Abstract
Dramatic structural and functional remodeling occurs in the postpartum brain for the establishment of maternal care, which is essential for the growth and development of young offspring. Glutamate and GABA signaling are critically important in modulating multiple behavioral performances. Large scale signaling changes occur in the postpartum brain, but it is still not clear to what extent the neurotransmitters glutamate and GABA change and whether the ratio of glutamate/GABA remains balanced. In this study, we examined the glutamate/GABA-glutamine cycle in the lateral septum (LS) of postpartum female mice. In postpartum females (relative to virgins), tissue levels of glutamate and GABA were elevated in LS and increased mRNA was found for the respective enzymes producing glutamate and GABA, glutaminase (Gls) and glutamate decarboxylase 1 and 2 (Gad1 and Gad2). The common precursor, glutamine, was elevated as was the enzyme that produces it, glutamate-ammonia ligase (Glul). Additionally, glutamate, GABA, and glutamine were positively correlated and the glutamate/GABA ratio was almost identical in the postpartum and virgin females. Collectively, these findings indicate that glutamate and GABA signaling are increased and that the ratio of glutamate/GABA is well balanced in the maternal LS. The postpartum brain may provide a useful model system for understanding how glutamate and GABA are linked despite large signaling changes. Given that some mental health disorders, including depression and schizophrenia display dysregulated glutamate/GABA ratio, and there is increased vulnerability to mental disorders in mothers, it is possible that these postpartum disorders emerge when glutamate and GABA changes are not properly coordinated.
Keywords: Gad1 (GAD67), Gad2 (GAD65), glutaminase, glutamate-ammonia ligase, glutamine synthetase, lactation
1. Introduction
The postpartum brain undergoes structural and functional reorganization to support multiple neuroendocrine and behavioral alterations required for parenting (Brunton and Russell, 2008; Kinsley and Amory-Meyer, 2011). The mechanisms underlying such a critical remodeling of the brain are complex and include changes in gene expression, synaptic plasticity, neurogenesis, morphology, structure, metabolism, and neurochemistry (Kinsley, 2008; Moltz et al., 1975; Salmaso et al., 2011; Shingo et al., 2003; Zhao et al., 2012a). Glutamate and GABA are critical excitatory and inhibitory CNS neurotransmitters and are involved in a broad variety of physiological events, including synaptic plasticity, neuroendocrine function, learning and memory, cell proliferation, and differentiation (Carver and Reddy, 2013; Ciceroni et al., 2010; Durand et al., 2008; Ogden et al., 2014; Zuure et al., 2013). Furthermore, the importance of glutamate and GABA signaling (i.e. excitation/inhibition balance) and its relationship with behavioral performance has been well established (Jocham et al., 2012; Yizhar et al., 2011).
In the CNS, the metabolic shuttle referred to as the glutamate/GABA-glutamine cycle between neurons and astrocytes supports the homeostasis of glutamate and GABA (Hertz, 2013; Schousboe et al., 2013), as neurons are not capable of synthesizing de novo glutamate and GABA from glucose (Hertz et al., 1999; Schousboe et al., 1997). In astrocytes, the cycle is initiated from the conversion of glutamate to glutamine by the astrocyte-specific enzyme glutamate-ammonia ligase (Glul, also known as glutamine synthetase–GS) (Martinez-Hernandez et al., 1977; Norenberg and Martinez-Hernandez, 1979). Astrocytic glutamine is subsequently transported into extracellular space and then imported to neurons via system transporters (Chaudhry et al., 2002; Jenstad et al., 2009; Solbu et al., 2010). In neurons, glutamine is metabolized to glutamate by the mitochondrial enzyme, glutaminase (Gls, also known as phosphate-activated glutaminase−Pag) (Kvamme et al., 2001). Neuronal glutamate is further converted to GABA by the rate-limiting enzymes glutamate decarboxylase 1 or 2 (Gad1 or Gad2) (also known as glutamic acid decarboxylase 1 and 2) (Martin and Tobin, 2000; Soghomonian and Martin, 1998). Alternatively, neuronal glutamate is released to extracellular space and taken up by astrocytes through the solute carrier family 1 (glial high affinity glutamate transporter), member 2 (Slc1a2, GLT-1, Eaat2) and member 3 (Slc1a3, GLAST, Eaat1) (Arriza et al., 1994; Pines et al., 1992). Finally, astrocytic glutamate is converted to glutamine by Glul, which completes the glutamate/GABA-glutamine cycle.
While GABA activity has been evaluated in the maternal brain, less research has focused on glutamate, and limited research has explored the concomitant dynamics of glutamate and GABA signaling in the postpartum brain. We recently found that Gad1 (also known as GAD67) and Gad2 (also known as GAD65), expression is increased in the postpartum lateral septum (LS) in an inbred strain of mice (Zhao et al., 2012a). In addition, our microarray study of whole septum (including LS) in the same strain identified Glul mRNA as upregulated in postpartum females (Zhao et al., 2012b). Interestingly, levels of glutamate, glutamine, and Glul were observed to be elevated in the cingulate cortex of postpartum rats compared with virgin females (Salmaso et al., 2011).
In this study, in order to determine to what extent glutamate and GABA signaling are altered and whether the ratio of glutamate to GABA changes, we systematically investigated the activity of glutamate/GABA-glutamine cycle in postpartum LS in outbred mice. We examined LS because GABA is remarkably abundant in this brain region (Castaneda et al., 2005; Onteniente et al., 1986; Panula et al., 1984) and we recently found that greater than 90% of the neurons in LS are GABA-positive (Zhao et al., 2013). Also, LS is critical in regulating maternal and non-maternal behaviors (Lee and Gammie, 2009; Sheehan et al., 2004; Singewald et al., 2011). As indicated above, there is an intimate crosstalk between GABA and glutamate signaling in other brain regions (Liang et al., 2006; Mora et al., 2008; Segovia et al., 1999), so an understanding of glutamate/GABA signaling in LS would provide missing information on the relationship of the glutamatergic excitation and GABAergic inhibition in this brain area. Based on the published data in literature, we hypothesized that the postpartum LS would display an elevated glutamate/GABA-glutamine cycle.
2. Results
2.1. Neuronal glutamate synthesis was enhanced in LS during the postpartum period
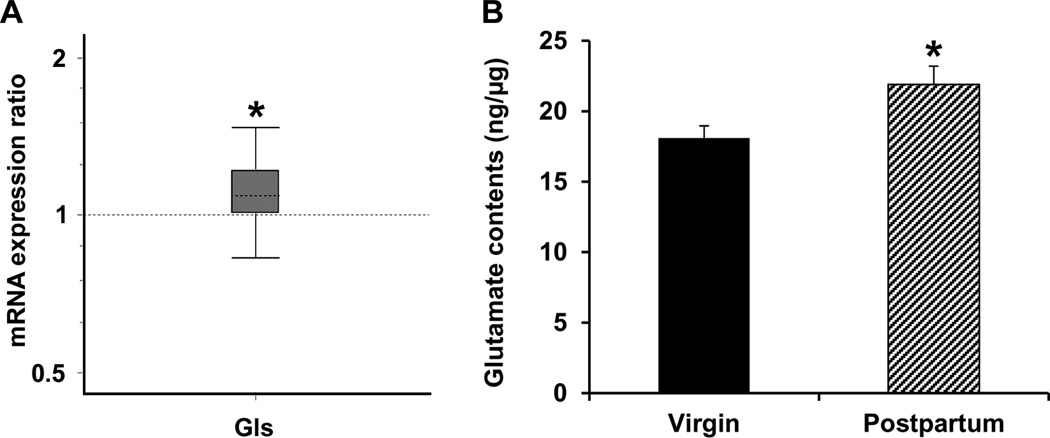
mRNA expression of Gls, the neuronal enzyme that catalyzes the conversion of glutamine to glutamate, was upregulated in LS of postpartum relative to virgin female mice (p = 0.031, Fig. 1A). In parallel with the enhanced expression of the enzyme responsible for the biosynthesis of glutamate in neurons, ELISA immunoassay showed that the tissue level of glutamate, the Gls-catalyzed reaction product, was elevated in LS of postpartum females compared to the virgin mice (p = 0.027, Fig. 1B).
Figure 1.
Quantitative real-time PCR analysis of Gls gene expression (A) as well as ELISA immunoassay of tissue level of glutamate (B) in LS. Relative expression distribution of mRNA (Y-axis) represented as a ratio of postpartum versus virgin mice (n = 12/group), was normalized against two reference genes Ppia and Ywhaz, and shown by box-and-whisker plot as medians (dashed lines), interquartile range (boxes) and ranges (whiskers). Ratio over one indicates gene that is more highly expressed in postpartum than in virgin mice. * P < 0.05 postpartum versus virgin mice.
2.2. Neuronal GABA synthesis was elevated in LS during the postpartum period
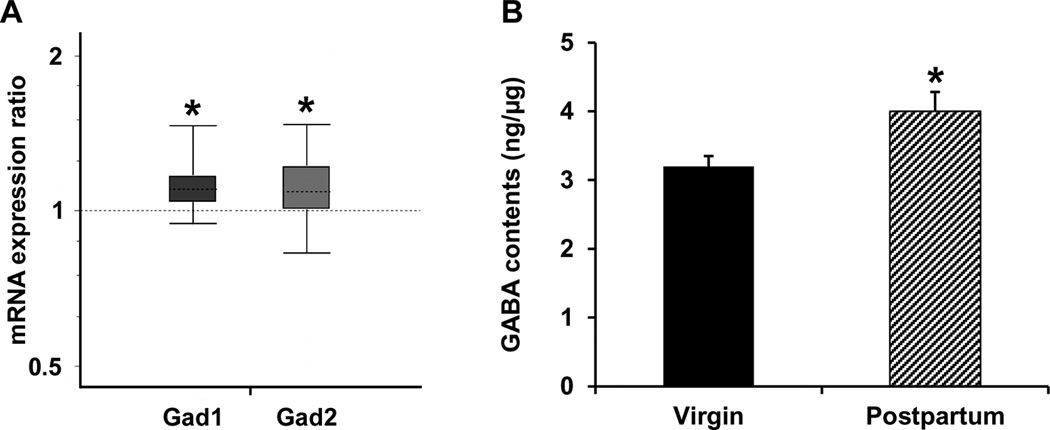
Consistent with our prior findings (Zhao et al., 2012a), expression of both Gad1 and Gad2 mRNAs was upregulated in LS of postpartum relative to virgin female mice (p < 0.001 for Gad1, p = 0.031 for Gad2, Fig. 2A). In parallel with the enhanced expression of enzymes (e.g., Gad1 and Gad2) that synthesize GABA in neurons, ELISA immunoassay showed that the tissue level of GABA was elevated in LS of postpartum females compared to the virgin mice (p = 0.023, Fig. 2B).
Figure 2.
Quantitative real-time PCR analysis of Gad1 and Gad2 gene expression (A) as well as ELISA immunoassay of tissue level of GABA (B) in LS. Relative expression distribution of mRNA (Y-axis) represented as a ratio of postpartum versus virgin mice (n = 12/group), was normalized against two reference genes Ppia and Ywhaz, and shown by box-and-whisker plot as medians (dashed lines), interquartile range (boxes) and ranges (whiskers). Ratios over one indicate genes that are more highly expressed in postpartum than in virgin mice. * P < 0.05 postpartum versus virgin mice.
2.3. Astrocytic glutamine synthesis was heightened in LS during the postpartum period
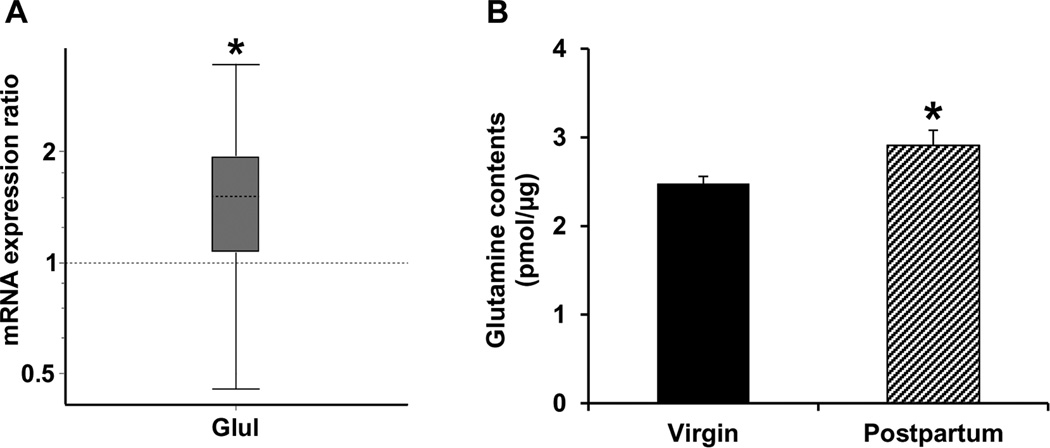
mRNA expression of Glul, the astrocytic enzyme that catalyzes the conversion of glutamate to glutamine, was upregulated in LS of postpartum relative to virgin female mice (p = 0.031, Fig. 3A). In parallel with the enhanced expression of enzyme that synthesizes glutamine in astrocytes, ELISA immunoassay showed that the tissue level of glutamine, the Glul-catalyzed reaction product, was elevated in LS of postpartum females compared to the virgin mice (p = 0.047, Fig. 3B). A summary of changes of the glutamate/GABA-glutamine cycle in LS during the postpartum period is shown in Fig. 4.
Figure 3.
Quantitative real-time PCR analysis of Glul gene expression (A) as well as ELISA immunoassay of tissue level of glutamine (B) in LS. Relative expression distribution of mRNA (Y-axis) represented as a ratio of postpartum versus virgin mice (n = 12/group), was normalized against two reference genes Ppia and Ywhaz, and shown by box-and-whisker plot as medians (dashed lines), interquartile range (boxes) and ranges (whiskers). Ratio over one indicates gene that is more highly expressed in postpartum than in virgin mice. * P < 0.05 postpartum versus virgin mice.
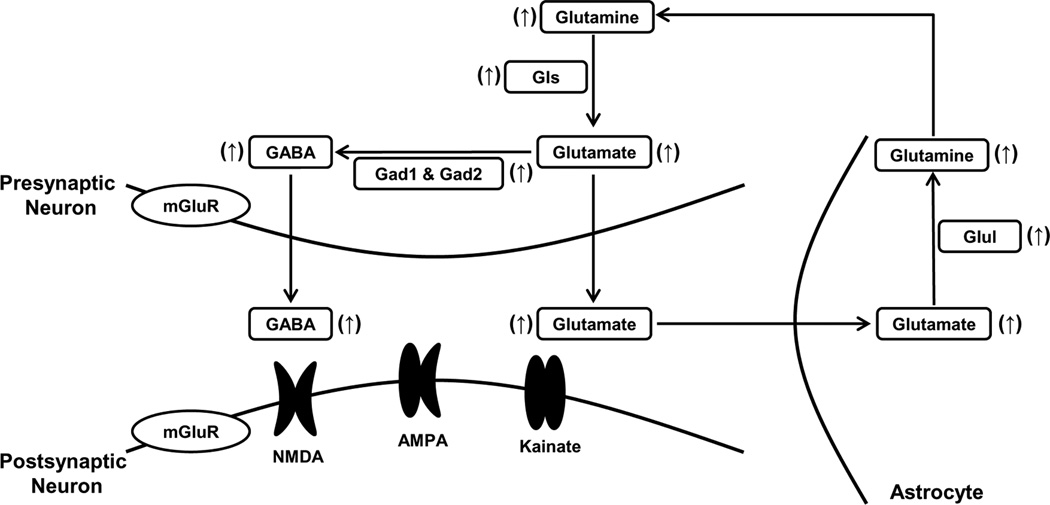
Figure 4.
Summary of the enhanced glutamate/GABA-glutamine cycle in LS during the postpartum period. ↑, increase. Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; Gad1, glutamate decarboxylase 1; Gad2, glutamate decarboxylase 2; Gls, glutaminase; Glul, glutamate-ammonia ligase; mGluR, metabotropic glutamate receptor; NMDA, N-methyl-D-aspartic acid.
2.4. The relationships among tissue levels of glutamate, GABA, and glutamine in LS during the postpartum period
To investigate the relationships among tissue contents of glutamate, GABA, and glutamine, we performed a correlational analysis. Pearson product-moment correlation (two-tailed) analysis revealed significant positive correlations between tissue levels of glutamate and GABA (r = 0.718, p = 0.001; Fig. 5A), glutamate and glutamine (r = 0.562, p = 0.015; Fig. 5B), and glutamine and GABA (r = 0.512, p = 0.03; Fig. 5C). Owing to a robust positive correlation between tissue levels of glutamate and GABA, we further compared the ratio of glutamate to GABA content (glutamate/GABA) between postpartum and virgin females. Independent- Samples T test showed that the ratio of glutamate to GABA content was highly similar and did not differ between postpartum and virgin females (p = 0.904; Fig. 5D).
Figure 5.
Scatterplot with linear regression showing the relationships between the tissue level of glutamate and GABA (A), glutamate and glutamine (B), glutamine and GABA (C) in LS, and bar graph showing the ratio of glutamate to GABA (glutamate/GABA) content in LS of virgin and postpartum female mice (D). Data were pooled from both postpartum and virgin females (n = 18). Pearson correlation analysis revealed significant positive correlations between glutamate and GABA (A), glutamate and glutamine (B), glutamine and GABA (C) in LS, while T test showed there was no difference in glutamate/GABA ratio between postpartum and virgin females (D).
3. Discussion
This study comprehensively investigated the activity of glutamate/GABA-glutamine cycle in the LS of the postpartum female mice. We found the activity of glutamate/GABA-glutamine cycle was significantly elevated as demonstrated by multifaceted alterations. First, the increased Gls and tissue level of glutamate indicated that neuronal synthesis of glutamate was elevated. Second, the increased Gad1 and Gad2 and tissue content of GABA demonstrated that neuronal synthesis of GABA was enhanced as well. Third, the increased Glul and tissue concentration of glutamine reflected that astrocytic synthesis of glutamine was heightened.
It is generally accepted that Gls is a reliable and specific marker for glutamatergic neurons (Akiyama et al., 1990). In neurons, Gls catalyzes the conversion of glutamine to glutamate (Kvamme et al., 2001). In this study, Gls transcript was found to be upregulated in the LS of postpartum mice. In the meantime, a parallel increase in glutamate was detected. These findings demonstrate that neuronal glutamate synthesis in the postpartum LS was elevated. Given that increased Gls in the LS has been linked to aggressive behavior induced by repeated anabolic/androgenic steroid exposure (Fischer et al., 2007), one possibility is that the increased Gls in the postpartum LS may contribute to this behavioral phenotype that occurs during the postpartum period, as maternal females actively protect offspring from intruders (Lonstein and Gammie, 2002). The increase in Gls was accompanied by a proportionate increase in glutamate, the Gls-synthesized product, indicating that Gls faithfully reflects glutamate synthesis in neurons, consistent with a previous observation that Gls inhibition reduced glutamate synthesis (Conti and Minelli, 1994).
Previous studies have reported on an increased level of GABA in distinct regions of the maternal brain (Kornblatt and Grattan, 2001; Rodriguez et al., 2004). Consistent with these observations, our recent work found upregulated Gad1 and Gad2 expression in the postpartum LS (Zhao et al., 2012a). The present study corroborated this finding and further extended this line of research by demonstrating a concomitant increase in tissue content of GABA. In the previous study GAD expression was assessed in a unique line of mouse that was previously selected for high maternal defense, and tissue level of GABA, the GAD-catalyzed reaction product, was not measured. Hence, it was not clear whether GAD expression changes in the selective mouse strain were strain-specific nature or whether upregulated GAD reflected an increased production of GABA. The present results in outbred mice demonstrate that the elevated GAD expression in the postpartum LS is not strain-specific and can be generalized to other mouse strains. Moreover, these findings provide direct evidence supporting that GAD, a reliable and specific marker for GABAergic neurons, truly reflects GABA synthesis, as changes in GAD coincide with changes in GABA.
Glul, a reliable and specific marker for astrocytes, is normally localized to the cytoplasm of most astrocytes where glutamate is metabolized to glutamine via catalyzation by Glul (Norenberg and Martinez-Hernandez, 1979). Little evidence in the published reports is available in relation to the changes in glutamate system during the postpartum period. The observed increase in Glul replicated our recent finding (Zhao et al., 2012b), and is in line with a previous report showing an upregulation of Glul in the cingulate cortex of postpartum females (Salmaso et al., 2011). We further showed that the upregulation of Glul was accompanied by a concomitant increase in glutamine, the product synthesized by Glul. These observations clearly demonstrate that glutamine synthesis in astrocytes is enhanced in the postpartum LS, and that Glul faithfully reflects glutamine synthesis. This concept is bolstered by findings that inhibition of Glul leads to a reduction in glutamine (Engelsen and Fonnum, 1985; Takahashi et al., 1991).
A novel and significant finding of the present study is the identification of a positive correlation between the levels of glutamate and GABA as well as a tightly coordinated ratio of glutamate/GABA within the glutamate/GABA-glutamine cycle. The concurrent measurements of glutamate, GABA, and glutamine in the same samples allowed us to assess the relationships among these amino acids. The glutamate/GABA ratio is thought to be tightly linked and even with aging it is maintained in some regions, such as striatum, but not in others, such as nucleus accumbens (Segovia et al., 1999). It is well established that glutamate/glutamine and GABA systems interplay to modulate glutamate and GABA neurotransmission. For example, modification of glutamate/glutamine production regulates GABA neurotransmission, including synaptic GABA release, GABA levels and GABA receptor subunit expression (Cremer et al., 2010; Liang et al., 2006; Sonnewald et al., 1993; Stransky, 1969). Within GABA signaling, auto feedback control has been proposed as GABA synthesis and GAD activity was inhibited by increased GABA concentrations (Manor et al., 1996; Rimvall and Martin, 1994; Sheikh and Martin, 1998). It is likely that multiple feedback mechanisms exist for a coordinated balance between glutamatergic excitation and GABAergic inhibition, but relatively few have been elucidated. Interestingly, a disruption of the balanced glutamate/GABA-glutamine cycle has been proposed as a pathological basis for a number of neurological and psychiatric disorders, such as depression (Sanacora et al., 2004), schizophrenia (Rose et al., 2013; West et al., 2003), Alzheimer’s disease (Bak et al., 2006) and epilepsy (Bacci et al., 2002; Petroff et al., 2002). What is notable from our findings is that although both glutamate and GABA show a large increase in signaling (about 25% for each) in the postpartum condition, the overall glutamate/GABA ratio remains tightly coordinated (Fig. 5D). From one perspective, the maternal brain could provide unique insights into how the glutamate/GABA ratio can be tightly tethered even in the face of large signaling changes and thus provide insight for how to fix a disrupted ratio in some individual with disorders. From a different perspective, these large changes in glutamate and GABA could provide the vulnerability in mothers, such that if changes are not made proportionally, then disorders could emerge, including postpartum depression.
GABA, the principal inhibitory neurotransmitter in the CNS is involved in mood-related events and aggressive behavior. Enhanced GABA signaling in LS has been intimately associated with reduced anxiety, fear, and stress responses. For instance, activation of GABA via intra-LS injection of GABAA receptor agonist produces an anxiolytic effect (Drugan et al., 1986; Pesold and Treit, 1996). Rats who displayed an attenuated response to stress show an increased GABA synthesis in LS (Herman et al., 2003). These findings support a role of LS GABA signaling in mood-related behaviors (Chozick, 1985). The postpartum period includes attenuated anxiety and fear, as well as a hyporesponsiveness to stress (Brunton et al., 2008; Neumann et al., 2000; Neumann, 2001). As elevated GABA signaling in LS reduces anxiety, fear and stress responses, there is a high likelihood that the observed increase in LS GABA synthesis may be implicated in these behavioral processes. GABA signaling in LS is also linked to aggression. Activation of GABAA receptor in LS increases aggression (McDonald et al., 2012). On the contrary, inhibition of GABA transmission in LS via microinfusion of GABAA receptor antagonist significantly disrupts offspring protection (also known as maternal aggression or maternal defense), while having no appreciable effect on other components of maternal behavior (Lee and Gammie, 2009), suggesting that elevated GABA in LS may promote maternal protective behavior. It should be noted that our findings of elevated postpartum GABA are consistent with GABA-mediated changes in maternal anxiety and offspring protection as increased GABA activity contributes to the attenuated postpartum anxiety and potentiated maternal defense (Lee and Gammie, 2009; Lonstein et al., 2014; Miller et al., 2010). Given that neuropeptides arginine vasopressin (AVP) and oxytocin (OXT) have been intimately linked to maternal anxiety and defense (Bosch, 2011; Lonstein et al., 2014; Nephew and Bridges, 2008; Nephew et al., 2010), it would be interesting to know whether there are functional interactions between GABA, AVP and OXT in the control of maternal care and/or offspring protection. In contrast to GABA, little information is available in the literature regarding the role of glutamate in LS, although the considerable involvement of glutamate is found in other brain regions in numerous behavioral processes, such as anxiety, stress responses and aggressive behavior (Bergink et al., 2004; Feldman and Weidenfeld, 1997; Kalinine et al., 2014). Future study addressing the behavioral functions of postpartum-associated changes in glutamate in LS is warranted.
4. Conclusions
In summary, this study demonstrates a large enhanced glutamate/GABA-glutamine cycle in the LS of postpartum female mice. Furthermore, during the postpartum period, a properly coordinated balance between glutamate and GABA signaling in the LS is maintained. As changes in enzyme expression paralleled expression changes in enzyme-catalyzed reaction products (e.g. glutamate, GABA and glutamine), the enzymes here serve as true reflectors of synthesis for glutamate, GABA and glutamine. To date, the functional and behavioral consequences of the enhanced glutamate/GABA-glutamine cycle in the postpartum LS remain to be fully understood. Given that dysregulation of glutamate/GABA ratio is linked to multiple disorders and that within the maternal brain we still see a virtually unaltered ratio despite ~25% increases in signaling, it is possible that the postpartum brain could provide important insights into how to rebalance glutamate/GABA ratios in some individuals with disorders.
5. Experimental procedures
5.1. Animals
Experimentally naïve, nulliparous female mice from outbred hsd:ICR strain (Mus domesticus) (Harlan, Madison, WI) were used in this study. After acclimation to the animal facility, female mice were housed individually with a breeder male (hsd:ICR strain) for 2 weeks to ensure pregnancy. In the meantime, virgin females were pair-housed to provide similar timing of housing and isolation. All mice were housed on a 12:12 light/dark cycle (lights on at 06:00 h CST) and at a controlled temperature (~22°C) with ad lib access to breeder chow (Harlan) and tap water. All mice were ~10 weeks old at the time of tissue collection. All procedures followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the University of Wisconsin.
5.2. Tissue microdissection for quantitative real-time PCR (qPCR) and enzyme-linked immunosorbent assay (ELISA)
On postpartum day 6 (parturition was designated as day 0 postpartum), postpartum and age-matched virgin mice were lightly anaesthetized with isoflurane and then decapitated. The whole brain was removed, fast frozen in isopentane on dry ice, and then stored at −80°C until sliced. Sections at a thickness of 150 µm were sliced on a cryostat and mounted on glass slides. Microdissection of frozen brain sections was made with Brain Punch Set from Stoelting (Stoelting, Wood Dale, IL, USA) under a dissecting microscope. LS was collected bilaterally from Bregma 1.045 to 0.02 mm (Fig. 6) according to The Allen Mouse Brain Atlas (reference atlas version 1, 2008), and pooled for each individual. Microdissections were flash frozen on dry ice and stored at −80°C until processing for qPCR analysis (N = 12 per group) and ELISA immunoassay (N = 9 per group). Immediately prior to decapitation or perfusion, virgin females were examined for stage of estrous cycle using a vaginal lavage (Drazen et al., 1999; Marcondes et al., 2002). Only diestrous females were included in this study.
Figure 6.
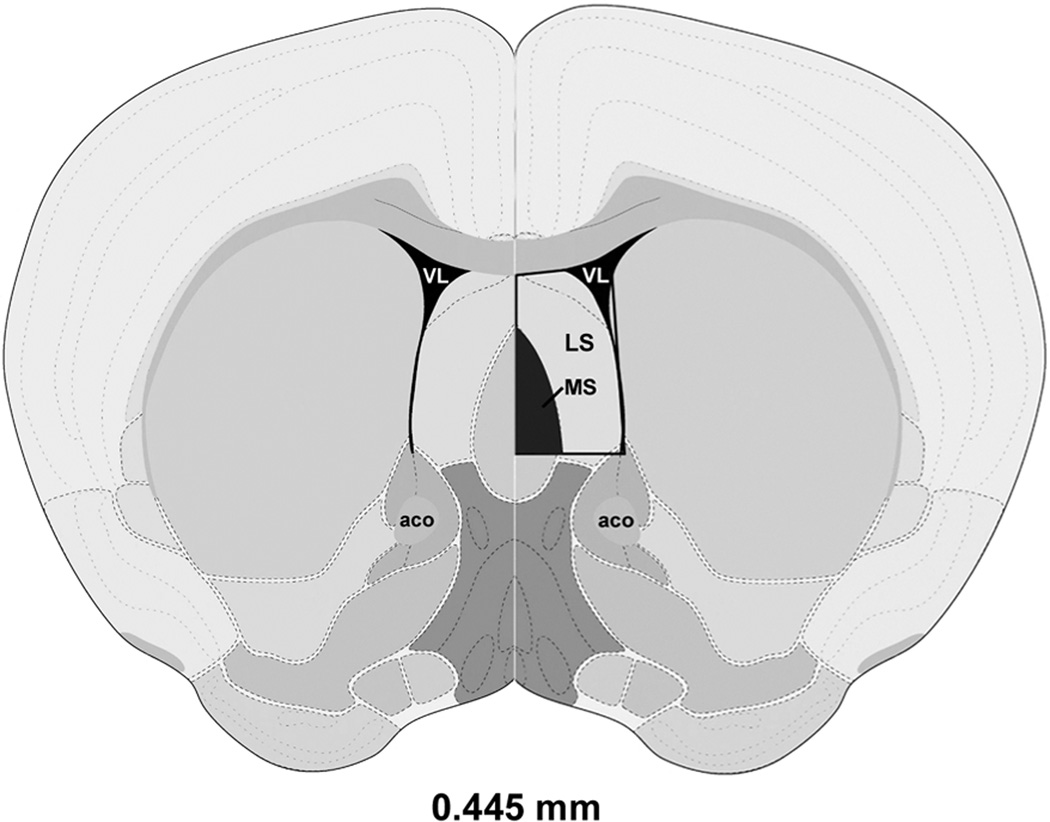
Schematic representation of a coronal section from the mouse LS in which expression of mRNA and protein immunoreactivity (boxed area with MS removed on the right side) were assessed. Image is adapted and modified from The Allen Mouse Brain Atlas (Reference Atlas Version 1, 2008). Distance relative to Bregma is indicated. Abbreviations: aco, anterior commissure, olfactory limb; LS, lateral septum; MS, medial septum; VL, lateral ventricle.
5.3. Gene expression analysis with qPCR
Expression of mRNAs for Gls, Gad1, Gad2, and Glul was analyzed using qPCR. For procedures of total RNA extraction and qPCR, we followed the protocol as described in detail previously (Zhao et al., 2012b). In brief, total RNA was extracted with an Aurum Total RNA Fatty and Fibrous Tissue Kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s specifications. With purified RNA, a SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA) was used to reverse transcribe 100 ng of RNA to cDNA in a T100 Thermal Cycler (Bio-Rad, Hercules, CA, USA). The cDNA was then amplified using a SsoFast EvaGreen Supermix kit in a CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA, USA) with the primers for target and reference genes Ppia and Ywhaz (Table 1) (Caldwell et al., 2008; Zhao et al., 2012b). Each sample was run in triplicate and standard amplification procedures were used. The cycling profile is as follows: an initial melting step at 95°C for 30 sec followed by 40 cycles of a 95°C melting step for 5 sec, a 58°C annealing step except Gad2 (at 57 °C) for 20 sec, and a 72°C elongation step for 20 sec. Following amplification, a standard curve was generated to assess the empirical PCR reaction efficiency, and a dissociation curve analysis was performed to insure specificity of PCR products. The expression ratio of mRNA of genes in postpartum relative to virgin (normalized against two reference genes, Ppia and Ywhaz) was calculated and analyzed using a relative expression software tool REST 2009 (Pfaffl et al., 2002).
Table 1.
Primer sequences for target and reference genes
| Symbol | NCBI accession number |
Forward primer | Reverse primer |
|---|---|---|---|
| Gad1 | NM_008077.4 | 5'-CTCAGGCTGTATGTCAGATGTTC-3' | 5'-AAGCGAGTCACAGAGATTGGTC-3' |
| Gad2 | NM_008078.2 | 5'-TCAACTAAGTCCCACCCTAAG-3' | 5'-CCCTGTAGAGTCAATACCTGC-3' |
| Gls | NM_001081081.2 | 5'-TTATGCCACTGTTTCTGCTG-3' | 5'-GGTTATCAAGTCCCTGACGG-3' |
| Glul | NM_008131.4 | 5'-TGAGAGAACCATCCTATTCACTG-3' | 5'-TAAGCAGTAATGAAGCTGAGACC-3' |
| Ppia | NM_008907.1 | 5'-TGCTGGACCAAACACAAACG-3' | 5'-GCCTTCTTTCACCTTCCCAAA-3' |
| Ywhaz | NM_011740.3 | 5'-TCCTTATTCCCTCTTGGCAG-3' | 5'-ATGGAAGCTACATTAGCGGTTT-3' |
Ppia primer sequences were described in published literature (Caldwell et al., 2008). Gad1, glutamate decarboxylase 1; Gad2, glutamate decarboxylase 2; Gls, glutaminase; Glul, glutamate-ammonia ligase; Ppia, peptidylprolyl isomerase A; Ywhaz, tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide.
5.4. ELISA immunoassay
Micropunched tissue was homogenized with ice-cold N-PER Neuronal Protein Extraction Reagent (Pierce Biotechnology, Rockford, IL, USA) containing 1 mM PMSF, a cocktail of protease inhibitor (P8340) and phosphatase inhibitor (P0044, Sigma, St Louis, MO, USA). Following tissue homogenization, samples were incubated on ice for 10 min and then centrifuged at 12,000 rpm for 10 min at 4°C to pellet the cell debris. The supernatant was collected for direct protein analysis. Tissue concentrations of glutamate, GABA, and glutamine were determined using Research ELISA kits for glutamate (IB89151, IBL-America, Minneapolis, MN, USA), GABA (IB89563, IBL-America, Minneapolis, MN, USA), and glutamine (CSB-E13328r, Cusabio Biotech, Wuhan, Hubei, China), respectively according to the manufacturer’s instructions. All samples were tested in duplicate in a single assay. Total protein concentration was measured using BCA Protein Assay (Pierce Biotechnology, Rockford, IL, USA). The final concentrations of glutamate, GABA, and glutamine were normalized against total protein and expressed as nanograms (for glutamate and GABA) or picomole (for glutamine) per micrograms protein.
5.5. Statistical Analysis
Statistical analyses were performed using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). Data on tissue levels of glutamate, GABA and glutamine were expressed as mean ± SEM and analyzed using Independent-Samples T test. The relationships among brain tissue levels of glutamate, GABA and glutamine were assessed using Pearson product-moment correlation. Overall level of statistical significance was set at P < 0.05.
Highlights.
Neuronal glutamate synthesis was enhanced in LS during the postpartum period.
Neuronal GABA synthesis was elevated in LS during the postpartum period.
Astrocytic glutamine synthesis was heightened in LS during the postpartum period.
There was a positive correlation between tissue levels of glutamate and GABA.
Ratio of glutamate to GABA did not differ between postpartum and virgin females.
Acknowledgments
Funding for this research was provided by National Institutes of Health Grant R01MH085642 to Stephen Gammie. We thank Terri Driessen and Sharon Stevenson for excellent technical and administrative support.
A list of Abbreviations
- GAD
glutamate decarboxylase
- Gad1 (GAD67)
glutamate decarboxylase 1
- Gad2 (GAD65)
glutamate decarboxylase 2
- Gls
glutaminase
- Glul
glutamate-ammonia ligase
- GS
glutamine synthetase
- LS
lateral septum
- Pag
phosphate-activated glutaminase
- Slc1a2 (GLT-1, Eaat2)
solute carrier family 1 (glial high affinity glutamate transporter), member 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akiyama H, Kaneko T, Mizuno N, McGeer PL. Distribution of phosphate-activated glutaminase in the human cerebral cortex. J. Comp. Neurol. 1990;297:239–252. doi: 10.1002/cne.902970207. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J. Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci A, Sancini G, Verderio C, Armano S, Pravettoni E, Fesce R, Franceschetti S, Matteoli M. Block of glutamate-glutamine cycle between astrocytes and neurons inhibits epileptiform activity in hippocampus. J. Neurophysiol. 2002;88:2302–2310. doi: 10.1152/jn.00665.2001. [DOI] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- Bergink V, van Megen HJ, Westenberg HG. Glutamate and anxiety. Eur. Neuropsychopharmacol. 2004;14:175–183. doi: 10.1016/S0924-977X(03)00100-7. [DOI] [PubMed] [Google Scholar]
- Bosch OJ. Maternal nurturing is dependent on her innate anxiety: the behavioral roles of brain oxytocin and vasopressin. Horm. Behav. 2011;59:202–212. doi: 10.1016/j.yhbeh.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nat. Rev. Neurosci. 2008;9:11–25. doi: 10.1038/nrn2280. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA, Douglas AJ. Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J. Neuroendocrinol. 2008;20:764–776. doi: 10.1111/j.1365-2826.2008.01735.x. [DOI] [PubMed] [Google Scholar]
- Caldwell KK, Sheema S, Paz RD, Samudio-Ruiz SL, Laughlin MH, Spence NE, Roehlk MJ, Alcon SN, Allan AM. Fetal alcohol spectrum disorder-associated depression: evidence for reductions in the levels of brain-derived neurotrophic factor in a mouse model. Pharmacol. Biochem. Behav. 2008;90:614–624. doi: 10.1016/j.pbb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, Reddy DS. Neurosteroid interactions with synaptic and extrasynaptic GABA(A) receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology (Berl) 2013;230:151–188. doi: 10.1007/s00213-013-3276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda MT, Sanabria ER, Hernandez S, Ayala A, Reyna TA, Wu JY, Colom LV. Glutamic acid decarboxylase isoforms are differentially distributed in the septal region of the rat. Neurosci. Res. 2005;52:107–119. doi: 10.1016/j.neures.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Edwards RH. The glutamine commute: take the N line and transfer to the A. J. Cell Biol. 2002;157:349–355. doi: 10.1083/jcb.200201070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chozick BS. The behavioral effects of lesions of the septum: a review. Int. J. Neurosci. 1985;26:197–217. doi: 10.3109/00207458508985617. [DOI] [PubMed] [Google Scholar]
- Ciceroni C, Mosillo P, Mastrantoni E, Sale P, Ricci-Vitiani L, Biagioni F, Stocchi F, Nicoletti F, Melchiorri D. mGLU3 metabotropic glutamate receptors modulate the differentiation of SVZ-derived neural stem cells towards the astrocytic lineage. Glia. 2010;58:813–822. doi: 10.1002/glia.20965. [DOI] [PubMed] [Google Scholar]
- Conti F, Minelli A. Glutamate immunoreactivity in rat cerebral cortex is reversibly abolished by 6-diazo-5-oxo-L-norleucine (DON), an inhibitor of phosphate-activated glutaminase. J. Histochem. Cytochem. 1994;42:717–726. doi: 10.1177/42.6.7910617. [DOI] [PubMed] [Google Scholar]
- Cremer CM, Bidmon HJ, Gorg B, Palomero-Gallagher N, Escobar JL, Speckmann EJ, Zilles K. Inhibition of glutamate/glutamine cycle in vivo results in decreased benzodiazepine binding and differentially regulated GABAergic subunit expression in the rat brain. Epilepsia. 2010;51:1446–1455. doi: 10.1111/j.1528-1167.2010.02562.x. [DOI] [PubMed] [Google Scholar]
- Drazen DL, Klein SL, Burnett AL, Wallach EE, Crone JK, Huang PL, Nelson RJ. Reproductive function in female mice lacking the gene for endothelial nitric oxide synthase. Nitric Oxide. 1999;3:366–374. doi: 10.1006/niox.1999.0251. [DOI] [PubMed] [Google Scholar]
- Drugan RC, Skolnick P, Paul SM, Crawley JN. Low doses of muscimol produce anticonflict actions in the lateral septum of the rat. Neuropharmacology. 1986;25:203–205. doi: 10.1016/0028-3908(86)90042-0. [DOI] [PubMed] [Google Scholar]
- Durand D, Pampillo M, Caruso C, Lasaga M. Role of metabotropic glutamate receptors in the control of neuroendocrine function. Neuropharmacology. 2008;55:577–583. doi: 10.1016/j.neuropharm.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Engelsen B, Fonnum F. The effect of methioninesulfoximine, an inhibitor of glutamine synthetase, on the levels of amino acids in the intact and decorticated rat neostriatum. Brain Res. 1985;338:165–168. doi: 10.1016/0006-8993(85)90261-6. [DOI] [PubMed] [Google Scholar]
- Feldman S, Weidenfeld J. Hypothalamic mechanisms mediating glutamate effects on the hypothalamo-pituitary-adrenocortical axis. J. Neural Transm. 1997;104:633–642. doi: 10.1007/BF01291881. [DOI] [PubMed] [Google Scholar]
- Fischer SG, Ricci LA, Melloni RH., Jr Repeated anabolic/androgenic steroid exposure during adolescence alters phosphate-activated glutaminase and glutamate receptor 1 (GluR1) subunit immunoreactivity in Hamster brain: correlation with offensive aggression. Behav. Brain Res. 2007;180:77–85. doi: 10.1016/j.bbr.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Renda A, Bodie B. Norepinephrine-gamma-aminobutyric acid (GABA) interaction in limbic stress circuits: effects of reboxetine on GABAergic neurons. Biol. Psychiatry. 2003;53:166–174. doi: 10.1016/s0006-3223(02)01449-x. [DOI] [PubMed] [Google Scholar]
- Hertz L, Dringen R, Schousboe A, Robinson SR. Astrocytes: glutamate producers for neurons. J. Neurosci. Res. 1999;57:417–428. [PubMed] [Google Scholar]
- Hertz L. The Glutamate-Glutamine (GABA) Cycle: Importance of Late Postnatal Development and Potential Reciprocal Interactions between Biosynthesis and Degradation. Front. Endocrinol (Lausanne) 2013;4:59. doi: 10.3389/fendo.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenstad M, Quazi AZ, Zilberter M, Haglerod C, Berghuis P, Saddique N, Goiny M, Buntup D, Davanger S, FM SH, Barnes CA, McNaughton BL, Ottersen OP, Storm-Mathisen J, Harkany T, Chaudhry FA. System A transporter SAT2 mediates replenishment of dendritic glutamate pools controlling retrograde signaling by glutamate. Cereb. Cortex. 2009;19:1092–1106. doi: 10.1093/cercor/bhn151. [DOI] [PubMed] [Google Scholar]
- Jocham G, Hunt LT, Near J, Behrens TE. A mechanism for value-guided choice based on the excitation-inhibition balance in prefrontal cortex. Nat. Neurosci. 2012;15:960–961. doi: 10.1038/nn.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinine E, Zimmer ER, Zenki KC, Kalinine I, Kazlauckas V, Haas CB, Hansel G, Zimmer AR, Souza DO, Muller AP, Portela LV. Nandrolone-induced aggressive behavior is associated with alterations in extracellular glutamate homeostasis in mice. Horm. Behav. 2014;66:383–392. doi: 10.1016/j.yhbeh.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Kinsley CH. The neuroplastic maternal brain. Horm. Behav. 2008;54:1–4. doi: 10.1016/j.yhbeh.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Amory-Meyer E. Why the maternal brain? J. Neuroendocrinol. 2011;23:974–983. doi: 10.1111/j.1365-2826.2011.02194.x. [DOI] [PubMed] [Google Scholar]
- Kornblatt JJ, Grattan DR. Lactation alters gamma-aminobutyric acid neuronal activity in the hypothalamus and cerebral cortex in the rat. Neuroendocrinology. 2001;73:175–184. doi: 10.1159/000054634. [DOI] [PubMed] [Google Scholar]
- Kvamme E, Torgner IA, Roberg B. Kinetics and localization of brain phosphate activated glutaminase. J. Neurosci. Res. 2001;66:951–958. doi: 10.1002/jnr.10041. [DOI] [PubMed] [Google Scholar]
- Lee G, Gammie SC. GABA(A) receptor signaling in the lateral septum regulates maternal aggression in mice. Behav. Neurosci. 2009;123:1169–1177. doi: 10.1037/a0017535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SL, Carlson GC, Coulter DA. Dynamic regulation of synaptic GABA release by the glutamate-glutamine cycle in hippocampal area CA1. J. Neurosci. 2006;26:8537–8548. doi: 10.1523/JNEUROSCI.0329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci. Biobehav. Rev. 2002;26:869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Maguire J, Meinlschmidt G, Neumann ID. Emotion and Mood Adaptations in the Peripartum Female: Complementary Contributions of GABA and Oxytocin. J. Neuroendocrinol. 2014;26:649–664. doi: 10.1111/jne.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor D, Rothman DL, Mason GF, Hyder F, Petroff OA, Behar KL. The rate of turnover of cortical GABA from [1-13C]glucose is reduced in rats treated with the GABA-transaminase inhibitor vigabatrin (gamma-vinyl GABA) Neurochem. Res. 1996;21:1031–1041. doi: 10.1007/BF02532413. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz. J. Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Martin DL, Tobin AJ. Mechanisms controlling GABA synthesis and degradation in the brain. In: M DL, O RW, editors. GABA in the nervous system: the view at fifty years Vol. Philadelphia, PA, USA: Lippincott, Williams & Wilkins; 2000. pp. 25–41. [Google Scholar]
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- McDonald MM, Markham CM, Norvelle A, Albers HE, Huhman KL. GABAA receptor activation in the lateral septum reduces the expression of conditioned defeat and increases aggression in Syrian hamsters. Brain Res. 2012;1439:27–33. doi: 10.1016/j.brainres.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SM, Piasecki CC, Peabody MF, Lonstein JS. GABA(A) receptor antagonism in the ventrocaudal periaqueductal gray increases anxiety in the anxiety-resistant postpartum rat. Pharmacol. Biochem. Behav. 2010;95:457–465. doi: 10.1016/j.pbb.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Moltz H, Rowland D, Steele M, Halaris A. Hypothalamic norepinephrine: concentration and metabolism during pregnancy and lactation in the rat. Neuroendocrinology. 1975;19:252–258. doi: 10.1159/000122445. [DOI] [PubMed] [Google Scholar]
- Mora F, Segovia G, Del Arco A. Glutamate-dopamine-GABA interactions in the aging basal ganglia. Brain Res. Rev. 2008;58:340–353. doi: 10.1016/j.brainresrev.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacol. Biochem. Behav. 2008;91:77–83. doi: 10.1016/j.pbb.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Byrnes EM, Bridges RS. Vasopressin mediates enhanced offspring protection in multiparous rats. Neuropharmacology. 2010;58:102–106. doi: 10.1016/j.neuropharm.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Torner L, Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 2000;95:567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Alterations in behavioral and neuroendocrine stress coping strategies in pregnant, parturient and lactating rats. Prog. Brain Res. 2001;133:143–152. doi: 10.1016/s0079-6123(01)33011-x. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Ogden KK, Khatri A, Traynelis SF, Heldt SA. Potentiation of GluN2C/D NMDA receptor subtypes in the amygdala facilitates the retention of fear and extinction learning in mice. Neuropsychopharmacology. 2014;39:625–637. doi: 10.1038/npp.2013.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onteniente B, Tago H, Kimura H, Maeda T. Distribution of gamma-aminobutyric acid-immunoreactive neurons in the septal region of the rat brain. J. Comp. Neurol. 1986;248:422–430. doi: 10.1002/cne.902480310. [DOI] [PubMed] [Google Scholar]
- Panula P, Revuelta AV, Cheney DL, Wu JY, Costa E. An immunohistochemical study on the location of GABAergic neurons in rat septum. J. Comp. Neurol. 1984;222:69–80. doi: 10.1002/cne.902220107. [DOI] [PubMed] [Google Scholar]
- Pesold C, Treit D. The neuroanatomical specificity of the anxiolytic effects of intraseptal infusions of midazolam. Brain Res. 1996;710:161–168. doi: 10.1016/0006-8993(95)01359-8. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Errante LD, Rothman DL, Kim JH, Spencer DD. Glutamate-glutamine cycling in the epileptic human hippocampus. Epilepsia. 2002;43:703–710. doi: 10.1046/j.1528-1157.2002.38901.x. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines G, Danbolt NC, Bjoras M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- Rimvall K, Martin DL. The level of GAD67 protein is highly sensitive to small increases in intraneuronal gamma-aminobutyric acid levels. J. Neurochem. 1994;62:1375–1381. doi: 10.1046/j.1471-4159.1994.62041375.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez C, Guillamon A, Pinos H, Collado P. Postpartum changes in the GABAergic system in the bed nucleus of the accessory olfactory tract. Neurochem. Int. 2004;44:179–183. doi: 10.1016/s0197-0186(03)00115-3. [DOI] [PubMed] [Google Scholar]
- Rose CF, Verkhratsky A, Parpura V. Astrocyte glutamine synthetase: pivotal in health and disease. Biochem. Soc. Trans. 2013;41:1518–1524. doi: 10.1042/BST20130237. [DOI] [PubMed] [Google Scholar]
- Salmaso N, Cossette MP, Woodside B. Pregnancy and maternal behavior induce changes in glia, glutamate and its metabolism within the cingulate cortex. PLoS One. 2011;6:e23529. doi: 10.1371/journal.pone.0023529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch. Gen. Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Westergaard N, Waagepetersen HS, Larsson OM, Bakken IJ, Sonnewald U. Trafficking between glia and neurons of TCA cycle intermediates and related metabolites. Glia. 1997;21:99–105. [PubMed] [Google Scholar]
- Schousboe A, Bak LK, Waagepetersen HS. Astrocytic Control of Biosynthesis and Turnover of the Neurotransmitters Glutamate and GABA. Front. Endocrinol (Lausanne) 2013;4:102. doi: 10.3389/fendo.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia G, Del Arco A, Mora F. Effects of aging on the interaction between glutamate, dopamine, and GABA in striatum and nucleus accumbens of the awake rat. J. Neurochem. 1999;73:2063–2072. [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res. Brain Res. Rev. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Sheikh SN, Martin DL. Elevation of brain GABA levels with vigabatrin (gammavinylGABA) differentially affects GAD65 and GAD67 expression in various regions of rat brain. J. Neurosci. Res. 1998;52:736–741. doi: 10.1002/(SICI)1097-4547(19980615)52:6<736::AID-JNR12>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- Singewald GM, Rjabokon A, Singewald N, Ebner K. The modulatory role of the lateral septum on neuroendocrine and behavioral stress responses. Neuropsychopharmacology. 2011;36:793–804. doi: 10.1038/npp.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol. Sci. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- Solbu TT, Bjorkmo M, Berghuis P, Harkany T, Chaudhry FA. SAT1, A Glutamine Transporter, is Preferentially Expressed in GABAergic Neurons. Front. Neuroanat. 2010;4:1. doi: 10.3389/neuro.05.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnewald U, Westergaard N, Schousboe A, Svendsen JS, Unsgard G, Petersen SB. Direct demonstration by [13C]NMR spectroscopy that glutamine from astrocytes is a precursor for GABA synthesis in neurons. Neurochem. Int. 1993;22:19–29. doi: 10.1016/0197-0186(93)90064-c. [DOI] [PubMed] [Google Scholar]
- Stransky Z. Time course of rat brain GABA levels following methionine sulphoximine treatment. Nature. 1969;224:612–613. doi: 10.1038/224612a0. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Koehler RC, Brusilow SW, Traystman RJ. Inhibition of brain glutamine accumulation prevents cerebral edema in hyperammonemic rats. Am. J. Physiol. 1991;261:H825–H829. doi: 10.1152/ajpheart.1991.261.3.H825. [DOI] [PubMed] [Google Scholar]
- West AR, Floresco SB, Charara A, Rosenkranz JA, Grace AA. Electrophysiological interactions between striatal glutamatergic and dopaminergic systems. Ann. N. Y. Acad. Sci. 2003;1003:53–74. doi: 10.1196/annals.1300.004. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Driessen T, Gammie SC. Glutamic acid decarboxylase 65 and 67 expression in the lateral septum is up-regulated in association with the postpartum period in mice. Brain Res. 2012a;1470:35–44. doi: 10.1016/j.brainres.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Saul MC, Driessen T, Gammie SC. Gene expression changes in the septum: possible implications for microRNAs in sculpting the maternal brain. PLoS One. 2012b;7:e38602. doi: 10.1371/journal.pone.0038602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Eisinger B, Gammie SC. Characterization of GABAergic neurons in the mouse lateral septum: a double fluorescence in situ hybridization and immunohistochemical study using tyramide signal amplification. PLoS One. 2013;8:e73750. doi: 10.1371/journal.pone.0073750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuure WA, Roberts AL, Quennell JH, Anderson GM. Leptin signaling in GABA neurons, but not glutamate neurons, is required for reproductive function. J. Neurosci. 2013;33:17874–17883. doi: 10.1523/JNEUROSCI.2278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]