Abstract
Aims
We aimed to clarify the associations of high-density lipoprotein cholesterol (HDL-C) subclasses with incident coronary heart disease (CHD) in two large primary prevention cohorts.
Methods
We measured cholesterol at baseline from the two major HDL subfractions (larger, more buoyant HDL2 and smaller, denser HDL3) separated by density gradient ultracentrifugation in 4114 (mean age 53.8 years; 64% female) African American participants from the Jackson Heart Study and 818 (mean age 57.3 years, 52% female) predominantly Caucasian participants from the Framingham Offspring Cohort Study. Multivariable adjusted hazard ratios (HRs) for HDL-C and its subclasses were derived from Cox proportional hazards regression models to estimate associations with incident CHD events including myocardial infarction, CHD death, and revascularization. Analyses were performed for each cohort separately and as a combined population.
Results
In models adjusted for cardiovascular risk factors for the combined population, HDL3-C (HR 0.76 per SD increase; 95% confidence interval (CI), 0.62–0.94; p = 0.01), rather than HDL2-C (HR 0.88 per SD; 95% CI, 0.72–1.09; p = 0.24) drove the inverse association of HDL-C (HR 0.79 per SD; 95% CI, 0.64–0.98; p = 0.03) with CHD. Similar associations were seen in multivariable analyses within each cohort including after adjusting for apolipoprotein A1 in the Jackson Heart Study.
Conclusion
Smaller, denser HDL3-C levels are primarily responsible for the inverse association between HDL-C and incident CHD in this diverse group of primary prevention subjects. These findings have important implications ranging from considerations of HDL biology to interpretations of clinical trials utilizing HDL-C therapeutics.
Keywords: High-density lipoprotein cholesterol, coronary heart disease, primary prevention
Introduction
Gofman’s work in the mid-20th century followed by the Framingham Heart Study confirmed the consistent, independent inverse association of cholesterol levels measured from high-density lipoprotein (HDL-C) with coronary heart disease (CHD) events.1–3 As a result, guidelines incorporate HDL-C as a variable in CHD risk calculation.4 A variety of post hoc analyses have suggested that raising HDL-C is associated with reductions in both cardiovascular events and atherosclerotic disease progression.5–8 However, recent large scale clinical trials evaluating therapies to raise HDL-C in those already on statin therapy have been discouraging.9–12 These recent data mandate a more nuanced analysis of the complex association between HDL and CHD.13,14
Underlying this complexity is the structural and functional heterogeneity of HDL. Investigations into the atheroprotective role of HDL have shown that in addition to “reverse cholesterol transport” there is evidence of anti-inflammatory, anti-oxidative, anti-apoptotic, anti-thrombotic, and endothelial function-enhancing properties.15,16 However, measures of HDL functionality are not clinically practical currently. Therefore, focus has shifted towards the HDL structure–function association given significant HDL heterogeneity and fluctuating metabolic functions secondary to differences in the proteome, lipidome, and microRNA content.17–21 A practical classification separates larger, more buoyant HDL2 from smaller, denser HDL3.
Previous studies examining the association of HDL subclasses with CHD have yielded conflicting results over whether HDL2-C or HDL3-C levels are the primary determinant of the inverse HDL-C association with CHD risk.22–25 As a result, questions remain over the clinical utility of these measurements.26 Notably, previous studies evaluating HDL2-C and HDL3-C were conducted in largely Caucasian and male populations.
We sought to evaluate the association of HDL-C subclasses with risk for incident CHD in African American men and women from the Jackson Heart Study (JHS) and in a subset of men and women from the predominantly Caucasian Framingham Offspring Cohort Study (FOCS).
Methods
The present study reports results of the HDL-C analysis from the two primary prevention cohorts within the Lipoprotein Investigators Collaborative (LIC). The LIC is described in the online Supplementary Material.
Study populations
The design and methods for the JHS have been described.27 Data and samples were collected from 5301 African American adults from the Jackson, Mississippi region with a primary objective to investigate the causes of cardiovascular disease in African Americans. Using fasting samples from the baseline visit in 2000–2004, lipoprotein cholesterol subfractions obtained by density gradient ultracentrifugation (VAP test, Atherotech, Birmingham, AL) and apolipoproteins A1 (apoA1) and B (apoB) were available in 4722 participants. We excluded 464 participants for missing variables of interest and another 144 participants who reported prevalent CHD. The primary analysis was performed in 4114 participants followed during 2000–2008 for incident CHD. Multiple imputation under a missing-at-random assumption confirmed robust results regardless of exclusions.
The design and methods of the FOCS have been described.28 The FOCS is a longitudinal study initiated in 1971 with 5135 Framingham Heart Study cohort offspring and offsprings’ spouses who were continuously followed for the development of CHD. As part of an exploratory analysis, we evaluated stored serum specimens from the sixth examination (1996–1997) of 818 randomly-selected FOCS participants who were followed for incident CHD over the subsequent eight years.
CHD was a composite of myocardial infarction (MI), coronary death, and revascularization (surgical bypass and percutaneous intervention). Events were obtained through a combination of active and passive ascertainment with adjudication by independent reviewers as previously described.27,29
Measurement of lipoproteins
Frozen (−70°C) serum specimens from JHS and FOCS were sent to the testing laboratory (Atherotech, Birmingham, AL) on dry ice by overnight express and kept frozen at −70°C until analysis. We measured cholesterol from the two major HDL subfractions (larger, more buoyant HDL2 and smaller, denser HDL3) and traditional lipid panel parameters (total cholesterol (TC), HDL-C, low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL-C)) separated by single vertical spin density gradient ultracentrifugation (Vertical Auto Profile (VAP) procedure) as detailed previously and in the online Supplementary Material.30 In the JHS, apoA1 and apoB were measured in the same laboratory by immunoassay (Abbott Architect/C8000); however, apoA1 and apoB were not available for the FOCS.
Statistical analyses
Across both cohorts, between those with and those without incident CHD, categorical variables were compared using Fisher exact or chi-square testing where appropriate. Continuous variables were reported as means with standard deviations or medians with 25th and 75th percentiles and compared by Student’s t-test or Kruskal–Wallis where appropriate. Restricted cubic spline curves adjusted for age, sex, BMI, smoking, systolic blood pressure (SBP) and diastolic blood pressure (DBP), lipid-altering medications, and diabetes were created to assess linearity assumptions.
We estimated hazard ratios (HRs) of HDL-C, HDL2-C, and HDL3-C for CHD using pre-specified multivariable-adjusted Cox proportional hazards models. In the JHS, all covariate results were from the baseline examination. Model 1 was adjusted for age, sex, BMI, smoking, SBP, DBP, lipid-altering medications, and diabetes; Model 2 included Model 1 adjustors and apoA1; Model 3 included Model 1 adjustors + apoA1 and apoB; and Model 4 was adjusted for Model 1 adjustors + non-HDL-C.
In the FOCS, all covariate values were from the sixth examination. Similar to JHS, Model 1 was adjusted for age, sex, BMI, smoking, SBP, DBP, lipid-altering medications and diabetes. Model 2 was based on the 2008 Framingham risk factors including age, sex, SBP, antihypertensive medications, smoking, and diabetes.29 Model 3 included Model 2 variables, but substituted diagnosed hypertension for antihypertensive medications. Similar to JHS, Model 4 included Model 1 adjustors + non-HDL-C.
For Model 1 and Model 4 above, homogeneity between each JHS and FOCS Cox regression coefficient was assessed by the Q-statistic. After verifying homogeneity (p > 0.25), a fixed-effect meta-analysis on the Cox coefficients was conducted to assess the overall effect of HDL-C subclasses on CHD.
Forest plots were created to visualize HRs and two-sided 95% confidence intervals (CIs) for a one standard deviation increase in each of the HDL variables for each study individually and for the meta-analysis.
Statistical analyses were coordinated across centers within the LIC study group using SAS V.9.3 (Cary, NC) and Stata V.13.1 (College Station, TX). All p-values are two-sided using an alpha of 0.05.
Results
Baseline characteristics
The baseline characteristics and lipids for each population are shown in Table 1. The online Supplementary Material includes an analysis of JHS participants including those with prevalent CHD (n = 4258).
Table 1.
Baseline risk factors and lipids of the Jackson Heart Study and Framingham Offspring Cohort Study participants.
| Variable | Jackson Heart Study (n = 4114) | Framingham Offspring Cohort (n = 818) | Combined populations (n = 4932) | p-valuea |
|---|---|---|---|---|
| Males | 1463 (36%) | 395 (48%) | 1858 (38%) | <0.0001 |
| Age, years | 53.8 (12.8) | 57.3 (9.5) | 54.4 (12.3) | <0.0001 |
| Diabetes | 678 (16%) | 58 (7%) | 736 (15%) | <0.0001 |
| Current smokers | 512 (12%) | 118 (14%) | 630 (13%) | 0.12 |
| Body mass index, kg/m2 | 31.7 (7.3) | 27.7 (5.0) | 31.1 (7.1) | <0.0001 |
| Waist circumference, cm | 100.4 (16.4) | 96.6 (13.1) | 99.8 (15.9) | <0.0001 |
| Systolic blood pressure, mmHg | 126.5 (18.0) | 126.2 (18.1) | 126.4 (18.0) | 0.67 |
| Diastolic blood pressure, mmHg | 79.1 (10.4) | 74.8 (9.3) | 78.4 (10.4) | <0.0001 |
| Lipid-altering medicationsb | 443 (11%) | 66 (8%) | 509 (10%) | 0.02 |
| Total cholesterol, mg/dl | 198.8 (40.5) | 201.5 (37.2) | 199.3 (40.0) | 0.06 |
| HDL-C, mg/dl | 53.6 (14.4) | 50.4 (13.2) | 53.1 (14.3) | <0.0001 |
| HDL2-C, mg/dl | 13.6 (6.4) | 11.1 (5.0) | 13.2 (6.3) | <0.0001 |
| HDL3-C, mg/dl | 40.1 (8.7) | 39.2 (8.7) | 39.9 (8.7) | 0.01 |
| Direct LDL-C, mg/dl | 122.8 (36.2) | 128.0 (32.0) | 123.7 (35.6) | <0.0001 |
| Triglycerides, mg/dl | 90 (67, 126) | 155 (126, 187) | 100 (72, 143) | 0.0001 |
| Non-HDL-C, mg/dl | 145.2 (38.6) | 151.2 (35.0) | 146.2 (38.1) | <0.0001 |
| apoA1, mg/dl | 147.8 (27.2) | – | – | – |
| apoB, mg/dl | 98.0 (24.6) | – | – | – |
| TC/HDL-C | 3.9 (1.1) | 4.2 (1.1) | 4.0 (1.1) | <0.0001 |
| apoB/apoA1 | 0.77 (0.37) | – | – | – |
Values are n (%), mean (SD), or median (25th, 75th percentile) where appropriate;
p-value for heterogeneity between populations;
Lipid-altering medications include statins, bile sequestrants, niacin derivatives, and fibric acid derivatives;
HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TC: total cholesterol; apoA1: apolipoprotein A1; apoB: apolipoprotein B
CHD events
In the 4114 participants from the JHS, there were 112 CHD events over a median follow-up of 5.7 years consisting of 21 CHD deaths, 63 MIs, and 28 revascularizations. In this sample of 818 participants from the FOCS, there were 34 events over a median follow-up of 8.0 years, including one CHD death, 18 MIs, and 15 revascularizations.
Association of HDL-C subclasses with CHD in JHS
In JHS, comparing unadjusted baseline characteristics (Table 2), those with CHD events were older with a lower BMI, had a higher SBP, lower DBP, higher prevalence of diabetes and higher use of lipid-lowering medications. Comparing unadjusted baseline lipids (Table 2), those with CHD events had lower HDL3-C and higher apoB levels, with no significant difference in HDL-C or HDL2-C.
Table 2.
Unadjusted baseline cardio-metabolic risk factors and lipids between those with and those without incident coronary heart disease in the Jackson Heart Study and the Framingham Offspring Cohort Study.
| Jackson Heart Study
|
Framingham Offspring Cohort Study
|
|||||
|---|---|---|---|---|---|---|
| Variable | No CHD (n = 4002) |
CHD (n = 112) |
p-valuea | No CHD (n = 784) |
CHD (n = 34) |
p-valuea |
| Males | 1,417 (35%) | 46 (41%) | 0.22 | 371 (47%) | 24 (71%) | 0.008 |
| Age, years | 53.5 (12.7) | 64.8 (9.9) | <0.0001 | 57.2 (9.5) | 61.6 (8.3) | 0.007 |
| Diabetes | 629 (16%) | 49 (44%) | <0.0001 | 52 (7%) | 6 (18%) | 0.03 |
| Current smoking status | 498 (12%) | 14 (13%) | 0.99 | 110 (14%) | 8 (24%) | 0.13 |
| Body mass index, kg/m2 | 31.8 (7.3) | 29.8 (6.1) | 0.001 | 27.7 (5.0) | 29.2 (4.1) | 0.07 |
| Waist circumference, cm | 100.4 (16.5) | 100.3 (13.5) | 0.96 | 96.4 (13.1) | 101.9 (11.9) | 0.02 |
| Systolic blood pressure, mmHg | 126.2 (17.9) | 135.1 (20.8) | <0.0001 | 125.8 (18.1) | 134.1 (16.8) | 0.009 |
| Diastolic blood pressure, mmHg | 79.1 (10.4) | 76.6 (11.9) | 0.03 | 74.8 (9.3) | 76.4 (9.2) | 0.33 |
| Lipid-altering medicationsb | 416 (10%) | 27 (24%) | <0.0001 | 59 (8%) | 7 (21%) | 0.02 |
| Total cholesterol, mg/dl | 198.7 (40.3) | 202.5 (47.5) | 0.40 | 201.0 (36.8) | 213.7 (44.8) | 0.05 |
| HDL-C, mg/dl | 53.7 (14.4) | 52.3 (15.3) | 0.33 | 50.7 (13.2) | 42.9 (10.3) | 0.0007 |
| HDL2-C, mg/dl | 13.5 (6.4) | 13.9 (6.6) | 0.58 | 11.2 (5.1) | 8.8 (3.0) | <0.0001 |
| HDL3-C, mg/dl | 40.1 (8.7) | 38.5 (9.4) | 0.05 | 39.5 (8.7) | 34.2 (7.5) | 0.0005 |
| Direct LDL-C, mg/dl | 122.8 (36.1) | 125.5 (40.2) | 0.42 | 127.2 (31.5) | 145.3 (37.1) | 0.001 |
| Triglycerides, mg/dl | 90 (67, 126) | 106 (81, 143) | 0.0002 | 154 (126, 185) | 175 (143, 207) | 0.003 |
| Non-HDL-C, mg/dl | 145.0 (38.5) | 150.2 (42.2) | 0.16 | 150.3 (34.6) | 170.8 (39.8) | 0.0008 |
| apoA1, mg/dl | 147.8 (27.2) | 146.9 (27.5) | 0.71 | – | – | – |
| apoB, mg/dl | 97.9 (24.5) | 102.9 (26.6) | 0.03 | – | – | – |
| TC/HDL-C | 3.9 (1.1) | 4.1 (1.1) | 0.15 | 4.2 (1.1) | 4.9 (1.2) | <0.0001 |
| apoB/apoA1 | 0.77 (0.37) | 0.82 (0.37) | 0.19 | – | – | – |
Values are n (%), mean (SD), or median (25th, 75th percentile) where appropriate;
p-value for heterogeneity between populations;
Lipid-altering medications include statins, bile sequestrants, niacin derivatives, and fibric acid derivatives.
Formal tests of linearity supported a linear association of HDL subclasses with CHD risk in each study. Spline curves in the JHS (Supplementary Material) demonstrate the overall trend of an inverse linear association of HDL-C and significant inverse linear association of HDL3-C with CHD. Forest plots are shown in Figure 1(a). In Model 1, the trend towards an inverse association of HDL-C with CHD (p = 0.13) was driven by the significant associations of HDL3-C with less CHD (p = 0.04), while HDL2-C was not associated with CHD (p = 0.61). When adjusting for apoA1 (Model 2), HDL3-C remained significantly associated with less CHD (p = 0.04), while associations of total HDL-C and HDL2-C with CHD were not significant (p ≥ 0.38). When accounting for apoB (Model 3), the HDL3-C association with CHD was attenuated (p = 0.10). Finally, substituting non-HDL-C for apolipoproteins in Model 4, HDL3-C (p = 0.04) accounted for the trend towards an inverse association of HDL-C with CHD events (p = 0.11).
Figure 1.
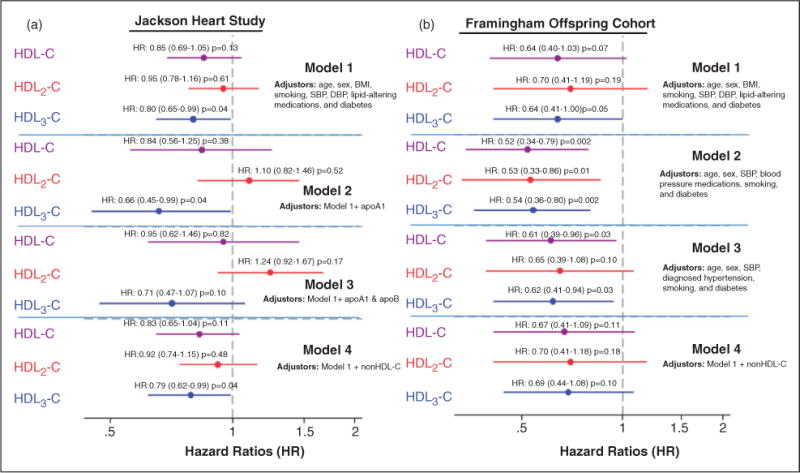
Individual study hazard ratios (HRs) of HDL-C, HDL2-C and HDL3-C for coronary heart disease events. (a) Jackson Heart Study. (b) Framingham Offspring Cohort Study. HRs are point estimates (95% confidence intervals) per SD increase in cholesterol. HR: hazard ratio; HDL-C: high-density lipoprotein cholesterol; SBP: systolic blood pressure; DBP: diastolic blood pressure; apoA1: apolipoprotein A1; apoB: apolipoprotein B
Association of HDL-C subclasses with CHD in FOCS
Comparing unadjusted baseline characteristics in the FOCS (Table 2), those with CHD were older, had higher proportions of males and diabetes, higher waist circumference, higher SBP, and higher use of lipid-lowering medications. Comparing baseline lipids, those with CHD had lower HDL2-C, lower HDL3-C, and higher LDL-C, non-HDL-C, and TC/HDL-C ratio.
Spline curves in the FOCS (Supplementary Material) demonstrate the overall trend of an inverse linear association of HDL-C and both subclasses with CHD. Forest plots of HRs (Figure 1(b)) from the various multivariable-adjusted models show an inverse association between one standard deviation increase in HDL3-C and CHD (p = 0.05). The association was consistent across models, but lost significance when adjusting for non-HDL-C in Model 4 (p = 0.10). When adjusting for Framingham variables in Model 2, HDL2-C was also inversely associated with CHD (p = 0.01).
Meta-analysis
Given similar Cox regression coefficients and HRs between JHS and FOCS for HDL-C, HDL2-C and HDL3-C (Q-statistic p ≥ 0.25), a meta-analysis was performed. Spline curves are shown in the online supplementary material. As seen in the forest plot (Figure 2), the inverse association of HDL-C with CHD risk was significant (Model 1p = 0.03; Model 4p = 0.03) and predominantly due to the HDL3-C subclass (Model 1p = 0.007; Model 4p = 0.01). The association between HDL2-C and CHD risk was not significant in either model (p ≥ 0.20).
Figure 2.
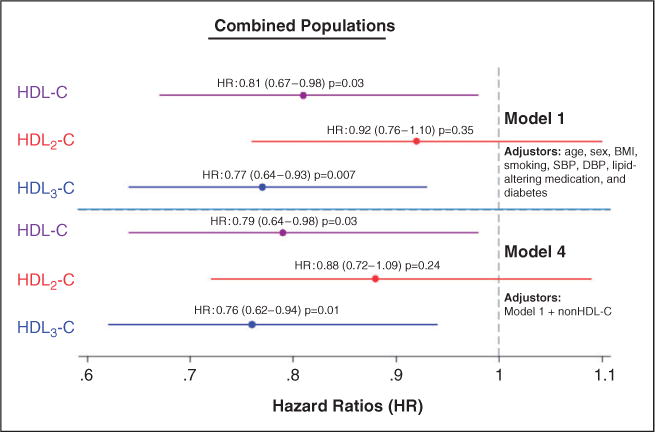
Hazard ratios (HR) of HDL-C, HDL2-C and HDL3-C for coronary heart disease events in meta-analysis of the Jackson Heart and Framingham Offspring Cohort studies. HRs are point estimates (95% confidence intervals) per SD increase in cholesterol. HR: hazard ratio; HDL-C: high-density lipoprotein cholesterol; SBP: systolic blood pressure; DBP: diastolic blood pressure
Sensitivity analyses
Notably, there was no significant interaction across gender in any of the models. Furthermore, there was no difference in the associations of HDL subclasses with risk when considering only hard CHD events (MI and cardiac death).
Discussion
We found the denser HDL3-C subclass to be the major determinant of the inverse association between HDL-C and incident CHD in a diverse primary prevention population. In the largest cohort study of African Americans to our knowledge, HDL2-C is not associated with CHD. Furthermore, these HDL-C subclass associations with CHD were independent of the major structural HDL lipoprotein, apoA1, in the JHS.
Prospective studies examining HDL-C subclasses
Previous prospective studies have generated conflicting results regarding the degree to which HDL2-C and HDL3-C contribute to the inverse association of HDL-C with CHD events. Initial studies in the 1960s suggested that incident CHD was inversely associated with both HDL2-C and HDL3-C in unadjusted analyses.1 In the Physician’s Health Study and Caerphilly and Speedwell Collaborative Heart Disease studies there were inverse associations of both HDL2-C and HDL3-C with CHD, with a suggestion of stronger HR for HDL3-C.24,25
In contrast, other prospective studies have suggested only HDL2-C as the main inverse predictor of CHD risk. In the Kuopio Ischemic Heart Disease Risk Factor Study, HDL-C and HDL2-C levels were inversely associated with risk of MI while HDL3-C did not maintain a significant association when adjusted for HDL2-C.22 Similarly, in the prospective Quebec Cardiovascular Study, HDL2-C remained statistically significant in the multivariable model while HDL3-C did not.23
The present study populations differ from previous prospective analyses of HDL2-C and HDL3-C in two important ways: the entirely African American demographic of the JHS cohort and the inclusion of women, both groups that have been significantly underrepresented in lipid research.31 This increase in diversity allowed for examination of a wide range of HDL-C, HDL2-C, and HDL3-C values. Additionally, the VAP method of ultracentrifugation was unique to the present study, providing greater consistency for comparison of results between the two study cohorts.
Given these differences from prior studies, our findings show that, amongst men and women of African American ethnicity, HDL3-C is the main inverse predictor of HDL-associated risk. Interestingly, contrary to findings in Caucasian populations, there is no association of HDL2-C levels with CHD in African Americans from the JHS. Our findings from the FOCS complement those from previous studies of Caucasian populations in that both HDL2-C and HDL3-C are inversely associated with risk, with stronger associations with HDL3-C, though the association is attenuated when adjusting for non-HDL-C. We found similar results in the LIC secondary prevention studies, also based on the VAP method.32
The major difference between the JHS and FOCS populations was the mild association of HDL2-C with decreased cardiovascular risk in only the FOCS sample, consistent with prior findings in Caucasian populations. The differences between the HDL2-C findings in JHS and FOCS may be due to known genetic polymorphisms in hepatic lipase activity, which specifically affect HDL2 and not HDL3 in African Americans.33,34 Also, the mean age of the JHS population was significantly younger than that of the FOCS, possibly leading to an underestimation of the impact of HDL-C and its subfractions on event rates. Nevertheless, the consistent finding across both cohorts is that HDL3-C is the chief determinant of the inverse HDL-C association with CHD with similar effect-sizes in both cohorts.
Atheroprotective biological considerations of HDL
While evidence of anti-inflammatory, anti-oxidant, anti-apoptotic, anti-thrombotic, and endothelial function-enhancing properties is growing, the role of HDL in reverse cholesterol transport remains a major focus of interest. HDL refers to a heterogeneous group of particles with fluctuating lipid and proteomic cargo. The biological complexities of the reverse cholesterol transport system suggest that a simple “snapshot” of cholesterol content within HDL species at one time point may be an inadequate surrogate for gauging reverse cholesterol transport efficiency.19 Nonetheless, our results suggest that higher cholesterol levels within the denser HDL3 particles may represent a more proficient reverse cholesterol transport system. Elevated HDL2-C may represent a saturated system that is not being efficiently offloaded, and is limited in further uptake of peripheral cholesterol.
Alternatively, proteomic analyses show distinct proteins affiliated with each HDL subclass, suggesting a mechanistic basis for the variety of putative atheroprotective functions attributed to HDL beyond reverse cholesterol transport.17,18,20,21 Our results are supported by recent data suggesting the potential for more atheroprotective HDL species within the broad HDL3 subclass.35 Further investigation into these complexities of HDL is necessary.
Effects of HDL therapies on subclasses
The epidemiological associations of HDL-C with CHD make it an attractive target for therapies to improve cardiovascular outcomes beyond the standard approach of apoB reduction with statin therapy. However, recent secondary prevention trials of niacin and cholesterol-ester transport protein (CETP) inhibitors added to statin therapy have been disappointing.9–12
Our findings emphasize the importance of HDL3-C levels and may provide insight into the negative results of these trials. Niacin and CETP inhibitors are effective therapies for raising total HDL-C; however, both therapies raise HDL2-C more so than HDL3-C.36,37 This suggests a saturation of HDL particles via alternative mechanisms, without necessarily increasing reverse cholesterol transport throughput. Alternatively, fibrates have been shown to raise HDL3-C more so than HDL2-C, though it remains uncertain whether fibrates reduce CHD risk in those on statin therapy.38–40 As trials of HDL-C altering therapies continue, HDL subclass analysis may provide further insights into the results.
Limitations
Our study has several limitations. First, the JHS is limited to one ethnic group in one geographic location, though this allowed for the largest analysis of HDL subclasses in African Americans, to our knowledge. Furthermore, the results were verified using the same measurement methodology in the predominantly Caucasian population from the FOCS.
Additionally, there are a variety of HDL subclassification methods with each providing differing resultant profiles with possible overlap across HDL subclasses. While we employed a validated method, whether the methodology used in prior studies provided comparable separation of HDL species is unclear. For example, a recent Women’s Health Study analysis found no association of smaller HDL particles with incident CHD.41 This apparent contrast with our findings highlights the possible heterogeneity within HDL measurement techniques.
Additionally, cross-sectional laboratory measurements may not reflect the accumulated lifetime exposure to risk factors or the efficiency of reverse cholesterol transport. Finally, the associations between HDL-C subclasses and functionality are unclear.
Conclusion
In summary, we found that HDL3-C levels were primarily responsible for the inverse association between HDL-C and incident CHD. Particularly noteworthy is that this finding was based on the first examination of this association in a large cohort of African American men and women and was consistent in a subset of predominantly Caucasian men and women from the FOCS. Further insight into potential biological mechanisms for the protective role of HDL may be gained by focusing on the HDL3 density subclass. Similar HDL-C subclassification could be useful in evaluations of results from recent and future trials of therapies directed at HDL-C.
Supplementary Material
Acknowledgments
The Jackson Heart Study is registered with ClinicalTrials.gov under the identifier: NCT00415415. The Framingham Offspring Cohort Study is registered under the National Heart, Lung, and Blood Institute sponsored website: http://www.framinghamheartstudy.org. Analyses were presented at AHA 2012 (oral) and ACC 2013 (poster) sessions. PHJ and PPT are co-first authors.
Funding
This work was supported by the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities (JHS; contracts HHSN2682 01300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C), with additional support from the National Institute on Biomedical Imaging and Bioengineering; the NIH/NHLBI (Framingham Heart Study; contract no. N01-HC-25195-06) in collaboration with Boston University; the NIH (training grants T32HL007227, T32HL07024 to PHJ and SSM); the Pollin Cardiovascular Prevention Fellowship; the Marie-Josee and Henry R Kravis endowed fellowship (to SSM).
Footnotes
Conflict of interest
None for PHJ, STL, MEG, MJB, AAK, AC. PPT: consultant and speaker’s bureau for Amgen, AstraZeneca, Kowa and Merck; consultant for Atherotech, Boehringer-Ingelheim, GSK, Liposcience; speaker’s bureau for Amarin, Genzyme. SSM and SRJ: co-inventors on a pending patent filed by Johns Hopkins University for a method of low-density lipoprotein cholesterol estimation. SRJ: medical advisory board for Atherotech. KRK: Atherotech Research Director; receives royalty from the University of Alabama at Birmingham. JMM and RBD were funded by Atherotech Research for this work.
References
- 1.Gofman JW, Young W, Tandy R. Ischemic heart disease, atherosclerosis, and longevity. Circulation. 1966;34:679–697. doi: 10.1161/01.cir.34.4.679. [DOI] [PubMed] [Google Scholar]
- 2.Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Hu G, Cui Y, Jousilahti P, et al. Joint effect of high-density lipoprotein cholesterol and low-density lipoprotein cholesterol on the risk of coronary heart disease. Eur J Prev Cardiol. 2013;20:89–97. doi: 10.1177/1741826711428242. [DOI] [PubMed] [Google Scholar]
- 4.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballantyne CM, Herd JA, Ferlic LL, et al. Influence of low HDL on progression of coronary artery disease and response to fluvastatin therapy. Circulation. 1999;99:736–743. doi: 10.1161/01.cir.99.6.736. [DOI] [PubMed] [Google Scholar]
- 6.Cui Y, Watson DJ, Girman CJ, et al. Effects of increasing high-density lipoprotein cholesterol and decreasing low-density lipoprotein cholesterol on the incidence of first acute coronary events (from the Air Force/Texas Coronary Atherosclerosis Prevention Study) Am J Cardiol. 2009;104:829–834. doi: 10.1016/j.amjcard.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg I, Goldbourt U, Boyko V, et al. Relation between on-treatment increments in serum high-density lipoprotein cholesterol levels and cardiac mortality in patients with coronary heart disease (from the Bezafibrate Infarction Prevention trial) Am J Cardiol. 2006;97:466–471. doi: 10.1016/j.amjcard.2005.09.078. [DOI] [PubMed] [Google Scholar]
- 8.Nicholls SJ, Tuzcu EM, Sipahi I, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA. 2007;297:499–508. doi: 10.1001/jama.297.5.499. [DOI] [PubMed] [Google Scholar]
- 9.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 10.Group HTC. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: Trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–1291. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Investigators A-H, Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 13.Heinecke J. HDL and cardiovascular-disease risk – time for a new approach? N Engl J Med. 2011;364:170–171. doi: 10.1056/NEJMe1012520. [DOI] [PubMed] [Google Scholar]
- 14.Shah PK. Jekyll and Hyde of HDL: A lipoprotein with a split personality. Eur Heart J. 2013;34:3531–3534. doi: 10.1093/eurheartj/eht382. [DOI] [PubMed] [Google Scholar]
- 15.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Movva R, Rader DJ. Laboratory assessment of HDL heterogeneity and function. Clin Chem. 2008;54:788–800. doi: 10.1373/clinchem.2007.101923. [DOI] [PubMed] [Google Scholar]
- 17.Vickers KC, Remaley AT. HDL and cholesterol: Life after the divorce? J Lipid Res. 2014;55:4–12. doi: 10.1194/jlr.R035964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaisar T, Pennathur S, Green PS, et al. Shotgun prote-omics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenson RS, Brewer HB, Jr, Davidson WS, et al. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinecke JW. The protein cargo of HDL: Implications for vascular wall biology and therapeutics. J Clin Lipidol. 2010;4:371–375. doi: 10.1016/j.jacl.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidson WS, Silva RA, Chantepie S, et al. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: Relevance to antioxidative function. Arterioscler Thromb Vasc Biol. 2009;29:870–876. doi: 10.1161/ATVBAHA.109.186031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salonen JT, Salonen R, Seppanen K, et al. HDL, HDL2, and HDL3 subfractions, and the risk of acute myocardial infarction. A prospective population study in eastern Finnish men. Circulation. 1991;84:129–139. doi: 10.1161/01.cir.84.1.129. [DOI] [PubMed] [Google Scholar]
- 23.Lamarche B, Moorjani S, Cantin B, et al. Associations of HDL2 and HDL3 subfractions with ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Arterioscler Thromb Vasc Biol. 1997;17:1098–1105. doi: 10.1161/01.atv.17.6.1098. [DOI] [PubMed] [Google Scholar]
- 24.Stampfer MJ, Sacks FM, Salvini S, et al. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med. 1991;325:373–381. doi: 10.1056/NEJM199108083250601. [DOI] [PubMed] [Google Scholar]
- 25.Sweetnam PM, Bolton CH, Yarnell JW, et al. Associations of the HDL2 and HDL3 cholesterol sub-fractions with the development of ischemic heart disease in British men. The Caerphilly and Speedwell Collaborative Heart Disease Studies. Circulation. 1994;90:769–774. doi: 10.1161/01.cir.90.2.769. [DOI] [PubMed] [Google Scholar]
- 26.Superko HR, Pendyala L, Williams PT, et al. High-density lipoprotein subclasses and their relationship to cardiovascular disease. J Clin Lipidol. 2012;6:496–523. doi: 10.1016/j.jacl.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Taylor HA, Jr, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: Design and methods of the Jackson Heart Study. Ethn Dis. 2005;15:S6–4–17. [PubMed] [Google Scholar]
- 28.Feinleib M, Kannel WB, Garrison RJ, et al. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 29.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni KR, Marcovina SM, Krauss RM, et al. Quantification of HDL2 and HDL3 cholesterol by the Vertical Auto Profile-II (VAP-II) methodology. J Lipid Res. 1997;38:2353–2364. [PubMed] [Google Scholar]
- 31.Peer N, Steyn K, Lombard C, et al. Alarming rise in prevalence of atherogenic dyslipidaemia in the black population of Cape Town: The Cardiovascular Risk in Black South Africans (CRIBSA) study. Eur J Prev Cardiol. 2013 Jul 23; doi: 10.1177/2047487313497865. [DOI] [PubMed] [Google Scholar]
- 32.Martin SS, Khokhar AA, May HT, et al. HDL cholesterol subclasses, myocardial infarction, and mortality in secondary prevention: The lipoprotein investigators collaborative. Eur Heart J. 2014 Jun 30; doi: 10.1093/eurheartj/ehu264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vega GL, Clark LT, Tang A, et al. Hepatic lipase activity is lower in African American men than in white American men: Effects of 5′ flanking polymorphism in the hepatic lipase gene (LIPC) J Lipid Res. 1998;39:228–232. [PubMed] [Google Scholar]
- 34.Juo SH, Han Z, Smith JD, et al. Promoter polymorphisms of hepatic lipase gene influence HDL(2) but not HDL(3) in African American men: CARDIA study. J Lipid Res. 2001;42:258–264. [PubMed] [Google Scholar]
- 35.Camont L, Lhomme M, Rached F, et al. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arterioscler Thromb Vasc Biol. 2013;33:2715–2723. doi: 10.1161/ATVBAHA.113.301468. [DOI] [PubMed] [Google Scholar]
- 36.Shepherd J, Packard CJ, Patsch JR, et al. Effects of nicotinic acid therapy on plasma high density lipoprotein subfraction distribution and composition and on apoli-poprotein A metabolism. J Clin Invest. 1979;63:858–867. doi: 10.1172/JCI109385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brousseau ME, Schaefer EJ, Wolfe ML, et al. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N Engl J Med. 2004;350:1505–1515. doi: 10.1056/NEJMoa031766. [DOI] [PubMed] [Google Scholar]
- 38.Sorisky A, Ooi TC, Simo IE, et al. Change in composition of high density lipoprotein during gemfibrozil therapy. Atherosclerosis. 1987;67:181–189. doi: 10.1016/0021-9150(87)90278-4. [DOI] [PubMed] [Google Scholar]
- 39.Chapman MJ, Ginsberg HN, Amarenco P, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: Evidence and guidance for management. Eur Heart J. 2011;32:1345–1361. doi: 10.1093/eurheartj/ehr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Group AS, Ginsberg HN, Elam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akinkuolie AO, Paynter NP, Padmanabhan L, et al. High-density lipoprotein particle subclass heterogeneity and incident coronary heart disease. Circ Cardiovasc Qual Outcomes. 2014;7:55–63. doi: 10.1161/CIRCOUTCOMES.113.000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


