Abstract
Although a number of tissue adhesives and sealants for surgical use are currently available, attaining a useful balance in high strength, high compliance, and low swelling has proven difficult. Recent studies have demonstrated that a 4-arm poly(propylene oxide)-poly(ethylene oxide) (PPO-PEO) block copolymer, Tetronic, can be chemically modified to form a hydrogel tissue adhesive21–23. Building on the success of these studies, the present study explored bi-functionalization of Tetronic with acrylates for chemical crosslinking of the hydrogel and N-hydroxysuccinimide (NHS) for reaction with tissue amines. The adhesive bond strengths of various uni- and bi-functional Tetronic blends (T1107 ACR: T1107 ACR/NHS) determined by lap shear testing ranged between 8 and 74 kPa, with the 75:25 (T1107 ACR: T1107 ACR/NHS) blend displaying the highest value. These results indicated that addition of NHS led to improvement of tissue bond strength over acrylation alone Furthermore, ex vivo pressure tests using the rat bladder demonstrated that the bi-functional Tetronic adhesive exhibited high compliance and maintained pressures under hundreds of filling and emptying cycles. Together, the results of the present study provided evidence that the bi-functional Tetronic adhesive with a proper blend ratio may be used to achieve an accurate balance in bulk and tissue bond strengths, as well as the compliance and durability for soft tissue such as the bladder.
Keywords: Poloxamine, Tissue Adhesive, Hydrogel, Mechanical Characterization, Functionalization
Introduction
With laparoscopic and robotic surgical techniques advancing, the need for an injectable surgical adhesive is growing1–3. To be effective, surgical adhesives for internal organs require bulk strength and compliance to avoid rips and tears, and adhesive strength to avoid leakage at the application site, while not hindering the natural healing process4. Although a number of tissue adhesives and sealants approved by the FDA for surgical use are currently available, attaining a useful balance in all of these qualities has proven difficult, particularly when considering applications involving highly expandable tissue, such as bladder and lung5. For example, biologically-derived adhesives, such as fibrin gels or bovine serum albumin with aldehyde crosslinker that are marketed by trade names such as Tisseel® and BioGlue®, do not actually eliminate the need for sutures due to their relatively low mechanical strengths6–9. In addition, these materials involve risk of possible viral transmission and hypersensitive reactions10. Due to these shortcomings of biologically-derived adhesives, developmental focus of a synthetic adhesive or sealant such as cyanoacrylate and hydrogel has become more prevalent. Although cyanoacrylates exhibit much greater mechanical strength than biologically-derived adhesives and are approved by FDA for specific internal applications11, they are considered unsuitable for internal, expandable organs due to high stiffness and evidence of cytotoxicity12–14. It has been shown that poly(ethylene glycol) (PEG) based adhesives, traded under Coseal® (Baxter) and Duraseal™ (Confluent Surgical), have satisfactory mechanical strength for some applications, for example, sealing lung air15, vascular, and dural leaks16. However, these hydrogels tend to swell following application in vivo, leading to decreases in mechanical strength over time and potential compression of surrounding organ or nerve17–19.
Because of the limitations with existing products, different polymers such as Tetronic (BASF), a 4-arm poly(propylene oxide)-poly(ethylene oxide) (PPO-PEO) block copolymer, are being characterized as a potential tissue adhesive20–23. Solutions of Tetronic T1107 can support reverse thermal gelation at physiological temperatures, which can be combined with covalent crosslinking to achieve a “tandem gelation” process24 making it ideal for use as a tissue adhesive. Cho and co-workers used the combination of non-covalent and covalent crosslinking with various blends of two acrylated Tetronics (T904 and T1107) to achieve tunable adhesive properties21. The authors suggested that the Tetronic adhesive binds to tissues via mechanical interdigitation to the surface microstructure and through covalent crosslinking of the terminal acrylates to free thiols present on the tissue ECM proteins21. More recently, Barrett et al. demonstrated that the addition of a catechol, specifically 3,4-dihydroxyphenyl-L-alanine (DOPA), an amino acid found in mussel adhesive proteins, enhances the tissue bond strength of Tetronic-based tissue adhesive22. DOPA-modified Tetronics were crosslinked by oxidizing DOPA to its reactive o-quinone form, which is capable of covalent coupling with catechols, amines, thiols, imidazoles, etc22. This approach takes advantage of the catechol’s ability to crosslink with other catechols for cohesive strength and the tissue surface for adhesive strength22. Although this study demonstrated improved adhesive bond strength over Cho et al, DOPA’s practical applications have been severely limited by its uneconomical extraction process and unsuccessful large scale production25.
To build on the success of these previous studies, and taking advantage of the 4-arm structure of Tetronic, the present study explored bi-functionalization of the polymer with acrylates for chemical crosslinking of the hydrogel for bulk strength and N-hydroxysuccinimide (NHS) for reaction with tissue amines to further improve the adhesive bond strength. The material and mechanical behaviors for various formulations with or without NHS were characterized to assess the adhesive’s applicability for the bladder and other expandable organs. Specifically, gelation and swelling behaviors were examined to confirm that the NHS modification did not compromise Tetronic’s tandem gelation properties. Lap shear testing was performed to quantify the bond strength to tissue. An ex vivo testing method using a custom pressure device was developed to determine the sealing capability of the Tetronic adhesive under hydrated conditions and to further analyze the compliance and durability of the adhesive.
Materials and Methods
Materials
Tetronics® T1107 (T1107, MW: 15 kDa, HLB: 18–23) was donated by BASF corporation (USA). Acryloyl chloride, Celite fine 500, 4-dimethylaminopyridine (DMAP), succinic anhydride, tetrahydrofuran (THF), N-hydroxysuccinimide (NHS), N, N-dicyclohexylcarbodiimide (DCC), and chloroform were purchased from Sigma-Aldrich (St. Louis, MO, USA). Toluene (HPLC grade), ethyl ether (anhydrous, BHT stabilized), and anhydrous sodium sulfate were purchased from Fisher Scientific (NJ, USA). Dichloromethane (DCM) (HPLC grade), triethylamine (TEA), ditiothreitol (DTT), sodium bicarbonate, calcium hydride and CDCl3 were obtained from Acros Organics (NJ, USA). DCM was dried with calcium hydride and stored over molecular sieves (Grade 514, Type 4A). All other chemicals were used as received.
Preparation of acrylated T1107 (T1107 ACR)
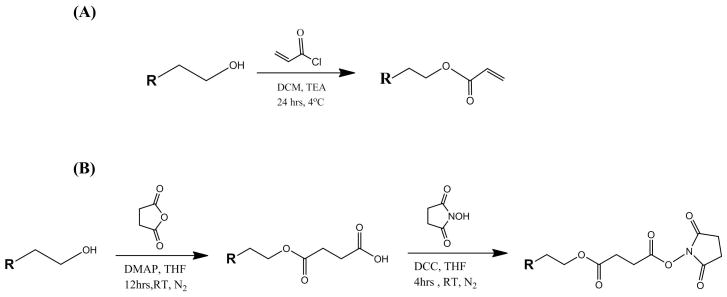
T1107 ACR was prepared by reacting raw T1107 with acryloyl chloride using published methods (Figure 1A)21. Briefly, 30g (2 mmol) of T1107 was dehydrated by azeotropic distillation with toluene for 2 hrs and dried T1107 was dissolved in 270 mL of dried DCM. Once the T1107 was completely dissolved and TEA (8 mmol) was added on an ice bath, acryloyl chloride (16 mmol) in 30 mL of dried DCM was added dropwise over 2 hrs. After the ice bath was removed, the reaction was allowed to continue at 4°C for 24 hrs. The reactant was filtered through Celite to remove TEA-HCl salt then a rotary evaporator was used to reduce dried DCM to one-tenth of its initial volume. The residue was precipitated in 500 mL of cold ether, recovered by filtration and dried under vacuum overnight. The product was dissolved in 300 mL of DCM for pH neutralization and washed with 10% w/v sodium bicarbonate. This was followed by water washes to remove the sodium bicarbonate and then water was removed with anhydrous sodium sulfate. The solution was concentrated using a rotary evaporator to remove DCM and precipitated with ethyl ether (−20°C). After washing three times with cold ethyl ether, the final product was recovered by filtration, dried for 48 hrs in a vacuum desiccator and stored at 4°C.
Figure 1.
Schematic of (A) acryation and (B) N-hydroxysuccinimide (NHS) reactions.
Preparation of bi-functional T1107 (T1107 ACR/NHS)
T1107 ACR/NHS was prepared by partial (2 of 4 arms) acrylation of 30g of T1107 (2 mmol) with reduced amounts of TEA (4 mmol) and acryloyl chloride (8 mmol) and further modified using a coupling reaction to replace the unreacted terminal hydroxyl groups with NHS (Figure 1B). Briefly, 10 g (1 mmol) of partially acrylated T1107, DMAP (0.3 mmol), and succinic anhydride (3.2 mmol) were dissolved in 250 mL of THF and reacted under nitrogen at room temperature for 12 hrs. After the reaction was complete, concentrated residue was precipitated in ethyl ether (−20°C), and washed three times using cold ethyl ether and chloroform. The intermediate product, T1107 ACR/COOH (1 mmol), NHS (2 mmol), and DCC (2.5 mmol) were dissolved in 250 mL of THF and reacted under nitrogen at room temperature for 4 hrs. After washing three times with cold ethyl ether and chloroform, the final product (T1107 ACR/NHS) was recovered by centrifugation, dried for 48 hrs in a vacuum desiccator and stored at 4°C.
Material analysis
Thermal gelation
Thermal gelation was evaluated by tube inversion testing, where 2 mL macromer solutions (30% w/v in 1X PBS) in 5 mL vials were placed in a temperature controlled water bath. Temperature of the water bath was increased 1°C every 5 min with samples being removed from bath and inverted at each time point. The time when the solution no longer flowed inside the tube was recorded as the gelation point.
Swelling behavior
Hydrogel solutions were prepared as described above and 100 μL samples were crosslinked with DTT between glass coverslips separated by 1 mm Teflon spacers. Hydrogel discs cured overnight at 37°C in humidified atmosphere and initial wet weight (Wwi) was measured. Each disc was lyophilized (FreeZone 4.5 Benchtop; Labconco Corp.) for 3 d, weighed (Wd1), and allowed to swell in PBS (pH = 7.4) at 37°C for 24 h. Excess buffer solution was blotted off then weighed (Ww1). Samples were lyophilized and weighed (Wd2). The volumetric swelling ratio (Q) was calculated as:
where ρp is the polymer density (1.04 g/ml), ρs is the solvent density (1 g/ml for PBS). The Equilibrium water content (EWC) was calculated as:
Mechanical analysis
T1107 ACR and T1107 ACR/NHS were dissolved in phosphate-buffered saline (PBS) on a shaker plate at 4°C for 24 hrs and various blends (100/0, 75/25, 50/50, 0/100) (T1107 ACR: T1107 ACR/NHS) were prepared at a final polymer concentration of 30% w/v. Tetronic® solutions (pH=7.4) were crosslinked by Michael-type addition reaction with dithiothreitol (DTT) solutions in PBS (1:1 thiol:acrylate molar ratio, pH=7.4).
Lap-Shear Testing
Bond strength of modified Tetronic® hydrogels adhesives was quantified following a protocol adapted from AS™ F2255-05. Briefly, dehydrated collagen sheets (DeWied International Inc., TX) were soaked in PBS for 1 hr and affixed on aluminum fixtures using SurgiSeal® cyanoacrylate adhesive (Adhezion Biomedical, PA), which was cured at room temperature for 24 hrs. The tissue samples were cut to size (4 cm X 1 cm) and rehydrated in 37°C water bath for 1 hr. Hydrogel mixture (100μL) was applied on the tissue surface and a second strip was placed on top to obtain a 1 cm X 1 cm contact area. The bonded tissue samples were wrapped in PBS soaked gauze and allowed to cure at 37°C for 1 hr. All samples were subjected to uniaxial strain at constant crosshead speed of 10mm min−1 until failure on MTS Synergie 100 (Eden Prairie, MN). The adhesive bond strength was calculated as maximum load divided by bonded area.
Pressure Testing
Maximum pressures held by punctured rat bladders sealed with modified Tetronic® adhesives were evaluated using a custom ex vivo device. Prior to testing, a small, approximately 2 mm puncture was made on the dome of the bladder (Pel-Freez Biologicals Inc., AR) using an 18 gage needle. Modified Tetronic®/DTT (25 μl, pH=7.4) was applied to cover the hole and allowed to cure for 1 h in 37°C hydrated condition. Using a Harvard Apparatus 11 Plus Pump (Harvard Apparatus, MA) and a 60 ml syringe, the specimen was subjected to either single loading (at a flow rate of 0.8 ml/min until failure) or cyclic loading (0–40 cmH2O at a flow rate of 0.5 ml/min for loading, and 2 ml/min for unloading, 5 hr duration or until failure). The device was controlled and pressure data were recorded using PC with a custom LabView code (National Instruments). Results were compared to a non-punctured rat bladder.
Statistical analysis
Quantitative data for volumetric swelling ratio, adhesive bond strength, pressure failure and cyclic pressures were compared by analysis of one-way analysis of variance (ANOVA) with Tukey’s Honestly Significant Difference (HSD) for post hoc testing, with p values of <0.05 considered statistically significant.
Results
1H-NMR (CDCl3) Characterization
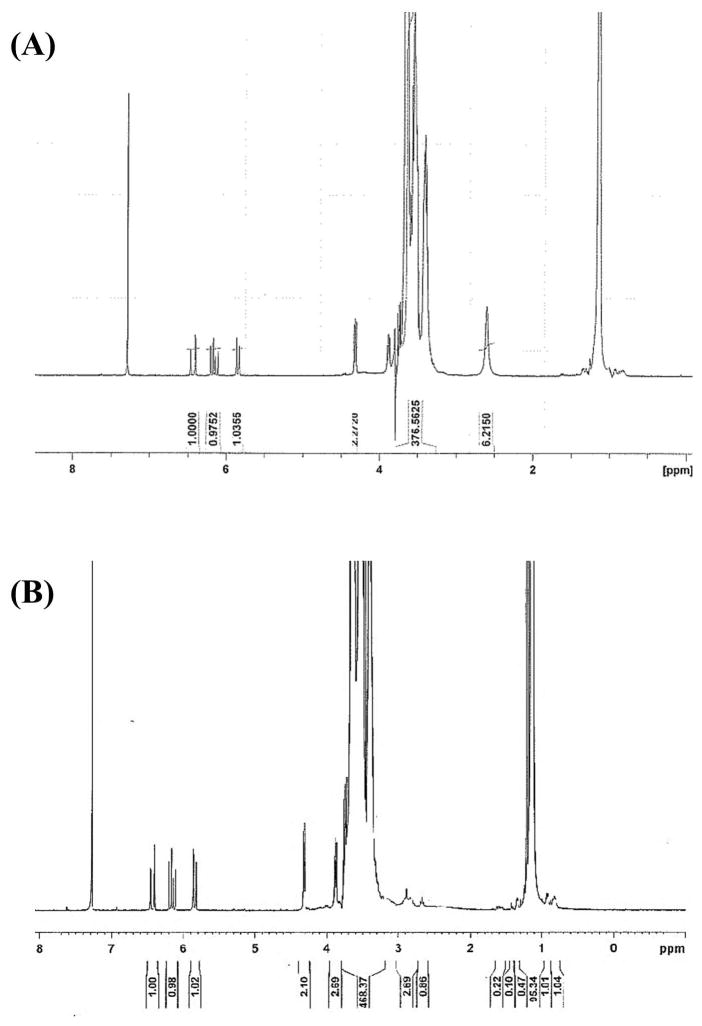
T1107 ACR: δ = 1.1 (m, PPO CH3), 3.4 (m, PPO CH), 3.54 (m, PPO CH2), 3.65 (m, PEO CH2), 4.32 (t, -CCH2OC(=O)-), 5.8 and 6.4 (d, 2H, acrylic –CH2), 6.15 (q, 1H, acrylic –CH) ppm. T1107 ACR/NHS: δ = 1.1 (m, PPO CH3), 2.85 (m, NHS CH2), 3.4 (m, PPO CH), 3.54 (m, PPO CH2), 3.65 (m, PEO CH2), 4.32 (t, -CCH2OC(=O)-), 5.8 and 6.4 (d, 2H, acrylic –CH2), 6.15 (q, 1H, acrylic –CH) ppm. The percent acrylation efficiency of T1107 ACR (T1107 ACR) and T1107 ACR NHS (T1107 ACR/NHS), calculated based on the ratio of integrals of the PEG backbone (δ = 3.5) and acrylate peaks (δ = 6.15–6.4) on NMR spectra (Figure 2), were consistently 80–95% and 60–65%, respectively in all samples used. The ratio of acrylate to activated PEG terminal methylene (δ = 4.32) peaks closely approximated the 3:2 theoretical value, confirming the efficient removal of unreacted acryloyl chloride. NHS efficiency, calculated based on the ratio of integrals of the acrylate peaks (δ = 6.15–6.4) and NHS peak (δ = 2.85), was 20–30%.
Figure 2.
Representative 1H-NMR spectra of (A) T1107 ACR and (B) T1107 ACR/NHS.
Material analysis
Thermal gelation
Thermal gelation tests were performed to verify that the modification of NHS did not change the thermosensitive properties of Tetronic. Sol-gel transition temperatures were determined by tube inversion tests for T1107 ACR (T1107 ACR) and T1107 ACR/NHS (T1107 ACR/NHS) blends. The 100:0 and 75:25 (T1107 ACR: T1107 ACR/NHS) gelled at similar temperatures, 20°C and 21°C, from the onset of temperature sweep starting at 1°C (1°C every 5 minutes), respectively (Table 1).
Table 1.
Material analysis data of thermal gelation, swelling behavior and equilibrium water content for various modified Tetronic blends (T1107 ACR: T1107 ACR/NHS).
| Tetronic Blend | Thermal Gelation | Thermal and Michael-type Addition Crosslinking | |
|---|---|---|---|
| Temperature (°C) | Volumetric Swelling Ratio | Equilibrium Water Content (%) | |
| 100:0 | 20 | 3.2 ± 0.79 | 66.4 ± 6.96 |
| 75:25 | 21 | 2.3 ± 0.48 | 52.96 ± 8.69 |
| T1107* | 22 | 5.5 ± 0.2 | NA |
| PEG* | ND | 10.8 ± 1.3 | NA |
Previously report data21. ND = not dectectable.
Swelling behavior
Thermosensitive swelling properties were investigated by quantifying the volumetric swelling ratios and equilibrium water content (Table 1). After allowing the hydrogel to swell at 37°C for 24 h, the weight of the hydrogel was compared to the initial hydrogel weight to determine the volumetric swelling ratio (Q). Tetronic 75:25 (T1107 ACR: T1107 ACR/NHS) blends showed a slightly lower swelling ratio and equilibrium water content compared to 100:0 (T1107 ACR: T1107 ACR/NHS) blend.
Mechanical analysis
Lap Shear Testing
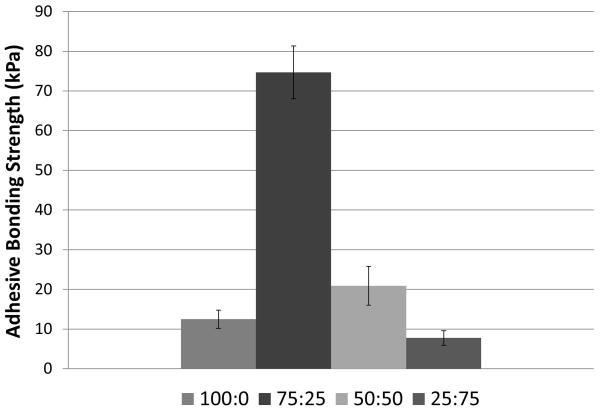
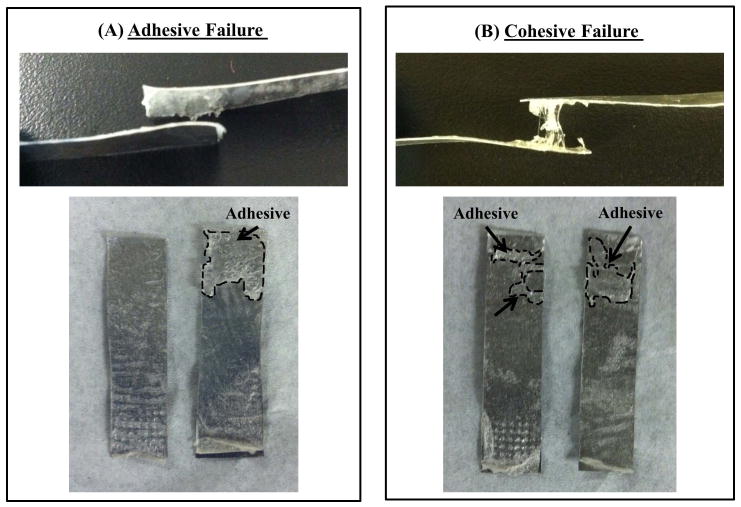
The adhesive bond strengths of various Tetronic blends, 100:0, 75:25, 50:50, 25:75 (T1107 ACR: T1107 ACR/NHS), were determined by normalizing the failure forces by the contact area based on lap shear testing of decellularized collagen tissue. The calculated strength values ranged between 8 and 74 kPa, with the 75:25 (T1107 ACR: T1107 ACR/NHS) blend displaying the highest average bond strength (Figure 3). Moreover, it should be noted that different Tetronic blends exhibited different modes of failure seen as adhesive failure and cohesive failure (Figure 4). While the blends with lower T1107 ACR/NHS content (100:0 and 75:25) mostly failed due to detachment of the adhesive from the tissue (adhesive failure), the blends with higher T1107 ACR/NHS content (50:50 and 0:100) mostly failed due to tearing of the adhesive (cohesive failure) (Table 2).
Figure 3.
Adhesive bond strengths of various Tetronic blends (T1107 ACR: T1107 ACR/NHS). Data are mean +/− standard deviation, analyzed using ANOVA followed by Tukey HSD test, n=6 per group. All are significantly (p<0.05) different from each other, except the comparison between 100:0 and 25:75.
Figure 4.
Images of specimens after lap shear testing. While the blends with lower T1107 ACR/NHS content (100:0 and 75:25) mainly failed due to detachment of the adhesive from the tissue (adhesive failure, A), the blends with higher T1107 ACR/NHS content (50:50 and 25:75) mainly failed due to tearing of the adhesive (cohesive failure, B).
Table 2.
Modes of failure for various modified Tetronic blends (T1107 ACR: T1107 ACR/NHS) during Lap Shear testing, n=6.
| Tetronic Blend | Adhesive Failure | Cohesive Failure |
|---|---|---|
| 100:0 | 6 | 0 |
| 75:25 | 5 | 1 |
| 50:50 | 2 | 4 |
| 25:75 | 2 | 4 |
Pressure Testing
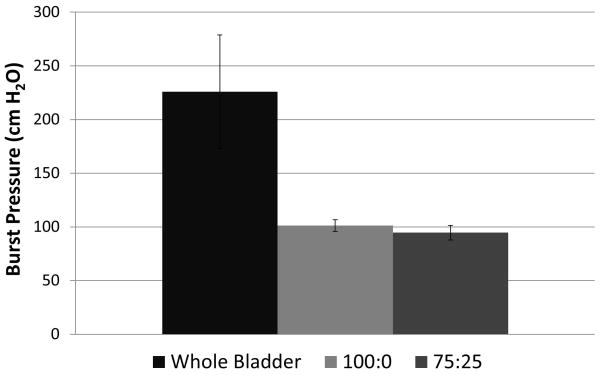
Pressure burst testing demonstrated that bladders sealed with 100:0 and 75:25 (T1107 ACR: T1107 ACR/NHS) blend Tetronic adhesives withstood similar average maximum pressures of 100 ± 5.4 cmH2O and 99 ± 6.8 cmH2O, respectively (Figure 5). However, these results were significantly (p < 0.05) lower compared to a non-punctured rat bladder, which withstood 225 ± 52.9 cmH2O before rupture.
Figure 5.
Maximum pressures for bladder puncture sealed with various Tetronic blends (T1107 ACR: T1107 ACR/NHS). Data are mean +/− standard deviation, analyzed using ANOVA followed by Tukey HSD test, *p<0.05, n=3.
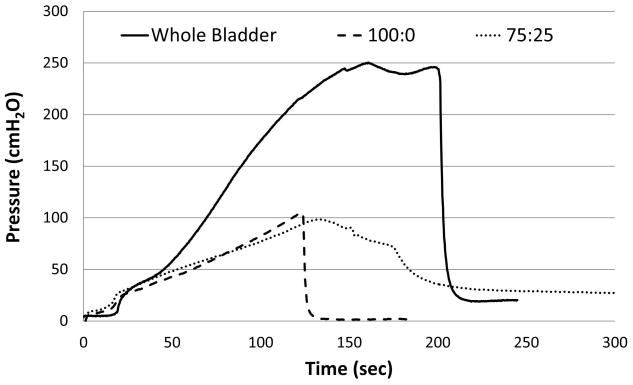
Further, the results of the pressure testing revealed that the pressure profiles during filling of the bladders sealed with the Tetronic adhesive resemble that of the non-punctured control bladder in the physiological pressure (0–50 cmH2O) range, but deviated at higher pressures (Figure 6). Specifically, the pressure-volume plot showed initially a slow rise, which was followed by a steady rise of the pressure. When the adhesive failed, an abrupt pressure loss was observed with the 100:0 (T1107 ACR: T1107 ACR/NHS) blend due to sudden separation of the adhesive from the tissue (Figure 6). In contrast, the bladder sealed with the 75:25 (T1107 ACR: T1107 ACR/NHS) blend exhibited a slow leak (Figure 6), which indicated that the adhesive sheared away from the tissue gradually.
Figure 6.
Pressure-Volume plots for bladder punctures sealed with various Tetronic blends (T1107 ACR: T1107 ACR/NHS). Data are representative curves from three separate experiments.
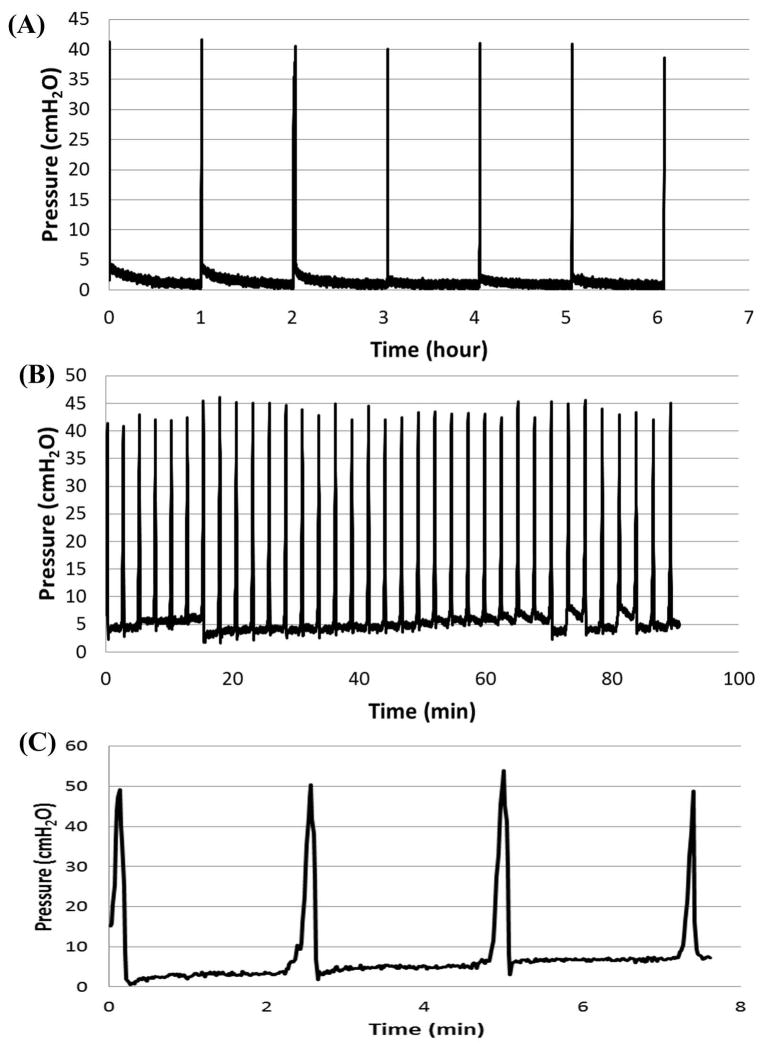
To determine the durability of the adhesive, cyclic pressure tests were performed and bladders sealed with the adhesive were subjected to numerous filling and voiding cycles, with varying time delays between each testing cycle. The results of the cyclic pressure testing provided evidence that Tetronic adhesive with 75:25 (T1107 ACR:T1107 ACR/NHS) blend sealed the puncture for hundreds of cycles of filling and emptying (0–40 cmH2O, 2.5 minute cycles) with over 24 h of testing (Figure 7A), while the 100:0 (T1107 ACR: T1107 ACR/NHS) blend was intact for an average of 4 cycles before the adhesive site failed (Figure 7C). The test results were similar when the time delay between each filling and voiding cycle was increased to 1 hr and run for 24 h (Figure 7B). Moreover, some bladder samples were subjected to a second round of cyclic pressure testing after being stored overnight in hydrated conditions at 37°C, and exhibited similar results in pressure retention under cyclic loading. In some instances, solution leakage occurred not through adhesive failure, but from thinning bladder walls after hours of tissue stretch due to cyclic loading.
Figure 7.
Cyclic pressure profile for punctured rat bladders sealed with modified Tetronic adhesive. Bladders sealed with the 75:25 (T1107 ACR: T1107 ACR/NHS) withstood numerous cylces at 2 min time delay (A) and 1 hr time delay (B) between each cycle while the ones sealed with the 100:0 (T1107 ACR: T1107 ACR/NHS) retained pressure for four cycles with 2 min time delay (C).
Discussion
Although a number of FDA-approved surgical adhesives are on the market, they are not mechanically adequate for internal wound repair and, thus, are often used as an adjunct to sutures at this time4. For this reason, the goal of the present study was to develop a tissue adhesive that exhibits a balance between bulk and tissue bond strength as well as the compliance and durability that meet the demand of the target organ. In pursuit of this goal, the present study synthesized and characterized a bi-functional Tetronic adhesive to determine its effectiveness. More specifically, we modified the terminal hydroxyl groups on the four arms of Tetronic (T1107, MW: 15kDa) by either acrylation of all four arms of the Tetronic (T1107 ACR) or acrylation of two arms of Tetronic and activation of the other two arms with NHS (T1107 ACR/NHS) (Figure 1). The results of the NMR spectroscopy confirmed conversion efficiencies to be constantly 85–94 acrylation percent for T1107 ACR (T1107 ACR) and 63–65 acrylation percent with 20–30 percent NHS modification for T1107 ACR/NHS (T1107 ACR/NHS) (Figure 2).
To verify that the addition of NHS did not alter its thermosensitive gelation and swelling characteristics that have been demonstrated previously21, sol-gel transition temperatures were determined by tube inversion tests for T1107 ACR (T1107 ACR) and T1107 ACR/NHS (T1107 ACR/NHS) blends, where each blend varies the amount of NHS contained within the hydrogel matrix. The specimens with the blend ratios of 100:0 and 75:25 (T1107 ACR: T1107 ACR/NHS) not containing the thiol crosslinker, DTT, gelled below physiological temperature (37°C) resulting in hydrogel gelation at 20°C and 21°C, respectively (Table 1). The tube inversion results were in agreement with previously published results21, and demonstrated that addition of NHS did not affect the thermosensitive gelation properties of T1107. Moreover, swelling tests using modified Tetronic hydrogel crosslinked with DTT at physiological temperature, 37 °C, for 24 h provided evidence that 75:25 (T1107 ACR: T1107 ACR/NHS) blend exhibited similar volumetric swelling ratio to the adhesive that did not contain NHS, 100:0 (T1107 ACR: T1107 ACR/NHS) blend and to previously reported values21. Further, there was no significant difference in equilibrium water content (%) of 100:0 and 75:25(T1107 ACR: T1107 ACR/NHS) blends. Together, the gelation and swelling results prove that the addition of NHS did not compromise the thermosensitive and tandem gelation properties of Tetronic.
The results of lap shear testing revealed that the bond strength of the 75:25 (T1107 ACR: T1107 ACR/NHS) blend formulation, 74 kPa, was greatest among all the blends tested in the present study (Figure 3). Although we realize that differences in lap shear testing conditions (e.g., curing for 1 vs. 24 hrs21 and with or without weight applied22) could influence the outcome, this value was a significant improvement over the bond strengths previously reported for other Tetronic-based adhesives (11–49 kPa)21, 22. More specifically, the 75:25 (T1107 ACR: T1107 ACR/NHS) blend exhibited a six-fold increase in bond strength when compared to the sample with no NHS (100:0), validating that the addition of NHS is beneficial in enhancing the adhesive’s bonding with the tissue. Adhesives with higher T1107 ACR/NHS content (25:75) demonstrated a decrease in bulk strength due to the lower amount of available acrylate groups for internal crosslinking. The increase in the bond strength and decrease in the bulk strengths due to the addition of NHS was further demonstrated by the observation that the different blends exhibit different modes of failure (Figure 4). The blends with lower T1107 ACR/NHS content (100:0 and 75:25) mainly failed due to detachment of the adhesive from the tissue (adhesive failure, Figure 4) indicating that bulk strength due to higher crosslinking density surpassed the bond strength to the tissue (Table 2). In contrast, the blends with higher T1107 ACR/NHS content (50:50 and 25:75) mainly failed due to tearing of the adhesive (cohesive failure, Figure 4) with strong adhesion to the tissues, but lower bulk strength due to lower crosslinking density (Table 2). These results confirmed that the NHS modification is beneficial in increasing adhesive bond strength, although additional adjustments of the balance between T1107 ACR/NHS and T1107 ACR contents might allow further fine tuning of cohesive and adhesive properties as needed.
Lap shear testing is one of the most common methods for quantifying adhesive bond strengths due to its simple geometrical design and highly repeatable test technique26. This testing is executed by applying the adhesive between two strips of tissue overlapping one another and subjecting to uniaxial loading to separate them. However, this loading condition does not completely simulate realistic surgical situations such as anastomosis of tissue segments or sealing of laceration and puncture wounds on hollow organs. For this reason, we developed a bladder inflation test to measure the adhesive’s sealing strength in hydrated conditions using an ex vivo system. By plotting the pressure-volume curves, we determined the compliance of the adhesive. Furthermore, by exposing the bladder to filling and voiding cycles, the durability of the adhesive was determined. Specifically, punctured rat bladders were sealed with modified Tetronic adhesive blends 100:0 and 75:25 (T1107 ACR: T1107 ACR/NHS) and inflated with saline in a saline bath. The results of burst tests demonstrated that both blends withstood pressures up to 100 cmH2O (Figure 5), which surpasses a typical voiding pressure for the human and rat bladder (40 cm H2O27). Although these burst pressures were lower than that of a non-punctured rat bladder, which withstood over 200 cmH2O, the pressure-volume curve of the bladders with adhesive resembled that of the non-punctured bladder (Figure 6). All samples exhibited a similar pattern in the pressure-volume curves characteristic of soft biological tissues: an initial nonlinear, toe region due to straightening of undulated collagen fibrils, followed by a linear region, and finally a region where failure occurred. Neither the intact bladder (control) nor the bladders sealed with the adhesive exhibited a steep rise in pressure during the tissue stretch indicating that the high compliance (low stiffness) of the bladder tissue was not compromised by the adhesive. Furthermore, the analysis of the pressure-volume curves further confirmed that the 75:25 (T1107 ACR: T1107 ACR/NHS) blend sample formed a stronger bond with the tissue. This was demonstrated by the gradual loss of pressure indicating that the 75:25 (T1107 ACR: T1107 ACR/NHS) blend adhesive slowly broken away from the tissue (Figure 6). In contrast, the 100:0 (T1107 ACR: T1107 ACR/NHS) blend displayed by a drastic pressure loss indicating the adhesive broke away from the tissue at once (Figure 6). In addition, to examine the durability of the adhesive following application to the tissue surface, the bladders sealed with Tetronic adhesives were subjected to numerous pressure cycles ranging 0–40 cmH2O. The results provided evidence that the 75:25 (T1107 ACR: T1107 ACR/NHS) adhesive maintained the seal during hundreds of filling and emptying cycles for 24 h (Figure 7A). Similar results were obtained even after the testing was stopped and the sample was stored overnight for additional cyclic pressure tests. In contrast, the seal on the punctured bladder formed by the 100:0 (T1107 ACR: T1107 ACR/NHS) blend was only intact for an average of 4 cycles before the adhesive site failed and started leaking (Figure 7C). Although the 75:25 blend exhibited a significantly higher shear adhesion strength than the 100:0 blend in lap shear tests (Figure 3), burst pressure tests measured similar maximum pressures (Figure 5). However, the difference in the performance of these two blends as tissue adhesives was clearly demonstrated by different modes of failure, sudden loss of pressure (100:0) vs. slow leak (75:25) (Figure 6), and by the significant difference in the numbers of cycles withstood, four (100:0) vs. hundreds (75:25) (Figure 7). Together, these results supported the idea that the NHS aided in enhancing the adhesive’s bonding strength to the tissue surface.
In conclusion, the present study demonstrated that the bi-functionalization of Tetronic adhesive with acrylation and addition of NHS led to improvement of tissue bond strength over acrylation alone. Moreover, high compliance and durability of the product were demonstrated by our ex vivo pressure tests. These results suggest that the bi-functional Tetronic adhesive with a proper blend ratio may be used to achieve an accurate balance in bulk and tissue bond strengths, as well as the compliance and durability for soft tissue such as the bladder. However, further testing, especially in vivo, is needed to demonstrate the efficacy of our bi-functional Tetronic tissue adhesive for various internal organ applications.
Acknowledgments
The authors wish to thank Robert Teague, Department of Electrical and Computer Engineering, Clemson University for assistance in Lab View coding. This study was funded by NIH grant EB008785.
References
- 1.Kaouk JH, Haber GP, Goel RK, Desai MM, Aron M, Rackley RR, Moore C, Gill IS. Single-port laparoscopic surgery in urology: Initial experience. Urology. 2008;71:3–6. doi: 10.1016/j.urology.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 2.Vallancien G, Cathelineau X, Baumert H, Doublet JD, Guillonneau B. Complications of transperitoneal laparoscopic surgery in urology: Review of 1,311 procedures at a single center. J Urol. 2002;168(1):23–26. [PubMed] [Google Scholar]
- 3.Sung GT, Gill IS. Robotic laparoscopic surgery: A comparison of the Da Vinvi and Zeus systems. Urology. 2001;58(6):893–898. doi: 10.1016/s0090-4295(01)01423-6. [DOI] [PubMed] [Google Scholar]
- 4.Reece TB, Maxey TS, Kron IL. A prospectus on tissue adhesives. Am J Surg. 2001;182:40S–44S. doi: 10.1016/s0002-9610(01)00742-5. [DOI] [PubMed] [Google Scholar]
- 5.Mehdizadeh M, Yang J. Design strategies and applications of tissue bioadhesives. Macromol Biosci. 2012;13(3):271–288. doi: 10.1002/mabi.201200332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radosevich M, Goubran HA, Burnouf T. Fibrin sealant: Scientific reationale, production methods, properties, and current clinical use. Vox Sang. 1997;72:133–143. doi: 10.1046/j.1423-0410.1997.7230133.x. [DOI] [PubMed] [Google Scholar]
- 7.Marcovich R, Williams AL, Rubin MA, Wolf JS. Comparison of 2-octyl cyanoacrylate adhesive, fibrin glue, and suturing for wound closure in the porcine urinary tract. Urology. 2001;57:806–810. doi: 10.1016/s0090-4295(00)01075-x. [DOI] [PubMed] [Google Scholar]
- 8.Zilling TL, Jansson O, Walther BS, et al. Sutureless small bowel anastomoses: Experimental study in pigs. Eur J Surg. 1999;165:61–68. doi: 10.1080/110241599750007522. [DOI] [PubMed] [Google Scholar]
- 9.LeMarie SA, Carter SA, Won T, Wang X, Conklin LD, Coselli JS. The threat of adhesive embolization: Bioglue leaks through needle holes in aortic tissue and prosthetic grafts. Ann Thorac Surg. 2005;80:106–111. doi: 10.1016/j.athoracsur.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Spotniz WD, Burks S. Hemostats, sealants, and adhesives: Components of the surgical toolbox. Transfusion. 2008;48:1502–1516. doi: 10.1111/j.1537-2995.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- 11.Chiesa R, Melissano G, Zangrillo A, Coselli JS, editors. Thoraco-abdominal aorta: Surgical and anesthetic management. New York, NY: Springer; 2011. [Google Scholar]
- 12.Leggat PA, Smith DR, Kedjarune U. Surgical applications of cyanoacrylate adhesives: A review of toxicity. Anz J Surg. 2007;77:209–213. doi: 10.1111/j.1445-2197.2007.04020.x. [DOI] [PubMed] [Google Scholar]
- 13.Trott AT. Cyanoacrylate tissue adhesive: An advance in wound care. JAMA. 1997;277:1559–1560. doi: 10.1001/jama.277.19.1559. [DOI] [PubMed] [Google Scholar]
- 14.Ciapetti G, Stea S, Cenni E, Sudanese A, Marraro D, Toni A, Pizzoferrato A. Cytotoxicity testing of cyanoacrylates using direct contact assay on cell cultures. Biomaterials. 1994;15(1):63–67. doi: 10.1016/0142-9612(94)90199-6. [DOI] [PubMed] [Google Scholar]
- 15.Gillinov AM, Lytle BW. A novel synthetic sealant to treat air leaks at cardiac reoperation. J Card Surg. 2001;16:255–257. doi: 10.1111/j.1540-8191.2001.tb00517.x. [DOI] [PubMed] [Google Scholar]
- 16.Boogaarts JD, Grotenhuis JA, Bertels RH, Beems T. Use of novel absorbable hydrogel for augmentation of dural repair: Results of a preliminary clinical study. Neurosurgery. 2005;57:146–151. doi: 10.1227/01.neu.0000164384.05351.59. [DOI] [PubMed] [Google Scholar]
- 17.Blackburn SL, Smyth MD. Hydrogel-induced cervicomedullary compression after posterior fossa decompression for Chiari malformation. J Neurosurg. 2007;106:302–304. doi: 10.3171/ped.2007.106.4.302. [DOI] [PubMed] [Google Scholar]
- 18.Package Insert. Coseal; Baxter: Mar, 2006. [Google Scholar]
- 19.Package Insert. Duraseal; Covidien: 2005. [Google Scholar]
- 20.Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. Eur J Pharm Biopharm. 2008;68(1):34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho E, Lee JS, Webb K. Formulation and characterization of poloamine-based hydrogels as tissue sealants. Acta Biomater. 2012;8:2223–2232. doi: 10.1016/j.actbio.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrett DG, Bushnell GC, Messersmith PB. Mechanically robust, negative-swelling, mussel-inspired tissue adhesives. Adv Healthc Mater. 2012:1–11. doi: 10.1002/adhm.201200316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balakrishnan N. Evaluating mechanical performance of hydrogel-based adhesives for soft tissue applications. Clemson University; 2013. All These Paper 1574:Tiger Prints. [Google Scholar]
- 24.Cellesi F, Tirelli N, Hubbell JA. Materials for cell encapsulation via a new tandem approach combining reverse thermal gelation and covalent crosslinking. Macromol Chem Phys. 2002;203:1466–1472. [Google Scholar]
- 25.Duarte AP, Coelho JF, Bordado JC, Cidade MT, Gil MH. Surgical adhesives: Systematic review of the main types and development forecast. Prog Polym Sci. 2012;37:1031–1050. [Google Scholar]
- 26.Sitterle VB, Sun W, Levenston ME. A modified lap test to more accurately estimate interfacial shear strength for bonded tissues. J Biomech. 2008;41:3260–3264. doi: 10.1016/j.jbiomech.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Asselt EA, Groen J, Van Mastrigt R. A comparative study of voiding in rat and guinea pig: Simultaneous measurement of flow rate and pressure. Amer Physiol Soc. 1995:R98–R103. doi: 10.1152/ajpregu.1995.269.1.R98. [DOI] [PubMed] [Google Scholar]