Abstract
The synthesis of 9-(β-D-1,3-dioxolan-4-yl)2,6-diaminopurine nucleoside phosphoramidate prodrugs as well as various 2-amino-6-carbamoylpurine dioxolane derivatives and their phosphoramidates prodrugs is reported. Their ability to block HIV and HBV replication along with their cytotoxicity toward HepG2, human lymphocyte, CEM and Vero cells was also assessed.
Keywords: β-D-1, 3-dioxolan-4-yl nucleosides, DAPD, Antiviral agent, Anti-HIV, Anti-HBV
1. Introduction
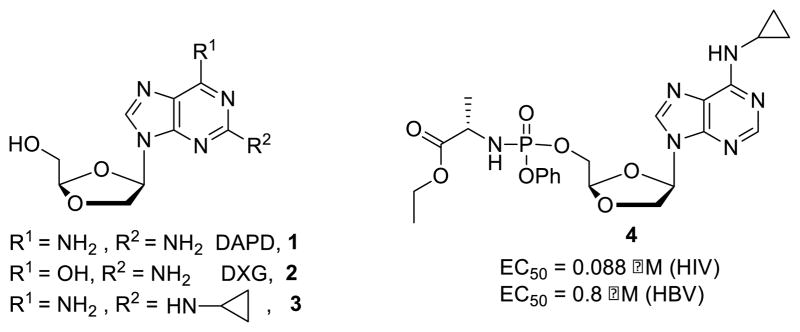
Nucleoside reverse transcriptase inhibitors (NTRI) are the backbone in fixed dose combinations now called highly active antiretroviral therapy (HAART) for human immunodeficiency virus type 1 (HIV-1).1 Despite the effectiveness of these drugs, resistance can result from their long-term use and latent toxicity remains an issue.2 Therefore, studies on novel nucleoside analogs with improved efficacy, resistance profile and safety are continuously needed to improve clinical outcome. Among all the modified nucleosides prepared over the years, β-D-1,3-dioxolan-4-yl nucleosides appeared to be a very promising family. Therefore, 9-(β-D-1,3-dioxolan-4-yl)2,6-diaminopurine (DAPD) 1, a prodrug of 9-(β-D-1,3-dioxolan-4-yl)guanine (DXG, 2), was evaluated for the treatment of HIV-1 infected persons in phase 2 clinical studies. However, the study was abandoned due to slow enrollment and high dosing regimens (500 mg twice a day). Over the years of nucleoside analog development, two main approaches have been utilized to improve the potency of a compound and potentially decrease the administered dose: A) Formation of a monophosphate prodrug to bypass the rate limiting first phosphorylation step.3 For instance, (−)-β-D-(2R,4R)-1,3-dioxolane-2-amino-6-aminopropyl purine nucleoside phosphoramidate prodrug 4 displayed submicromolar activities against HBV and HIV while its corresponding nucleoside 3 was devoid of activity (Figure 1).4 B) Conversion of hydrophilic functional groups (such as amino or hydroxy) into corresponding lipophilic groups (such as amides or esters) in order to improve cell penetration. Thus, L-1,3-dixolane-cytidine (L-OddC) bearing a fatty acid group at its N4-position demonstrated significantly improved antitumor activity (170-fold) in vitro when compared to the parent nucleoside.5 Fatty acyl derivatives of (−)-2′,3′-dideoxy-3′-thiacytidine (3TC) and (−)-2′,3′-dideoxy-5-fluoro-3′-thiacytidine [(−)-FTC] were 36- and 24-fold, respectively, more potent against HIV when compared to 3TC and (−)-FTC.6
Figure 1.
Herein, we combine both approaches to potentially improve the antiviral potency of DAPD. Thus, we report the synthesis and antiviral evaluation of DAPD phosphoramidate prodrugs as well as their 2-amino-6-carbamoylpurine derivatives as a potential strategy to increase intracellular delivery of DXG-TP.
2. Results and discussion
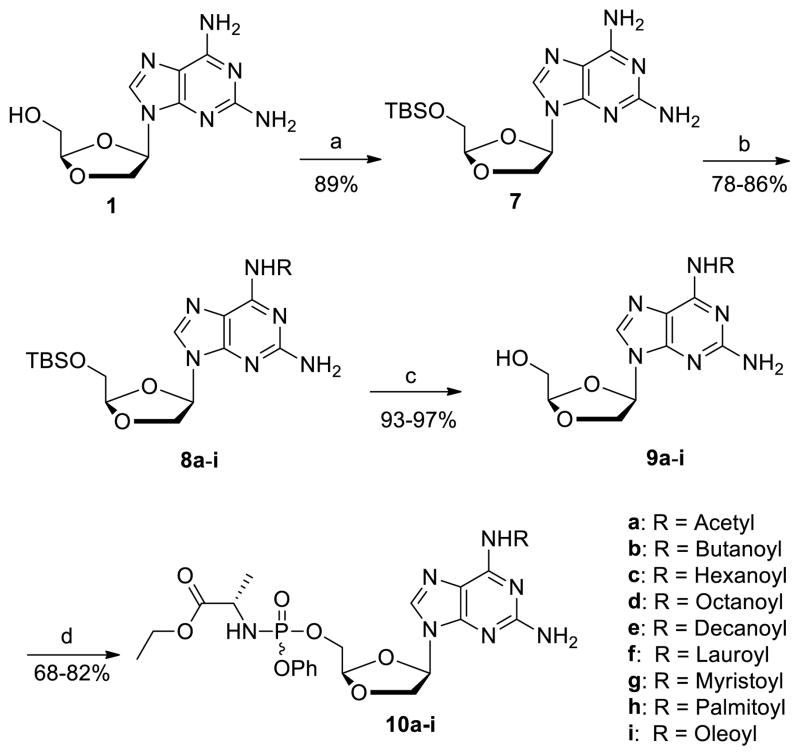
We first envisaged to prepare the desired 2-amino-6-carbamoylpurine dioxolane nucleoside phosphoramidate prodrugs 10a-i from DAPD in a 2 steps sequence by 1) formation of the phosphoramidate prodrug 5; 2) introduction of the carbamoyl moiety. However, reaction of DAPD 1 with phosphoramidate chloride 67 in the presence of t-BuMgCl afforded DAPD prodrug 5 in only 45% yield (Scheme 1). To further complicate this approach, acylation reactions with 5 would either proceed to only about 50% or when forced gave a substantial amount of the N2,N6-diacylated product. These low yields combined with the difficulty of purifying 5 using repeated tedious silica gel column chromatography lead us to design an alternative sequence which also allowed us to prepare and evaluate the 2-amino-6-carbamoylpurine dioxolanes 9a-i (Scheme 2).
Scheme 1.
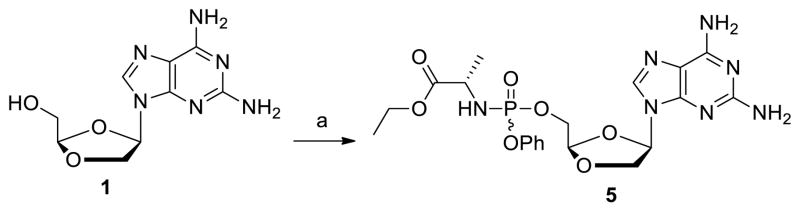
Reagents and reaction conditions: a) (EtOAlaNH)P(=O)(OPh)Cl 6, t-BuMgCl, THF, −78 °C then rt, 8 h.
Scheme 2.
Reagents and reaction conditions: a) TBSCl, imidazole, pyridine, 0 °C then rt, 12 h; b) RCl, NMI, CH2Cl2, 0 °C then rt, 12 h; c) Et3N-3HF, THF, rt, 12 h; d) (NHAlaOEt)P(=O)(OPh)Cl 6, NMI, −78 °C then rt, 8–12 h.
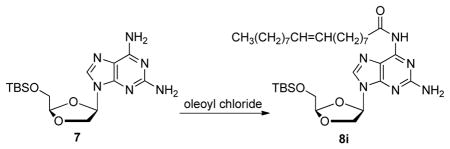
Thus, DAPD 1 was reacted with t-butyldimethylsilyl chloride (TBSCl) in the presence of imidazole in pyridine to give 5′-O-TBS-2,6-diaminopurine dioxolane 7 in 89% yield. With 7 in hand, its chemoselective N6-acylation was investigated using oleoyl chloride as the model system for optimization (Table 1). Interestingly, the reaction of 7 with oleoyl chloride (1.1 eq) in the presence of pyridine (entry 2), DMAP (entry 4), Et3N (entry 6), imidazole (entry 7) or their combination (entries 3 and 5) lead to the formation of 5′-O-TBS-2-amino-6-oleoylpurine dioxolane 8i in poor to moderate yields along with N2,N6-diacylated DAPD in ca. 5–25% yields. On the other hand, treatment of 7 with oleic acid chloride (1.1 eq) in the presence of N-methylimidazole (entry 1),8 provided the desired compound 8i in 80% yield with less than 5 % of the N2,N6-diacylated byproduct. Using these optimized conditions, compound 7 was reacted with a variety of acyl chlorides to give the corresponding 5′-O-TBS-2-amino-6-carbamoylpurine dioxolane derivatives 8a-i in 78–86% isolated yields. The final phenyloxy phosphoramidate dioxolane derivatives (10a-i) were obtained after removal of the 5′-silyl group with Et3N-3HF and subsequent coupling with phosphoramidate chloride reagent 6.
Table 1.
Condition for optimization of the N6-acylation of 7
| ||
|---|---|---|
| Entry | Conditions | Yield (%) |
| 1 | NMI, CH2Cl2, 12 h | 80 |
| 2 | pyridine, 12 h | Tracea |
| 3 | DMAP (0.1 eq), pyridine, 12 h | 5a |
| 4 | DMAP (2.0 eq), CH2Cl2, 12 h | 43a |
| 5 | DMAP (0.1 eq), Et3N (2.0 eq), CH2Cl2, 12 h | 18a |
| 6 | Et3N (2.0 eq), CH2Cl2, 12 h | 21a |
| 7 | imidazole (2.0 eq), CH2Cl2, 12 h | 8b |
Reactions provided N2,N6-diacylated compounds in 20–30% yields and ca. 5–15 % of starting material (7) was recovered;
Most of 7 was recovered.
Both anti-HIV and anti-HBV potency of DAPD monophosphate prodrug 5, as well as 2-amino-6-carbamoylpurine dioxolanes (9a-i) and their phosphoramidate dioxolane prodrugs (10a-i) were evaluated in primary human lymphocytes and HepG2 cells, respectively. In addition, cytotoxicity was determined in human peripheral blood mononuclear (PBM) cells, human lymphoblastoid CEM, African Green monkey Vero cells and HepG2 cells.9 The antiviral activity and cytotoxicity are summarized in Table 2. Generating phosphoramidate prodrug of DAPD 5 barely increased DAPD’s potency against HIV (EC50 of 0.45 vs 0.25 μM) but, more interestingly, boosted its potency against HBV by a factor of 40 (EC50 of 0.3 vs 12 μM). C1 to C11 N6-carbamoyl derivative 9a-f, 9i and their corresponding prodrugs 10a-f, 10i displayed either equal or less potency than DAPD against HIV and HBV. On the other hand, phosphoramidate prodrugs 10g and 10h, with EC50’s of 0.041 and 0.083 μM respectively, were 3 to 11 times more potent than DAPD 1 or DAPD prodrug 5 against HIV while their anti-HBV potency was in the same range as DAPD prodrug 5. Unfortunately, these compounds also displayed cytotoxicity in various cell lines that ranged from a CC50 of 7.5 to 71.0 μM.
Table 2.
In vitro antiviral activity and cytotoxicity of compounds 9a-i and phosphoramidate prodrugs 5, 10a-i.a
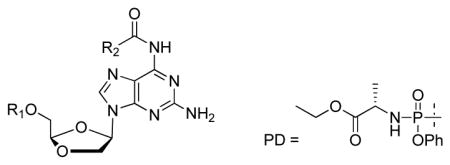
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmpd | R1 | R2 | Anti-HIV-1 activity (μM) | Anti-HBV activity (μM) | Cytotoxicity, CC50 (μM) | |||||
|
| ||||||||||
| EC50 | EC90 | EC50 | EC90 | PBM | CEM | Vero | HepG2 | |||
| AZT | NA | NA | 0.0037 | 0.047 | > 10 | > 10 | > 100 | 14.0 | 56.0 | > 100 |
| 1 | H | NA | 0.45 | 7.6 | 12.0 | > 100 | > 100 | > 100 | > 100 | > 100 |
| 5 | PD | NA | 0.25 | 1.5 | 0.30 | 2.8 | 67.0 | > 100 | > 100 | > 100 |
| 9a | H | CH3 | 0.56 | 12.0 | > 10 | > 10 | > 100 | > 100 | > 100 | > 100 |
| 10a | PD | CH3 | 19.0 | 93.0 | 9.3 | > 10 | > 100 | > 100 | > 100 | > 100 |
| 9b | H | CH3(CH2)2 | 9.2 | 38.0 | > 10 | > 10 | > 100 | > 100 | > 100 | > 100 |
| 10b | PD | CH3(CH2)2 | 1.2 | 15.0 | 3.3 | > 10 | > 100 | > 100 | > 100 | > 100 |
| 9c | H | CH3(CH2)4 | 34.0 | > 100 | > 10 | > 10 | > 100 | > 100 | > 100 | > 100 |
| 10c | PD | CH3(CH2)4 | 2.2 | 33.0 | 1.3 | > 10 | > 100 | 89 | > 100 | > 100 |
| 9d | H | CH3(CH2)6 | 21 | 56.0 | > 10 | > 10 | > 100 | > 100 | > 100 | > 100 |
| 10d | PD | CH3(CH2)6 | 0.42 | 6.6 | 0.55 | ≥ 10 | > 100 | 16.0 | 87.0 | 9.5 |
| 9e | H | CH3(CH2)8 | 26.0 | 65.0 | > 10 | > 10 | > 100 | 65.0 | > 100 | > 100 |
| 10e | PD | CH3(CH2)8 | 1.5 | 9.4 | 0.43 | ≥ 10 | 67.0 | 13.0 | 13.0 | 9.5 |
| 9f | H | CH3(CH2)10 | 3.6 | 18.0 | > 10 | > 10 | 60.0 | 13 | 53.0 | > 100 |
| 10f | PD | CH3(CH2)10 | 0.98 | 2.9 | 0.34 | ≥ 10 | 14.0 | 12.0 | 45.0 | 71.0 |
| 9g | H | CH3(CH2)12 | 6.5 | 27.0 | > 10 | > 10 | > 100 | 4.6 | 7.7 | 56.0 |
| 10g | PD | CH3(CH2)12 | 0.041 | 0.89 | 0.39 | 8.8 | 9.9 | 7.5 | 13.0 | 57.0 |
| 9h | H | CH3(CH2)14 | 1.2 | 6.0 | > 10 | > 10 | > 100 | > 100 | 90 | > 100 |
| 10h | PD | CH3(CH2)14 | 0.083 | 0.50 | 0.33 | 7.9 | 7.9 | 12 | > 100 | ND |
| 9i | H | CH3(CH2)7CH=CH(CH2)7 | 3.5 | 13.0 | > 10 | > 10 | 76 | 13 | 19 | 11 |
| 10i | PD | CH3(CH2)7CH=CH(CH2)7 | 0.77 | 2.1 | 0.1 | 5.8 | 10 | 10 | 13 | ND |
In conclusion, this work confirms the potential of the phosphoramidate prodrug approach to reveal or enhance the antiviral activity of nucleosides. In our hands, DAPD phosphoramidate prodrug 5 was 40 times more potent than DAPD against HBV. Combination of this approach with the introduction of long fatty ester chains known to mask polar groups allowed us to even further increase their potency against HIV. However, this augmentation of potency came, in this case, with an increase of cellular toxicity.
V. Experimentals
General
Nuclear magnetic resonance (NMR) spectra (1H, 13C and 31P) were recorded on a Varian Unity Plus 400 MHz and a Bruker Ascend™ 400 MHz Fourier transform spectrometer at rt, with tetramethylsilane (TMS) as an internal standard. Chemical shifts (δ) are reported in parts per million (ppm), and signals are quoted as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), br (broad), dd (doublet of doublets), or ddd (doublet of doublets of doublets). The phosphoramidates are an approximate 50:50 mixture of diastereomers (RP/SP) and the 13C NMR data is reported as observed, that is, some carbon signals overlap. High-resolution mass spectra (HRMS) were recorded on a Micromass Autospec high-resolution mass spectrometer with electrospray ionization (ESI). Thin-layer chromatography (TLC) was performed on 0.25 mm silica gel. Purifications were carried out on silica gel column chromatography (60 Å, 63–200 μm, or 40–75 μm).
(2S)-ethyl 2-(((((2R,4R)-4-(2-amino-6-hydroxy-9H-purin-9-yl)-1,3-dioxolan-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate (5)
To a solution of DAPD (0.10 g, 0.40 mmol) in THF (10 mL) was added t-butylmagnesium chloride (1.19 mL, 1.0 M in THF, 1.19 mmol) at −78 °C under argon atmosphere. After stirred for 30 min, the reaction mixture was additionally stirred at rt for 30 min. To the solution was added phosphoramide chloride 6 (0.24 g, 0.80 mmol) at −78 °C under argon atmosphere. The reaction temperature was warmed to rt over 1 h and then stirred for 6 h. The mixture was quenched with saturated ammonium chloride (1.0 mL) at 0 °C, and then stirred for 10 min. The resulting solution was adsorbed on silica gel and purified by silica gel column chromatography (CH2Cl2:MeOH = 20:1 to 10:1 v/v) to give compound 5 in 45% yield (0.09 g, 0.18 mmol). 1H NMR (400 MHz, CD3OD): δ 7.99-7.98 (s, 1H), 7.37-7.27 (m, 2H), 7.23-7.13 (m, 3H), 6.37-6.33 (m, 1H), 5.36-5.32 (m, 1H), 4.63-4.60 (m, 1H), 4.38-4.30 (m, 3H), 4.14-4.06 (m, 2H), 3.76-3.71 (m, 1H), 1.35-1.19 (m, 6H); 13C NMR (100 MHz, CD3OD) δ 173.6, 160.6, 156.2, 151.3, 150.6, 135.8, 129.2, 124.6, 119.9, 112.6, 103.3, 79.6, 70.6, 64.6, 60.9, 50.1, 18.9, 13.0; 31P NMR (162 MHz, CD3OD) δ 3.91, 3.64; MS-ESI+ m/z 508 (M+H+); HRMS-ESI+ calcd for C20H27N7O7P (M+H+) 508.1708, found 508.1704.
9-((2R,4R)-2-(((tert-Butyldimethylsilyl)oxy)methyl)-1,3-dioxolan-4-yl)-9H-purine-2,6-diamine (7)
To a solution of DAPD (2.0 g, 7.93 mmol) in 25 mL of anhydrous pyridine was added imidazole (0.65 g, 9.52 mmol) and TBDMSCl (1.20 g, 7.93 mmol) at 0 °C under N2 atmosphere. After stirring for 12 h at rt, the reaction mixture was treated with 10 mL of MeOH, stirred for 30 min at rt and then concentrated under reduced pressure. The residue was purified on silica gel column chromatography (CH2Cl2 to CH2Cl2: MeOH = 10:1 v/v) to give compound 7 (2.58 g, 7.06 mmol) in 89% yield. 1H NMR (400 MHz, DMSO-d6) δ 7.85 (s, 1H), 6.98 (br, 2H), 6.20 (dd, J = 1.6, 5.6 Hz, 1H), 6.05 (br, 2H), 5.04 (t, J = 3.6 Hz, 1H), 4.45 (dd, J = 1.6, 10.0 Hz, 1H), 4.19 (dd, J = 1.6, 5.6 Hz, 1H), 3.79 (d, J = 3.2 Hz, 2H), 0.84 (s, 9H), 0.03 (s, 3H), 0.02 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 159.8, 155.7, 151.5, 134.9, 112.6, 104.7, 78.6, 70.5, 63.1, 25.8, 18.2, −5.37, −5.39; MS-ESI+ m/z 367 (M+H+); HRMS-ESI+ calcd for C15H31N6O3Si (M+H+) 367.1907, found 367.1908.
(Z)-N-(2-Amino-9-((2R,4R)-2-(((tert-butyldimethylsilyl) oxy)methyl)-1,3-dioxolan-4-yl)-9H-purin-6-yl)octadec-8-enamide (8i)
To a solution of compound 7 (2.70 g, 7.37 mmol) in 50 mL of anhydrous CH2Cl2 was added N-methylimidazole (2.0 eq, 1.21 g, 14.7 mmol) and oleoyl chloride (1.1 eq, 3.05 g, 8.11 mmol) at 0 °C under N2 atmosphere. After stirring for 12 h at rt, the reaction mixture was treated with 10 mL of MeOH, stirred for 30 min at rt and then concentrated under reduced pressure. The residue was purified on silica gel column chromatography (hexane: EtOAc = 4:1 to 1:4 v/v) to give compound 8i (3.72 g, 5.90 mmol) in 80 % yield. 1H NMR (400 MHz, CDCl3) δ 8.66 (br, 1H), 8.07 (s, 1H), 6.34 (dd, J = 1.6, 5.2 Hz, 1H), 5.36-5.32 (m, 2H), 5.16 (br, 2H), 5.12 (t, J = 3.2 Hz, 1H), 4.42 (dd, J = 1.2, 9.6 Hz, 1H), 4.22 (dd, J = 5.2, 9.6 Hz, 1H), 3.95-3.88 (m, 2H), 2.79 (t, J = 7.6 Hz, 2H), 2.20-1.98 (m, 4H), 1.78-1.70 (m, 2H), 1.40-1.24 (m, 20H), 0.91-0.85 (m, 12H), 0.08 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 173.3, 159.7, 153.1, 149.9, 138.0, 130.1, 129.9, 129.95, 116.0, 106.0, 79.4, 71.7, 63.5, 38.0, 32.1, 29.93, 29.90, 29.7, 29.54, 29.48,, 29.3, 27.39, 27.36, 26.1, 25.0, 22.8, 18.7, 14.3, −5.17, −5.19; MS-ESI+ m/z 631(M+H+); HRMS-ESI+ calcd for C33H58N6O4NaSi (M+Na+) 653.4188, found 653.4181.
N-(2-Amino-9-((2R,4R)-2-(((tert-butyldimethylsilyl) oxy)methyl)-1,3-dioxolan-4-yl)-9H-purin-6-yl)acetamide (8a)
Compound 8a was prepared using the same procedure as for compound 8i; yield: 78%; 1H NMR (400 MHz, CDCl3) δ 8.55 (s, 1H), 8.07 (s, 1H), 6.34 (dd, J = 1.6, 5.2 Hz, 1H), 5.12 (t, J = 3.2 Hz, 1H), 5.03 (br, 2H), 4.42 (dd, J = 1.2, 10.0 Hz, 1H), 4.23 (dd, J = 5.2, 10.0 Hz, 1H), 3.94-3.86 (m, 2H), 2.56 (s, 3H), 0.90 (s, 9H), 0.09 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 170.7, 159.6, 153.0, 149.8, 138.2, 115.9, 106.0, 79.5, 71.8, 63.5, 39.3, 26.1, 25.9, 18.8, −5.13, −5.16; MS-ESI+ m/z 409 (M+H+); HRMS-ESI+ calcd for C17H29N6O4Si (M+H+) 409.2008, found 409.2014.
N-(2-Amino-9-((2R,4R)-2-(((tert-butyldimethylsilyl) oxy)methyl)-1,3-dioxolan-4-yl)-9H-purin-6-yl)butyramide (8b)
Compound 8b was prepared using the same procedure as for compound 8i; yield: 84%; 1H NMR (400 MHz, CD3OD) δ 8.29 (s, 1H), 6.45 (d, J = 4.0 Hz, 1H), 5.07 (t, J = 2.4 Hz, 1H), 4.42 (d, J = 10.0 Hz, 1H), 4.20 (dd, J = 5.2, 10.0 Hz, 1H), 3.92-3.84 (m, 2H), 2.41 (t, J = 5.2 Hz, 2H), 1.71-1.65 (m, 2H), 0.96 (t, J = 7.2 Hz, 3H), 0.87 (s, 9H), 0.03 (s, 3H), 0.02 (s, 3H); 13C NMR (100 MHz, CD3OD) δ 175.7, 157.7, 154.6, 151.4, 140.2, 116.8, 107.0, 81.5, 73.0, 64.1, 40.2, 26.6, 20.1, 19.6, 14.2, −5.1, −5.2; MS-ESI+ m/z 437 (M+H+); HRMS-ESI+ calcd for C19H33N6O4Si (M+H+) 437.2327, found 437.2327.
N-(2-Amino-9-((2R,4R)-2-(((tert-butyldimethylsilyl) oxy)methyl)-1,3-dioxolan-4-yl)-9H-purin-6-yl)hexanamide (8c)
Compound 8c was prepared using the same procedure as for compound 8i; yield: 85%; 1H NMR (400 MHz, CDCl3) δ 8.85 (s, 1H), 8.09 (s, 1H), 6.35 (dd, J = 1.2, 5.2 Hz, 1H), 5.20 (br, 2H), 5.12 (t, J = 3.2 Hz, 1H), 4.41 (dd, J = 1.2, 9.6 Hz, 1H), 4.22 (dd, J = 5.2, 9.6 Hz, 1H), 3.94-3.87 (m, 2H), 2.77 (t, J = 7.6 Hz, 2H), 1.78-1.70 (m, 2H), 1.40-1.32 (m, 4H), 0.95-0.88 (m, 12H), 0.09 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 173.2, 159.8, 152.9, 150.0, 138.0, 115.8, 106.0, 79.5, 71.8, 63.4, 37.9, 31.6, 26.1, 24.8, 22.7, 18.8, 14.2, −5.1, −5.2; MS-ESI+ m/z 465 (M+H+); HRMS-ESI+ calcd for C21H37N6O4Si (M+H+) 465.2461, found 465.2460.
N-(2-Amino-9-((2R,4R)-2-(((tert-butyldimethylsilyl) oxy)methyl)-1,3-dioxolan-4-yl)-9H-purin-6-yl)octanamide (8d)
Compound 8d was prepared using the same procedure as for compound 8i; yield: 82%; 1H NMR (400 MHz, CDCl3) δ 8.98 (s, 1H), 8.09 (s, 1H), 6.35 (dd, J = 1.2, 4.8 Hz, 1H), 5.33 (br, 2H), 5.12 (t, J = 3.2 Hz, 1H), 4.42 (dd, J = 1.2, 10.0 Hz, 1H), 4.22 (dd, J = 4.8, 10.0 Hz, 1H), 3.92-3.86 (m, 2H), 2.77 (t, J = 7.6 Hz, 2H), 1.76-1.69 (m, 2H), 1.42-1.28 (m, 8H), 0.92-0.84 (m, 12H), 0.08 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 173.3, 159.9, 153.0, 150.0, 138.0, 115.9, 105.9, 79.4, 71.7, 63.5, 37.9, 31.8, 29.3, 29.2, 26.0, 25.1, 22.7, 18.7, 14.2, −5.21, −5.24; MS-ESI+ m/z 493(M+H+); HRMS-ESI+ calcd for C23H41N6O4Si (M+H+) 493.2946, found 493.2953.
N-(2-Amino-9-((2R,4R)-2-(((tert-butyldimethylsilyl) oxy)methyl)-1,3-dioxolan-4-yl)-9H-purin-6-yl)decanamide (8e)
Compound 8e was prepared using the same procedure as for compound 8i; yield: 85%; 1H NMR (400 MHz, CDCl3) δ 8.93 (s, 1H), 8.06 (s, 1H), 6.32 (dd, J = 1.2, 5.2 Hz, 1H), 5.32 (br, 2H), 5.09 (t, J = 3.2 Hz, 1H), 4.40 (dd, J = 1.2, 9.6 Hz, 1H), 4.18 (dd, J = 5.2, 9.6 Hz, 1H), 3.90-3.82 (m, 2H), 2.75 (t, J = 7.6 Hz, 2H), 1.74-1.66 (m, 2H), 1.36-1.16 (m, 12H), 0.88-0.82 (m, 12H), 0.05 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 173.4, 159.8, 153.1, 150.0, 138.0, 116.0, 105.9, 79.3, 71.7, 63.5, 37.9, 32.0, 29.6, 29.4, 26.0, 25.0, 22.8, 18.7, 14.3, −5.22, −5.25; MS-ESI+ m/z 521(M+H+); HRMS-ESI+ calcd for C25H45N6O4Si (M+H+) 521.3267, found 521.3266.
N-(2-Amino-9-((2R,4R)-2-(((tert-butyldimethylsilyl) oxy)methyl)-1,3-dioxolan-4-yl)-9H-purin-6-yl)dodecanamide (8f)
Compound 8f was prepared using the same procedure as for compound 8i; yield: 74%; 1H NMR (400 MHz, CDCl3) δ 9.09 (s, 1H), 8.08 (s, 1H), 6.32 (d, J = 4.4 Hz, 1H), 5.34 (br, 2H), 5.10 (t, J = 3.2 Hz, 1H), 4.39 (d, J = 10.0 Hz, 1H), 4.20 (dd, J = 5.6, 10.0 Hz, 1H), 3.92-3.84 (m, 2H), 2.70 (t, J = 7.6 Hz, 2H), 1.74-1.66 (m, 2H), 1.38-1.18 (m, 16H), 0.90-0.82 (m, 12H), 0.06 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 173.1, 159.9, 152.9, 150.0, 138.0, 116.0, 105.9, 79.4, 71.8, 63.4, 37.9, 32.1, 29.8, 29.7, 29.6, 29.5, 29.4, 26.0, 25.1, 22.8, 18.7, 14.3, −5.22, −5.25; MS-ESI+ m/z 549(M+H+); HRMS-ESI+ calcd for C27H49N6O4Si (M+H+) 549.3583, found 549.3579.
N-(2-Amino-9-((2R,4R)-2-(((tert-butyldimethylsilyl) oxy)methyl)-1,3-dioxolan-4-yl)-9H-purin-6-yl)tetradecanamide (8g)
Compound 8g was prepared using the same procedure as for compound 8i; yield: 82%; 1H NMR (400 MHz, CDCl3) δ 8.66 (br, 1H), 8.08 (s, 1H), 6.34 (dd, J = 1.2, 4.8 Hz, 1H), 5.14-5.11 (m, 3H), 4.42 (dd, J = 1.2, 9.6 Hz, 1H), 4.22 (dd, J = 5.2, 10.0 Hz, 1H), 3.93-3.86 (m, 2H), 2.79 (t, J = 7.6 Hz, 2H), 1.75-1.69 (m, 2H), 1.40-1.20 (m, 20H), 0.92-0.85 (m, 12H), 0.09 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 173.3, 159.7, 153.0, 150.0, 138.0, 116.0, 106.0, 79.4, 71.8, 63.5, 38.0, 32.1, 29.84, 29.71, 29.65, 29.55, 29.51, 26.1, 25.1, 22.9, 18.8, 14.3, −5.15, −5.18; MS-ESI+ m/z 577 (M+H+); HRMS-ESI+ calcd for C29H53N6O4Si (M+H+) 577.3889, found 577.3892.
N-(2-Amino-9-((2R,4R)-2-(((tert-butyldimethylsilyl) oxy)methyl)-1,3-dioxolan-4-yl)-9H-purin-6-yl)palmitamide (8h)
Compound 8h was prepared using the same procedure as for compound 8i; yield: 86%; 1H NMR (400 MHz, CDCl3) δ 10.30 (br, 1H), 8.11 (s, 1H), 8.07 (s, 1H), 7.20-6.80 (br, 1H), 6.35 (d, J = 4.8 Hz, 1H), 5.13 (t, J = 3.2 Hz, 1H), 4.46 (dd, J = 0.8, 9.6 Hz, 1H), 4.23 (dd, J = 5.2, 10.0 Hz, 1H), 3.94-3.86 (m, 2H), 2.97 (br, 2H), 1.77-1.70 (m, 2H), 1.44-1.20 (m, 24H), 0.92-0.84 (m, 12H), 0.09 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 176.8, 157.0, 153.6, 149.9, 138.0, 116.3, 106.0, 79.7, 71.9, 63.5, 37.4, 32.1, 29.9, 29.8, 29.78, 29.5, 26.1, 25.3, 22.8, 18.7, 14.3, −5.18, −5.20; MS-ESI+ m/z 605 (M+H+); HRMS-ESI+ calcd for C31H57N6O4Si (M+H+) 605.4210, found 605.4205.
(Z)-N-(2-Amino-9-((2R,4R)-2-(hydroxymethyl)-1,3-dioxolan-4-yl)-9H-purin-6-yl)octadec-8-enamide (9i)
To a solution of compound 8i (2.20 g, 3.49 mmol) in 50 mL of anhydrous THF was added Et3N-3HF (5.0 eq, 0.36 g, 4.60 mmol) at 0 °C under N2 atmosphere. After stirring for 12 h at rt, the reaction mixture was concentrated under reduced pressure. The residue was purified on silica gel column chromatography (CH2Cl2 to CH2Cl2: MeOH = 20:1 v/v) to give compound 9i (1.71 g, 3.32 mmol) in 95% yield. 1H NMR (400 MHz, DMSO-d6) δ 10.10 (s, 1H), 8.08 (s, 1H), 6.43 (br, 2H), 6.27 (dd, J = 1.2, 5.6 Hz, 1H), 5.38-5.29 (m, 2H), 5.14 (t, J = 6.4 Hz, 1H), 5.04 (t, J = 3.2 Hz, 1H), 4.49 (dd, J = 1.2, 9.6 Hz, 1H), 4.20 (dd, J = 5.6, 10.0 Hz, 1H), 3.59 (dd, J = 3.2, 6.0 Hz, 1H), 2.53-2.48 (m, 2H), 2.04-1.92 (m, 4H), 1.60-1.50 (m, 2H), 1.36-1.18 (m, 22H), 0.86-0.81 (m, 3H); 13C NMR (100 MHz, DMSO-d6) δ 171.6, 159.9, 153.7, 150.0, 137.7, 129.6, 116.8, 105.5, 78.7, 70.4, 61.1, 36.1, 31.3, 29.13, 29.09, 28.83, 28.73, 28.69, 28.62, 28.58, 26.62, 26.57, 24.81, 22.09, 13.95; MS-ESI+ m/z 517 (M+H+); HRMS-ESI+ calcd for C27H45N6O4 (M+H+) 517.3501, found 517.3497.
N-(2-Amino-9-((2R,4R)-2-(hydroxymethyl)-1,3-dioxolan-4-yl)-9H-purin-6-yl)acetamide (9a)
Compound 9a was prepared using the same procedure as for compound 9i; yield: 93%; 1H NMR (400 MHz, DMSO-d6) δ 9.87 (s, 1H), 8.16 (s, 1H), 7.27 (br, 2H), 6.30 (d, J = 3.6 Hz, 1H), 5.14 (t, J = 5.4 Hz, 1H), 5.05 (t, J = 3.2 Hz, 1H), 4.53 (dd, J = 1.2, 9.6 Hz, 1H), 4.22 (dd, J = 4.8, 9.6 Hz, 1H), 3.60 (m, 2H), 2.20 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 173.7, 156.1, 153.1, 150.0, 137.8, 115.5, 105.5, 79.0, 70.5, 61.1, 24.7; MS-ESI+ m/z 295 (M+H+); HRMS-ESI+ calcd for C11H15N6O4 (M+H+) 295.1148, found 295.1149.
N-(2-Amino-9-((2R,4R)-2-(hydroxymethyl)-1,3-dioxolan-4-yl)-9H-purin-6-yl)butyramide (9b)
Compound 9b was prepared using the same procedure as for compound 9i; yield: 94%; 1H NMR (400 MHz, DMSO-d6) δ 10.12 (s, 1H), 8.09 (s, 1H), 6.45 (br, 2H), 6.27 (d, J = 4.4 Hz, 1H), 5.14 (t, J = 6.0 Hz, 1H), 5.04 (t, J = 3.2 Hz, 1H), 4.51 (d, J = 9.6 Hz, 1H), 4.20 (dd, J = 5.2, 9.6 Hz, 1H), 3.59 (dd, J = 3.2, 5.2 Hz, 2H), 1.62-1.56 (m, 2H), 0.91 (t, J = 7.6 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 171.6, 156.0, 153.7, 150.0, 137.7, 116.9, 105.5, 78.7, 70.4, 38.0, 18.3, 13.6; MS-ESI+ m/z 323 (M+H+); HRMS-ESI+ calcd for C13H19N6O4 (M+H+) 323.1463, found 323.1462.
N-(2-Amino-9-((2R,4R)-2-(hydroxymethyl)-1,3-dioxolan-4-yl)-9H-purin-6-yl)hexanamide (9c)
Compound 9c was prepared using the same procedure as for compound 9i; yield: 96%; 1H NMR (400 MHz, CD3OD) δ 8.21 (s, 1H), 6.34 (dd, J = 1.2, 4.8 Hz, 1H), 5.16 (t, J = 2.8 Hz, 1H), 4.52 (dd, J = 1.2, 9.6 Hz, 1H), 4.25 (dd, J = 4.8, 10.0 Hz, 1H), 3.82-3.74 (m, 2H), 2.56 (t, J = 7.6 Hz, 2H), 1.75-1.67 (m, 2H), 1.40-1.30 (m, 4H), 0.92 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CD3OD) δ 174.6, 161.8, 154.6, 150.9, 140.0, 116.5, 107.1, 81.3, 72.5, 62.4, 38.3, 32.6, 26.1, 23.6, 14.1; MS-ESI+ m/z 351 (M+H+); HRMS-ESI+ calcd for C15H23N6O4 (M+H+) 351.1781, found 351.1775.
N-(2-Amino-9-((2R,4R)-2-(hydroxymethyl)-1,3-dioxolan-4-yl)-9H-purin-6-yl)octanamide (9d)
Compound 9d was prepared using the same procedure as for compound 9i; yield: 95%; 1H NMR (400 MHz, CD3OD) δ 8.22 (s, 1H), 6.36 (dd, J = 1.2, 5.2 Hz, 1H), 5.11 (t, J = 2.4 Hz, 1H), 4.51 (dd, J = 1.2, 10.0 Hz, 1H), 4.25 (dd, J = 5.6, 10.0 Hz, 1H), 3.82-3.74 (m, 2H), 2.57 (t, J = 7.2 Hz, 2H), 1.75-1.66 (m, 2H), 1.44-1.26 (m, 8H), 0.90 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CD3OD) δ 174.7, 161.9, 154.7, 151.1, 140.1, 116.6, 107.2, 81.3, 72.5, 62.4, 38.4, 33.0, 30.42, 30.35, 26.4, 23.8, 14.6; MS-ESI+ m/z 379 (M+H+); HRMS-ESI+ calcd for C17H27N6O4 (M+H+) 379.2091, found 379.2088.
N-(2-Amino-9-((2R,4R)-2-(hydroxymethyl)-1,3-dioxolan-4-yl)-9H-purin-6-yl)decanamide (9e)
Compound 9e was prepared using the same procedure as for compound 9i; yield: 97%; 1H NMR (400 MHz, CD3OD) δ 8.20 (s, 1H), 6.34 (dd, J = 1.2, 5.2 Hz, 1H), 5.11 (t, J = 2.4 Hz, 1H), 4.51 (dd, J = 1.2, 10.0 Hz, 1H), 4.25 (dd, J = 5.6, 10.0 Hz, 1H), 3.82-3.74 (m, 2H), 2.56 (t, J = 7.6 Hz, 2H), 1.74-1.68 (m, 2H), 1.42-1.24 (m, 12H), 0.90 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CD3OD) δ 174.6, 161.9, 154.6, 151.0, 140.0, 116.5, 107.2, 81.3, 72.5, 62.4, 38.4, 33.2, 30.75, 30.68, 30.56, 30.45, 26.4, 23.9, 14.6; MS-ESI+ m/z 407 (M+H+); HRMS-ESI+ calcd for C19H31N6O4 (M+H+) 407.2398, found 407.2401.
N-(2-Amino-9-((2R,4R)-2-(hydroxymethyl)-1,3-dioxolan-4-yl)-9H-purin-6-yl)dodecanamide (9f)
Compound 9f was prepared using the same procedure as for compound 9i; yield: 93%; 1H NMR (400 MHz, CD3OD) δ 8.37 (s, 1H), 6.52 (d, J = 4.0 Hz, 1H), 5.12 (t, J = 2.4 Hz, 1H), 4.49 (dd, J = 1.2, 10.0 Hz, 1H), 4.26 (dd, J = 5.2, 10.0 Hz, 1H), 3.84-3.76 (m, 2H), 2.48 (t, J = 7.2 Hz, 2H), 1.76-1.68 (m, 2H), 1.46-1.22 (m, 16H), 0.90 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CD3OD) δ 174.5, 157.7, 154.6, 152.5, 140.2, 116.9, 107.2, 81.6, 72.9, 62.3, 38.3, 33.2, 30.9, 30.8, 30.7, 30.6, 30.5, 26.8, 23.9, 14.6; MS-ESI+ m/z 435 (M+H+); HRMS-ESI+ calcd for C21H34N6O4Na (M+Na+) 457.2541, found 457.2538.
N-(2-Amino-9-((2R,4R)-2-(hydroxymethyl)-1,3-dioxolan-4-yl)-9H-purin-6-yl)tetradecanamide (9g)
Compound 9g was prepared using the same procedure as for compound 9i; yield: 93%; 1H NMR (400 MHz, CDCl3) δ 8.98 (br, 1H), 8.01 (s, 1H), 6.28 (d, J = 4.8 Hz, 1H), 5.45 (br, 2H), 5.21 (s, 1H), 4.49 (d, J = 9.6 Hz, 1H), 4.25 (dd, J = 5.2, 10.0 Hz, 1H), 3.97-3.88 (m, 2H), 2.68 (t, J = 7.2 Hz, 2H), 1.74-1.66 (m, 2H), 1.40-1.20 (m, 22H), 0.89 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 173.2, 159.8, 152.6, 149.7, 138.5, 115.4, 106.0, 79.8, 71.3, 61.6, 37.9, 33.9, 32.1, 29.88, 29.77, 29.72, 29.55, 25.1, 22.9, 14.3; MS-ESI+ m/z 463 (M+H+); HRMS-ESI+ calcd for C23H39N6O4 (M+H+) 463.3021, found 463.3027.
N-(2-Amino-9-((2R,4R)-2-(hydroxymethyl)-1,3-dioxolan-4-yl)-9H-purin-6-yl)palmitamide (9h)
Compound 9h was prepared using the same procedure as for compound 9i; yield: 96%; 1H NMR (400 MHz, DMSO-d6) δ 9.84 (s, 1H), 8.16 (s, 1H), 7.26 (br, 1H), 6.29 (d, J = 4.4 Hz, 1H), 5.15 (t, J = 6.4 Hz, 1H), 5.05 (t, J = 3.2 Hz, 1H), 4.52 (d, J = 9.6 Hz, 1H), 4.21 (dd, J = 5.6, 9.6 Hz, 1H), 3.61-3.59 (m, 2H), 2.47 (t, J = 7.2 Hz, 2H), 1.53 (t, J = 5.8 Hz, 2H), 1.30-1.17 (m, 28H), 0.84 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 186.5, 156.1, 153.0, 150.0, 137.7, 115.5, 105.5, 78.9, 70.5, 61.1, 47.3, 36.2, 33.9, 31.3, 29.1, 29.0, 28.9, 28.7, 24.9, 22.1, 14.0; MS-ESI+ m/z 491 (M+H+); HRMS-ESI+ calcd for C25H43N6O4 (M+H+) 491.3340, found 491.3340.
(2S)-Ethyl 2-(((((2R,4R)-4-(2-amino-6-((Z)-octadec-8-enamido)-9H-purin-9-yl)-1,3-dioxolan-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate (10i)
To a solution of compound 9i (0.052 g, 0.100 mmol) in 10 mL of anhydrous THF was added phosphoramidate chloride 6 (2.0 eq, 0.058 g, 0.20 mmol) and N-methylimidazole (4.0 eq, 0.033 g, 0.40 mmol) at −78 °C under argon atmosphere. After stirring for 12 h at rt, the solution was concentrated under reduced pressure. The residue was dissolved in EtOAc (20 mL) and washed with aqueous 0.5 M HCl solution (5 mL × 2) and brine (10 mL). The solution was dried over Na2SO4 for 3 h at rt and filtered. The filtrate was concentrated under reduced pressure and purified on silica gel column chromatography (CH2Cl2: MeOH = 40:1 to 20:1 v/v) to give compound 10i (0.062 g, 0.081 mmol) in 81 % yield. 1H NMR (400 MHz, CDCl3) δ 8.37 (s, 0.5H), 8.34 (s, 0.5H), 7.93 (s, 1H), 7.33-7.10 (m, 5H), 6.35-6.33 (m, 1H), 5.39-5.30 (m, 3H), 5.07 (br, 2H), 4.60-4.55 (m, 1H), 4.47-4.25 (m, 3H), 4.18-4.10 (m, 2H), 4.03-3.91 (m, 1H), 3.84-3.65 (m, 1H), 2.84-2.77 (m, 2H), 2.02-1.98 (m, 4H), 1.78-1.71 (m, 2H), 1.44-1.20 (m, 27H), 0.89-0.86 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 173.7, 173.2, 159.8, 153.1, 150.7, 150.0, 138.0, 130.2, 129.8, 125.2, 120.3, 120.2, 116.1, 103.8, 103.7, 80.0, 70.8, 65.2, 64.9, 61.8, 50.4, 38.1, 32.1, 29.9, 29.7, 29.6, 29.5, 29.4, 27.4, 25.0, 22.9, 21.3, 21.2, 14.3; 31P NMR (162 MHz, CDCl3) δ 3.41, 3.15; MS-ESI+ m/z 772 (M+H+); HRMS-ESI+ calcd for C38H59N7O8P (M+H+) 772.4183, found 772.4157.
(2S)-Ethyl 2-(((((2R,4R)-4-(6-acetamido-2-amino-9H-purin-9-yl)-1,3-dioxolan-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate (10a)
Compound 10a was prepared using the same procedure as for compound 10i; yield: 68%; 1H NMR (400 MHz, CD3OD) δ 8.20 (s, 0.5H), 8.19 (s, 0.5H), 7.33-7.24 (m, 2H), 7.20-7.17 (m, 1H), 7.15-7.12 (m, 2H), 6.54-6.50 (m, 1H), 5.35-5.30 (m, 1H), 4.65-4.61 (m, 1H), 4.38-4.29 (m, 3H), 4.13-4.03 (m, 2H), 3.92-3.68 (m, 1H), 2.26 (br, 3H), 1.32-1.30 (m, 2H), 1.22-1.16 (m, 6H); 13C NMR (100 MHz, CD3OD) δ 186.0, 175.1, 157.8, 154.7, 152.1, 151.4, 141.5, 139.9, 130.9, 126.3, 121.5, 121.4, 117.0, 105.5, 104.9, 81.5, 72.3, 66.0, 62.5, 51.6, 24.8, 20.5, 14.6; 31P NMR (162 MHz, CD3OD) δ 5.19, 4.91; MS-ESI+ m/z 550 (M+H+); HRMS-ESI+ calcd for C22H29N7O8P (M+H+) 550.1823, found 550.1810.
(2S)-Ethyl 2-(((((2R,4R)-4-(2-amino-6-butyramido-9H-purin-9-yl)-1,3-dioxolan-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate (10b)
Compound 10b was synthesized using the same procedure as 10i; yield: 79%; 1H NMR (400 MHz, CD3OD) δ 8.21 (s, 0.5H), 8.19 (s, 0.5H), 7.34-7.21 (m, 2H), 7.20-7.18 (m, 1H), 7.16-7.10 (m, 2H), 6.56-6.53 (m, 1H), 5.35-5.30 (m, 1H), 4.64-4.60 (m, 1H), 4.39-4.30 (m, 3H), 4.14-4.03 (m, 2H), 3.92-3.69 (m, 1H), 2.47 (t, J = 7.2 Hz, 2H), 1.78 (m, 2H), 1.32-1.31 (m, 2H), 1.23-1.19 (m, 3H), 1.04-0.99 (m, 3H); 13C NMR (100 MHz, CD3OD) δ 175.7, 175.1, 157.8, 154.7, 152.2, 151.7, 141.5, 139.9, 130.8, 126.3, 121.5, 121.3, 117.0, 104.9, 81.5, 72.4, 66.0, 62.4, 51.6, 40.2, 30.9, 20.5, 14.6; 31P NMR (162 MHz, CD3OD) δ 5.23, 4.96; MS-ESI+ m/z 578 (M+H+); HRMS-ESI+ calcd for C24H33N7O8P (M+H+) 578.2130, found 578.2123.
(2S)-Ethyl 2-(((((2R,4R)-4-(2-amino-6-hexanamido-9H-purin-9-yl)-1,3-dioxolan-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate (10c)
Compound 10c was prepared using the same procedure as for compound 10i; yield: 75%; 1H NMR (400 MHz, CD3OD) δ 8.10 (s, 0.5H), 8.08 (s, 0.5H), 7.32-7.07 (m, 5H), 6.39-6.36 (m, 1H), 5.32-5.30 (m, 1H), 4.66-4.64 (m, 1H), 4.36-4.27 (m, 3H), 4.14-4.00 (m, 2H), 3.91-3.66 (m, 1H), 2.58-2.53 (m, 2H), 1.76-1.66 (m, 2H), 1.40-1.26 (m, 6H), 1.23-1.14 (m, 4H), 0.94-0.90 (m, 3H); 13C NMR (100 MHz, CD3OD) δ 175.0, 174.5, 161.9, 154.8, 152.1, 151.0, 139.6, 130.8, 126.2, 121.5, 121.2, 116.5, 104.8, 81.1, 72.0, 66.0, 62.4, 51.5, 38.4, 32.7, 26.1, 23.7, 20.6, 20.5, 14.6, 14.5; 31P NMR (162 MHz, CD3OD) δ 5.07, 4.82; MS-ESI+ m/z 606 (M+H+); HRMS-ESI+ calcd for C26H37N7O8P (M+H+) 606.2447, found 606.2436.
(2S)-Ethyl 2-(((((2R,4R)-4-(2-amino-6-octanamido-9H-purin-9-yl)-1,3-dioxolan-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate (10d)
Compound 10d was prepared using the same procedure as for compound 10i; yield: 72%; 1H NMR (400 MHz, CD3OD) δ 8.10 (s, 0.5H), 8.09 (s, 0.5H), 7.32-7.07 (m, 5H), 6.40-6.37 (m, 1H), 5.34-5.31 (m, 1H), 4.66-4.64 (s, 1H), 4.34-4.28 (m, 3H), 4.14-4.00 (m, 2H), 3.91-3.66 (m, 1H), 2.58-2.52 (m, 2H), 1.75-1.67 (m, 2H), 1.44-1.26 (m, 9H), 1.23-1.15 (m, 5H), 0.91-0.88 (m, 3H); 13C NMR (100 MHz, CD3OD) δ 175.0, 174.5, 161.9, 154.8, 152.1, 151.0, 139.6, 130.8, 126.2, 121.5, 121.2, 116.5, 104.8, 81.1, 72.0, 66.0, 62.4, 51.5, 38.4, 32.7, 26.1, 23.7, 20.6, 20.5, 14.6; 31P NMR (162 MHz, CD3OD) δ 5.10, 4.85; MS-ESI+ m/z 634 (M+H+); HRMS-ESI+ calcd for C28H41N7O8P (M+H+) 634.2758, found 634.2749.
(2S)-Ethyl 2-(((((2R,4R)-4-(2-amino-6-decanamido-9H-purin-9-yl)-1,3-dioxolan-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate (10e)
Compound 10e was prepared using the same procedure as for compound 10i; yield: 76%; 1H NMR (400 MHz, CD3OD) δ 8.09 (s, 0.5H), 8.08 (s, 0.5H), 7.31-7.07 (m, 5H), 6.39-6.35 (m, 1H), 5.33-5.30 (m, 1H), 4.66-4.63 (m, 1H), 4.35-4.27 (m, 3H), 4.14-4.00 (m, 2H), 3.92-3.65 (m, 1H), 2.58-2.53 (m, 2H), 1.76-1.66 (m, 2H), 1.42-1.26 (m, 13H), 1.23-1.15 (m, 5H), 0.91-0.88 (m, 3H); 13C NMR (100 MHz, CD3OD) δ 175.0, 174.5, 161.9, 154.7, 152.1, 151.0, 139.6, 130.8, 126.2, 121.5, 121.2, 116.4, 104.8, 81.1, 72.0, 66.0, 62.4, 51.5, 38.5, 33.2, 30.7, 30.5, 26.4, 23.9, 20.6, 20.5, 14.6; 31P NMR (162 MHz, CD3OD) δ 5.04, 4.79; MS-ESI+ m/z 662 (M+H+); HRMS-ESI+ calcd for C30H45N7O8P (M+H+) 662.3067, found 662.3062.
(2S)-Ethyl 2-(((((2R,4R)-4-(2-amino-6-dodecanamido-9H-purin-9-yl)-1,3-dioxolan-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate (10f)
Compound 10f was prepared using the same procedure as for compound 10i; yield: 70%; 1H NMR (400 MHz, CDCl3) δ 9.84 (br, 1H), 8.05 (s, 0.5H), 8.04 (s, 0.5H), 7.30-7.08 (m, 5H), 6.89 (br, 2H), 6.38 (m, 1H), 5.34-5.31 (m, 1H), 4.58-4.60 (s, 1H), 4.43-4.26 (m, 3H), 4.19-3.90 (m, 3H), 2.88 (br, 2H), 1.78-1.70 (m, 2H), 1.43-1.18 (m, 23H), 0.89-0.86 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 173.7, 156.8, 153.7, 150.7, 150.2, 137.9, 129.8, 125.2, 120.3, 120.2, 116.3, 103.8, 80.0, 71.3, 65.2, 61.8, 50.4, 47.9, 37.5, 32.1, 29.9, 29.8, 29.6, 25.3, 22.9, 21.1, 21.0, 14.3; 31P NMR (162 MHz, CDCl3) δ 3.40, 3.16; MS-ESI+ m/z 690 (M+H+); HRMS-ESI+ calcd for C32H49N7O8P (M+H+) 690.3379, found 690.3375.
(2S)-Ethyl 2-(((((2R,4R)-4-(2-amino-6-tetradecanamido-9H-purin-9-yl)-1,3-dioxolan-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate (10g)
Compound 10g was prepared using the same procedure as for compound 10i; yield: 72%; 1H NMR (400 MHz, CD3OD) δ 8.11 (s, 0.5H), 8.09 (s, 0.5H), 7.32-7.07 (m, 5H), 6.41-6.37 (m, 1H), 5.34-5.31 (m, 1H), 4.66-4.63 (m, 1H), 4.36-4.28 (m, 3H), 4.14-4.00 (m, 2H), 3.91-3.66 (m, 1H), 2.58-2.53 (m, 2H), 1.78-1.68 (m, 2H), 1.43-1.24 (m, 21H), 1.23-1.15 (m, 5H), 0.91-0.88 (m, 3H); 13C NMR (100 MHz, CD3OD) δ 175.1, 174.6, 162.0, 154.8, 152.1, 151.0, 139.7, 130.8, 126.2, 121.5, 121.3, 116.4, 104.9, 81.1, 72.0, 66.0, 62.4, 51.6, 38.5, 33.2, 30.95, 30.92, 30.89, 30.8, 30.6, 30.4, 26.4, 23.9, 20.6, 20.5, 14.6; 31P NMR (162 MHz, CD3OD) δ 5.14, 4.88; MS-ESI+ m/z 718 (M+H+); HRMS-ESI+ calcd for C34H53N7O8P (M+H+) 718.3703, found 718.3690.
(2S)-Ethyl 2-(((((2R,4R)-4-(2-amino-6-palmitamido-9H-purin-9-yl)-1,3-dioxolan-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate (10h)
Compound 10h was prepared using the same procedure as for compound 10i; yield: 78%; 1H NMR (400 MHz, CDCl3) δ 9.97 (br, 1H), 8.08 (s, 0.5H), 8.07 (s, 0.5H), 7.30-7.08 (m, 5H), 6.92 (br, 2H), 6.40-6.38 (m, 1H), 5.34-5.30 (m, 1H), 4.58-4.56 (s, 1H), 4.42-4.24 (m, 3H), 4.22-4.07 (m, 2H), 4.03-3.90 (m, 1H), 2.88 (br, 2H), 1.77-1.70 (m, 2H), 1.44-1.17 (m, 31H), 0.89-0.86 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 173.7, 156.8, 153.7, 150.7, 150.1, 137.9, 129.8, 125.1, 120.3, 120.2, 116.3, 103.7, 80.0, 71.3, 65.2, 61.7, 50.4, 39.3, 37.5, 32.1, 29.9, 29.84, 29.81, 29.76, 29.5, 25.3, 22.9, 21.1, 21.0, 14.3; 31P NMR (162 MHz, CDCl3) δ 3.42, 3.19; MS-ESI+ m/z 746 (M+H+); HRMS-ESI+ calcd for C36H57N7O8P (M+H+) 746.4012, found 746.4001.
Biological testing
Compounds were evaluated against HIV-1 and HBV as previously reported.8 Cytotoxicity was also determined in various cell systems as described previously.8
Supplementary Material
Acknowledgments
We thank Tyana Singletary for excellent technical assistance. This work was supported in part by NIH grant 5P30-AI-50409 (CFAR), and by the Department of Veterans Affairs. Dr. Schinazi is the founder and a major shareholder of RFS Pharma, LLC. Emory received no funding from RFS Pharma, LLC to perform this work and vice versa.
References and notes
- 1.a) Huryn DM, Okabe M. Chem Rev. 1992;92:1745–1768. [Google Scholar]; b) Garg R, Gupta SP, Gao H, Babu MS, Debnath AK, Hansch C. Chem Rev. 1999;99:3525–3601. doi: 10.1021/cr9703358. [DOI] [PubMed] [Google Scholar]; c) De Clercq E. J Clin Virol. 2001;22:73–78. doi: 10.1016/s1386-6532(01)00167-6. [DOI] [PubMed] [Google Scholar]; d) Mehellou Y, De Clercq E. J Med Chem. 2010;53:521–538. doi: 10.1021/jm900492g. [DOI] [PubMed] [Google Scholar]
- 2.Related reviews: Miller V. Antiviral Ther. 2001;6(suppl 2):1–9.Wainberg MA, White AJ. Antiviral Ther. 2001;6(suppl 2):11–19.Shafer RW. Clin Microbiol Rev. 2002;15:247–277. doi: 10.1128/CMR.15.2.247-277.2002.Gallant JE, Gerondelis PZ, Wainberg MA, Shulman NS, Haubrich RH, Clair MS, Lanier ER, Hellmann NS, Richman DD. Antiviral Ther. 2003;8:489–506.Deeks SG. Lancet. 2003;362:2002–2011. doi: 10.1016/S0140-6736(03)15022-2.Wainberg MA, Turner DJ. Acquir Immune Defic Syndr. 2004;37(suppl 1):S36–43. doi: 10.1097/01.qai.0000137005.63376.6e.Rezende LF, Prasad VR. Int J Biochem Cell Biol. 2004;36:1716–1734. doi: 10.1016/j.biocel.2004.02.025.
- 3.a) Richman DD, Fischl MA, Grieco MH. N Engl J Med. 1987;317:192–197. doi: 10.1056/NEJM198707233170402. [DOI] [PubMed] [Google Scholar]; b) Starnes MC, Cheng YC. J Biol Chem. 1987;262:988–991. [PubMed] [Google Scholar]; c) Larder B, Darby G, Richman D. Science. 1989;243:1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]; d) Petersen EA, Ramirez-Ronda CH, Hardy WD. J Infect Dis. 1995;171(suppl 2):S131–S139. doi: 10.1093/infdis/171.supplement_2.s131. [DOI] [PubMed] [Google Scholar]; e) Yahi N, Tamalet C, Tourres C, Tivoli N, Fantini J. J Biomed Sci. 2000;7:507–513. doi: 10.1007/BF02253366. [DOI] [PubMed] [Google Scholar]; f) Mocroft A, Phillips AN, Friis-Moller N, Colebunders R, Johnson AM, Hirschel B, Marc-Saint T, Staub T, Clotet B, Lundgren JD. Antiviral Ther. 2002;7:21–30. [PubMed] [Google Scholar]; g) Little SJ, Holte S, Routy JP, Daar ES, Markowitz M, Collier AC. N Engl J Med. 2002;347:385–394. doi: 10.1056/NEJMoa013552. [DOI] [PubMed] [Google Scholar]; h) Tamalet C, Fantini J, Tourres C, Yahi N. AIDS. 2003;17:2383–2388. doi: 10.1097/01.aids.0000076341.42412.59. [DOI] [PubMed] [Google Scholar]; i) Turner D, Brenner B, Wainberg MA. J Antimicrob Chemother. 2004;53:53–57. doi: 10.1093/jac/dkh009. [DOI] [PubMed] [Google Scholar]; j) Marcelin AG, Delaugerre C, Wirden M, Viegas P, Simon A, Katlama C, Calvez V. J Med Virol. 2004;72:162–165. doi: 10.1002/jmv.10550. [DOI] [PubMed] [Google Scholar]; k) Imamichi T. Curr Pharm Des. 2004;10:4039–4053. doi: 10.2174/1381612043382440. [DOI] [PubMed] [Google Scholar]; l) Martinez-Picado J, Martinez MA. Virus Res. 2008;134:104–123. doi: 10.1016/j.virusres.2007.12.021. [DOI] [PubMed] [Google Scholar]; m) Menéndez-Arias L. Virus Res. 2008;134:124–146. doi: 10.1016/j.virusres.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 3.For a recent review on the synthesis of nucleoside phosphate and phosphonate prodrugs: Pradere U, Garnier-Amblard EC, Coats SJ, Amblard F, Schinazi RF. Chem Rev. 2014 doi: 10.1021/cr5002035. Article asap.
- 4.Bondana L, Detorio M, Bassit L, Tao S, Montero CM, Singletary TM, Zhang H, Zhou L, Cho JH, Coats SJ, Schinazi RF. ACS Med Chem Lett. 2013;4:747–751. doi: 10.1021/ml4001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radi M, Adema AD, Daft JR, Cho JH, Hoebe EK, Alexander LEMM, Peters GJ, Chu CK. J Med Chem. 2007;50:2249–2253. doi: 10.1021/jm0612923. [DOI] [PubMed] [Google Scholar]
- 6.a) Agarwal HK, Chhikara BS, Hanley MJ, Ye G, Doncel GF, Parang K. J Med Chem. 2012;55:4861–4871. doi: 10.1021/jm300492q. [DOI] [PubMed] [Google Scholar]; b) Agarwal HK, Chhikara BS, Bhavaraju S, Mandal D, Doncel GF, Parang K. Mol Pharma. 2013;10:467–476. doi: 10.1021/mp300361a. [DOI] [PubMed] [Google Scholar]
- 7.McGuigan C, Thiery JC, Daverio F, Jiang WG, Davies G, Mason M. Bioorg Med Chem. 2005;13:3219–3227. doi: 10.1016/j.bmc.2005.02.041. [DOI] [PubMed] [Google Scholar]
- 8.Cho JH, Amblard F, Coats SJ, Schinazi RF. Tetrahedron. 2011;67:5487–5493. doi: 10.1016/j.tet.2011.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Schinazi RF, Sommadossi JP, Saalmann V, Cannon DL, Xie MW, Hart GC, Smith GA, Hahn EF. Antimicrob Agents Chemother. 1990;34:1061. doi: 10.1128/aac.34.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Stuyver LJ, Lostia S, Adams M, Mathew J, Pai BS, Grier J, Tharnish P, Choi Y, Chong Y, Choo H, Chu CK, Otto MJ, Schinazi RF. Antimicrob Agents Chemother. 2002;46:3854. doi: 10.1128/AAC.46.12.3854-3860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.