Abstract
The distribution expansion of important human visceral leishmaniasis (HVL) and sporadic cutaneous leishmaniasis (SCL) vector species, Phlebotomus perfiliewi and P. perniciosus, throughout central Tunisia is a major public health concern. This study was designed to investigate if the expansion of irrigation influences the abundance of sand fly species potentially involved in the transmission of HVL and SCL located in arid bioclimatic regions. Geographic and remote sensing approaches were used to predict the density of visceral leishmaniasis vectors in Tunisia. Entomological investigations were performed in the governorate of Sidi Bouzid, located in the arid bioclimatic region of Tunisia. In 2012, sand flies were collected by CDC light traps located at nine irrigated and nine non-irrigated sites to determine species abundance. Eight species in two genera were collected. Among sand flies of the subgenus Larroussius, P. perfiliewi was the only species collected significantly more in irrigated areas. Trap data were then used to develop Poisson regression models to map the apparent density of important sand fly species as a function of different environmental covariates including climate and vegetation density. The density of P. perfiliewi is predicted to be moderately high in the arid regions. These results highlight that the abundance of P. perfiliewi is associated with the development of irrigated areas and suggests that the expansion of this species will continue to more arid areas of the country as irrigation sites continue to be developed in the region. The continued increase in irrigated areas in the Middle East and North Africa region deserves attention, as it is associated with the spread of L. infantum vector P. perfiliewi. Integrated vector management strategies targeting irrigation structures to reduce sand fly vector populations should be evaluated in light of these findings.
Keywords: human visceral leishmaniasis, Phlebotomus perfiliewi, integrated vector management, remote sensing approaches
1. Introduction
Visceral and cutaneous leishmaniasis in the Middle East and North Africa region are important vector-borne diseases, accounting for 15% of the global leishmaniasis burden (World Health Organization, WHO 2008). Rapid urbanization and environmental changes, especially in arid regions of the Middle East and North Africa, have resulted in an increase in the incidence of leishmaniasis (WHO 2002). Specifically, Tunisia, during the last twenty years, has experienced the development of more than 10,000 wells in the arid bioclimatic areas (Ben Salah et al. 2000). During this time, there has been an increase in visceral leishmaniasis in arid bioclimatic regions of the country where previously, few human cases were described. Each year in Tunisia, there are 100 to 160 cases of human visceral leishmaniasis (HVL) (Alvar et al. 2012). The highest prevalence of HVL, 33%, is observed in the governorate of Kairouan, an arid bioclimatic area located in central Tunisia (Bouratbine et al. 1998).
In Tunisia, as in other Western Mediterranean countries, Leishmania infantum is the etiological agent of HVL and sporadic cutaneous leishmaniasis (SCL) and transmitted by sand fly species of the subgenus Larroussius mainly Phlebotomus perniciosus Newstead and Phlebotomus perfiliewi Parrot. HVL and SCL are historically endemic in northern Tunisia in humid, sub-humid and semi-arid bio-geographical areas (Ben Rachid et al. 1983, Aoun et al. 2000). However, from 1982 to 1991, more than 200 cases of HVL were reported from areas located in arid central and southern Tunisia (Ben Salah et al. 2000, Ayadi et al. 1991, Besbes et al. 1994). Sporadic cutaneous leishmaniasis was previously limited to the northern Tunisia (Ben Rachid et al. 1983) but in 2006 (Ben Said et al. 2006) the spread of SCL towards central Tunisia was documented. Likewise, canine visceral leishmaniasis (CVL), until 1988, was limited to the northern Tunisian Ridge but is now endemic in the governorate of Sidi Bouzid, located in the center of Tunisia (Diwani et al. 2008, Keddous 1988). Evidence of the extension of HVL and SCL as well as CVL toward the center and the south of the country was shown based on earlier epidemiological studies (Ben Salah et al. 2000, Ayadi et al. 1991, Besbes et al. 1994, Ben Said et al. 2006, Diwani et al. 2008). However, recent entomological surveys suggest that the extension of HVL, SCL, and CVL is due to geographical expansion of P. perniciosus and P. perfiliewi toward the center of Tunisia (Zhioua et al. 2007).
Phlebotomus perfiliewi is one of the main vectors of HVL and SCL in Italy and Algeria (Maroli et al. 1987, Izri and Belazzoug 1993). In Tunisia, P. perfiliewi is possibly a vector of L. infantum but this role remains to be confirmed (Dancesco et al. 1970). Phlebotomus perniciosus is the principal vector of the viscerotropic L. infantum zymodeme MON 1, etiologic agent of HVL and CVL in Tunisia (Ben Ismail 1993, Chargi et al. 2013). Moreover, in Italy, P. perniciosus was shown to be naturally infected with L. infantum zymodeme MON 72 (Maroli et al. 1994). Phlebotomus longicuspis Nutzulescu is a vector for L. infantum in Algeria (Berdjane-Brouk et al. 2012) and suspected vector in Morocco (Benabdennbi et al. 1991) and in Tunisia (Zhioua et al. 2007). Though the distribution of these species is usually closely related to bioclimate, populations occurring in central and southern Tunisia may be related to land use changes that provide a more suitable niche for these important Leishmania vectors (Barhoumi et al. 2012).
In the humid zone located in the northwest part of Tunisia, P. perfiliewi is the most abundant species followed by P. perniciosus (Zhioua et al. 2007). In the sub-humid and semi-arid bioclimate, P. perniciosus is the most dominant species (Zhioua et al. 2007). The geographical distribution of both species is no longer limited to the north but they are also found in the center of Tunisia characterized by an arid bioclimate (Zhioua et al. 2007, Barhoumi et al. 2012). The relative high density of P. perniciosus observed in the arid bioclimatic zones is associated with the emergence of several foci of HVL in central Tunisia (Zhioua et al. 2007). Entomological findings of abundant populations of P. longicuspis in southern Tunisia have also been associated with HVL cases, but this role remains to be confirmed (Zhioua et al. 2007).
The purpose of this study was to compare the abundance of sand fly species potentially involved in the transmission of HVL between irrigated and non-irrigated zones located in arid bioclimatic areas of central Tunisia. The distribution expansion of these important HVL, SCL and CVL vector species is most likely attributed to the increase in irrigation throughout Tunisia. In Greece, wells were shown to be resting and a potential breeding sites for the important HVL vectors, P. tobbi and P. neglectus (Chaniotis and Tselentis 1996). Furthermore, remote sensing and geographic information system (GIS) analysis was used to predict the density of these species related to land use, land change and environmental conditions that could serve to identify potential risk areas for human infection.
2. Materials and Methods
2.1 Study area
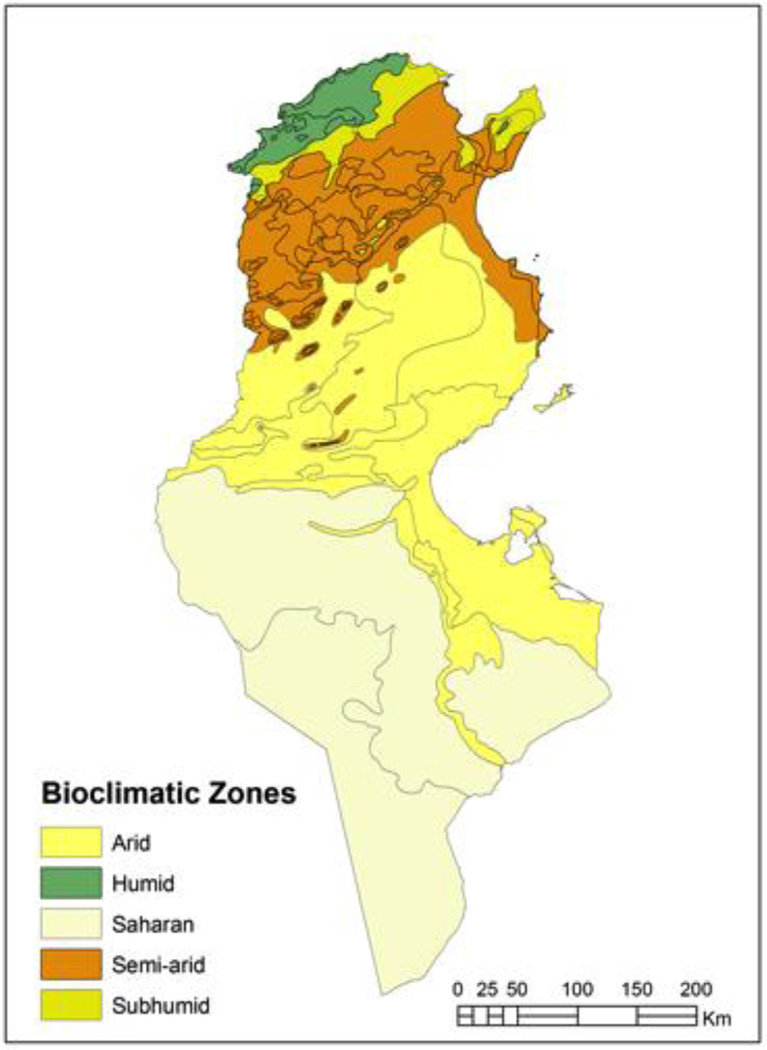
The sand fly field investigations were performed in the governorate of Sidi Bouzid, an arid bioclimatic area located in central Tunisia. The modelling of sand fly density considered all of Tunisia. Tunisia covers a wide bioclimatic range from the Mediterranean climate with its rainy winter in the north to the Saharan climate in the south (Figure 1). The Tunisian Ridge separates the northern part of the country from the south. The latter is a range of hills, which runs from north-east to south-west for some 220 km and marks the bioclimatic boundary between the Mediterranean north and the dry steppe of central Tunisia. Between the northern slopes of the Tunisian Ridge and the chains of hills in the south are extensive plateaus, called the High Tell. The Sahara is separated from the central steppe land by a series of salted areas called chotts (Ghrab et al. 2006).
Figure 1.
Bioclimatic map of Tunisia. Tunisia has a wide climatic range from a Mediterranean climate with its rainy winter in the north to a Saharan climate in the south.
2.2. Field investigation
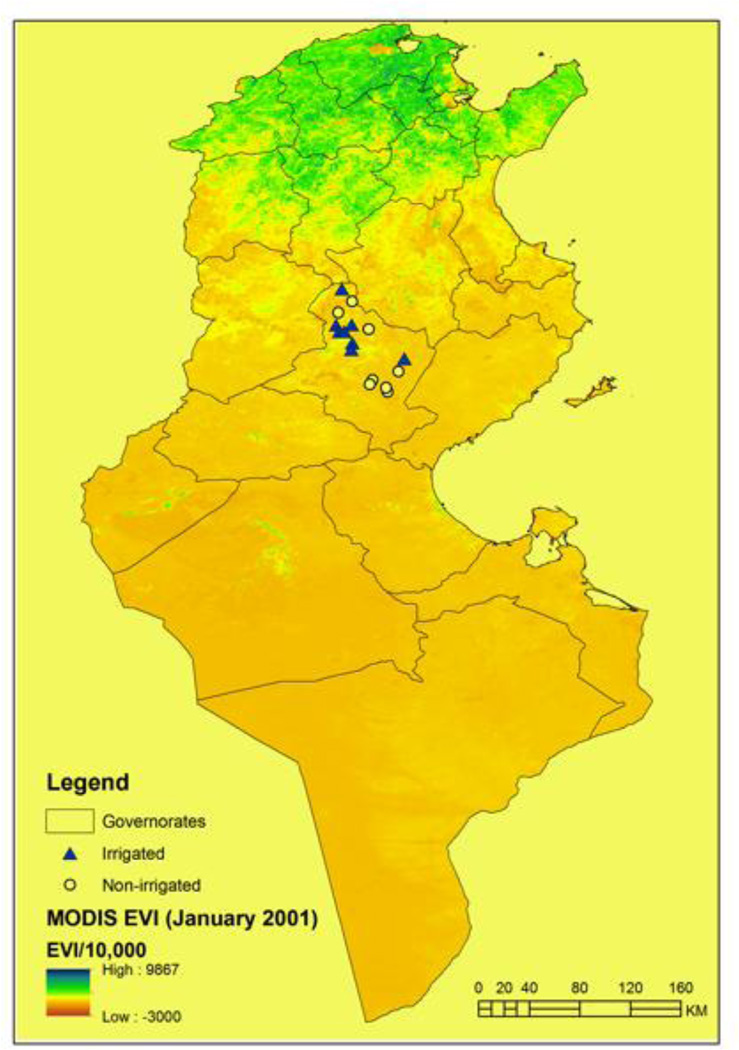
Sampling of sand flies was performed in 18 sites (9 irrigated and 9 non-irrigated) within the governorate of Sidi Bouzid (Figure 2). Sand flies were collected inside houses and in animal shelters located in the peri-domestic areas using CDC light traps. Two traps (one inside the house and one inside the animal shelter) were placed, from dusk to dawn, one night per site during September to October 2012. A total of 8 trapping nights were performed per site. Collection of sand flies was performed during the peak activity period of several sand fly species in Tunisia and in surrounding countries including P. perniciousis, P. perfiliewi, P. longicuspis, P. papatasi Scopoli, and P. langeroni Nutzulescu (Chelbi et al. 2007). Collected sand flies were placed in 70% ethanol and later mounted on glass slides in Mark André medium (Abonnenc 1972), then identified to species by using the identification keys of Croset et al. (1978). The dominance of each species was measured by its relative abundance (number of a sand fly species by the total number of sand flies caught per bioclimatic zone). A negative binomial analysis with significance set at P < 0.05 was used to determine the influence of irrigation on species abundance.
Figure 2.
The 16 sand fly sampling sites in the governorate of Sidi Bouzid, Tunisia.
2.3. Mapping apparent sand fly density using environmental covariates
Histograms of the count data for two important vectors of HVL and SCL, P. perniciosus, and P. perfiliewi, revealed overdispersed Poisson distributions consistent with similar results of sand fly vectors obtained by Gálvez et al. (2010). We therefore used Poisson regression models to estimate the apparent density estimates of these sand fly species as a function of different environmental covariates, which we obtained from a range of bioclimatic and landscape greenness data. Given overdispersed nature of the count data, we selected a Poisson regression model to fit the data and estimate beta coefficients. Bioclimatic data layers were derived from the WorldClim database (Hijmans et al. 2005). Greenness covariates were obtained from satellite vegetation index imagery provided by the Moderate Resolution Imaging Spectroradiometer (MODIS) sensor on board the Terra polar-orbiting satellite. Specifically, we selected the Enhanced Vegetation Index (EVI) for our regression models as it reduces soil background and atmospheric effects relative to the more commonly used normalized difference vegetation index or NDVI (Huete et al. 2002). EVI is a sensitive indicator of photosynthetic activity, which is generally limited by soil moisture in arid and semi-arid parts of Tunisia (Latiri et al. 2010). Therefore, we assumed that changes in EVI were driven by soil moisture fluctuations resulting from either rainfall or water inputs through crop irrigation. Various phenological metrics including the annual maximum, annual mean, annual minimum, and standard deviation, a measure of seasonality, were derived from time series of 16-day composites of 250 m EVI imagery for the years 2011 and 2012. The phenological metrics and bioclimatic data were extracted from raster files using GIS software for each of the 18 sites where sand fly vectors had been collected in central Tunisia during 2012. We included 2011 EVI metrics in the analysis as the previous year’s moisture availability may produce inter-annual carry-over effects (or lags) that may influence sand fly population and fitness in subsequent breeding seasons. The resolution of the WorldClim data was 1 km, thus we regridded the data to 250 m and extracted bioclimatic data values from bioclimatic grid squares that coincided with each of the 18 sites.
Each of these derived EVI phenology metrics and bioclimatic variables was considered as a model covariate with model selection guided by the Automatic Linear Modeling (ALM) module in IBM SPSS Statistics (version 19) software. The ALM selects important predictors and provides the Akaike’s Information Criterion (AIC), which was used to assess individual model performance. Combinations of important covariates with AIC values < 100 were further analyzed for colinearity by examining Pearson correlation matrices such that less important, intercorrelated covariates (i.e., p < 0.05) were eliminated from Poisson models. Poisson regressions were implemented in the General Linear Models suite of SPSS 19 and the Wald Chi-Squared statistic was used to evaluate the significance of model parameters (betas and intercepts). Significant parameters (p < 0.05) were retained and included in functions with the general form
where ADi is apparent density for species i, a is the estimated intercept, and β1…βn are model coefficients obtained through Poisson regressions for n covariates entered into the models and X1 through Xn are model covariates. The models determined through this general approach (Table 1) to estimate AD were then used to produce gridded surfaces depicting apparent sand fly densities at 250 m resolution, consistent with the original MODIS EVI imagery. Apparent sand fly density maps were evaluated in light of known distributions of these vectors as shown by Zhioua et al. (2007).
Table 1.
Poisson regression parameters used in the apparent density models for two sand fly vectors in Tunisia
| Species | Intercept | β1 | β2 | β3 | X1 | X2 | X3 |
|---|---|---|---|---|---|---|---|
| P. perfiliewi | −4.667 | 0.494 | −98.376 | Bio18 | EVI min 2011 | ||
| P. perniciosus | 4.553 | −43.102 | 10.618 | EVI min 2011 | EVI max 2011 |
All parameters significant at p < 0.05.
3. Results
3.1. Field investigations
A total of 2,463 sand flies were collected representing eight species in two genera: Phlebotomus and Sergentomyia. Five of the eight species were collected in the irrigated sites and all eight species were collected in the non-irrigated sites. Three of the eight species only appear once in the non-irrigated sites: P. alexandri, P. sergenti, and S. fallax. Among sand flies of the subgenus Larroussius, P. perfiliewi was the only species collected significantly more in irrigated areas (P < 0.001; Table 2).
Table 2.
Abundance of sand fly species in Rmilia (non-irrigated) and El Feta (irrigated) areas in the governorate of Sidi Bouzid during the activity season of 2012.
| Rmilia | El Feta | ||||
|---|---|---|---|---|---|
| Species | Mean | Se | Mean | Se | p |
| P. longicuspis | 29.1 | 7.8 | 57.6 | 15.6 | 0.074 |
| P. papatasi | 39.1 | 11.9 | 68.8 | 29.3 | 0.280 |
| P. perfiliewi | 2.3 | 0.4 | 43.7 | 28.6 | <0.001 |
| P. perniciosus | 48.7 | 26.6 | 34.1 | 16.5 | 0.627 |
| S. minuta | 7.0 | 3.7 | 3.8 | 1.0 | 0.300 |
P signifiant at <0.05
3.2. Mapping apparent sand fly density using environmental covariates
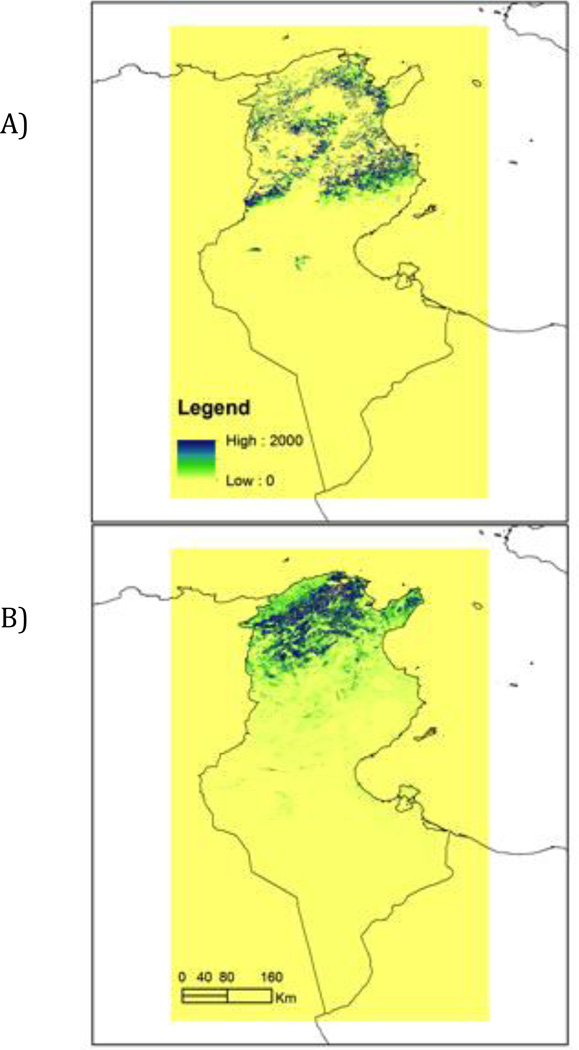
Figure 3 highlights the predicted density of the two important Leishmania vectors using a Poisson regression model with three independent covariates: minimum EVI (2011), minimum EVI (2012) and SD EVI (2011) and sand fly vector data from the current report and Zhioua et al. (2007). The apparent density maps of P. perfiliewi and P. perniciosus provide good qualitative agreement between our knowledge of species distribution patterns and density in Tunisia.
Figure 3.
Poisson regression predicated density of two important Leishmania vectors: A) P. perfiliewi and B) P. perniciosus. Models used three independent covariates: minimum EVI (2011), minimum EVI (2012), and SD EVI (2011) and sand fly vector distributions reported in the present report and Zhioua et al. 2007.
4. Discussion
Sand fly species diversity in the governorate of Sidi Bouzid is high, with eight species collected from the 18 sites sampled. These results highlight that the abundance of the important HVL and SCL vector, P. perfiliewi, is associated with development of irrigated areas in arid regions of Tunisia. The density of P. perfiliewi is predicted to be moderately high in the north central arid region of Tunisia. In 2006, P. perfiliewi only represented 5% of the collections in the arid central region of Tunisia (Zhioua et al. 2007). Another entomological study conducted in 2006 found P. perfiliewi populations to be rare in the sub-humid, semi-arid, and arid regions of Tunisia (Ghrab et al. 2006). However, this species was the most predominant species collected in humid areas of Tunisia (Zhioua et al. 2007, Ghrab et al. 2006). In the current study, P. perfiliewi represents 11% of the total collection in arid areas. Since P. perfiliewi is considered a potential vector of HVL and SCL in humid and sub-humid regions of Tunisia, the continued expansion of this species poses a public health risk.
Our results support the findings of Zhioua et al. (2007), that the distribution of P. perniciosus is expanding towards the center and southern arid areas of Tunisia. Previous entomological studies had found P. perniciousus to be the most abundant Larroussis species in semi-arid bioclimatic zones and much less abundant in sub-humid and arid areas (Ghrab et al. 2006, Ben-Ahmed et al. 2009). Based on our overall field collections, this species abundance is not associated with the development of irrigation as it was collected frequently in both irrigated and non-irrigated areas. It is important to note that in a recent study comparing two sites, one irrigated and one non-irrigated, P. pernicious was found to be more abundant at the irrigated sites (Barhoumi et al. 2012). Entomological transects performed in 1980, 1999, and in 2006 showed a relative abundance of P. perniciosus in the arid bioclimatic zone of 0.3% (n = 11,724), 9% (n = 1,087), and 20% (n = 1,024), respectively (Zhioua et al. 2007, Ghrab et al. 2006, Rioux et al. 1986). The relative abundance of P. perniciosus, in the eastern coast of Tunisia, was reported to be 31% and is considered one of the most important vectors in HVL transmission (Boudabous et al. 2009). These studies clearly show the extension of P. pernicious toward the arid areas located in central Tunisia. In the current study, P. perniciosus represents 17% of the total collection in the governorate of Sidi Bouzid. More importantly L. infantum was isolated from three field-collected P. perniciosus from a previously non-endemic region of central Tunisia (Chargi et al. 2013). The detection of L. infantum in unfed P. perniciosus where cases of HVL were recorded confirms the establishment of the life cycle transmission in the central region of Tunisia (Chargi et al. 2013). However, based on our environmental covariate analysis the density of this species is heavily concentrated in the northern humid, sub-humid and semiarid areas of Tunisia with a lower density occurring in arid areas. Aridity seems to be a limiting factor for its geographical distribution (Croset et al. 1978). Therefore, due to the development of irrigation in arid areas, P. perniciosus has become the second most abundant sand fly species after P. papatasi (Zhioua et al. 2007).
Phlebotomus longicuspis is the predominant species in the Saharan bioclimatic zone with a relative abundance of 60% (Zhioua et al. 2007). Ayadi et al. [8] reported 12 cases of HVL from Gafsa and Tozeur, both sites are located in the Saharan bioclimatic zone. Similar results were reported from southern Morocco where, P. longicuspis is the most abundant species in the arid bioclimatic zone and therefore, it is suspected to be the only vector of HVL in this area (Deureure et al. 1986, Rioux et al. 1997). The abundance and density of P. longicuspis could be responsible for the extension of HVL in southern Tunisia (Zhioua et al. 2007).
Phlebotomus papatasi, vector of Leishmania major, etiologic agent of zoonotic cutaneous leishmaniasis (ZCL) (Ben Ismail et al. 1987) was the most abundant species collected throughout this study. Therefore, Sidi Bouzid is the most endemic governorate for ZCL (Chelbi et al. 2007, Chelbi et al. 2009) nationwide. We did not find P. papatasi abundance to be associated with irrigated areas, which is consistent with a previous study showing no association of this species abundance with irrigation in Tunisia (Barhoumi et al. 2012). Zhioua et al. (2007) found the abundance of P. papatasi to be highest in arid and Saharan regions (62.7 % and 33.6 %, respectively) of Tunisia with the lowest abundance occurring in humid regions (0.2%) (Zhioua et al. 2007). Concomitantly, the incidence of ZCL is highest in the arid and Saharan areas of Tunisia (Chelbi et al. 2009). Aridity appears to be a limiting factor for the geographical distribution of P. papatasi (Cross et al. 1996, Chelbi et al. 2009).
Combining logistic or Poisson regression models with remote sensing and GIS provides significant power to be used to estimate the apparent density of sand fly vectors as a function of different environmental covariates (Ben-Ahmed et al. 2009, Cross et al. 1996). In particular, Table 1 shows that beta coefficients for annual minimum EVI, the standard deviation of EVI (a proxy of moisture seasonality), and annual maximum EVI provided significant results in our Poisson regressions; whereas bioclimatic covariates, with the exception of precipitation during the warmest quarter, did not add to model performance. The apparent density of HVL and SCL vectors is consistent with what is known about the distribution of P. perfiliewi and P. perniciosus in Tunisia and subsequently it provides useful insights concerning their ecological boundaries by using the Poisson regression models in combination with GIS. Mapping the potential distribution of P. perfiliewi and P. pernicious is a prerequisite for the development of a risk map of HVL and SCL in Tunisia.
Until recently, water wells or irrigation structures have not been a recognized breeding or resting habitat for some sand fly species of medical and veterinary importance (Biocca and Constantine 1986, Chaniotis and Tselentis 1996). However, because these structures provide a dark, moist habitat and protection from unfavorable weather conditions, these structures should not be overlooked for their potential to serve as an important habitat for a variety of sand fly species (Warburg and Faiman 2011). The phlebotomine fauna of central and southern Tunisia should be investigated to validate the predictive density models developed in this report and confirm the spread and establishment of HVL, SCL, and CVL in non-endemic areas. Irrigation in arid areas increases soil moistures and subsequently provides suitable ecological niches for the establishment of P. perfiliewi. Moreover, the continued increase in irrigated areas in the Middle Eastern and North African arid region deserves attention, as it is associated with the spread of important Leishmania vectors (Zhioua et al. 2007, Barhoumi et al. 2012).
HVL has been historically endemic in northern Tunisia with its southward distribution limited by the Tunisian Ridge (Ben Rachid et al. 1983, Croset et al. 1978, Anderson 1938, Chadli et al. 1968). From 1982 to 1991, more than 200 cases of HVL were reported from areas located in central and southern Tunisia (Ben Salah et al. 2000, Ayadi et al. 1991, Besbes et al. 1994). Similarly, CVL was limited to the Northern Tunisian Ridge (Diwani et al. 2008, Bouratbine et al. 2005). Since 1985, CVL became endemic in the governorates situated in the center of Tunisia (Keddous 1988), Ben Said et al. 1992, Chargui et al. 2012). Of a total of 142 cases of HVL reported during 1993, 47 (33%) originated from the arid governorate of Kairouan situated in Central Tunisia (Bouratbine et al. 1998). Among all nationwide HVL cases reported in 2011 and 2012, 72.2%, and 67.5% originated from governorates located in central Tunisia (DSSB 2011, DSSB 2012). Clearly, the main foci of HVL cases are now in the southern areas of the Tunisian Ridge located in central Tunisia where the governorate of Kairouan is bordering the governorate of Sidi Bouzid (Ben Salah et al. 2000). The spread of HVL from the north to south is mostly related to the increase in irrigated areas in arid regions of central Tunisia allowing the establishment of a stable cycle of sand fly vectors, L. infantum, dogs, and subsequently humans (Rioux et al. 1986, Zhioua et al. 2007).
The rapid spread of sand fly vectored pathogens to non-endemic areas require the development of integrated vector management strategies for prevention and control of sand flies. Identifying habitats of sand fly vectors is of major importance for the development of these strategies. Sand fly control has often proved to be unsuccessful largely because their larval development habitats and adult behavior patterns are unknown (Müller and Schlein 2011). Identifying the role that wells and irrigation structures play in the lifecycle of sand fly vectors could serve as a target site for application of control measures. New vector control methods, such as attractive toxic sugar baits, show promise for reducing sand fly vector populations (Müller and Schlein 2011) and could be applied easily to well and cistern structures (Qualls et al. 2012). Targeted control efforts in structures that create a favorable ecological niche for the establishment of a stable life cycle of important L. infantum, sand fly vectors should be evaluated in light of our findings.
Highlights.
Investigated the impact of irrigation on the distribution of leishmaniasis vectors
Remote sensing approaches were used to predict the density of sand fly vectors
The density of P. perfiliewi is predicted to be high in arid regions of Tunisia
P. perfiliewi is associated with the establishment of irrigation
Acknowledgments
Financial Support:
The research was supported by the by Pasteur Institute of Tunis, Tunisia and the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM09334501. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- World Health Organization (WHO) Urbanization: An increasing risk factor for Leishmaniasis. Wkly. Epidemiol. Record. 2002;77:365–372. [PubMed] [Google Scholar]
- World Health Organization (WHO) Report of the Consultative Meeting on Cutaneous Leishmaniasis. 2008 www.who.int/leishmaniasis/resources/Cutaneous_leish_cm_2008.pdf)
- Abonnenc E. Les phlébotomes de la région éthiopienne (Diptera, Psychodidae) Mémoires de l’ORSTOM. 1972;55:289. [Google Scholar]
- Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M the WHO leishmaniasis control team. Leishmaniasis worldwide and global estimates of its incidence. Plose one. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. Chronique du kala azar. Arch. Inst. Pasteur Tunis. 1938;27:97–104. [Google Scholar]
- Aoun K, Bouratbine A, Harrat Z, Guizani I, Mokni M, Bel Hadg Ali S, Ben Osman A, Belkaid M, Dellagi K, Ben Ismail R. Données épidémiologiques et parasitologiques concernant la leishmaniose cutanée sporadique du nord Tunisien. Bull Soc. Pathol. Exot. 2000;93:101–103. [PubMed] [Google Scholar]
- Ayadi A, Ben Ismail R, Ben Rachid MS. Extension de l’aire de transmission du Kala Azar à Leishmania infantum (Nicolle 1908) vers le centre et le sud de la Tunisie. Arch. Inst. Pasteur Tunis. 1991;68:269–273. [PubMed] [Google Scholar]
- Barhoumi W, Chelbi I, Zhioua E. Effects of the development of irrigation systems in the arid areas on the establishment of Phlebotomus (Larroussius) perfiliewi Parrot, 1939. Bull Soc. Pathol. Exot. 2012;105:403–405. doi: 10.1007/s13149-012-0261-x. [DOI] [PubMed] [Google Scholar]
- Ben Ismail R. Incrimination de Phlebotomus perniciosus comme vecteur de Leishmania infantum. Arch. Inst. Pasteur. Tunis. 1993;70:91–110. [Google Scholar]
- Ben Ismail R, Gramiccia M, Gradoni L, Helal H, Ben Rachid MS. Isolation of Leishmania major from Phlebotomus papatasi in Tunisia. Trans. Roy. Soc. Trop. Med. Hyg. 1987;81:749. doi: 10.1016/0035-9203(87)90018-6. [DOI] [PubMed] [Google Scholar]
- Ben Rachid MS, Hamza B, Tabbane C, Gharbi R, Jedidi H, Ben Said M. Etat actuel des leishmanioses en Tunisie. Ann. Soc. Belg. Méd. Trop. 1983;63:29–40. [PubMed] [Google Scholar]
- Ben Said M, Jaiem A, Smoorenburg M, Semiao-Santos SJ, Ben Rachid MS, El Harith A. La leishmaniose canine dans le région d’Enfidha (Tunisie Centrale) Bull Soc. Pathol. Exot. 1992;85:159–163. [PubMed] [Google Scholar]
- Ben Said M, Guerbouj S, Saghrouni F, Fathallah-Mili A, Guizani I. Occurrence of Leishmania infantum cutaneous leishmaniasis in central Tunisia. Trans. Roy. Soc. Trop. Med. Hyg. 2006;100:521–526. doi: 10.1016/j.trstmh.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Ben Salah A, Ben Ismail R, Amri F, Chlif S, Ben Rzig F, Karrat H, Hadhri H, Hassouna M, Dellagi K. Investigation of the spread of human visceral leishmaniasis in central Tunisia. Trans. Roy. Soc. Trop. Med. Hyg. 2000;94:382–386. doi: 10.1016/s0035-9203(00)90112-3. [DOI] [PubMed] [Google Scholar]
- Ben-Ahmed KB, Aoun K, Jeddi F, Ghrab J, El-Aroui MA, Bouratbine A. Visceral leishmaniasis in Tunisia: spatial distribution and association with climatic factors. Am. J. Trop. Med. Hyg. 2009;8:40–45. [PubMed] [Google Scholar]
- Benabdennbi I, Pesson B, Cadi-Soussi M, et al. Morphological and isoenzymatic differentiation to sympatric populations of Phlebotomus perniciosus and Phlebotomus longicuspis (Diptera: Psychodidae) in northern Morocco. J. Med. Entomol. 1999;36:116–120. doi: 10.1093/jmedent/36.1.116. [DOI] [PubMed] [Google Scholar]
- Berdjane-Brouk Z, Charrel RN, Hamrioui B, et al. First detection of Leishmania infantum DNA in Phlebotomus longicuspis Nitzulescu, 1930 from visceral leishmaniasis endemic focus in Algeria. Parasit. Res. 2012;111:419–422. doi: 10.1007/s00436-012-2858-1. [DOI] [PubMed] [Google Scholar]
- Besbes A, Pousse H, Ben Said M, Kharrat H, Chenimi L. Leishmanioses viscérales infantiles du centre Tunisien (221 cas) Méd. Mal. Infect. 1994;24:628–634. [Google Scholar]
- Biocca E, Constantine R. I pozzi come possibilli focolai larvali di flebotomi nell’Isola di Zante. Annali dell’ Instuto Superiore di Sanita. 1986;22:59–60. [Google Scholar]
- Boudabous R, Amor S, Khayeck F, Marzouk M, Bdira S, Mezhoud H, Azaiez R, Sfar M, Babba H. The Phlebotomine fauna (Diptera: Culicidae) of the eastern coast of Tunisia. J. Med. Entomol. 2009;46:1–8. doi: 10.1603/033.046.0101. [DOI] [PubMed] [Google Scholar]
- Bouratbine A, Aoun K, Chahed MK, Ben Ismail R. Données épidémiologiques sur la leishmaniose viscerale infantile en Tunisie en 1993. Méd. Mal. Infect. 1998;28:446–447. [Google Scholar]
- Bouratbine A, Aoun K, Gharbi M, Houas N, Zaroui J, Harrat Z, Baba H, Darghouth MA. Données épidémiologiques, cliniques et parasitologiques sur la leishmaniose générale canine en Tunisie. Bull Soc. Pathol. Exot. 2005;98:359–362. [PubMed] [Google Scholar]
- Chadli A, Ben Rachid MS, Fhaïel A. Chronique des leishmanioses en Tunisie. Arch. Inst. Pasteur Tunis. 1968;45:1–14. [Google Scholar]
- Chaniotis B, Tselentis Y. Water wells as habitat of sand fly (Diptera: Culicidae) vectors of visceral leishmaniasis in Greece. 1996;33:269–270. doi: 10.1093/jmedent/33.2.269. [DOI] [PubMed] [Google Scholar]
- Chargi N, Haouas N, Slama D, Gorcii M, Jaouadi K, Essabbah-Aguir N, Mezhoud H, Babba H. Transmission of visceral leishmaniasis in a previously non-endemic region of Tunisia: Detection of Leishmania DNA in Phlebotomus perniciosus. J. Vec. Ecol. 2013;38:1–5. doi: 10.1111/j.1948-7134.2013.12000.x. [DOI] [PubMed] [Google Scholar]
- Chargui N, Houas N, Slama D, Gorcii M, Jaouadi K, Essabah-Aguir N, Mezhoud H, Baba H. Transmission of visceral leishmaniasis in a previously non-endemic region of Tunisia: Detection of Leishmmania DNA in Phlebotomus perniciosus. J. Vect. Ecol. 2012;38:1–5. doi: 10.1111/j.1948-7134.2013.12000.x. [DOI] [PubMed] [Google Scholar]
- Chelbi I, Derbali M, AL-Ahmadi Z, Zaafouri B, El Fahem A, Zhioua E. Phenology of Phlebotomus papatasi (Diptera: Psychodidae) relative to the seasonal prevalence of zoonotic cutaneous leishmaniasis in Central Tunisia. J. Med. Entomol. 2007;44:385–388. doi: 10.1603/0022-2585(2007)44[385:poppdp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Chelbi I, Kaabi B, Bejaoui M, Derbali M, Zhioua E. Spatial correlation between Phlebotomus papatasi Scopoli (Diptera: Psychodidae) and incidence of zoonotic cutaneous leishmaniasis in Tunisia. J. Med. Entomol. 2009;46:400–402. doi: 10.1603/033.046.0229. [DOI] [PubMed] [Google Scholar]
- Croset H, Rioux JA, Master M, Bayar N. Les phlébotomes de la Tunisie (Diptera, Phlebotominae). Mise au point systématique, chorologique et éthologique. Ann. Parasitol. Hum. Comp. 1978;53:711–749. [PubMed] [Google Scholar]
- Cross ER, Newcomb WW, Tucker CJ. Use of weather data and remote sensing to predict the geographic and seasonal distribution of Phlebotomus papatasi in Southwest Asia. Am. J. Trop. Med. Hyg. 1996;54:530–536. doi: 10.4269/ajtmh.1996.54.530. [DOI] [PubMed] [Google Scholar]
- Thomson MC, Elnaiem DA, Anshford RW, Connor SJ. Towards a kala azar risk map for Sudan: mapping the potential distribution of Phlebotomus orientalis using digital data of environmental variables. Trop. Med. Int. Health. 1999;4:105–113. doi: 10.1046/j.1365-3156.1999.00368.x. [DOI] [PubMed] [Google Scholar]
- Dancesco P, Dedet JP, Ben Osman F, Chadli A. Les phlébotomes capturés dans des foyers de leishmaniose canine à Tunis. Rôle probable de Phlebotomus perniciosus et Phlebotomus perfiliewi dans la transmission. Arch. Inst. Pasteur Tunis. 1970;52:65–88. [Google Scholar]
- Deureure J, Velez ID, Partlong F. La leishmaniose viscérale autochtone au Maroc méridional. Présence de Leishmania infantum MON-1 chez le chien en zone présaharienne. Leishmania Taxonomie et Phylogenèse. Applications écoépidémiologiques International Colloquium CNRS/INSERM; IMEEE; July 1984; Montpellier, France. 1986. pp. 421–425. [Google Scholar]
- Diwani F, Ben Alay Bouafif N, Bettaib J, Louzir H, Jedidi S, Ftaiti A, Zaatour A, Jomma I, Dellagi K, Ben Ismail R, Ben Salah A. Dogs Leishmania infantum infection from endemic region of the North of Tunisia: A prospective study. Arch. Inst. Pasteur Tunis. 2008;85:55–61. [PubMed] [Google Scholar]
- DSSB. Direction Nationale de Soin de Santé de Base. Ministry of Health of Tunisia. Bull. Epidémiol. 2011 [Google Scholar]
- DSSB. Direction Nationale de Soin de Santé de Base. Ministry of Health of Tunisia. Bull. Epidémiol. 2012 [Google Scholar]
- Gálvez R, Descalzo MA, Guerrero I, Miró G, Molina R. Mapping the current distribution and predicted spread of the leishmaniasis sand fly vector in the Madrid Region (Spain) based on environmental variables and expected climate change. Vector-borne Zoonot. Dis. 2010;11:799–806. doi: 10.1089/vbz.2010.0109. [DOI] [PubMed] [Google Scholar]
- Ghrab J, Rhim A, Bhach-Hamba D, Chahed MK, Aoun K, Nouira S, Bouratbine A. Phlebotominae (Diptera: Psychodidae) of human leishmaniasis sites in Tunisia. Parasite. 2006;13:23–33. doi: 10.1051/parasite/2006131023. [DOI] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. [Google Scholar]
- Huete A, Didan K, Miura T, Rodriguez EP, Gao X, Ferreira LG. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002;83:195–213. [Google Scholar]
- Izri M, Belazzoug S. Phlebotomus (Larroussius) perfiliewi naturally infected with dermatropic Leishmania infantum at Tenes, Algeria. Tran Royal Soc Trop Med Hyg. 1993;87:399. doi: 10.1016/0035-9203(93)90011-e. [DOI] [PubMed] [Google Scholar]
- Keddous A. Etude d’un réservoir canin. M. D. Thesis. Medical School of Tunis; 1988. Enquête éco-épidémiologique sur le kala azar dans le gouvernorat de Sidi Bouzid; p. 152. [Google Scholar]
- Latiri K, Lhomme JP, Annabi M, Setter TL. Wheat production in Tunisia: Progress, inter-annual variability and relation to rainfall. European J. Agron. 2010;33:33–42. [Google Scholar]
- Maroli M, Gramiccia M, Gradoni L. Natural infections of sand fly Phlebotomus perfiliewi with Leishmania infantum in a cutaneous leishmaniasis focus of the Abruzzi region, Italy. Trans R Soc Trop Med Hyg. 1987;81:596–598. doi: 10.1016/0035-9203(87)90420-2. [DOI] [PubMed] [Google Scholar]
- Maroli M, Gramiccia M, Gradoni L, Troiani M, Ascione R. Natural infection of Phlebotomus perniciosus with an enzymatic variant of Leishmania infantum in the Campania region of Italy. Acta Trop. 1994;57:333–335. doi: 10.1016/0001-706x(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Müller GC, Schlein Y. Different methods of using attractive sugar baits (ATSB) for the control of Phlebotomus papatasi. J. Vector Ecol. 2011;36:S64–S70. doi: 10.1111/j.1948-7134.2011.00113.x. [DOI] [PubMed] [Google Scholar]
- Qualls WA, Xue RD, Revay EE, Allan SA, Müller GC. Implications for operational control of adult mosquito production in cisterns and wells in St. Augustine, FL using attractive sugar baits. Acta Trop. 2012;122:284–290. doi: 10.1016/j.actatropica.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Rioux JA, Lanotte G, Petter F, Deureure J, Akalay O, Pratlong F, Velez ID, Fikri NB, Maazoun R, Denial M, Jarry DM, et al. Les leishmanioses cutanées du bassin Méditerranéen occidental, de l’identification enzymatique à l’analyse éco épidémiologique. L’expemple de trois «foyers» tunisien, marocain et français. Leishmania Taxonomie et Phylogénèse. Applications éco-épidémiologiques. International Colloquium CNRS/INSERM; IMEEE; Juillet, 1984; Montpellier. 1986. pp. 471–478. [Google Scholar]
- Rioux JA, Akalay O, Perieres J, Deureure J, Mahjour J, Le Houerou HN, Leger N, Desjeux P, Gallego M, et al. L’évaluation éco-épidémiologique du «risque leishmanien au Sahara atlantique marocain. Intérêt heuristique de la relation phlébotomes - bioclimats». Ecologia Mediter. 1997;23:73–92. [Google Scholar]
- Warburg A, Faiman R. Research priorities for the control of phlebotomine sand flies. J. Vector Ecol. 2011;36:S10–S16. doi: 10.1111/j.1948-7134.2011.00107.x. [DOI] [PubMed] [Google Scholar]
- Zhioua E, Kaabi B, Chelbi I. Entomological investigations following the spread of visceral leishmaniasis in Tunisia. J. Vector Ecol. 2007;32:371–374. doi: 10.3376/1081-1710(2007)32[371:eiftso]2.0.co;2. [DOI] [PubMed] [Google Scholar]