Abstract
Anaplastic thyroid carcinoma is the least common form of thyroid cancer; however, it accounts for the majority of deaths associated with this family of malignancies. A number of genetically engineered immunocompetent mouse models recapitulating the genetic and histological features of anaplastic thyroid cancer have been very recently generated and represent an invaluable tool to dissect the mechanisms involved in the progression from indolent, well-differentiated tumors to aggressive, undifferentiated carcinomas and to identify novel therapeutic targets. In this review, we focus on the relevant characteristics associated with these models and on what we have learned in terms of anaplastic thyroid cancer biology, genetics, and response to targeted therapy.
Keywords: Thyroid Cancer, Papillary Thyroid Cancer, Vemurafenib, BRAF Inhibitor, Anaplastic Thyroid Carcinoma
Introduction
Anaplastic thyroid cancer (ATC) is one of the most aggressive human neoplasms, with a median survival of less than 6 months after diagnosis [38]. While ATC might constitute only 5 % of all thyroid malignancies, it accounts for the majority of thyroid cancer deaths [10, 40]. As this cancer presents as a rapidly growing tumor mass in the neck area, eradication surgery is one approach to control its progression. However, this approach commonly fails due to invasion into the surrounding tissues [10]. In fact, metastasis is not the main cause of death in ATC, and most patients die from the locally invasive tumor, which compromises airway passages [35]. Although there have been reports of cases successfully treated with multimodal therapeutic approaches, much more research is needed in this area, as ATC is almost invariably refractory to treatment [48, 58].
It is now accepted that ATC often arises from pre-existing, less aggressive, well-differentiated thyroid carcinomas (DTC), namely papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC), through a process of dedifferentiation, where epithelial and thyroid differentiation markers are lost [27, 38]. Histologically, ATC presents as a solid mass composed of large pleomorphic and bizarre giant cells, spindle cells, or squamoid cells, accompanied by areas of necrosis and hemorrhage [38]. The presence of many mitotic figures and atypical mitoses is also observed, which points to the high rate of cell proliferation in these tumors [1, 38].
The rarity of ATC has been for a long time a major obstacle in the study of the mechanistic features responsible for the aggressiveness and lethality of this disease. However, as slowly accumulating patient data converge on a number of signaling pathways that appear to be crucial in the establishment and development of ATC, genetically engineered mouse models based on such alterations have emerged in the past few years with the purpose of offering a physiological tool in the study of this malignancy. These mouse models of ATC are now allowing researchers a closer look at the mechanistic pathways responsible for aggressiveness and drug resistance observed in ATC, and they will thus continue to be invaluable tools (Table 1).
Table 1.
Available mouse models of anaplastic thyroid carcinoma (ATC)
| Model | Cre | Tumor type | ATC penetrance | Cellular features | Metastasis | Preclinical studies |
|---|---|---|---|---|---|---|
| RET/PTC1 +;Trp53 −/− | N/A | PTC/ATC | 60 % at 11–14 weeks. Difficult to determine due to extra-thyroidal tumors | Pleomorphic spindle, polyhedral, or multinucleated giant cells. Mitotic figures | Only 1 case found (lungs) | N/A |
| Kras G12D;Trp53 −/−− | TPO-Cre | PTC/PDTC/ATC | 40 % | Pleomorphic spindle or epithelioid cells | 28 % to the lungs | Inhibition of MEK by GSK1120212 and of anti-apoptotic Bcl2 family members by Obatoclax in allografts from tumor-derived cell lines |
| Thrb PV/PV; Kras G12D | TPO-Cre | FTC/ATC | 63 % between 2 and 5 months | Spindle cell anaplasia | 31 % between 2 and 5 months | N/A |
| Braf CA/+;Trp53 R270H/+ | TPO-CreER | PTC/PDTC/ATC | 50 % PDTC or ATC | Tall cell morphology with focal necrosis. Spindle cells and pleomorphic giant cell pattern | 19 % to the lungs | Inhibition of BRAF by PLX4720 and of MEK by PD0325901 |
| Braf CA/+;Pik3ca Lat/+ | TG-CreER | PTC/ATC | 80 % | Polygonal giant cells and/or spindle cell pattern | Not reported | N/A |
| Pten −/−;Trp53 −/− | TPO-Cre | FTC/ATC | 90 % | Spindle cell morphology, giant, cells, osteoclast-like, multinucleated cells, and areas of osseous metaplasia | 28 % to the lungs Sporadic to the liver | Inhibition of PLK1 by GSK461364A in allografts from tumor-derived cell lines |
| Thrb PV/PV;Pten +/− | N/A | FTC/ATC | 50 % after 7 months 62 % after 21 weeks HFD | Spindle cell anaplasia | 80 % to the lungs | N/A |
Two signaling pathways have been shown to be commonly deregulated in ATC: the PI3K and the MAPK cascades. Differentiated thyroid tumors also show deregulations in these signaling axes; however, additional mutations, such as the mutation or deletion of TRP53, are characteristically found in ATC suggesting a stepwise progression from DTC to ATC [31, 39]. Thus, much effort has been put into investigating the role of these pathways in thyroid anaplastic tumors, as they represent the the driving force behind their establishment and progression and a key to finding targets that will allow for the development of effective therapeutic approaches.
RET/PTC
Although this review focuses on ATC mouse models carrying mutations or deletions affecting the PI3K and MAPK pathways, it is also important to mention that the earliest model to present the characteristic ATC phenotype was generated through thyroid-specific expression of the RET/PTC1 oncogene in combination with a homozygous deletion of Trp53 [30]. These RET/PTC1 +;Trp53 −/− mice developed papillary thyroid tumors, which showed signs of progression to ATC, including solid areas composed of pleomorphic spindle, polyhedral, or multinucleated giant cells. The incidence of ATC was higher in mice with a homozygous deletion of p53 in comparison to heterozygous mice, and this increased with age. At 14–17 weeks, 30 % of all RET/PTC1 +;Trp53 −/− mice presented with ATC features, and a local invasion was frequently observed [30]. While this model might be useful in the study of ATC, some limitations are readily observed, one of them being the fact that all of the RET/PTC1 +; Trp53 −/− mice had to be removed from the study by 17 weeks of age, due to extra-thyroidal tumors caused by the systemic Trp53 deletion. In fact, while it is known that deficiency of Trp53 is not enough to drive tumorigenesis in the thyroid [2], it is sufficient to initiate tumors in other tissues [2, 30]. A second major disadvantage of this model is that while the RET/PTC1 translocation is one of the most common mutations associated with PTC, there is lack of evidence showing that human papillary thyroid tumors with RET/PTC1 can progress to ATC [53, 57].
RAS-MAPK Signaling Pathway
The mitogen-activated protein kinase (MAPK) pathway encompasses different signaling systems that are responsible for cell growth, proliferation, differentiation, migration, and apoptosis. One of these systems is the RAS → RAF → MEK → ERK1/2 cascade, which is deregulated in one third of all human cancers [11, 33]. Additionally, 70 % of thyroid cancers harbor mutations that can activate the MAPK pathway [11, 29, 31]. RAS GTPases act as switches, turning on signaling cascades upon stimulation of receptors at the membrane. Common point mutations in these proteins, which often occur in codons 12, 13, or 61, block GTP hydrolysis, thus rendering the protein constitutively active [11]. There are three human RAS genes (H-RAS, N-RAS, and K-RAS), and activating points mutations in these genes have been found in many different types of cancer. A study on the significance of RAS mutations in 107 thyroid cancer patients showed that 74.3 % patients with RAS-mutated tumors died as a result of their disease in comparison to 31.9 % patients with tumors lacking these mutations [15]. RAS mutations (most frequently N-RAS) are found in ATC with a variable frequency ranging from 8–60 % depending on the sample series [15, 17, 39] RAF proteins are direct effectors of RAS, and B-RAF is the strongest activator of MEK and the most commonly mutated of the RAF proteins [11]. B-RAF has been reported to be mutated in approximately 7 % of all cancers; nevertheless, mutations in B-RAF are found in approximately 50 % of papillary thyroid tumors and 25–44 % of ATC cases [11, 23, 47, 49]. The most common B-RAF mutation, V600E, involves a change in the activation loop which induces constitutive activation [11, 39]. Both RAS and B-RAF activating mutations have been shown to confer drug resistance to thyroid carcinoma cell lines [43]. Therefore, mouse models of ATC based on alterations of key proteins of the RAS-MAPK signaling pathway allow a clinically and physiologically appropriate study of the role of this pathway in the progression of human thyroid cancer and therapy resistance.
RAS Models
A recently described mouse strain that carries a deletion of Trp53 and expresses Kras G12D specifically in the thyroid through the TPO-cre transgene models tumor progression from PTC to poorly differentiated thyroid cancer (PDTC) and ATC. These mice develop tumors starting at 5 months of age with full penetrance. Notably, 40 % of tumors developing in these mice contain areas of ATC, characterized by a pleomorphic spindle cell pattern and a high mitotic index, with atypical mitosis and large areas of tumor necrosis [7, 8, 59]. Twenty-eight percent of mutants had lung metastases, an important feature considering that the most common sites for metastasis in ATC are the lungs [4]. Expression of epithelial-to-mesenchymal transition (EMT) markers and loss of thyroid differentiation markers such as Tpo, Tg, and Slc5a is observed, paralleling the increment of the ATC component [8]. All these features are hallmarks of human ATC [37]. Interestingly, these tumors show no immunoreactivity for activated AKT, suggesting that activated RAS is not functionally linked to the PI3K pathway in this model.
MEK inhibition in cell lines established from these tumors causes growth inhibition brought by an arrest in the G1 phase; however, cell death is not observed. Further investigation has shown that cell death resistance is associated with overexpression of Bcl2a1 and Mcl1, two of the anti-apoptotic members of the Bcl2 family of proteins [8]. Deregulation in the expression of the anti-apoptotic members of this family has been reported in many different types of cancer [62]. BCL2A1 is involved in resistance to BRAF inhibition in melanoma [21], and MCL1 has been reported to be expressed in undifferentiated thyroid cancer [5]. In fact, analysis of a large expression-profiling dataset derived from human thyroid cancers [16] reveals that overexpression of MCL1 and BCL2A1 is common among more aggressive forms of thyroid cancer [8, 16]. Accordingly, treatment of Kras G12D;Trp53 −/− cell lines with a pan-inhibitor of the anti-apoptotic members of the Bcl2 family, Obatoclax, shows a drastic increase in cell death and synergize with the MEK inhibitor GSK1120212 both in vitro and in vivo, in syngeneic allograft models [8]. Whether this inhibitor cocktail has efficacy on autochthonous tumors in Kras G12D;Trp53 −/− mice remains to be seen [5, 21]. Thus, this model lends itself for further investigation of cell death resistance pathways, a critical area in understanding ATC drug resistance.
Mice expressing a mutant thyroid hormone receptor β (ThrbPV/PV) spontaneously develop well-differentiated follicular thyroid cancer [56]. When this mutation is combined with thyroid-specific activation of Kras, compound mutants develop aggressive carcinomas with frequent presence (>60 %) of areas of anaplasia [63]. Furthermore, lung metastases are observed in over 30 % of mice. Interestingly, tumors with anaplastic foci display increased levels of MYC and reduced levels of the pro-apoptotic molecule, BIM, suggesting a direct link between the aggressive and drug resistance features of ATC and the expression levels of these markers.
BRAFV600E Models
Mice with a thyroid-specific BRAFV600E mutation mediated by TPO-CreER upon tamoxifen treatment develops PTC 12 weeks post induction [34]. When this model is crossed to an inducible Trp53 mutant allele, the resulting TPO-CreER;Braf CA/+;Trp53 R270H/+ model develop PTC that progresses to ATC in 50 % of the cases. Furthermore, homozygous deletion of Trp53 in these mice or the presence of the R270H point mutant combined with the loss of the wt Trp53 allele accelerates the appearance of ATC and decreases the median survival in these mice [34]. The 6-month latency after induction of the BRAF mutation and loss of p53 suggests not only that additional oncogenic events need to take place before the appearance of ATC but also that this model will be of use for studying the transition from a well-differentiated tumor to a fully dedifferentiated neoplasm. These tumors show ATC characteristic features such as solid growth, spindle cell morphology, and pleomorphic giant cell pattern. Furthermore, 19 % of these animals develop lung micrometastases [4, 34]. Contrary to other BRAF V600E thyroid cancer models showing highly upregulated TSH, this model presents a modest elevation of TSH in comparison to control mice. Notably, TSH suppression mediated by T4 supplementation does not alter the overall tumor phenotype, suggesting that TSH signaling does not contribute to the phenotype. This is important because elevated levels of TSH are not observed in human thyroid cancer [2, 50].
Gene expression profiling of these ATCs revealed signatures of proliferation and EMT in comparison to the PTCs, which corresponds to what is also observed in human ATC. Further interrogation of the signaling mechanisms involved in the transition from PTC to ATC in these mice showed increased levels of phospho-Akt(S473) and phospho-S6(S235/236) only in the ATC compartments. These findings suggest that the PI3K and mTOR signaling might become activated during progression to ATC in this model; and this is a common event observed in human ATC [31, 34].
Vemurafenib/PLX4032 is a potent BRAF inhibitor with selectivity against the wild-type BRAF, BRAFV600E, and c-RAF-1. This drug was approved by the FDA in 2011 for the treatment of advanced metastatic melanoma [42]. The particular case of a 51-year old man with BRAF-mutated ATC who responded successfully to vemurafenib treatment has been reported [51]. This finding makes a case for the consideration of vemurafenib in ATC treatment in patients who test positive for the BRAFV600E mutation [51]. The efficacy of this BRAF inhibitor was tested on the TPO-CreER;Braf CA/+;Trp53 R270H/+ model. Upon confirmation of the presence of ATC, mice were treated with PLX4720, a more bioavailable sister compound to vemurafenib. The treated group showed a statistically significant overall survival. However, upon closer examination, this was due to a decrease in the PTC but not in the ATC component size. Furthermore, IHC showed that both treated and control tumors were positive for phospho-Erk staining, suggesting incomplete MAPK pathway inhibition, and for pAKT [34]. Since phase I clinical trials for vemurafenib, it has been known that an incomplete inhibition of the MAPK pathway is achieved even at the maximum tolerated doses [14]. Furthermore, the ability of tumor cells to activate compensatory signaling pathways or mutate Ras and reactivate the MAPK pathway has also been heavily reported. Thus, combination treatments including the use of a MEK inhibitor together with a BRAF inhibitor might be one strategy to completely block this oncogenic pathway [22, 45]. In the TPO-CreER;Braf CA/+;Trp53 R270H/+ model, a combination treatment of PLX4720 and PD0325901, a MEK inhibitor, showed a dramatic tumor suppressive effect, where three out of four ATC-bearing animals showed complete regression [34]. Since the combination of MAPK and PI3K signaling inhibition was not investigated in this model, this remains a key opportunity for future studies [34].
An additional Braf V600E-based mouse model was developed with a thyroid-specific expression of Braf V600E along with the expression of a constitutively active Pik3ca, encoding the p110α catalytic subunit of PI3K (Pik3ca Lat). Tissue-specific recombination was achieved through a TG-Cre ER transgene. By 8.5 months of age, 71 % of TG-CreER;Braf CA/+;Pik3ca Lat/+ mice develop palpable tumors, become severely sick, and require euthanasia. These tumors present aggressive and invasive PTC morphology. Moreover 80 % of them show presence of polygonal giant cells and/or spindle cell pattern, which is a characteristic of human ATC. These features are never observed in single mutants. Additionally, areas of ATC within these tumors show an EMT phenotype with loss of E-cadherin and gain of Vimentin, along with the loss of expression of TTF-1 [9], similar to human ATC [13, 37]. In order to further characterize the collaboration between an activating mutation in BRAF and the PI3K signaling pathway, a similar model of ATC was generated substituting the mutation in PI3K for a homozygous deletion of Pten. These TG-CreER;Braf CA/+;Pten −/− mice develop ATC between 3.5 and 8 months after tamoxifen treatment. The latency period in these mice is shorter than in the TG-CreER;Braf CA/+;Pik3ca Lat/+ model [9]. It is important to note that while the activating mutation in Pik3ca alone does not have an effect in the mouse thyroid, the deletion of Pten causes hyperplasia and FTC [3, 61], suggesting either that Pten function can still restrain constitutively active PI3K signaling, or that Pten exerts PI3K-independent tumor suppressive functions. A more mechanistic characterization of the TG-CreER;Braf CA/+;Pik3ca Lat/+ and TG-CreER;Braf CA/+;Pten −/− models is needed to study the interplay of these pathways in ATC, validating and extending in vivo the lessons learned from human ATC cells lines.
PI3K Signaling Pathway
The PI3K signaling pathway is known to play a crucial role in glucose uptake, cell growth and proliferation, cell adhesion and motility, and survival [40, 60]. Activation of receptor tyrosine kinases (RTK) at the membrane by external factors activate PI3K, which then phosphorylates phosphatidylinositol-4,5-bisphosphate to produce phosphatidylinositol-3,4,5-trisphosphate (PIP3). PIP3 allows AKT to be localized to the membrane where it becomes activated by PDK1. Once activated, AKT is able to target downstream effectors that will promote a plethora of biological effects. PTEN is a negative regulator of this signaling pathway, since it dephosphorylates PIP3 and terminates the PI3K-AKT signal [60]. Activating mutations in the p110α catalytic subunit of PI3K have been found in 25–40 % of cancers. PIK3CA is mutated in 15–25 % of all ATC cases, and its amplification is found in 40 % of ATC. Furthermore, loss of function alterations in PTEN is found in 4–16 % of ATC [47]. RAS is known to activate the PI3K signaling pathway, and the crosstalk between these two systems has been well documented. In fact, 81.3 % of ATC have been found to carry genetic alterations that could activate both pathways [31]. Recent studies have shown that blocking these two essential pathways in aggressive thyroid cancer cells allow for their sensitization to novel targeted therapies and traditional chemotherapeutic compounds [20, 36].
Pten Loss Models
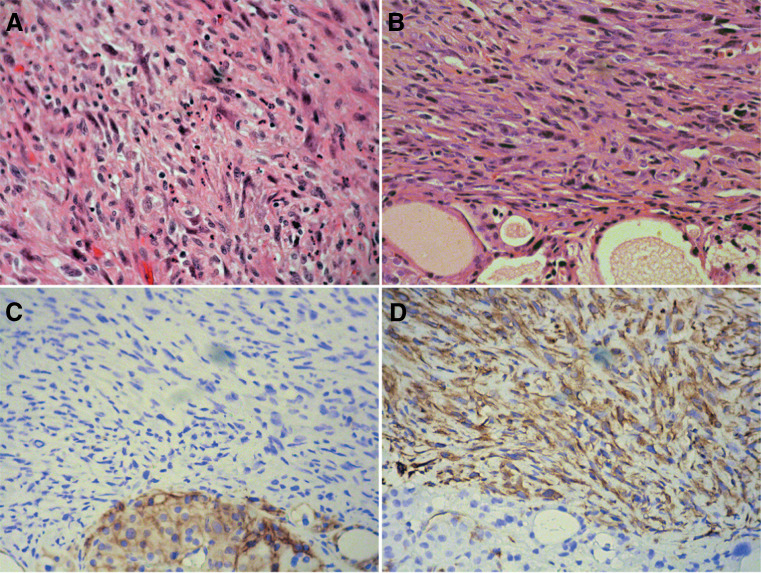
The Pten −/−;Trp53 −/− mouse model of ATC, driven by the TPO-Cre transgene, develops anaplastic thyroid tumors after 8–10 months of age. These tumors cause severe tracheal compression and invade into adjacent tissues. The presence of remnants of well-differentiated follicular thyroid carcinoma intermixed with ATC and the fact that glands from younger mice only show the presence, well-differentiated tumors suggest a progression mechanism from FTC to ATC. The ATC component of these tumors presents spindle cell morphology, giant osteoclast-like cells, and areas of osseous metaplasia (Fig. 1). Twenty-eight percent of these mice show metastases in the lungs; less often metastases are also observed in the liver [2, 4]. Anaplastic tumors show loss of thyroid differentiation markers such as Tpo, Tg, and Slc5a5 and the expression of EMT markers such as Vimentin, in accordance with human ATC [2, 37, 54]. Aneuploidy is an additional characteristic in common between these murine tumors and human ATC [2, 28]. Interestingly, IHC analysis reveals that the levels of activated Akt are higher in the well-differentiated areas of the tumors in comparison with the anaplastic component. The relevance and consequences of this finding, which is in keeping with similar data obtained in human ATCs [32], remain to be explored. Nevertheless, cell lines derived from these mice show sensitivity to AKT inhibition.
Fig. 1.
Human (a) and mouse (b) ATC display similar morphological features, including the establishment of EMT, as shown by loss of E-cadherin (c) and expression of Vimentin (d)
Genome-wide expression profiling of tumors from Pten −/−;Trp53 −/− mice revealed a unique signature correlated with the development of ATC. Murine ATCs showed higher expression level of genes involved in metabolic remodeling, cell motility and invasiveness, leukocyte recruitment, and EMT. Notably, cross-comparison of these findings to gene expression datasets from human ATCs showed a strong similarity between the mouse and human ATC profiles [2]. Commonalities between the two systems are likely to identify promising therapeutic targets, as exemplified by the upregulation of PLK1 [52]. Human ATCs display a strong uptake of glucose, suggesting a dependence on glycolysis for survival, a characteristic of some tumors that is defined as the Warburg effect [44]. In accordance to this, ATCs in Pten −/−;Trp53 −/− mice show a higher uptake of glucose in comparison to control mice but also upregulate genes involved in the establishment of the Warburg effect such as Hif1α, Hexokinase 2, and Pyruvate Kinase M2 and display a dramatic increase of lactate content. Most importantly, cell lines derived from these tumors were effectively inhibited by glycolytic inhibitors, which showed a synergistic effect when combined with doxorubicin, a drug currently used in the treatment of human ATC [2]. A major strength of this model lies in its thoroughly characterized morphological and physiological similarities to the human counterpart. This slow progression makes this model suitable for the study of the transition between well-differentiated thyroid cancer and ATC. On the other hand, the unpredictability of the time of tumor dedifferentiation is a limitation for preclinical studies. The development of several cell lines derived from these tumors, however, allows the establishment and preclinical use of syngeneic allograft models, partially addressing this issue [52].
Another mouse model presenting areas of ATC was generated by the simultaneous deletion of Pten and the expression of a dominant negative mutation of the thyroid hormone receptor-β. These Thrb PV/PV Pten +/− mice develop tumors with characteristics of FTC, which progress to display undifferentiated nests in 50 % of mice older than 7 months, and metastasize to the lungs in about 80 % of cases [19]. When Thrb PV/PV Pten +/− mice were fed a high-fat diet (HFD) to induce obesity, 62 % of them developed anaplasia by 21 weeks of age, in comparison to 24 % in mice fed a low-fat diet (LFD). Histopathological analysis of tumors developed by the HFD group showed loss of differentiation and the presence of spindle cell anaplasia. Further analysis of these tumors also showed they stained more positively for Ki-67 than tumors in the LFD group [26, 40]. Furthermore, TSH levels of HFD and LFD mice did not show any differences, a characteristic consistent with the lack of increased TSH in advanced human thyroid cancer [7, 26]. Mechanistically, it was suggested that tumor progression depended on increased levels of leptin, an adipocyte-derived hormone upregulated in obesity, and that leptin acts throughthe JAK2-STAT3 signaling pathway, which is critical in cell survival and proliferation [6, 26, 41]. This mouse model presented with high levels of leptin upon HFD and displayed upregulation of the JAK2-STAT3 pathway in comparison to LFD controls [26]. However, the fact that the JAK2-STAT3 pathway is also activated by several factors other than leptin calls for additional in depth analysis of this model [12, 24, 26, 55]. The limitations of this strain as an ATC model lie principally in the degree of anaplasia observed. Even when the original Thrb PV/PV presented anaplasia in 35 % of cases, this feature is restricted to small patches within the tumor [56]. While the concomitant deletion of one Pten allele accelerates tumorigenesis and the HFD make the anaplasia appear more readily, the level of dedifferentiation was still very limited [18, 25, 26, 46].
Conclusions
The rarity and aggressiveness of human ATC have been insurmountable barriers for research efforts focused on understanding the mechanisms of ATC development and designing rationale therapeutic approaches. The recent generation of different ATC mouse models encompassing most of the spectrum of ATC-associated mutations has opened new doors in the study of this malignancy. These models represent new tools that are helping understand the mechanisms involved in tumor progression and drug resistance. Particular enthusiasm is generated by models in which recombination can be induced postnatally, bypassing the proliferative wave that expands the thyrocytes pool between thyroid specification and 3–4 weeks after birth. A more detailed characterization of some of these models is still needed; for example, no data are available on the crosstalk between ATC cells and their microenvironment and on the role immune cells infiltrating these tumors have in sustaining or countering tumor growth and spread. Nevertheless, expression profiling studies have already unveiled possible roles for specific signaling pathways in the establishment and progression of ATC. Future research will have to exploit these models to design and test innovative therapeutic modalities.
Acknowledgments
Related work in the authors’ laboratory is supported by NIH grants to ADC (CA128943, CA167839, CA172012) and by the Irma T. Hirschl Career Scientist Award.
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- 1.Aldinger KA, Samaan NA, Ibanez M, Hill CS., Jr Anaplastic carcinoma of the thyroid: a review of 84 cases of spindle and giant cell carcinoma of the thyroid. Cancer. 1978;41(6):2267–2275. doi: 10.1002/1097-0142(197806)41:6<2267::AID-CNCR2820410627>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Antico Arciuch VG, Russo MA, Dima M, Kang KS, Dasrath F, Liao XH, Refetoff S, Montagna C, Di Cristofano A. Thyrocyte-specific inactivation of p53 and Pten results in anaplastic thyroid carcinomas faithfully recapitulating human tumors. Oncotarget. 2011;2(12):1109–1126. doi: 10.18632/oncotarget.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antico-Arciuch VG, Dima M, Liao XH, Refetoff S, Di Cristofano A. Cross-talk between PI3K and estrogen in the mouse thyroid predisposes to the development of follicular carcinomas with a higher incidence in females. Oncogene. 2010;29(42):5678–5686. doi: 10.1038/onc.2010.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besic N, Gazic B. Sites of metastases of anaplastic thyroid carcinoma: autopsy findings in 45 cases from a single institution. Thyroid. 2013;23(6):709–713. doi: 10.1089/thy.2012.0252. [DOI] [PubMed] [Google Scholar]
- 5.Branet F, Brousset P, Krajewski S, Schlaifer D, Selves J, Reed JC, Caron P. Expression of the cell death-inducing gene bax in carcinomas developed from the follicular cells of the thyroid gland. J Clin Endocrinol Metab. 1996;81(7):2726–2730. doi: 10.1210/jcem.81.7.8675602. [DOI] [PubMed] [Google Scholar]
- 6.Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG, Jr, Rossetti L. Critical role of STAT3 in leptin’s metabolic actions. Cell Metab. 2006;4(1):49–60. doi: 10.1016/j.cmet.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakravarty D, Santos E, Ryder M, Knauf JA, Liao XH, West BL, Bollag G, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011;121(12):4700–4711. doi: 10.1172/JCI46382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champa D, Russo MA, Liao XH, Refetoff S, Ghossein RA, Di Cristofano A. Obatoclax overcomes resistance to cell death in aggressive thyroid carcinomas by countering Bcl2a1 and Mcl1 overexpression. Endocr Relat Cancer. 2014;21(5):755–767. doi: 10.1530/ERC-14-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charles RP, Silva J, Iezza G, Phillips WA, McMahon M. Activating BRAF and PIK3CA mutations cooperate to promote anaplastic thyroid carcinogenesis. Mol Cancer Res. 2014;12(7):979–986. doi: 10.1158/1541-7786.MCR-14-0158-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornett WR, Sharma AK, Day TA, Richardson MS, Hoda RS, van Heerden JA, Fernandes JK. Anaplastic thyroid carcinoma: an overview. Curr Oncol Rep. 2007;9(2):152–158. doi: 10.1007/s11912-007-0014-3. [DOI] [PubMed] [Google Scholar]
- 11.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26(22):3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 12.Di Cristofano A. Obesity and thyroid cancer: is leptin the (only) link? Endocrinology. 2013;154(8):2567–2569. doi: 10.1210/en.2013-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabbro D, Diloreto C, Beltrami CA, Belfiore A, Dilauro R, Damante G. Expression of thyroid-specific transcription factors Ttf-1 and Pax-8 in human thyroid neoplasms. Cancer Res. 1994;54(17):4744–4749. [PubMed] [Google Scholar]
- 14.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363(9):809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Rostan G, Zhao HY, Camp RL, Pollan M, Herrero A, Pardo J, Ran W, Carcangiu ML, Costa J, Tallini G. ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol. 2003;21(17):3226–3235. doi: 10.1200/JCO.2003.10.130. [DOI] [PubMed] [Google Scholar]
- 16.Giordano TJ, Kuick R, Thomas DG, Misek DE, Vinco M, Sanders D, Zhu Z, et al. Molecular classification of papillary thyroid carcinoma: distinct BRAF, RAS, and RET/PTC mutation-specific gene expression profiles discovered by DNA microarray analysis. Oncogene. 2005;24(44):6646–6656. doi: 10.1038/sj.onc.1208822. [DOI] [PubMed] [Google Scholar]
- 17.Guerra A, Di Crescenzo V, Garzi A, Cinelli M, Carlomagno C, Tonacchera M, Zeppa P, Vitale M. Genetic mutations in the treatment of anaplastic thyroid cancer: a systematic review. BMC Surg. 2013;13(Suppl 2):S44. doi: 10.1186/1471-2482-13-S2-S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guigon CJ, Fozzatti L, Lu CX, Willingham MC, Cheng SY. Inhibition of mTORC1 signaling reduces tumor growth but does not prevent cancer progression in a mouse model of thyroid cancer. Carcinogenesis. 2010;31(7):1284–1291. doi: 10.1093/carcin/bgq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guigon CJ, Zhao L, Willingham MC, Cheng SY. PTEN deficiency accelerates tumour progression in a mouse model of thyroid cancer. Oncogene. 2009;28(4):509–517. doi: 10.1038/onc.2008.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunda V, Bucur O, Varnau J, Vanden Borre P, Bernasconi MJ, Khosravi-Far R, Parangi S. Blocks to thyroid cancer cell apoptosis can be overcome by inhibition of the MAPK and PI3K/AKT pathways. Cell Death Dis. 2014;5:e1104. doi: 10.1038/cddis.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haq R, Yokoyama S, Hawryluk EB, Jonssond GB, Frederick DT, McHenry K, Porter D, et al. BCL2A1 is a lineage-specific antiapoptotic melanoma oncogene that confers resistance to BRAF inhibition. Proc Natl Acad Sci U S A. 2013;110(11):4321–4326. doi: 10.1073/pnas.1205575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holderfield M, Nagel TE, Stuart DD. Mechanism and consequences of RAF kinase activation by small-molecule inhibitors. Br J Cancer. 2014;111(4):640–645. doi: 10.1038/bjc.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung CK, Little MP, Lubin JH, Brenner AV, Wells SA, Jr, Sigurdson AJ, Nikiforov YE. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab. 2014;99(2):E276–E285. doi: 10.1210/jc.2013-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim WG, Choi HJ, Kim WB, Kim EY, Yim JH, Kim TY, Gong G, Kim SY, Chung N, Shong YK. Basal STAT3 activities are negatively correlated with tumor size in papillary thyroid carcinomas. J Endocrinol Invest. 2012;35(4):413–418. doi: 10.3275/7907. [DOI] [PubMed] [Google Scholar]
- 25.Kim WG, Guigon CJ, Fozzatti L, Park JW, Lu CX, Willingham MC, Cheng SY. SKI-606, an Src inhibitor, reduces tumor growth, invasion, and distant metastasis in a mouse model of thyroid cancer. Clin Cancer Res. 2012;18(5):1281–1290. doi: 10.1158/1078-0432.CCR-11-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim WG, Park JW, Willingham MC, Cheng SY. Diet-induced obesity increases tumor growth and promotes anaplastic change in thyroid cancer in a mouse model. Endocrinology. 2013;154(8):2936–2947. doi: 10.1210/en.2013-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitamura Y, Shimizu K, Nagahama M, Sugino K, Ozaki O, Mimura T, Ito K, Ito K, Tanaka S. Immediate causes of death in thyroid carcinoma: clinicopathological analysis of 161 fatal cases. J Clin Endocrinol Metab. 1999;84(11):4043–4049. doi: 10.1210/jcem.84.11.6115. [DOI] [PubMed] [Google Scholar]
- 28.Klemi PJ, Joensuu H, Eerola E. DNA aneuploidy in anaplastic carcinoma of the thyroid gland. Am J Clin Pathol. 1988;89(2):154–159. doi: 10.1093/ajcp/89.2.154. [DOI] [PubMed] [Google Scholar]
- 29.Knauf JA, Fagin JA. Role of MAPK pathway oncoproteins in thyroid cancer pathogenesis and as drug targets. Curr Opin Cell Biol. 2009;21(2):296–303. doi: 10.1016/j.ceb.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 30.La Perle KMD, Jhiang SM, Capen CC. Loss of p53 promotes anaplasia and local invasion in ret/PTC1-induced thyroid carcinomas. Am J Pathol. 2000;157(2):671–677. doi: 10.1016/S0002-9440(10)64577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z, Hou P, Ji M, Guan H, Studeman K, Jensen K, Vasko V, El-Naggar AK, Xing M. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. 2008;93(8):3106–3116. doi: 10.1210/jc.2008-0273. [DOI] [PubMed] [Google Scholar]
- 32.Marlow LA, von Roemeling CA, Cooper SJ, Zhang Y, Rohl SD, Arora S, Gonzales IM, et al. Foxo3a drives proliferation in anaplastic thyroid carcinoma through transcriptional regulation of cyclin A1: a paradigm shift that impacts current therapeutic strategies. J Cell Sci. 2012;125(Pt 18):4253–4263. doi: 10.1242/jcs.097428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773(8):1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McFadden DG, Vernon A, Santiago PM, Martinez-McFaline R, Bhutkar A, Crowley DM, McMahon M, Sadow PM, Jacks T. p53 constrains progression to anaplastic thyroid carcinoma in a Braf-mutant mouse model of papillary thyroid cancer. Proc Natl Acad Sci U S A. 2014;111(16):E1600–E1609. doi: 10.1073/pnas.1404357111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, van Heerden JA, Goellner JR. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001;130(6):1028–1034. doi: 10.1067/msy.2001.118266. [DOI] [PubMed] [Google Scholar]
- 36.Milosevic Z, Pesic M, Stankovic T, Dinic J, Milovanovic Z, Stojsic J, Dzodic R, Tanic N, Bankovic J (2014) Targeting RAS-MAPK-ERK and PI3K-AKT-mTOR signal transduction pathways to chemosensitize anaplastic thyroid carcinoma. Transl Res [DOI] [PubMed]
- 37.Montemayor-Garcia C, Hardin H, Guo Z, Larrain C, Buehler D, Asioli S, Chen H, Lloyd RV. The role of epithelial mesenchymal transition markers in thyroid carcinoma progression. Endocr Pathol. 2013;24(4):206–212. doi: 10.1007/s12022-013-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neff RL, Farrar WB, Kloos RT, Burman KD. Anaplastic thyroid cancer. Endocrinol Metab Clin North Am. 2008;37(2):525–538. doi: 10.1016/j.ecl.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Nikiforov YE. Genetic alterations involved in the transition from well-differentiated to poorly differentiated and anaplastic thyroid carcinomas. Endocr Pathol. 2004;15(4):319–327. doi: 10.1385/EP:15:4:319. [DOI] [PubMed] [Google Scholar]
- 40.Parenti R, Salvatorelli L, Magro G. Anaplastic thyroid carcinoma: current treatments and potential new therapeutic options with emphasis on TfR1/CD71. Int J Endocrinol. 2014;2014:685396. doi: 10.1155/2014/685396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140(2):197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patrawala S, Puzanov I. Vemurafenib (RG67204, PLX4032): a potent, selective BRAF kinase inhibitor. Future Oncol. 2012;8(5):509–523. doi: 10.2217/fon.12.31. [DOI] [PubMed] [Google Scholar]
- 43.Piscazzi A, Costantino E, Maddalena F, Natalicchio MI, Gerardi AM, Antonetti R, Cignarelli M, Landriscina M. Activation of the RAS/RAF/ERK signaling pathway contributes to resistance to sunitinib in thyroid carcinoma cell lines. J Clin Endocrinol Metab. 2012;97(6):E898–E906. doi: 10.1210/jc.2011-3269. [DOI] [PubMed] [Google Scholar]
- 44.Poisson T, Deandreis D, Leboulleux S, Bidault F, Bonniaud G, Baillot S, Auperin A, et al. 18F-fluorodeoxyglucose positron emission tomography and computed tomography in anaplastic thyroid cancer. Eur J Nucl Med Mol Imaging. 2010;37(12):2277–2285. doi: 10.1007/s00259-010-1570-6. [DOI] [PubMed] [Google Scholar]
- 45.Puzanov I, Burnett P, Flaherty KT. Biological challenges of BRAF inhibitor therapy. Mol Oncol. 2011;5(2):116–123. doi: 10.1016/j.molonc.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puzianowska-Kuznicka M, Krystyniak A, Madej A, Cheng SY, Nauman J. Functionally impaired TR mutants are present in thyroid papillary cancer. J Clin Endocrinol Metab. 2002;87(3):1120–1128. doi: 10.1210/jcem.87.3.8296. [DOI] [PubMed] [Google Scholar]
- 47.Ragazzi M, Ciarrocchi A, Sancisi V, Gandolfi G, Bisagni A, Piana S. Update on anaplastic thyroid carcinoma: morphological, molecular, and genetic features of the most aggressive thyroid cancer. Int J Endocrinol. 2014;2014:790834. doi: 10.1155/2014/790834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Regalbuto C, Frasca F, Pellegriti G, Malandrino P, Marturano I, Di Carlo I, Pezzino V. Update on thyroid cancer treatment. Future Oncol. 2012;8(10):1331–1348. doi: 10.2217/fon.12.123. [DOI] [PubMed] [Google Scholar]
- 49.Ricarte-Filho JC, Ryder M, Chitale DA, Rivera M, Heguy A, Ladanyi M, Janakiraman M, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69(11):4885–4893. doi: 10.1158/0008-5472.CAN-09-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rinaldi S, Plummer M, Biessy C, Tsilidis KK, Ostergaard JN, Overvad K, Tjonneland A, et al. Thyroid-stimulating hormone, thyroglobulin, and thyroid hormones and risk of differentiated thyroid carcinoma: the EPIC study. J Natl Cancer Inst. 2014;106(6):dju097. doi: 10.1093/jnci/dju097. [DOI] [PubMed] [Google Scholar]
- 51.Rosove MH, Peddi PF, Glaspy JA. BRAF V600E inhibition in anaplastic thyroid cancer. N Engl J Med. 2013;368(7):684–685. doi: 10.1056/NEJMc1215697. [DOI] [PubMed] [Google Scholar]
- 52.Russo MA, Kang KS, Di Cristofano A (2013) The PLK1 Inhibitor GSK461364A is effective in poorly differentiated and anaplastic thyroid carcinoma cells, independent of the nature of their driver mutations. Thyroid [DOI] [PMC free article] [PubMed]
- 53.Santoro M, Carlomagno F, Hay ID, Herrmann MA, Grieco M, Melillo R, Pierotti MA, et al. Ret oncogene activation in human thyroid neoplasms is restricted to the papillary cancer subtype. J Clin Invest. 1992;89(5):1517–1522. doi: 10.1172/JCI115743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2009;16(1):17–44. doi: 10.1677/ERC-08-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song L, Turkson J, Karras JG, Jove R, Haura EB. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene. 2003;22(27):4150–4165. doi: 10.1038/sj.onc.1206479. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki H, Willingham MC, Cheng SY. Mice with a mutation in the thyroid hormone receptor beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid. 2002;12(11):963–969. doi: 10.1089/105072502320908295. [DOI] [PubMed] [Google Scholar]
- 57.Tallini G, Santoro M, Helie M, Carlomagno F, Salvatore G, Chiappetta G, Carcangiu ML, Fusco A. RET/PTC oncogene activation defines a subset of papillary thyroid carcinomas lacking evidence of progression to poorly differentiated or undifferentiated tumor phenotypes. Clin Cancer Res. 1998;4(2):287–294. [PubMed] [Google Scholar]
- 58.Wein RO, Weber RS. Anaplastic thyroid carcinoma: palliation or treatment? Curr Opin Otolaryngol Head Neck Surg. 2011;19(2):113–118. doi: 10.1097/MOO.0b013e328343af3d. [DOI] [PubMed] [Google Scholar]
- 59.Williams ED, Abrosimov A, Bogdanova T, Demidchik EP, Ito M, LiVolsi V, Lushnikov E, et al. Thyroid carcinoma after Chernobyl latent period, morphology and aggressiveness. Br J Cancer. 2004;90(11):2219–2224. doi: 10.1038/sj.bjc.6601860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xing M. Genetic alterations in the phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer. Thyroid. 2010;20(7):697–706. doi: 10.1089/thy.2010.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeager N, Klein-Szanto A, Kimura S, Di Cristofano A. Pten loss in the mouse thyroid causes goiter and follicular adenomas: insights into thyroid function and Cowden disease pathogenesis. Cancer Res. 2007;67(3):959–966. doi: 10.1158/0008-5472.CAN-06-3524. [DOI] [PubMed] [Google Scholar]
- 62.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27(50):6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 63.Zhu X, Zhao L, Park JW, Willingham MC, Cheng SY. Synergistic signaling of KRAS and thyroid hormone receptor beta mutants promotes undifferentiated thyroid cancer through MYC up-regulation. Neoplasia. 2014;16(9):757–769. doi: 10.1016/j.neo.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]