Abstract
Depressed sarcoplasmic reticulum (SR) calcium cycling, reflecting impaired SR Ca-transport and Ca-release, is a key and universal characteristic of human and experimental heart failure. These SR processes are regulated by multimeric protein complexes, including protein kinases and phosphatases as well as their anchoring and regulatory subunits that fine-tune Ca-handling in specific SR sub-compartments. SR Ca-transport is mediated by the SR Ca-ATPase (SERCA2a) and its regulatory phosphoprotein, phospholamban (PLN). Dephosphorylated PLN is an inhibitor of SERCA2a and phosphorylation by protein kinase A (PKA) or calcium-calmodulin-dependent protein kinases (CAMKII) relieves these inhibitory effects. Recent studies identified additional regulatory proteins, associated with PLN, that control SR Ca-transport. These include the inhibitor-1 (I-1) of protein phosphatase 1 (PP1), the small heat shock protein 20 (Hsp20) and the HS-1 associated protein X-1 (HAX1). In addition, the intra-luminal histidine-rich calcium binding protein (HRC) has been shown to interact with both SERCA2a and triadin. Notably, there is physical and direct interaction between these protein players, mediating a fine-cross talk between SR Ca-uptake, storage and release. Importantly, regulation of SR Ca-cycling by the PLN/SERCA interactome does not only impact cardiomyocyte contractility, but also survival and remodeling. Indeed, naturally occurring variants in these Ca-cycling genes modulate their activity and interactions with other protein partners, resulting in depressed contractility and accelerated remodeling. These genetic variants may serve as potential prognostic or diagnostic markers in cardiac pathophysiology.
1. INTRODUCTION
Cardiovascular disease is the leading cause of morbidity and mortality worldwide, with heart failure representing the fastest growing subcategory over the past decades. Aberrant Ca handling is a hallmark of heart failure, which is partially attributed to alterations in the function of the sarcoplasmic reticulum (SR). In cardiomyocytes, coordinated mobilization of cytosolic Ca by the SR is principally responsible for each cycle of cardiac contraction and relaxation. Ca is also an integral signaling molecule for numerous other cellular processes including survival and cell death. As such, the dysregulation of Ca handling observed in heart failure is linked to impaired cardiac muscle performance as well as tissue viability.
The level of intracellular Ca is regulated by a balance between the release of Ca into the cytosol and the removal of Ca by the combined action of several proteins in the cell. Cytosolic Ca is sequestered into the SR lumen by the sarcoplasmic reticulum Ca-ATPase (SERCA2a), whose activity is reversibly regulated by phospholamban (PLN), a 52 amino acid phosphoprotein [1]. Dephosphorylated PLN interacts with SERCA2a and inhibits the pumping activity, whereas phosphorylation of PLN by PKA and CAMKII during β-adrenergic stimulation relieves the inhibitory effects and augments the contractile parameters. Restoration of contractility to basal levels is mediated by protein phosphatase 1 (PP1), which dephosphorylates PLN [2,3,4]. Interestingly, PP1 is regulated by two PKA phosphoproteins, inhibitor-1 (I-1) and the small heat shock protein 20, Hsp20. Phosphorylation of inhibitor-1 or Hsp20 during β-adrenergic stimulation results in increases in their inhibitory activity for PP1, allowing for amplification of the stimulatory effects of PKA-phosphorylation in cardiomyocytes [1].
Recently, two other regulators of SR Ca-transport were identified. One of them is the small anti-apoptotic HS-1 associated protein X-1 (HAX1), which interacts with PLN and regulates SR Ca-cycling and contractility [5]. The other one is the histidine-rich calcium binding protein, HRC, which interacts with SERCA2a as well as the ryanodine receptor Ca release complex [6], mediating regulation of both SR Ca-uptake and release [7].
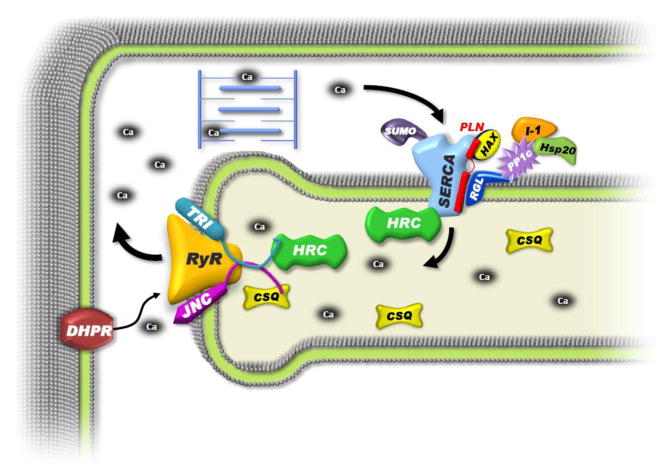
Thus, there is a multimeric SR Ca-transport ensemble composed of the regulatory partners: inhibitor-1/PP1/Hsp20, which are anchored to PLN through the regulatory subunit (RGL) of PP1 [8] and the transport complex of HAX/PLN/SERCA/HRC (Fig. 1).
Figure 1. Schematic Representation of the PLN/SERCA Interactome.
In the classical view of EC-coupling, membrane depolarization activates sarcolemmal DHPR, causing an influx of calcium that activates the RyR complex, releasing calcium from the SR store. This calcium triggers myofilament contraction and is then transported back into the SR by SERCA2a, which is regulated by its binding partner phospholamban (PLN). However, this model is expanding with the identification of novel interacting and regulatory partners generating a much larger “interactome.” At the center of this scheme is the classical PLN/SERCA-transport complex. HAX-1 binds to PLN and stabilizes its inhibitory interaction with SERCA2a. PLN also interacts with the RGL, the regulatory subunit of protein phosphatase 1 (PP1c), which anchors this enzyme along with its regulators inhibitor-1 (I-1) and Hsp20 to PLN/SERCA, thereby modulating SERCA2a function through PLN phosphorylation. SERCA2a is also regulated post transcriptionally by the small ubiquitin-related modifier (SUMO-1), improving SERCA2a protein stability and activity. The histidine rich calcium binding protein (HRC) is a multi-functional protein that binds and inhibits SERCA2a as well as modulates RyR function through its interaction with triadin (TRI). The SR calcium release complex is comprised of RyR, TRI, junctin (JNC) and calsequestrin (CSQ).
2. SR Calcium Cycling in Cardiac Contractility and Survival
2.1 Sarcoplasmic Reticulum Ca-ATPase (SERCA)
SERCA is a 110 kD transmembrane protein, that belongs to a family of highly conserved proteins. SERCA2a is primarily expressed in the heart and is the mediator of calcium uptake by the SR, initiating relaxation. In human and experimental heart failure, the expression levels and enzymatic activity of SERCA2a are significantly decreased and these may underlie the depressed SR Ca-cycling [1, 9]. The functional significance of alterations in SERCA2a levels has been examined using mouse models with overexpression or ablation of SERCA2a. Transgenic overexpression of SERCA2a resulted in significantly enhanced contractile parameters under baseline condition, which remained preserved under pressure overload without affecting mortality [10]. On the other hand, SERCA2a gene knock-out resulted in early embryonic lethality, while heterozygous mice exhibiting depressed function, survived without signs of heart disease [11].
Since early lethality of the targeted ablation of SERCA2a did not allow investigation of cardiac function, an inducible model with cardiac-specific deletion of SERCA2a was generated in order to gain insight into the mechanisms of SERCA2a deficiency [12]. Surprisingly, 4 weeks after inducible SERCA2a ablation in adult mice only moderately impaired cardiac function was observed with a relatively small reduction in both systolic and diastolic performance. These findings under major reduction of SERCA2a protein, indicate that SR-independent Ca mechanism(s) could compensate for SERCA2a depletion [12]. However, 7–10 weeks after inducible SERCA2a gene ablation, the mice developed substantially impaired myocardial relaxation and diastolic dysfunction and died from overt heart failure [12]. Recently, Heinis et al. [13] used isolated whole hearts from the inducible SERCA2a deficient mice to further delineate the mechanisms contributing to progressive SERCA2a deficiency. Surprisingly, heart performance was practically normal with SERCA2a protein levels at 32% of control hearts at one week after initiating down-regulation of SERCA2a [13]. Therefore, down-regulation of SERCA2a in the adult heart allows function to be maintained for a limited time before going into failure [13]. Although the underlying mechanisms are still unclear, the modest increases in the expression and activity of the L-type Ca channel, the Na/Ca exchanger, the plasma membrane Ca-ATPase [14] and elevated serum norepinephrine in SERCA2a deficient mice may collectively enhance trans-sarcolemmal Ca-transport and maintain the SERCA2a deficient cardiac function for a limited time.
In failing hearts, the depressed calcium cycling has been associated with reduced SERCA2a expression and increased inhibition by dephosphorylated PLN [1, 15]. Consequently, adenoviral-mediated gene transfer of SERCA2a and increases in pump levels resulted in improved contractility in animal models of heart failure as well as isolated failing human cardiac myocytes [16]. With the generation of adeno-associated gene vectors, SERCA2a expression in small and large animal models of heart failure indicated promise as a therapeutic target [17]. Indeed, the first clinical trial of SERCA2a gene therapy in humans, the CUPID trial (Calcium Upregulation by Percutaneous administration of gene therapy In cardiac Disease) was performed in 39 patients with New York Heart Association (NYHA) class III/IV heart failure and has provided promising results. The adeno-associated SERCA2a gene treated patients revealed improvement or stabilization in NYHA class, stress test, peak maximum oxygen consumption (VO2 max), cardiac biomarker N-terminal pro-hormone brain natriuretic peptide (NT-proBNP) levels, and left ventricular (LV) end-systolic volumes compared with the placebo group. Furthermore, there were no increases in adverse cardiovascular events, laboratory abnormalities or arrhythmias and the duration of hospitalizations was decreased [18]. More importantly, the expression of SERCA2a persisted after 3 years of initial gene delivery and continued to exert a long term cardiac benefit in these patients with advanced heart failure [19]. Thus, adeno-associated virus-mediated delivery of SERCA2a appears safe and effective for treating heart failure [18, 20]. Even though the CUPID trial demonstrated that SERCA2a is a critical target in the pathogenesis of heart failure, larger studies are needed to establish adeno-associated SERCA2a gene delivery as a treatment concept for advanced heart failure. As a result, this approach has now moved on to phase 3 trial, including an international study in 250 patients [19].
Interestingly, it has also been demonstrated that SERCA2a may be post-translationally modified by the small ubiquitin-related modifier (SUMO-1, Fig. 1), which binds SERCA2a and improves protein stability and activity [21]. In heart failure, SERCA2a expression levels and SUMOylation of SERCA2a are down-regulated. Indeed, knockdown of SUMO-1 by short hairpin RNA resulted in an accelerated deterioration of cardiac function, while adenovirus-mediated over-expression of SUMO1 in a small animal model of heart failure improved myocardial function and increased the survival rate similar to SERCA2a gene transfer [21]. By contrast, knockdown of SERCA2a resulted in severe contractile dysfunction, which could not be rescued by SUMO-1 overexpression [21].
The promising results in rodents prompted further evaluation of the effects of SUMO-1 gene transfer in a porcine model of ischemia induced heart failure [22]. SUMO-adeno-associated gene transfer via coronary infusion significantly improved cardiac function and prevented dilatation of LV volumes, compared to controls. Thus, these findings support further translational studies, using SUMO-1 gene transfer in human clinical trials either alone or in combination with SERCA2a for treatment of heart failure.
2.2 Phospholamban: The Reversible Regulator of SERCA2a and Cardiac Function
In cardiac muscle, the enzymatic activity of SERCA2a is regulated by a small phosphoprotein, called phospholamban. Phospholamban is a 52-amino acid protein that can be phosphorylated by PKA and CAMKII and dephosphorylated by protein phosphatase 1. This results in reversible regulation of SERCA2a and cardiac contractility (Fig. 2). PLN exists in equilibrium between its monomeric and pentameric forms. The functional inhibition by PLN can effectively be described by a simple schematic, wherein the binding of a PLN monomer inhibits SERCA2a function and the PLN pentamer acts as an inactive reservoir that is unable to interact with SERCA2a [23] (Fig. 2). Phosphorylation of PLN relieves the inhibition by blocking the binding interaction with SERCA2a as well as increasing pentamer stability [24, 25]. However, several recent studies have challenged these mechanistic concepts, proposing that while phosphorylation of PLN enhances pentamer stability, phosphorylated monomers can still interact with SERCA2a with a diminished affinity [26, 27]. Additionally, X-ray crystallography studies have shown that the PLN pentamer has the ability to interact with SERCA2a [28]. The functional importance of these observations has yet to be determined.
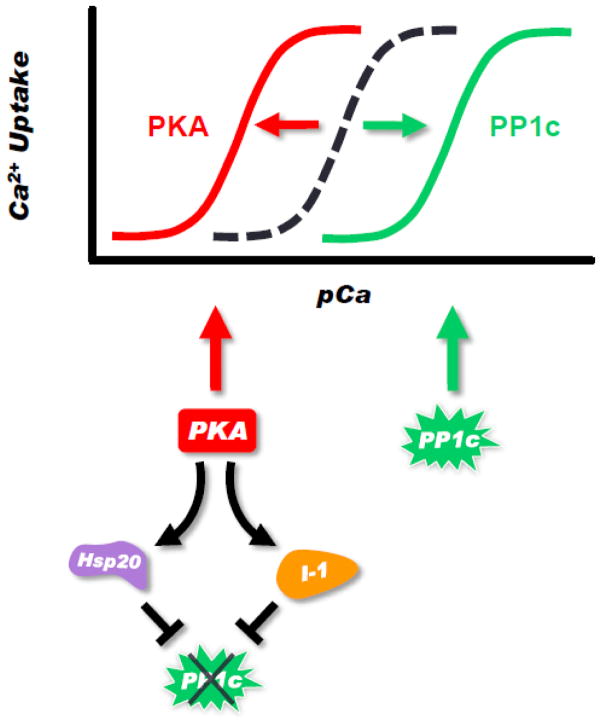
Figure 2. Amplification of PKA Effects at the Level of PLN.
PLN phosphorylation increases the apparent calcium affinity of SERCA2a. This is achieved by: a) PKA direct phosphorylation of PLN; and b) PKA phosphorylation of inhibitor-1 (I-1) and Hsp20, which inhibit PP1 and sustain PLN phosphorylation. Dephosphorylation of PLN occurs by protein phosphatase 1 (PP1c), which decreases the calcium affinity of SERCA2a. The balance of PKA and PP1 activities controls the phosphorylation status of PLN, leading to the leftward (PKA) or rightward (PP1) shifts in the calcium response curve of SERCA2a.
The role of PLN in cardiac function was elucidated by the generation and characterization of genetically-altered animal models. Cardiac-specific overexpression of PLN inhibited SR Ca uptake, Ca-load and contractile parameters [29]. However, isoproterenol stimulation and phosphorylation of PLN relieved these inhibitory effects [29]. Accordingly, PLN ablation resulted in significantly enhanced Ca cycling and myocardial contractility with no gross developmental abnormalities. The elevated contractile parameters were associated with increased affinity of SERCA2a for Ca [30]. Furthermore, it was found that the hyperdynamic cardiac function in PLN knockout (PLN-KO) mice persisted through the aging process [31]. The persistence of increased contractility over the long term did not induce any pathological or adverse functional consequences [31], suggesting that phospholamban may constitute an important target for treatment in heart disease.
In addition, analyses of wild type, heterozygous and homozygous PLN-KO mice at the sub-cellular, cellular, organ and intact animal levels revealed that the relative PLN levels correlated with the affinity of SERCA2a for Ca and with the rates of relaxation and contraction in cardiomyocytes, intact hearts and intact mice [30, 32]. These findings suggested that the PLN levels are key regulators of SR function and cardiac contractility. Indeed, increases in PLN levels by gene transfer in isolated cardiomyocytes or by cardiac overexpression in transgenic mice significantly depressed SERCA2a activity, intracellular calcium handling and contractile parameters [29, 33, 34]. Thus, the endogenous PLN levels are not sufficiently high to fully inhibit SERCA2a at the functional level. Accordingly, antisense strategies to decrease PLN levels in human myocytes from failing hearts improved sarcoplasmic reticulum Ca-transport, cardiomyocyte calcium handling and contractile parameters [33]. Furthermore, overexpression of a dominant negative mutant of PLN has been reported to enhance SERCA2a activity and contractility [35]. Taken together, these studies suggest that: a) PLN is a key regulator of SERCA2a activity; b) the PLN/SERCA2a ratio is a critical determinant of basal contractility; and c) there are some spare SERCA2a pumps that are not functionally inhibited by PLN.
The prominent role of PLN in regulation of Ca-cycling and excitation-contraction coupling was further supported by the identification of naturally occurring genetic variations in the human PLN gene. There have been several identified naturally occurring mutations in the coding region of PLN in heart failure patients [36, 37, 38, 39]. The R9C substitution in PLN is associated with decreased phosphorylation of the endogenous PLN and consequently, chronic inhibition of SERCA2a, leading to dilated cardiomyopathy [36]. The affected heterozygous carriers die at a young age [36]. Interestingly, no homozygous R9C carries were identified in this DCM population. Another PLN mutation (T116G) results in a stop codon (L39stop) and is associated with death in teen years for homozygous patients [37]. However, the heterozygous carriers exhibit asymptomatic cardiac hypertrophy without alterations in cardiac function. A third mutation results in deletion of amino acid 14 in PLN (R14del) and heterozygous carriers develop left ventricular dilation, dysfunction, episodic ventricular arrhythmias and death by mid-age [38]. Interestingly, this PLN mutation is present in 15% of dilated cardiomyopathy (DCM) and 12% of arrhythmiogenic right ventricular cardiomyopathy (ARVC) Dutch patients [40]. Overexpression of R14del-PLN in the mouse results in super-inhibition of SERCA2a, early cardiac pathology and death [38]. However, expression of this human mutant in the absence of endogenous WT-PLN results in its miss-routing to sarcolemma and interaction with NKA, leading to cardiac remodeling [41]. In addition, 2 more substitutions at Arg9 were identified in a heart failure cohort, namely R9L and R9H [39]. Thus, there are human mutations in the PLN coding region that are associated with predisposition to dilated cardiomyopathy and sudden death. Furthermore, mutations have also been identified in the promoter region of the PLN gene, which are associated with increased [42, 43] or decreased promoter activity, [44] presumably leading to alterations in PLN levels and possible remodeling or cardiomyopathy.
2.3 The Anti-apoptotic Protein HAX-1 as a Regulator of Cardiac Function
The HS-1 associated protein X-1 (HAX-1) was considered mainly a mitochondrial protein [1, 45] until recently, when we identified HAX-1 as a novel interacting partner of PLN (Fig. 3) [45]. Detailed biochemical studies identified the minimal binding domains between HAX-1 (residues 203–245) and PLN (residues 16–22). Interestingly, phosphorylation of PLN or increases in calcium levels result in dissociation of HAX-1 from PLN, indicating the physiological relevance of this interaction (Fig. 3). In HEK cells, HAX-1 localizes to mitochondria but upon co-expression of PLN, it also co-localizes with PLN at the endoplasmic reticulum [45].
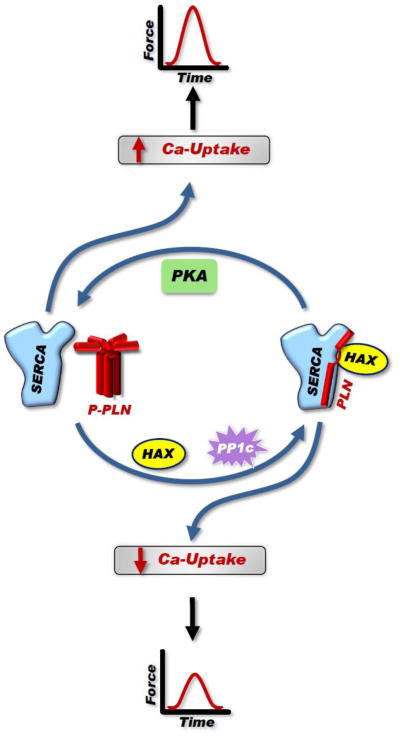
Figure 3. Regulation of SERCA2a Activity by PLN.
PLN exists in equilibrium between its monomeric and pentameric forms. Monomeric PLN is mostly the active inhibitory unit that binds to SERCA2a when dephosphorylated by PP1, while the pentamer acts as the monomeric reservoir. Some studies indicate that pentameric PLN may still interact with SERCA2a but not inhibit it. SERCA2a bound by monomeric PLN has reduced affinity for calcium in comparison to SERCA2a not bound by PLN. The PLN inhibition of SERCA2a results in decreased SR calcium load, which is available for release and decreased force of contraction. PLN phosphorylation favors pentamer formation, relieving SERCA2a inhibition and resulting in increased SR calcium load and enhanced contractility. Therefore, the balance of activity between PKA and PP1 modulate SERCA2a function and force generation. In addition, HAX-1 binds to PLN and promotes monomer formation, stabilizing the PLN interaction with SERCA2a and reducing calcium uptake and force.
Cardiac overexpression of HAX-1 resulted in increased abundance of PLN monomers, the inhibitory species, and decreased SERCA2a activity, which reflected depressed Ca-kinetics and contractile parameters (Fig. 3). However, the interaction of HAX-1 with PLN and its inhibitory effects were relieved upon PKA-phosphorylation of PLN by isoproterenol-stimulation (Fig. 3) [5]. Interestingly, human mutations associated with loss of HAX-1 protein have been identified and these result in severe neutropenia, a rare immunodeficiency disease [46]. In the mouse, ablation of HAX-1 associates with a short life-span due to progressive loss of neuronal cells [46]. HAX-1 has been reported to regulate cell survival in multiple tissues and it was recently shown that cardiac overexpression of HAX-1 reduced cardiac infarct size and improved contractile recovery after ischemia/reperfusion ex vivo and in vivo [47]. The protective effects of HAX-1 were mediated by formation of a regulatory complex between HAX-1 and Hsp90, resulting in inhibition of ER stress-induced cell death response through the IRE-1 signaling pathway. Moreover, HAX-1 recruited Hsp90 to PLN/SERCA2a, suggesting a “functional coupling” between ER stress signaling elements and calcium homeostasis in cardiac myocytes [47]. Notably, HAX-1 does not inhibit the other two ER response-elicited pathways (PERK or ATF-6). Thus, protection by HAX-1 may be partially mediated by the ER stress pathway promoting cell survival.
2.4 The Role of Inhibitor-1 in Cardiac Function
There are two main classes of Ser/Thr phosphatases (PP): PP1 and PP2. Their activity is primarily controlled by their subcellular localization and accessory subunits, organized in macromolecular signaling complexes. PP1 is mainly anchored to the SR by its regulatory RGL subunit, which physically interacts with PLN [8] and facilitates PP1 regulation of SR Ca-transport (Fig. 1 and 2). The PLN-type-1 phosphatase (PP1) activity is significantly elevated in human and experimental heart failure [48]. These increases have been suggested to be a contributing factor in the depressed cardiac function and remodeling. Indeed, cardiac overexpression of the catalytic subunit or PP1c in mouse models at similar levels as those observed in human failing hearts resulted in depressed function, remodeling, heart failure and early death [3]. Thus, it was suggested that inhibition of this enzyme by its endogenous inhibitor 2 (I-2) and inhibitor 1 (I-1) may hold promise for targeting the increased PP1 activity in heart failure. Inhibitor-2 is a thermostable phospho-protein, which was originally isolated from skeletal muscle [49]. Phosphorylation of I-2 causes a conformational change in the protein, which activates the PP1c/I-2 complex [50, 51]. Inhibitor-2 has been shown to increase cardiac contractility by augmenting Ca-cycling in a transgenic model [52]. Indeed, gene delivery of I-2 in a heart failure model prevented heart failure progression and prolonged survival [53]. In addition, increased activity of I-1 exhibited therapeutic promise in heart failure as well. The activity of I-1 is regulated by PKA-phosphorylation of its T35 residue, resulting in potent inhibition of PP1 and increased phosphorylation of PLN [4]. Inactivation of I-1 occurs through dephosphorylation of T35 by calcineurin (PP2B), and/or phosphorylation of S67 and T75 by PKC [54, 55].
The role of I-1 in heart function was elucidated by various genetically altered mouse models. The I-1 knock-out (KO) mouse presented with increased PP1 activity, had depressed cardiac contractility under basal conditions and attenuated β-adrenergic responses [3]. These effects were associated with decreased levels of phosphorylation on Serine 16 and Threonine 17 of PLN [3]. Accordingly, overexpression of a truncated (AA: 1–65) and constitutively active (T35D) form of I-1 (I-1c) was associated with inhibition of PP1 activity, increased Ser16 and Thr17 phosphorylation of PLN and attenuated hypertrophic response, delaying the onset of heart failure upon aortic constriction [4]. In addition, cardiac-specific and inducible expression of I-1c in the adult heart also revealed enhanced basal cardiac function, which was associated with increases in PLN phosphorylation levels [56]. Furthermore, under stress conditions (transverse aortic constriction, in vivo ischemia/reperfusion or chronic β-adrenergic stimulation), either conventional or inducible expression of I-1c was associated with increased PLN phosphorylation and increased SR Ca-transport [4, 56]. This enhanced SR calcium cycling improved the heart’s ability to accommodate the hypertrophic stimulus, delaying the progression from hypertrophy to failure and impact cell survival under stress conditions. Thus, targeting I-1c may be beneficial in alleviating the detrimental effects of heart failure, through specific modulation of the PLN-coupled PP1 activity.
Importantly, recent studies suggest that the increased activity of constitutively active I-1 by induction of its expression in the adult mouse heart, can maintain PLN hyper-phosphorylation through the aging process (up to 20 months) without any apparent cardiac remodeling or compromised survival [57]. In addition, gene-delivery studies using adeno-associated virus AAV9 indicated that long-term expression of I-1c in rat failing hearts maintains PLN phosphorylation, enhances contractility and attenuates remodeling [57]. These findings formed the basis for additional gene-transfer studies in a swine model of heart failure, induced by myocardial infarction [58]. Intracoronary injection of AAV9.I-1c prevented further deterioration of cardiac function and led to a decrease of scar size [58]. These studies indicate that inhibition of PP1 by active inhibitor-1 in heart failure may improve hemodynamic function and improve cardiac remodelling. In addition, a human polymorphism in inhibitor-1 is associated with attenuated contractile response of cardiomyocytes to β-adrenergic stimulation [59], suggesting that these carriers may specifically benefit from inhibitor-1 therapy in heart failure.
2.5 Role of Hsp20 in SR Ca-Cycling
Heat shock proteins (Hsps) are known to enhance cell survival under various stress conditions. We initially isolated and cloned Hsp20 from mouse LV myocytes in our efforts to distinguish between the phosphoproteins involved in the short-term beneficial effects of β-adrenergic stimulation vs. those mediating maladaptive responses over the long-term. Prolonged β-agonist stimulation of cardiomyocytes specifically induced the expression and phosphorylation of Hsp20 [60]. This 17-kDa chaperone protein belongs to a family of at least 10 different small Hsps, which transiently increase to serve as chaperones and enhance the survival of cells subjected to stress. Importantly, Hsp20 is the only small heat shock protein that is phosphorylated by PKA/PKG at Ser16 [60, 61]. Hsp20 is constitutively expressed in different tissues but is mainly abundant in smooth, skeletal and cardiac muscle. In smooth muscle, Hsp20 mediates relaxation, while its role in skeletal muscle is less well-defined [62].
Studies in cardiac muscle indicate that acute increases of Hsp20 levels or activity in isolated cardiomyocytes resulted in enhanced contractile parameters and Ca-transients [60]. Furthermore, cardiac specific overexpression of Hsp20 in transgenic mouse hearts significantly increased cardiac function in intact animals, and enhanced Ca-kinetics and contractile parameters in isolated cardiomyocytes. Accordingly, knockdown of Hsp20 by antisense RNA or microRNA-320 resulted in depressed contractility. The increased Ca-cycling by Hsp20 was associated with inhibition of the protein phosphatase 1 (PP1), associated with PLN, and specific increases in the phosphorylation of PLN, which relieved inhibition of SERCA2a (Fig. 1 and 2) [63]. Indeed, adenoviral expression of Hsp20 in cardiomyocytes from a mouse model expressing the non-phosphorylatable form of PLN (S16A/T17A) abrogated the increases in contractile parameters, confirming the notion that the inotropic effects of Hsp20 in cardiomyocytes are mediated through the PP1/PLN axis. The inhibitory effects of Hsp20 on PP1 were mediated by direct physical interaction between these two proteins [49]. Thus, regulation of PP1 by Hsp20 may provide another mechanism by which the cell achieves increases in basal contractile parameters and their responses to β-agonists (Hsp20 acts as an amplifier of β-adrenergic signaling) (Fig. 2). In addition, Hsp20 protects against β-agonist and hypoxic-induced apoptosis [61, 64], introducing an additional role of Hsp20 function in the heart. Contractile recovery post ischemia/reperfusion injury was also increased in hearts with increased Hsp20 expression and infarct size was significantly reduced in Hsp20 hearts. Interestingly, the levels of Hsp20 and its phosphorylation are significantly increased during ischemia/reperfusion and heart failure, which may represent a compensatory response to cardiac stress. Indeed, overexpression of a mutant Hsp20 form that cannot be phosphorylated (S16A) prevented the cardioprotective effects of Hsp20, resulting in increased apoptosis and infarct size [65]. Furthermore, we have recently identified a human mutation in Hsp20 (P20L), which was associated with reduced phosphorylation at S16 and abrogation of the Hsp20 beneficial effect on cardioprotection [66]. Given the potential of Hsp20 in enhancing both contractility and protection under pathological stress, it is interesting to propose that targeting Hsp20 in heart disease may have a potential dual benefit.
2.6 The Role of HRC in the Heart
The histidine-rich calcium binding protein (HRC) is a 170-kDa protein and it has a single isoform. HRC resides in the lumen of the SR in skeletal and cardiac muscle and has low affinity but high capacity for Ca-binding [67]. In early studies, HRC was shown to interact with triadin [68]. Triadin is a single span membrane protein, which was first identified in rabbit skeletal SR [68] and is part of the quaternary Ca-release complex (junctin, triadin, calsequestrin, and RyR) [69]. It has been demonstrated that HRC binds triadin’s luminal KEKE motif, which is capable of interacting with calsequestrin and the ryanodine receptor [70], implicating HRC in the regulation of SR Ca release [71]. Importantly, we discovered that HRC also interacts with SERCA2a (Fig. 1 and 4A) and regulates SR Ca-uptake in the heart [6, 7, 72].
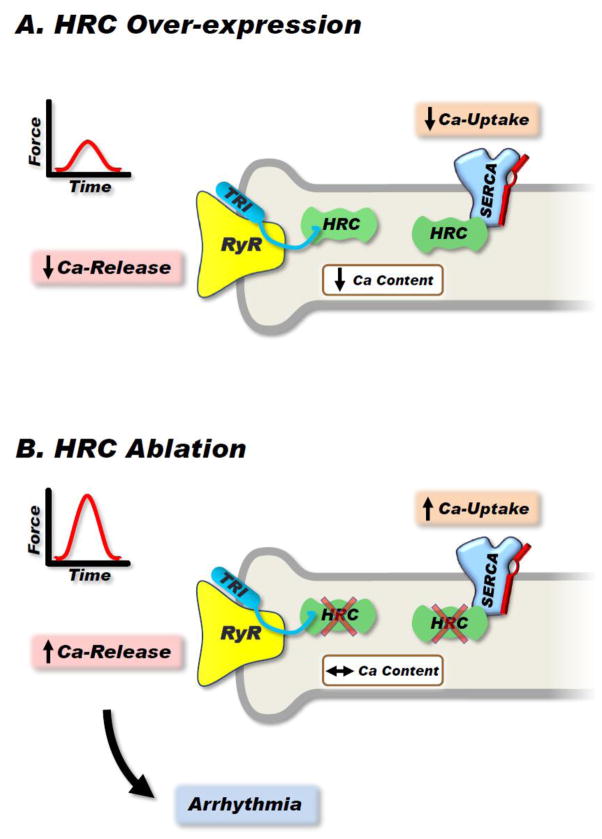
Figure 4. Effects of HRC on Calcium Handling.
HRC is a multifunctional protein that can bind and inhibit SERCA2a as well as modulate RyR function through its interaction with triadin. (A): Overexpression of HRC inhibits SERCA2a activity, reducing SR calcium uptake as well as controlling calcium release through RyR. The resultant effect is decreased contractility. (B): Ablation of HRC increases SERCA2a activity but also enhances RyR opening, leading to increased calcium leak and arrhythmia occurrence.
The binding domains of HRC for SERCA2a and triadin are different and calcium appears to regulate these interactions. Acute in vitro overexpression of HRC in adult rat cardiomyocytes or chronic cardiac specific overexpression of HRC in the mouse depressed SR Ca-uptake and relaxation [72]. The underlying mechanism involved inhibition of SERCA2a maximal rates of Ca-uptake. In the long-term, the inhibition of SR Ca-uptake led to the development of cardiac hypertrophy and congestive heart failure in the HRC transgenic mouse model [72]. Interestingly, HRC ablation (HRC-KO) enhanced cardiomyocyte Ca-cycling but induced aberrant Ca-release from the SR (Fig. 4B) [7]. This resulted in increased frequency of Ca-sparks and Ca-waves. Furthermore, after-contractions developed in cardiomyocytes under stress conditions of high-frequency stimulation (5 Hz) in the presence of isoproterenol. Upon transverse-aortic constriction (TAC), the HRC-KO mouse showed significantly deteriorated cell contractility and Ca-cycling, severe hypertrophy and fibrosis. The KOs developed pulmonary edema and exhibited decreased survival after TAC [7]. Thus, HRC appears to play an essential role in maintaining the integrity of cardiac function.
Importantly, we identified a human HRC polymorphism (Ser96Ala), which is associated with lethal arrhythmias in dilated cardiomyopathy (DCM) [73]. Heterozygosity (Ser96Ala) was associated with increased risk for sudden death and homozygosity (Ala96Ala) further augmented this risk in DCM [73]. Thus, Ala96Ala HRC may serve as an independent predictor of susceptibility to arrhythmogenesis in the setting of DCM. The underlying mechanisms include: a) aberrant Ca-cycling and depressed SR Ca-load; b) altered interactions of HRC with SERCA2a and triadin associated with impaired function and increased Ca-leak by the RyR; and c) increased Ser2814 phosphorylation of RyR by the CAMK II, leading to lethal arrhythmias under stress conditions [74], consistent with the phenotype in human carriers.
3. Conclusions
In summary, it has been 20 years since the mouse PLN gene was ablated and the key role of this protein was revealed in the heart. Subsequent studies have shown that our original simple understanding of PLN, serving as the sole physiological brake of SERCA2a and contractility, has evolved with identification of additional interacting partners. These include Hax-1, RGL, PP1, Inhibitor-1 and Hsp20. In addition, PLN/SERCA2a interacts with HRC in the lumen of the SR. Proper cardiac function is maintained through this “regulatory SR Ca-transport ensemble”, coordinating a fine crosstalk between SR Ca-uptake, storage and release as well as cell survival or death. Disturbances in the activity of any of these players in the SR interactome may contribute to depressed cardiac function and remodeling especially under stress conditions. Thus, restoring their impaired function may hold promise as therapeutic strategy in heart failure.
Highlights.
SR Ca-handling defects are a hallmark of human and experimental heart failure
PLN regulates SERCA2a activity and PLN/SERCA2a ratio is vital for basal contractility
SR Ca-transport is modulated with additional regulatory proteins associated with PLN
SERCA2a/PLN regulatome mediates cross-talk between SR Ca uptake, storage and release
Targeting SERCA2a/PLN is a promising strategy for treatment of heart failure
Acknowledgments
This work was supported by: NIH grants HL-26057 and HL-64018 to E. G. K. The authors are grateful to Ms. Erica Vanderbilt for her excellent assistance.
Footnotes
Disclosures:
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kranias EG, Hajjar RJ. Modulation of Cardiac Contractility by the Phopholamban/SERCA2a Regulatome. Circ Res. 2012;110:1646–1660. doi: 10.1161/CIRCRESAHA.111.259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steenaart NA, Ganim JR, Di Salvo J, Kranias EG. The phospholamban phosphatase associated with cardiac sarcoplasmic reticulum is a type 1 enzyme. Arch Biochem Biophys. 1992;293(1):17–24. doi: 10.1016/0003-9861(92)90359-5. [DOI] [PubMed] [Google Scholar]
- 3.Carr AN, Schmidt AG, Suzuki Y, del Monte F, Sato Y, Lanner C, et al. Type 1 phosphatase, a negative regulator of cardiac function. Mol Cell Biol. 2002;22(12):4124–35. doi: 10.1128/MCB.22.12.4124-4135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pathak A, del Monte F, Zhao W, Shultz JE, Lorenz JN, Bodi I, et al. Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase 1. Circ Res. 2005;96(7):756–66. doi: 10.1161/01.RES.0000161256.85833.fa. [DOI] [PubMed] [Google Scholar]
- 5.Zhao W, Waggoner JR, Zhang ZG, Lam CK, Han P, Qian J, et al. The anti-apoptotic protein HAX-1 is a regulator of cardiac function. Proc Natl Acad Sci. 2009;106(49):20776–81. doi: 10.1073/pnas.0906998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arvanitis DA, Vafiadaki E, Fan GC, Mitton BA, Gregory KN, del Monte F, et al. Histidine-rich Ca2+-binding protein interacts with sarcoplasmic reticulum Ca2+-ATPase. Am J Physiol Heart Circ Physiol. 2007;293:H1581–H1589. doi: 10.1152/ajpheart.00278.2007. [DOI] [PubMed] [Google Scholar]
- 7.Park CS, Chen S, Lee H, Cha H, Oh JG, Hong S, et al. Targeted ablation of the histidine-rich Ca(2+)-binding protein (HRC) gene is associated with abnormal SR Ca(2+)-cycling and severe pathology under pressure-overload stress. Basic Res Cardiol. 2013;108(3):344. doi: 10.1007/s00395-013-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vafiadaki E, Arvanitis DA, Sanoudou D, Kranias EG. Identification of a Protein Phosphatase 1/Phospholamban Interactome that is Regulated by cAMP-dependent Phosphorylation. PloS One. 2013;8(11):e80867. doi: 10.1371/journal.pone.0080867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer M, Schillinger W, Pieske B, Holubarsch C, Heilmann C, Posival H, et al. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation. 1995;92:778–84. doi: 10.1161/01.cir.92.4.778. [DOI] [PubMed] [Google Scholar]
- 10.He H, Giordano FJ, Hilal-Dandan R, Choi D, Rockman HA, McDonough PM, et al. Overexpression of the rat sarcoplasmic reticulum Ca2+ ATPase gene in the heart of transgenic mice accelerates calcium transients and cardiac relaxation. J Clin Invest. 1997;100:380–389. doi: 10.1172/JCI119544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji Y, Lalli MJ, Babu GJ, Xu Y, Kirkpatrick DL, Liu LH, et al. Disruption of a single copy of the SERCA2 gene results in altered Ca2+ homeostasis and cardiomyocyte function. J Biol Chem. 2000;275:38073–38080. doi: 10.1074/jbc.M004804200. [DOI] [PubMed] [Google Scholar]
- 12.Anderson KB, Birkeland JAK, Finsen AV, Louch WE, Sjaastad I, Wang Y, et al. Moderate heart dysfunction in mice with inducible cardiomyocyte-sepcific excision of the Serca2 gene. J Mol Cell Cardiol. 2009;47:180–7. doi: 10.1016/j.yjmcc.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Heinis FI, Andersson KB, Christensen G, Metzger JM. Prominent Heart Organ-Level Performance Deficits in a Genetic Model of Targeted Severe and Progressive SERCA2 Deficiency. PloS One. 2013;8(11):e79609. doi: 10.1371/journal.pone.0079609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louch WE, Hougen K, Mørk HK, Swift F, Aronsen JM, Sjaastad I, et al. Sodium accumulation promotes diastolic dysfunction in end-stage heart failure following Serca2 knockout. J Physiol. 2010;588:465–478. doi: 10.1113/jphysiol.2009.183517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dash R, Frank KF, Carr AN, Moravec CS, Kranias EG. Gender influences on sarcoplasmic reticulum Ca2+-handling in failing human myocardium. J Mol Cell Cardiol. 2001;33(7):1345–53. doi: 10.1006/jmcc.2001.1394. [DOI] [PubMed] [Google Scholar]
- 16.del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, et al. Improvement of survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca2+- ATPase in a rat model of heart. Circulation. 2001;104:1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zsebo KM, Yaroshinsky A, Rudy JJ, Wagner K, Greenberg B, Jessup M, et al. Long-Term Effects of AAV1/SERCA2a Gene Transfer in Patients With Severe Heart Failure: Analysis of Recurrent Cardiovascular Events and Mortality. Circ Res. 2014;114:101–108. doi: 10.1161/CIRCRESAHA.113.302421. [DOI] [PubMed] [Google Scholar]
- 20.Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase ½ clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E, et al. SUMO1-dependent modulation of SERCA2a in heart failure. Nature. 2011;477(7366):601–605. doi: 10.1038/nature10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tilemann L, Lee A, Ishikawa K, Aguero J, Rapti K, Santos-Gallego C, et al. SUMO-1 Gene Transfer Improves Cardiac Function in a Large-Animal Model of Heart Failure. Sci Transl Med. 2013;5:211ra159. doi: 10.1126/scitranslmed.3006487. [DOI] [PubMed] [Google Scholar]
- 23.MacLennan DH, Kimura Y, Toyofuku T. Sites of regulatory interaction between calcium ATPases and phospholamban. Ann N Y Acad Sci. 1998;853:31–42. doi: 10.1111/j.1749-6632.1998.tb08254.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Akin BL, Stokes DL, Jones LR. Cross-linking of C-terminal residues of phospholamban to the Ca2+ pump in cardiac sarcoplasmic reticulum to probe spatial and functional interactions within the transmembrane domain. J Biol Chem. 2006;281(20):14163–72. doi: 10.1074/jbc.M601338200. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Akin BL, Jones LR. Mechanism of reversal of phospholamban inhibition of the cardiac Ca2+-ATPase by protein kinase A and by anti-phospholamban monoclonal antibody 2D12. J Biol Chem. 2007;282(29):20968–76. doi: 10.1074/jbc.M703516200. [DOI] [PubMed] [Google Scholar]
- 26.Hou Z, Kelly EM, Robia SL. Phosphomimetic mutations increase phospholamban oligomerization and alter the structure of its regulatory complex. J Biol Chem. 2008;283(43):28996–9003. doi: 10.1074/jbc.M804782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pallikkuth S, Blackwell DJ, Hu Z, Hou Z, Zieman DT, Svensson B, et al. Phosphorylated phospholamban stabilizes a compact conformation of the cardiac calcium-ATPase. Biophys J. 2013;105(8):1812–21. doi: 10.1016/j.bpj.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glaves JP, Trieber CA, Ceholski DK, Stokes DL, Young HS. Phosphorylation and mutation of phospholamban alter physical interactions with the sarcoplasmic reticulum calcium pump. J Mol Biol. 2011;405(3):707–23. doi: 10.1016/j.jmb.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadambi VJ, Ponniah S, Harrer JM, Hoit BD, Dorn GW, 2nd, Walsh RA, et al. Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice. J Clin Invest. 1996;97:533–539. doi: 10.1172/JCI118446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, et al. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res. 1994;75:401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- 31.Slack JP, Grupp IL, Dash R, Holder D, Schmidt A, Gerst MJ, et al. The Enhanced Contractility of the Phospholamban-deficient Mouse Heart Persists with Aging. J Mol Cell Cardiol. 2001;33:1031–1040. doi: 10.1006/jmcc.2001.1370. [DOI] [PubMed] [Google Scholar]
- 32.Lorenz JN, Kranias EG. Regulatory effects of phospholamban on cardiac function in intact mice. Am J Physiol. 1997;273:H2826–H2831. doi: 10.1152/ajpheart.1997.273.6.H2826. [DOI] [PubMed] [Google Scholar]
- 33.del Monte F, Harding SE, Dec GW, Gwathmey JK, Hajjar RJ. Targeting phospholamban by gene transfer in human heart failure. Circulation. 2002;105:904–907. doi: 10.1161/hc0802.105564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brittsan AG, Carr AN, Schmidt AG, Kranias EG. Maximal inhibition of SERCA2 Ca(2+) affinity by phospholamban in transgenic hearts overexpressing a non-phosphorylatable form of phospholamban. J Biol Chem. 2000;275:12129–35. doi: 10.1074/jbc.275.16.12129. [DOI] [PubMed] [Google Scholar]
- 35.Ziolo MT, Martin JL, Bossuyt J, Bers DM, Pogwizs SM. Adenoviral gene transfer of mutant phospholamban rescues contractile dysfunction in failing rabbit myocytes with relatively preserved SERCA function. Circ Res. 2005;96:815–817. doi: 10.1161/01.RES.0000163981.97262.3b. [DOI] [PubMed] [Google Scholar]
- 36.Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, et al. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science. 2003;299(5611):1410–1413. doi: 10.1126/science.1081578. [DOI] [PubMed] [Google Scholar]
- 37.Haghighi K, Kolokathis F, Pater L, Lynch RA, Asahi M, Gramolini AO, et al. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest. 2003;111:869–876. doi: 10.1172/JCI17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haghighi K, Kolokathis F, Gramolini AO, Waggoner JR, Pater L, Lynch RA, et al. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc Natl Acad Sci USA. 2006;103:1388–1393. doi: 10.1073/pnas.0510519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medeiros A, Biagi DG, Sobreira TJ, de Oliveira PS, Negrao CE, Mansur AJ, et al. Mutations in the human phospholamban gene in patients with heart failure. Am Heart J. 2011;162:1088–95. doi: 10.1016/j.ahj.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 40.van der Zwaag PA, van Rijsingen IA, Asimaki A, Jongbloed JD, van Veldhuisen DJ, Wiesfeld AC, et al. Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur J Heart Fail. 2012;14(11):1199–207. doi: 10.1093/eurjhf/hfs119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haghighi K, Pritchard T, Bossuyt J, Waggoner JR, Yuan Q, Fan GC, et al. The human phospholamban Arg14-deletion mutant localizes to plasma membrane and interacts with the Na/K-ATPase. J Mol Cell Cardiol. 2012;52(3):773–82. doi: 10.1016/j.yjmcc.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minamisawa S, Sato Y, Tatsuguchi Y, Fujino T, Imamura S, Uetsuka Y, et al. Mutation of the phospholamban promoter associated with hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2003;304:1–4. doi: 10.1016/s0006-291x(03)00526-6. [DOI] [PubMed] [Google Scholar]
- 43.Haghighi K, Chen G, Sato Y, Fan GC, He S, Kolokathis F, et al. A human phospholamban promoter polymorphism in dilated cardiomyopathy alters transcriptional regulation by glucocorticoids. Hum Mutat. 2008;29:640–7. doi: 10.1002/humu.20692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medin M, Hermida-Prieto M, Monserrat L, Laredo R, Rodriguez-Rey JC, Fernandez X, et al. Mutational screening of phospholamban gene in hypertrophic and idiopathic dilated cardiomyopathy and functional study of the PLN -42 C>G mutation. Eur J Heart Fail. 2007;9:37–43. doi: 10.1016/j.ejheart.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Vafiadaki E, Sanoudou D, Arvanitis DA, Catino DH, Kranias EG, Kontrogianni-Konstantopoulos A. Phospholamban interacts with HAX-1, a mitochondrial protein with anti-apoptotic function. J Mol Biol. 2007;367:65–79. doi: 10.1016/j.jmb.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 46.Chao JR, Parganas E, Boyd K, Hong CY, Opferman JT, Ihle JN. Hax1-mediated processing of HtrA2 by Parl allows survival of lymphocytes and neurons. Nature. 2008;452(7183):98–102. doi: 10.1038/nature06604. [DOI] [PubMed] [Google Scholar]
- 47.Lam CK, Zhao W, Cai W, Vafiadaki E, Zhang Z, Ren X, et al. Novel Role of HAX-1 in Ischemic Injury Protection: Involvement of Hsp90. Circ Res. 2013;112(1):79–89. doi: 10.1161/CIRCRESAHA.112.279935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neumann J, Eschenhagen T, Jones LR, Linck B, Schmitz W, Scholz H, et al. Increased expression of cardiac phosphatases in patients with end-stage heart failure. J Mol Cell Cardiol. 1997;29(1):265–72. doi: 10.1006/jmcc.1996.0271. [DOI] [PubMed] [Google Scholar]
- 49.Huang FL, Glinsmann WH. Separation and characterization of two phosphorylase phosphatase inhibitors from rabbit skeletal muscle. Eur J Biochem. 1976;70:419–26. doi: 10.1111/j.1432-1033.1976.tb11032.x. [DOI] [PubMed] [Google Scholar]
- 50.Picking WD, Kudlicki W, Kramer G, Hardesty B, Vandenheede JR, Merlvede W, et al. Fluorescence studies on the interaction of inhibitor 2 and okadaic acid with the catalytic subunit of type 1 phosphoprotein phosphatases. Biochemistry. 1991;30:10280–7. doi: 10.1021/bi00106a028. [DOI] [PubMed] [Google Scholar]
- 51.Sakashita G, Shima H, Komatsu M, Urano T, Kikuchi A, Kikuchi K. Regulation of type 1 protein phosphatase/inhibitor-2 complex by glycogen synthase kinase-3beta in intact cells. J Biochem. 2003;133:165–71. doi: 10.1093/jb/mvg020. [DOI] [PubMed] [Google Scholar]
- 52.Kirchhefer U, Baba HA, Boknik P, Breeden KM, Mavila N, Bruchert N, et al. Enhanced cardiac function in mice overexpressing protein phosphatase inhibitor-2. Cardiovasc Res. 2005;68:98–108. doi: 10.1016/j.cardiores.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 53.Yamada M, Ikeda Y, Yano M, Yoshimura K, Nishino S, Aoyama H, et al. Inhibition of protein phosphatase 1 by inhibitor-2 gene delivery ameliorates heart failure progression in genetic cardiomyopathy. FASEB J. 2006;20(8):1197–9. doi: 10.1096/fj.05-5299fje. [DOI] [PubMed] [Google Scholar]
- 54.El-Armouche A, Bednorz A, Pamminger T, Ditz D, Didie M, Dobrev D, et al. Role of calcineurin and protein phosphatase-2A in the regulation of phosphatase inhibitor-1 in cardiac myocytes. Biochem Biophys Res Commun. 2006;346(3):700–6. doi: 10.1016/j.bbrc.2006.05.182. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez P, Mitton B, Nicolaou P, Chen G, Kranias EG. Phosphorylation of human inhibitor-1 at Ser67 and/or Thr75 attenuates stimulatory effects of protein kinase A signaling in cardiac myocytes. Am J of Physiol Heart Circ Physiol. 2007;293(1):H762–9. doi: 10.1152/ajpheart.00104.2007. [DOI] [PubMed] [Google Scholar]
- 56.Nicolaou P, Rodriguez P, Ren X, Zhou X, Qian J, Sadayappan S, et al. Inducible expression of active protein phosphatase-1 inhibitor-1 enhances basal cardiac function and protects against ischemia/reperfusion injury. Circ Res. 2009;104:1012–1020. doi: 10.1161/CIRCRESAHA.108.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pritchard TJ, Kawase Y, Haghighi K, Anjak A, Cai W, Jiang M, et al. Active Inhibitor-1 maintains protein hyper-phosphorylation in aging hearts and halts remodeling in failing hearts. PLoS One. 2013;8(12):e80717. doi: 10.1371/journal.pone.0080717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fish KM, Ladage D, Kawase Y, Karakikes I, Jeong D, Ly H, et al. AAV9.I-1c delivered via direct coronary infusion in a porcine model of heart failure improves contractility and mitigates adverse remodeling. Circ Heart Fail. 2013;6:310–7. doi: 10.1161/CIRCHEARTFAILURE.112.971325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen G, Zhou X, Nicolaou P, Rodriguez P, Song G, Mitton B, et al. A human polymorphism of protein phosphatase-1 inhibitor-1 is associated with attenuated contractile response of cardiomyocytes to beta-adrenergic stimulation. FASEB J. 2008;22(6):1790–6. doi: 10.1096/fj.07-097428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chu G, Egnaczyk GF, Zhao W, Jo SH, Fan GC, Maggio JE, et al. Phosphoproteome analysis of cardiomyocytes subjected to beta-adrenergic stimulation: identification and characterization of a cardiac heat shock protein p20. Circ Res. 2004;94(2):184–93. doi: 10.1161/01.RES.0000107198.90218.21. [DOI] [PubMed] [Google Scholar]
- 61.Fan GC, Ren X, Qian J, Yuan Q, Nicolaou P, Wang Y, et al. Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury. Circulation. 2005;111(14):1792–9. doi: 10.1161/01.CIR.0000160851.41872.C6. [DOI] [PubMed] [Google Scholar]
- 62.Dreiza CM, Komalavilas P, Furnish EJ, Flynn CR, Sheller MR, Smoke CC, et al. The small heat shock protein, HSPB6, in muscle function and disease. Cell Stress Chaperones. 2010;15(1):1–11. doi: 10.1007/s12192-009-0127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qian J, Vafiadaki E, Florea SM, Singh VP, Song W, Lam CK, et al. Small heat shock protein 20 interacts with protein phosphatase-1 and enhances sarcoplasmic reticulum calcium cycling. Circ Res. 2011;108:1429–1438. doi: 10.1161/CIRCRESAHA.110.237644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fan GC, Chu G, Kranias EG. Hsp20 and its cardioprotection. Trends Cardiovasc Med. 2005;15(4):138–41. doi: 10.1016/j.tcm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 65.Qian J, Ren X, Wang X, Zhang P, Jones WK, Molkentin JD, et al. Blockade of Hsp20 phosphorylation exacerbates cardiac ischemia/reperfusion injury by suppressed autophagy and increased cell death. Circ Res. 2009;105(12):1223–31. doi: 10.1161/CIRCRESAHA.109.200378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicolaou P, Knöll R, Haghighi K, Fan GC, Dorn GW, 2nd, Hasenfub G, et al. Human mutation in the anti-apoptotic heat shock protein 20 abrogates its cardioprotective effects. J Biol Chem. 2008;283(48):33465–71. doi: 10.1074/jbc.M802307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hofmann SL, Goldstein JL, Orth K, Moomaw CR, Slaughter CA, Brown MS. Molecular cloning of a histidine-rich Ca2+-binding protein of sarcoplasmic reticulum that contains highly conserved repeated elements. J Biol Chem. 1989;264:18083–90. [PubMed] [Google Scholar]
- 68.Sacchetto R, Turcato F, Damiani E, Margreth A. Interaction of triadin with histidine-rich Ca(2+)-binding protein at the triadic junction in skeletal muscle fibers. J Muscle Res Cell Motil. 1999;20(4):403–415. doi: 10.1023/a:1005580609414. [DOI] [PubMed] [Google Scholar]
- 69.Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem. 1997;272:23389–97. doi: 10.1074/jbc.272.37.23389. [DOI] [PubMed] [Google Scholar]
- 70.Lee HG, Kang H, Kim DH, Park WJ. Interaction of HRC (histidine-rich Ca2+-binding protein) and triadin in the lumen of sarcoplasmic reticulum. J Biol Chem. 2001;276:39533–8. doi: 10.1074/jbc.M010664200. [DOI] [PubMed] [Google Scholar]
- 71.Gyorke I, Hester N, Jones LR, Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J. 2004;86:2121–8. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gregory KN, Ginsburg KS, Bodi I, Hahn H, Marreez YM, Song Q, et al. Histidine-rich Ca binding protein: a regulator of sarcoplasmic reticulum calcium sequestration and cardiac function. J Mol Cell Cardiol. 2006;40(5):653–665. doi: 10.1016/j.yjmcc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 73.Arvanitis DA, Sanoudou D, Kolokathis F, Vafiadaki E, Papalouka V, Kontrogianni-Konstantopoulos A, et al. The Ser96Ala variant in histidine-rich calcium-binding protein is associated with life-threatening ventricular arrhythmias in idiopathic dilated cardiomyopathy. Eur Heart J. 2008;29(20):2514–25. doi: 10.1093/eurheartj/ehn328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh VP, Arvanitis DA, Ren X, Gao X, Haghighi K, Gilbert M, et al. Abnormal calcium cycling and cardiac arrhythmias associated with the human Ser96Ala genetic variant of histidine-rich calcium-binding protein. J Am Heart Assoc. 2013;2(5):e000460. doi: 10.1161/JAHA.113.000460. [DOI] [PMC free article] [PubMed] [Google Scholar]