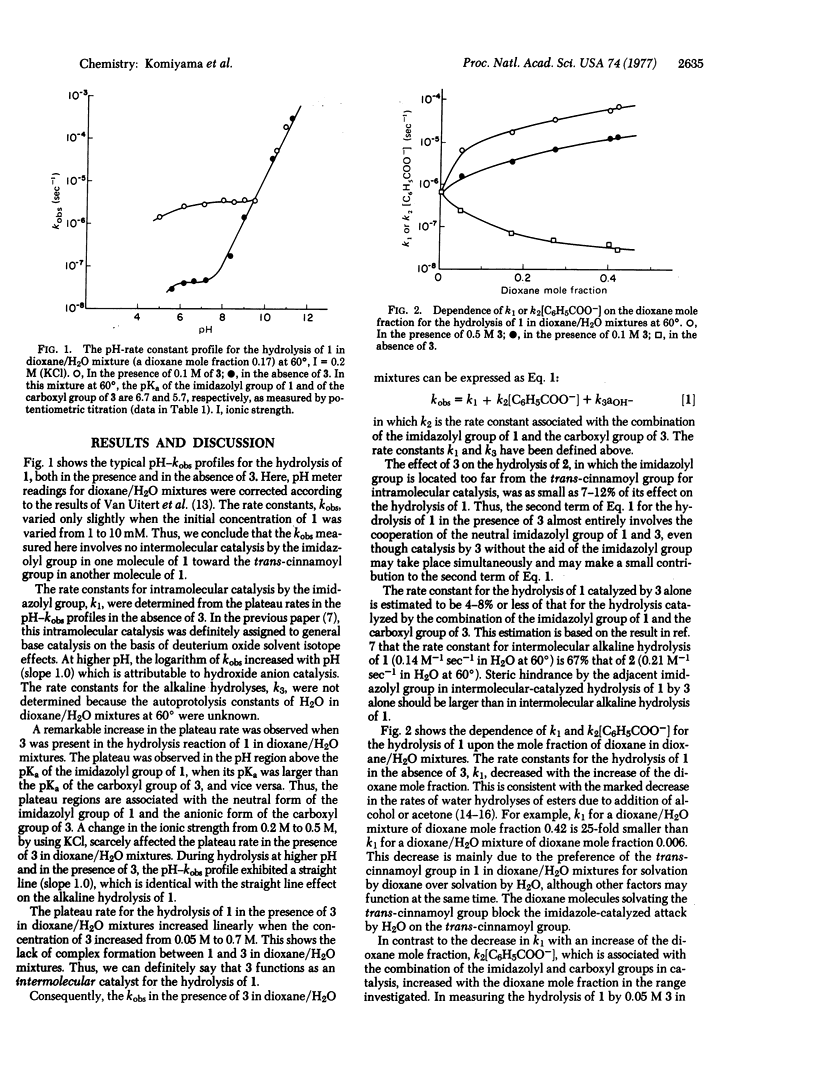
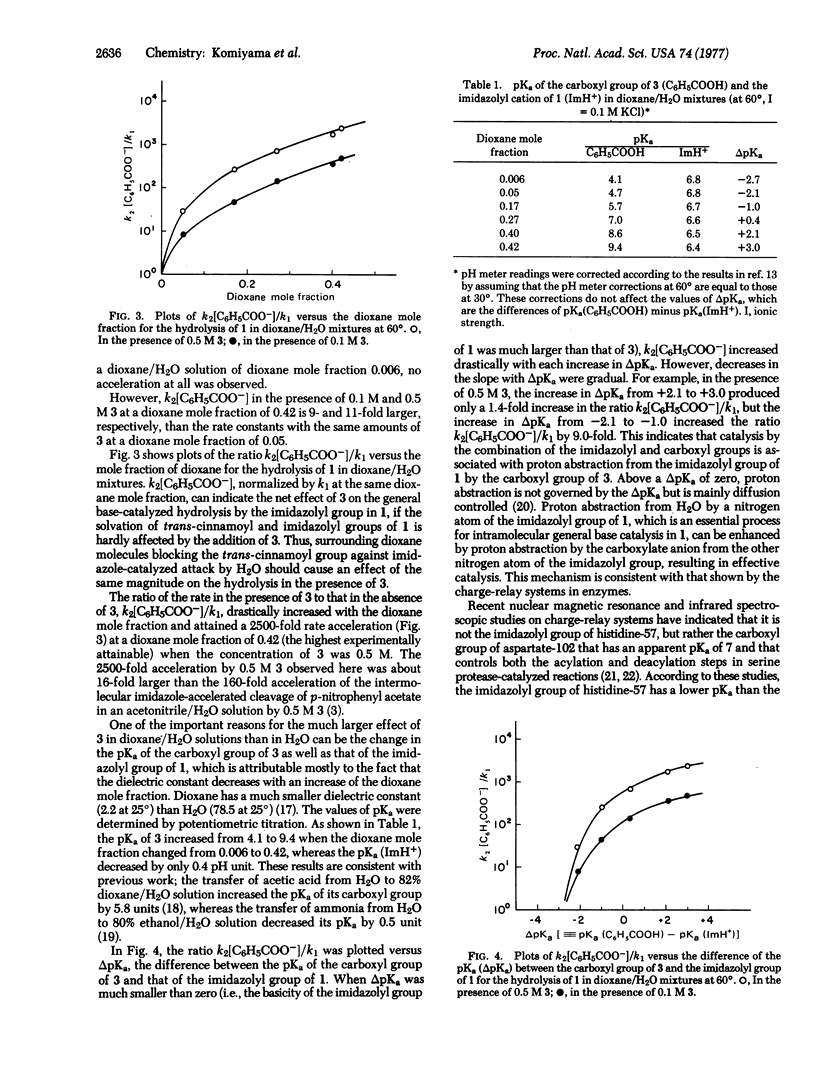
The effect of benzoate anion on intramolecular general base-catalyzed ester hydrolysis by the imidazolyl group in endo-5-[4′(5′)-imidazolyl]bicyclo[2.2.1]hept-endo-2-yl trans-cinnamate was examined in dioxane/H2O solutions. Benzoate anion exhibited a remarkable acceleration of the intramolecular general base-catalyzed hydrolysis of endo-5-[4′(5′)-imidazolyl]bicyclo[2.2.1]hept-endo-2-yl trans-cinnamate by the imidazolyl group. The rate of hydrolysis in the presence of the benzoate anion increased with the dioxane mole fraction and was proportional to the concentration of benzoate anion. On the other hand, the rate of hydrolysis of endo-5-[4′(5′)-imidazolyl]bicyclo[2.2.1]hept-endo-2-yl trans-cinnamate in the absence of benzoate anion decreased with the dioxane mole fraction. Thus, the ratio of the rate in the presence of benzoate anion to that in the absence of benzoate anion drastically increased with the dioxane mole fraction and attained a 2500-fold rate acceleration at a dioxane mole fraction of 0.42 (the highest experimentally attainable) when the concentration of benzoate anion was 0.5 M. The proposed mechanism involves proton abstraction by the benzoate anion from the imidazolyl group, followed by proton abstraction by the imidazolyl group from H2O, resulting in effective general base-catalysis of hydrolysis. The results of the present paper provide support for the “charge-relay” system in serine proteases.
Keywords: enzyme-like rate, benzoate anion, endo-endo structure, dioxane-H2O mixture
Full text
PDF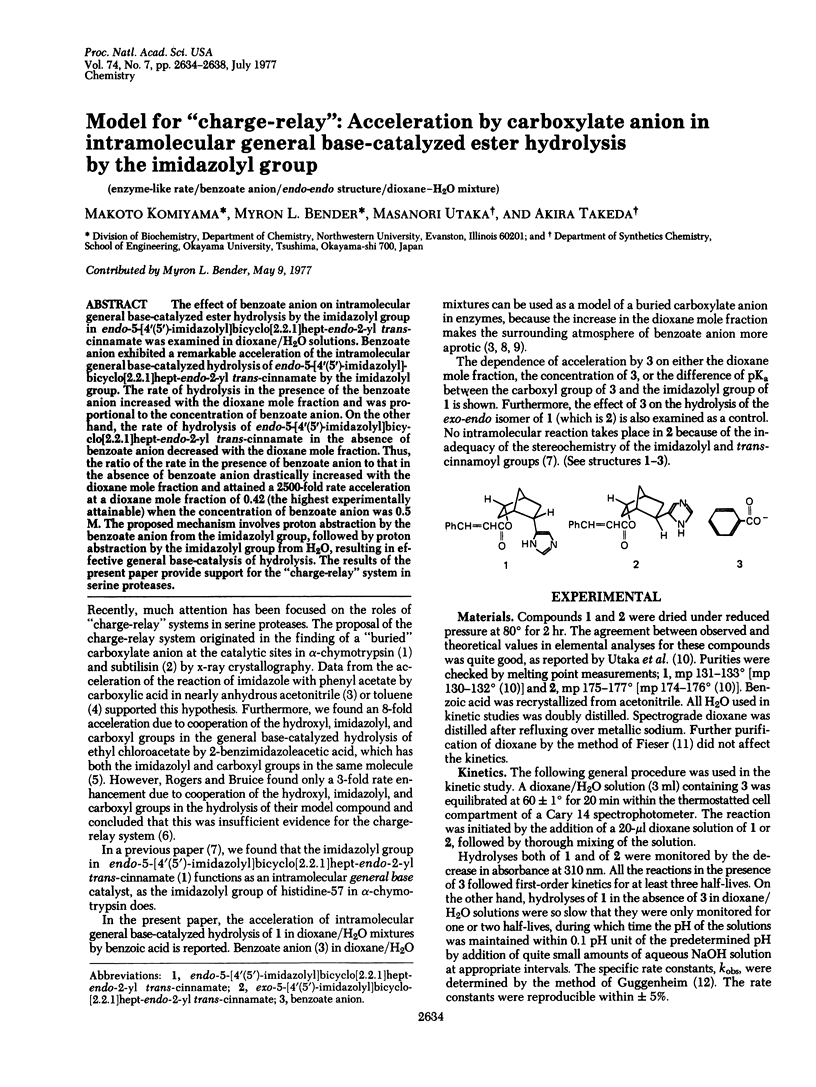


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blow D. M., Birktoft J. J., Hartley B. S. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969 Jan 25;221(5178):337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Smallcombe S. H., Whitaker D. R., Richards J. H. Carbon nuclear magnetic resonance studies of the histidine residue in alpha-lytic protease. Implications for the catalytic mechanism of serine proteases. Biochemistry. 1973 Nov 6;12(23):4732–4743. doi: 10.1021/bi00747a028. [DOI] [PubMed] [Google Scholar]
- Koeppe R. E., 2nd, Stroud R. M. Mechanism of hydrolysis by serine proteases: direct determination of the pKa's of aspartyl-102 and aspartyl-194 in bovine trypsin using difference infrared spectroscopy. Biochemistry. 1976 Aug 10;15(16):3450–3458. doi: 10.1021/bi00661a009. [DOI] [PubMed] [Google Scholar]
- Komiyama M., Roesel T. R., Bender M. L. Intramolecular general base-catalyzed ester hydrolyses by the imidazolyl group. Proc Natl Acad Sci U S A. 1977 Jan;74(1):23–25. doi: 10.1073/pnas.74.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G. A., Bruice T. C. Synthesis and evaluation of a model for the so-called "charge-relay" system of the serine esterases. J Am Chem Soc. 1974 Apr 17;96(8):2473–2481. doi: 10.1021/ja00815a028. [DOI] [PubMed] [Google Scholar]
- Scheiner S., Kleier D. A., Lipscomb W. N. Molecular orbital studies of enzyme activity: I: Charge relay system and tetrahedral intermediate in acylation of serine proteinases. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2606–2610. doi: 10.1073/pnas.72.7.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. S., Alden R. A., Kraut J. Structure of subtilisin BPN' at 2.5 angström resolution. Nature. 1969 Jan 18;221(5177):235–242. doi: 10.1038/221235a0. [DOI] [PubMed] [Google Scholar]


